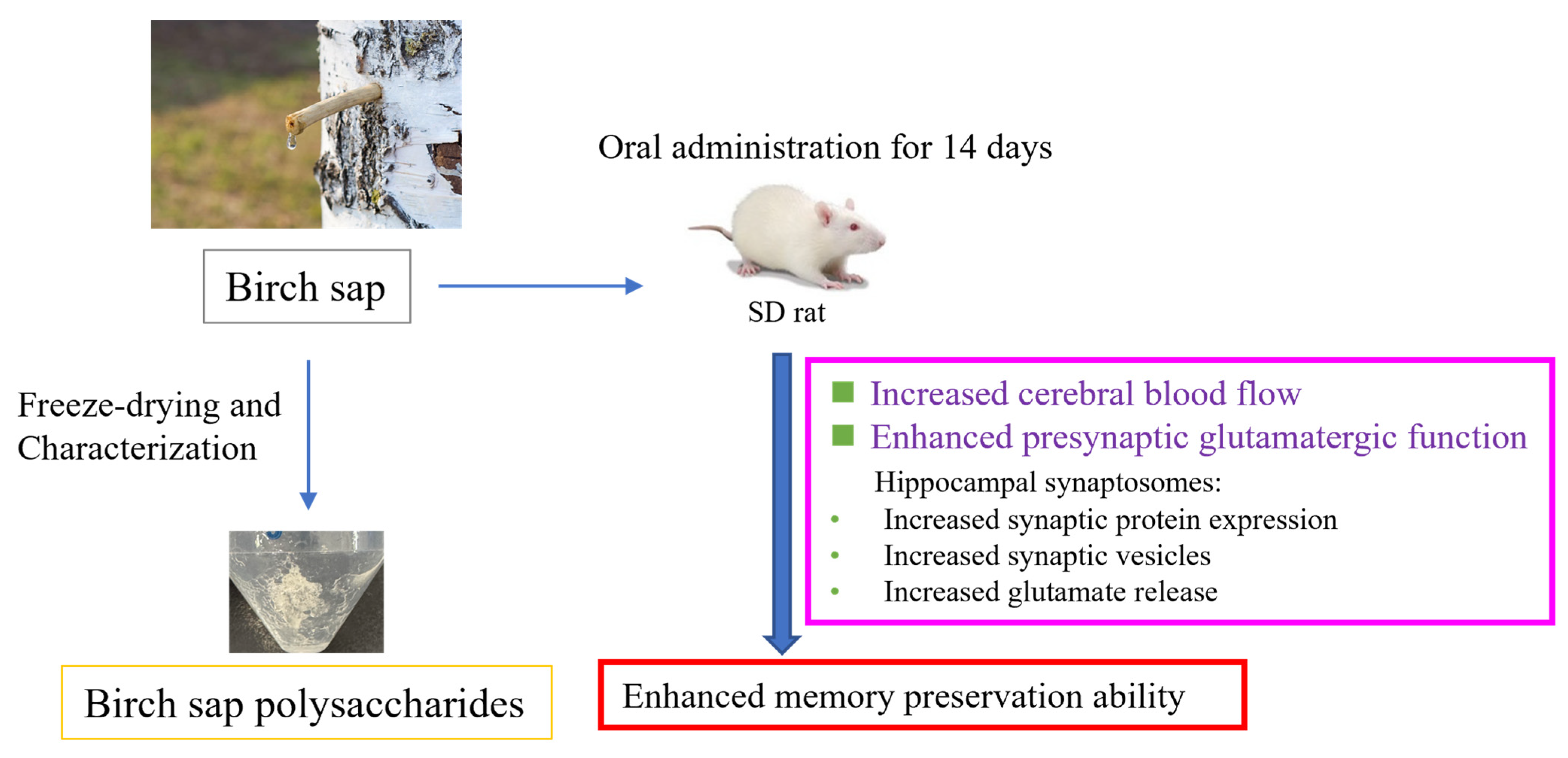
Birch Sap Preserves Memory Function in Rats by Enhancing Cerebral Blood Flow and Modulating the Presynaptic Glutamatergic System in the Hippocampus
Abstract
1. Introduction
2. Results
2.1. Characterization of Oligosaccharides from Birch Sap
2.2. Behavioral Evaluation of Spatial Memory in Rats
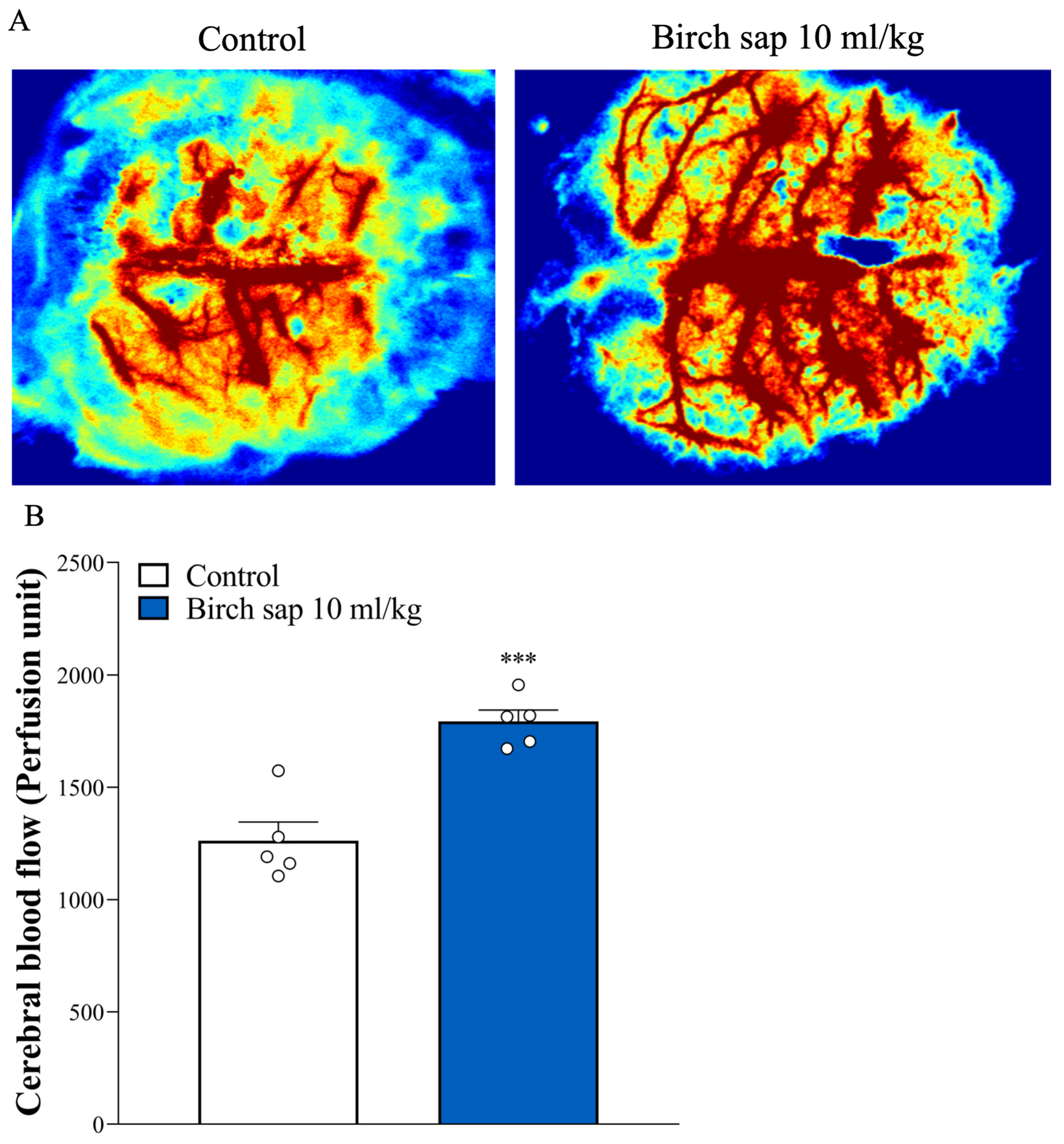
2.3. Evaluation of Cerebral Blood Flow in Rats
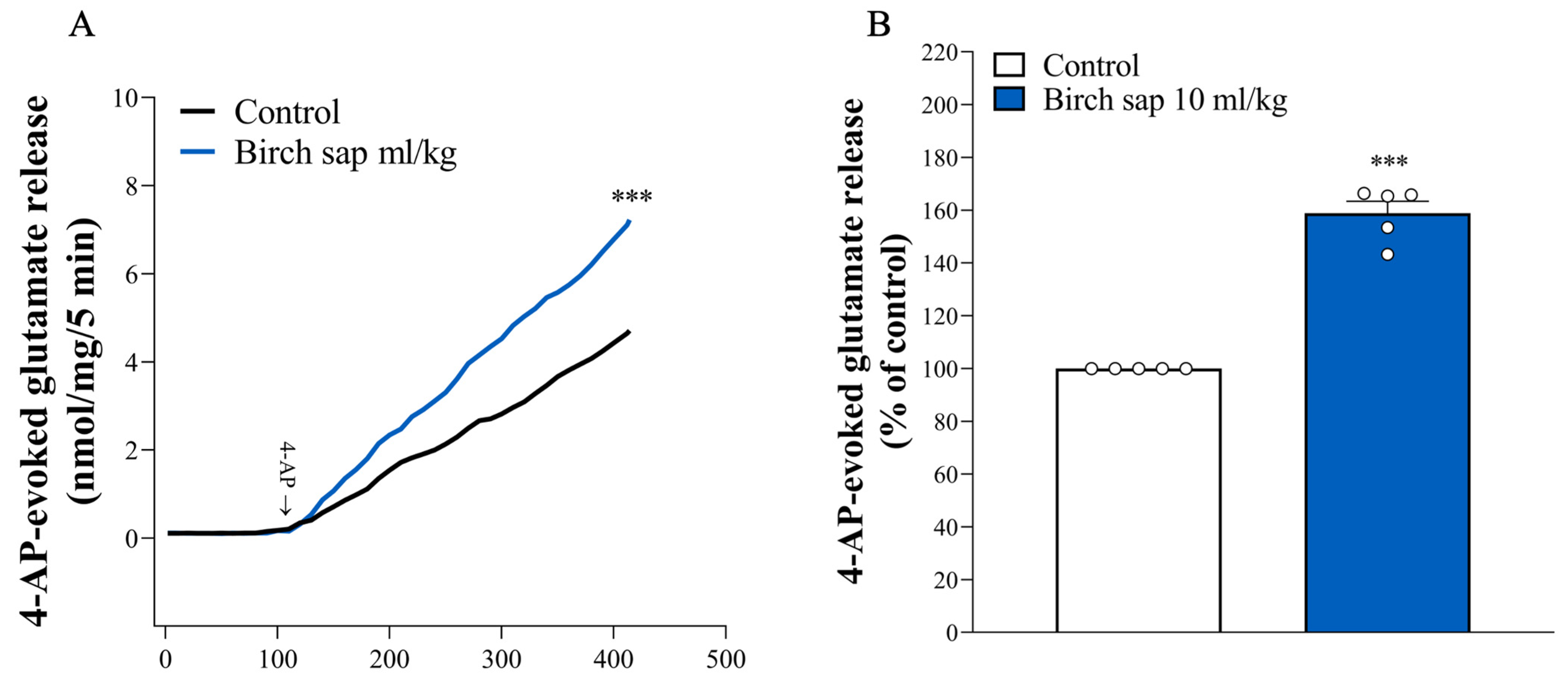
2.4. Assessment of Glutamate Release in Hippocampal Synaptosomes
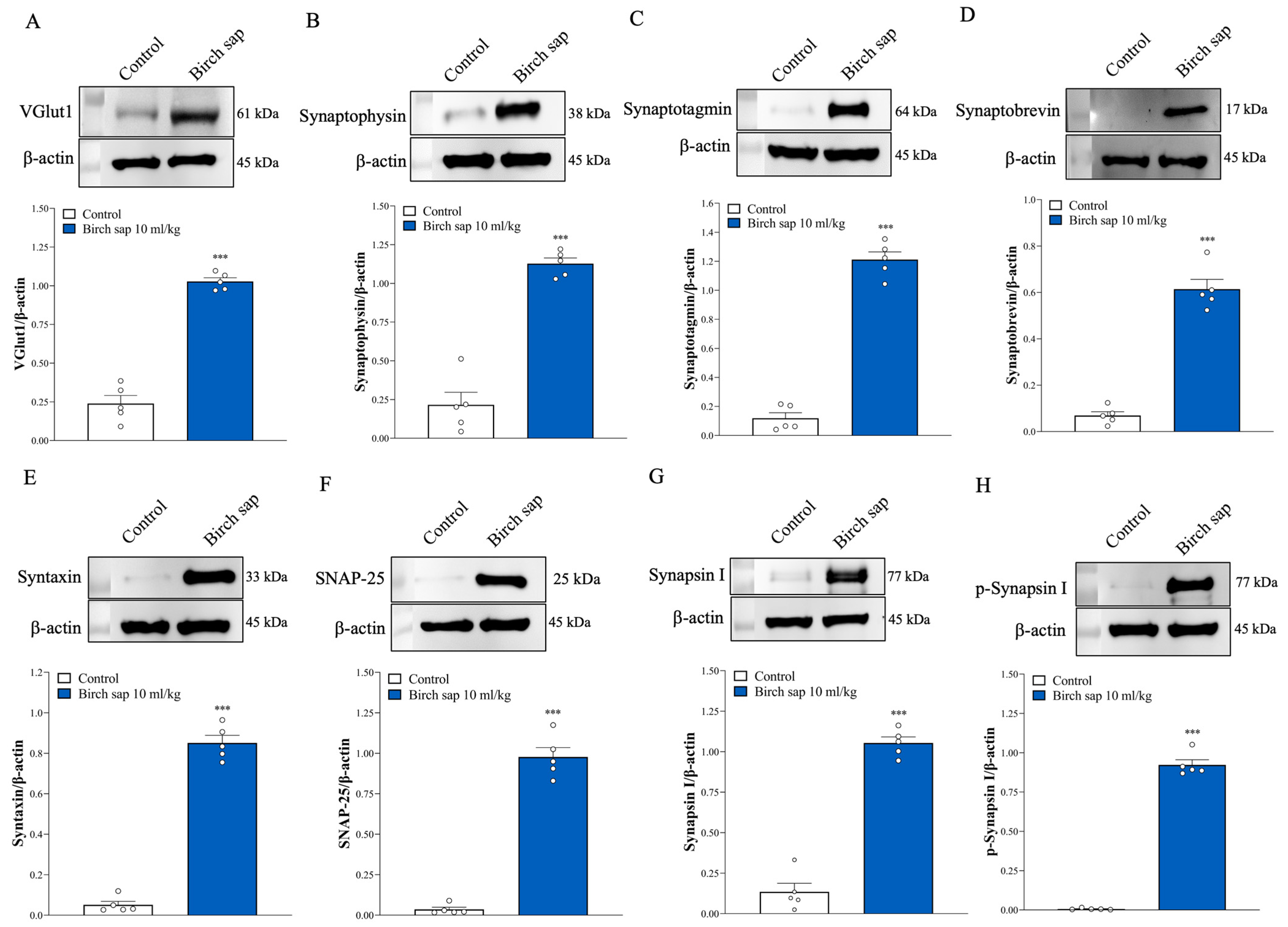
2.5. Assessment of Synaptic Protein Expression in Hippocampal Synaptosomes
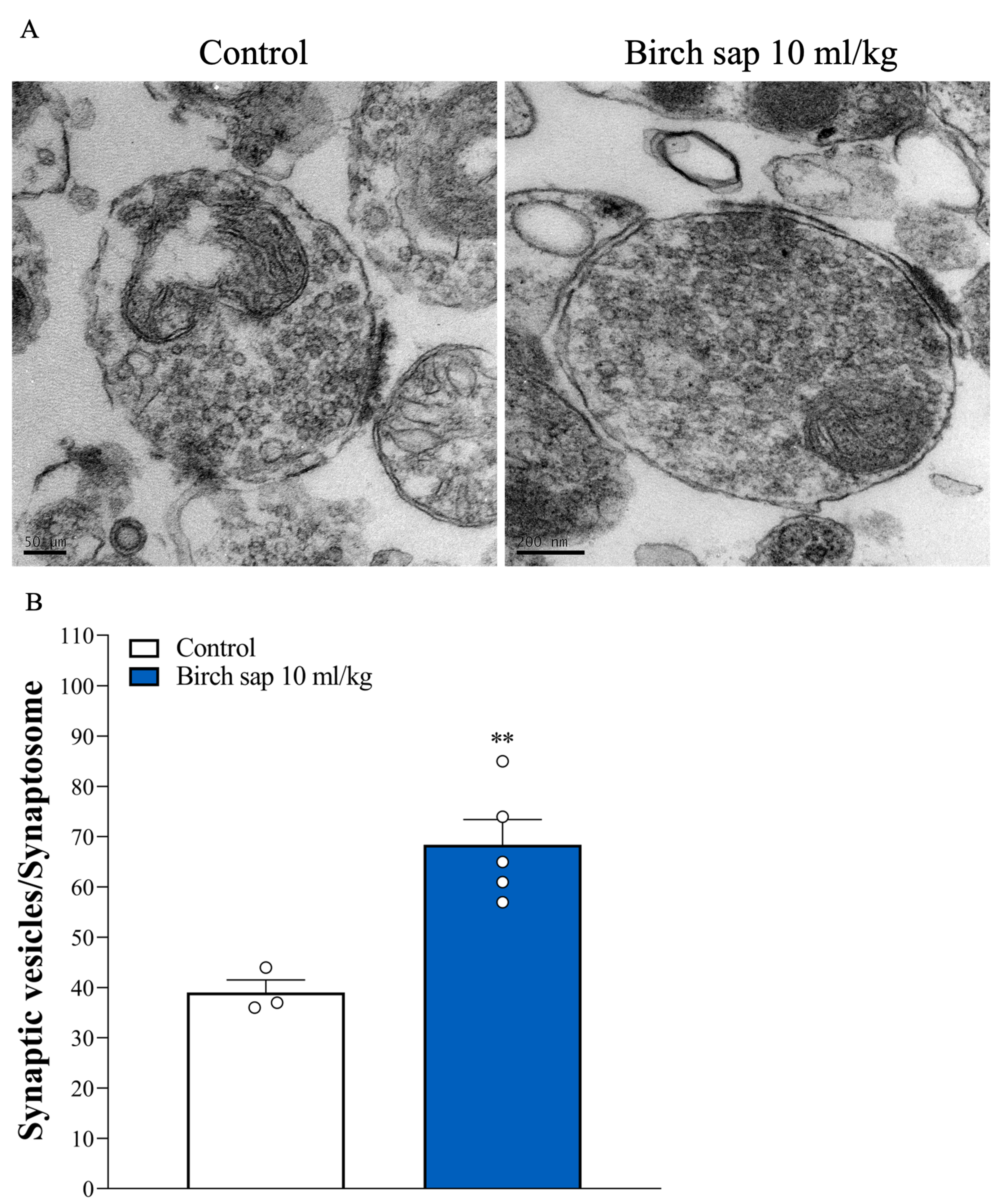
2.6. Assessment of Synaptic Vesicle Numbers in Hippocampal Synaptosomes
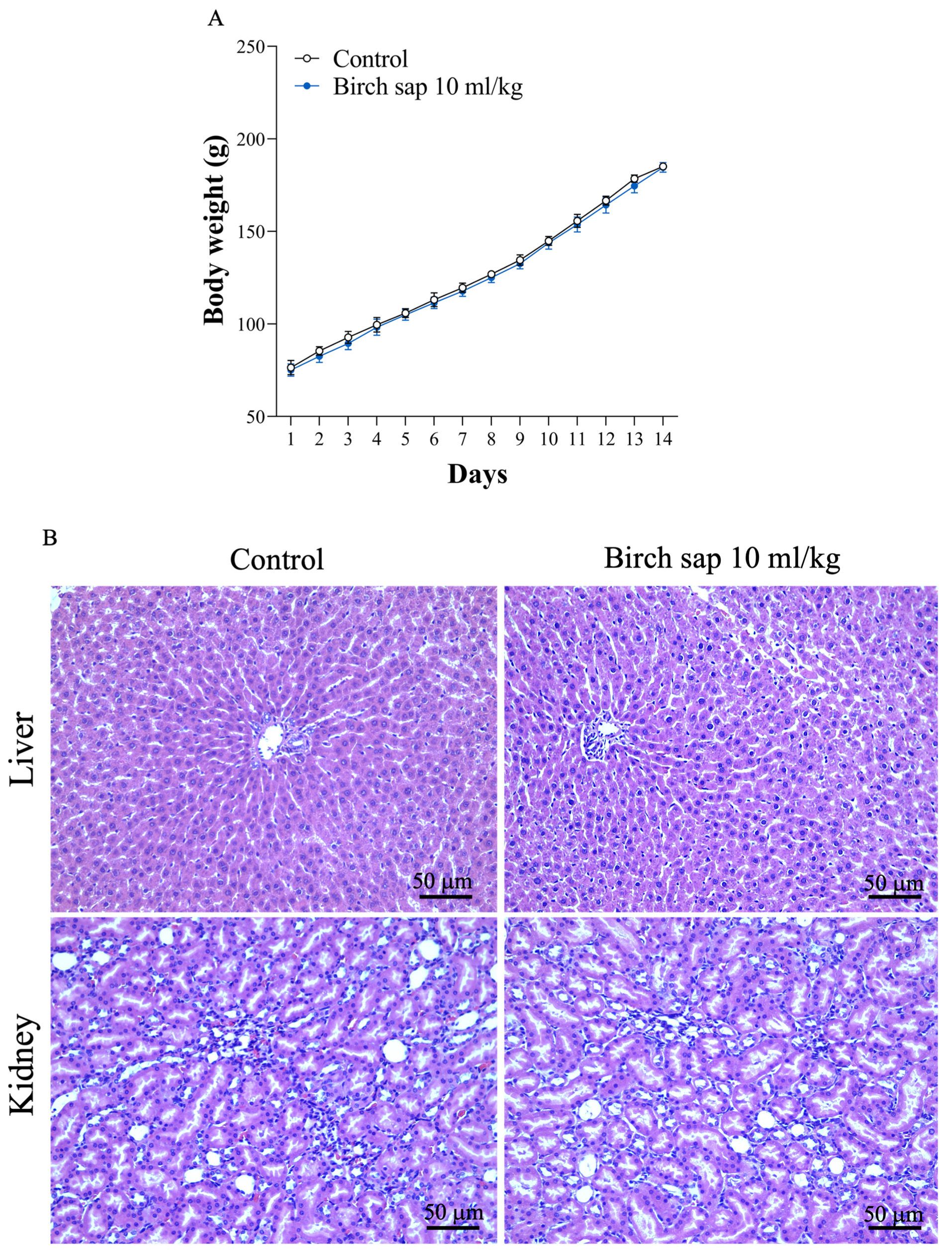
2.7. Evaluation of Birch Sap Effect on Body Weight, Liver, and Kidney Morphology in Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Analysis of Birch Sap Polysaccharides
4.2.1. The Freeze-Dried Procedure for the Liquid Polysaccharide
4.2.2. Molecular Weight Analysis
4.2.3. Monosaccharide Analysis
4.3. Animals
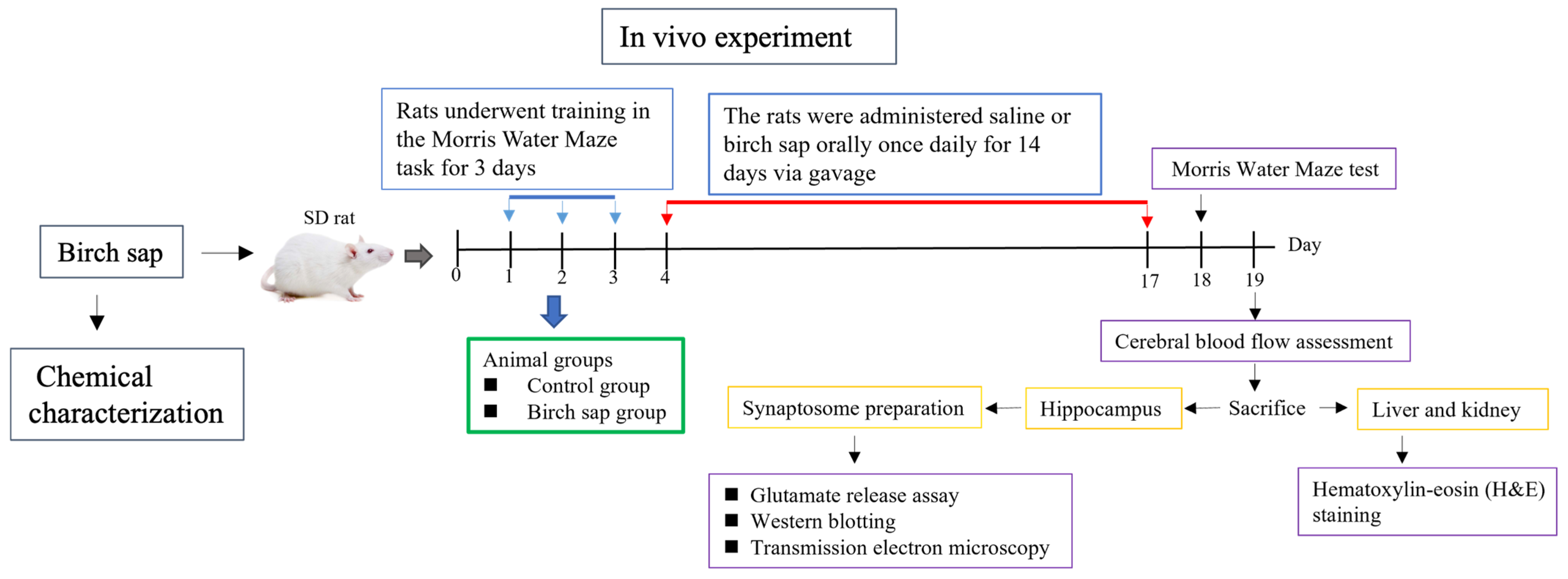
4.4. Experimental Protocol
4.5. MWM Test
4.6. Cerebral Blood Flow Assessment
4.7. H&E Staining
4.8. Preparation of Synaptosomes
4.9. Glutamate Release Assay
4.10. TEM
4.11. Western Blotting Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L., Jr.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M. Understanding brain diseases: From receptor dysregulation to neurodegeneration, neuroinflammation and memory impairment. Curr. Neuropharmacol. 2023, 21, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Reis, J.; Teixeira, J.; Borges, F. Alzheimer's disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res. Rev. 2014, 15, 116–145. [Google Scholar] [CrossRef]
- Lorca, C.; Mulet, M.; Arévalo-Caro, C.; Sanchez, M.; Perez, A.; Perrino, M.; Bach-Faig, A.; Aguilar-Martínez, A.; Vilella, E.; Gallart-Palau, X.; et al. Plant-derived nootropics and human cognition: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5521–5545. [Google Scholar] [CrossRef]
- Piva, A.; Benvegnù, G.; Negri, S.; Commisso, M.; Ceccato, S.; Avesani, L.; Guzzo, F.; Chiamulera, C. Whole plant extracts for neurocognitive disorders: A narrative review of neuropsychological and preclinical studies. Nutrients 2024, 16, 3156. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, B.Y.; Kim, Y.J.; Kim, J.H.; Jeong, S.J. Bauhinia coccinea extract prevents memory loss induced by scopolamine through activation of antiapoptotic and antioxidant pathways in mice. Sci. Rep. 2025, 15, 4037. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, K.; Yang, J.; Zhang, Y.; You, P.; Yan, L.; Weng, Y. Effects of ginseng on cognitive function: A systematic review and meta-analysis. Phytother. Res. 2024, 38, 6023–6034. [Google Scholar] [CrossRef]
- Svanberg, I.; Sõukand, R.; Łuczaj, Ł.; Kalle, R.; Zyryanova, O.; Dénes, A.; Papp, N.; Nedelcheva, A.; Šeškauskaitė, D.; Kołodziejska, I.; et al. Uses of tree saps in northern and eastern parts of Europe. Acta Soc. Bot. Pol. 2012, 81, 343–357. [Google Scholar] [CrossRef]
- Keita, D.; Léger, G.; Bordenave, N. Rheological and water binding properties of xanthan, guar and ultra-finely milled oatmeal in white birch sap: Influence of sap minor constituents. Food Res. Int. 2021, 147, 110478. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- McEntee, W.J.; Crook, T.H. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Balschun, D.; Moechars, D.; Callaerts-Vegh, Z.; Vermaercke, B.; Van Acker, N.; Andries, L.; D'Hooge, R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb. Cortex 2010, 20, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.L.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol. Aging 2011, 32, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Pecsok, M.K.; Robinson, H.; Atkins, A.; Calkins, M.E.; Elliott, M.A.; Mordy, A.; Stifelman, J.; Gur, R.C.; Moberg, P.J.; Nanga, R.P.R.; et al. Mapping hippocampal glutamate in healthy aging with in vivo glutamate-weighted CEST (GluCEST) imaging. Front. Aging Neurosci. 2024, 16, 1535158. [Google Scholar] [CrossRef]
- Roalf, D.R.; Sydnor, V.J.; Woods, M.; Wolk, D.A.; Scott, J.C.; Reddy, R.; Moberg, P.J. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol. Aging 2020, 95, 240–249. [Google Scholar] [CrossRef]
- Robbins, T.W.; Murphy, E.R. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol. Sci. 2006, 27, 141–148. [Google Scholar] [CrossRef]
- Horovitz, D.J.; Askins, L.A.; Regnier, G.M.; McQuail, J.A. Age-related synaptic signatures of brain and cognitive reserve in the rat hippocampus and parahippocampal regions. Neurobiol. Aging 2025, 148, 80–97. [Google Scholar] [CrossRef]
- Burgess, N.; Maguire, E.A.; O'Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef]
- Nobis, L.; Manohar, S.G.; Smith, S.M.; Alfaro-Almagro, F.; Jenkinson, M.; Mackay, C.E.; Husain, M. Hippocampal volume across age: Nomograms derived from over 19,700 people in UK Biobank. Neuroimage Clin. 2019, 23, 101904. [Google Scholar] [CrossRef]
- Wolters, F.J.; Zonneveld, H.I.; Hofman, A.; van der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Cerebral perfusion and the risk of dementia: A population-based study. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef]
- Mokhber, N.; Shariatzadeh, A.; Avan, A.; Saber, H.; Babaei, G.S.; Chaimowitz, G.; Azarpazhooh, M.R. Cerebral blood flow changes during aging process and in cognitive disorders: A review. Neuroradiol. J. 2021, 34, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Lin, T.Y.; Pan, T.L.; Wang, P.W.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Asiatic acid prevents cognitive deficits by inhibiting calpain activation and preserving synaptic and mitochondrial function in rats with kainic acid-induced seizure. Biomedicines 2021, 9, 284. [Google Scholar] [CrossRef]
- Hsu, S.K.; Lu, C.W.; Chiu, K.M.; Lee, M.Y.; Lin, T.Y.; Wang, S.J. Mangiferin depresses vesicular glutamate release in synaptosomes from the rat cerebral cortex by decreasing synapsin I phosphorylation. Eur. J. Pharmacol. 2023, 950, 175772. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, D.; Zhao, W.; Wang, P.; Dai, Y.; Dong, M.; Wang, J.; Zhao, Y.; Zhao, X. Structural and functional characterization of exopolysaccharide from Leuconostoc citreum BH10 discovered in birch sap. Carbohydr. Res. 2024, 535, 108994. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Wang, W.; Liu, Y.; Meng, Y.; Wang, Y.; Fan, M.; Cai, C. Ganoderma lucidum polysaccharide alleviates cognitive dysfunction by inhibiting neuroinflammation via NLRP3/NF-κB signaling pathway. J. Ethnopharmacol. 2025, 338, 119065. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, F.; Mo, W.; Li, H.; Zhu, D.; He, Z.; Ma, X. A new plant active polysaccharide from nicotiana improves the lead-led impairment of spatial memory in mice by modulating the gut microbiota and IL-6. Foods 2024, 13, 3177. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, S.Y.; Chen, Y.Y.; Chang, H.Y.; Chiang, I.C.; Yen, G.C. Polysaccharides extracted from common buckwheat (Fagopyrum esculentum) attenuate cognitive impairment via suppressing RAGE/p38/NF-κB signaling and dysbiosis in AlCl3-treated rats. Int. J. Biol. Macromol. 2024, 276, 133898. [Google Scholar] [CrossRef]
- He, C.; Jiang, J.; Liu, J.; Zhou, L.; Ge, Y.; Yang, Z. Pseudostellaria heterophylla polysaccharide mitigates Alzheimer's-like pathology via regulating the microbiota-gut-brain axis in 5 × FAD mice. Int. J. Biol. Macromol. 2024, 270, 132372. [Google Scholar] [CrossRef]
- Cox, M.F.; Hascup, E.R.; Bartke, A.; Hascup, K.N. Friend or foe? Defining the role of glutamate in aging and Alzheimer's disease. Front. Aging 2022, 3, 929474. [Google Scholar] [CrossRef]
- Hascup, K.N.; Hascup, E.R. Altered neurotransmission prior to cognitive decline in AβPP/PS1 mice, a model of Alzheimer's disease. J. Alzheimers Dis. 2015, 44, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.R.; Kang, J.; Hueske, E.V.; Leung, T.; Varoqui, H.; Murnick, J.G.; Erickson, J.D.; Liu, G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J. Neurosci. 2005, 25, 6221–6234. [Google Scholar] [CrossRef] [PubMed]
- Tordera, R.M.; Totterdell, S.; Wojcik, S.M.; Brose, N.; Elizalde, N.; Lasheras, B.; Del Rio, J. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1). Eur. J. Neurosci. 2007, 25, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Greengard, P.; Valtorta, F.; Czernik, A.J.; Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 1993, 259, 780–785. [Google Scholar] [CrossRef]
- Nicholls, D.G. Presynaptic modulation of glutamate release. Prog. Brain Res. 1998, 116, 15–22. [Google Scholar] [CrossRef]
- Lynch, M.A.; Voss, K.L.; Rodriguez, J.; Bliss, T.V. Increase in synaptic vesicle proteins accompanies long-term potentiation in the dentate gyrus. Neuroscience 1994, 60, 1–5. [Google Scholar] [CrossRef]
- Mullany, P.; Lynch, M.A. Changes in protein synthesis and synthesis of the synaptic vesicle protein, synaptophysin, in entorhinal cortex following induction of long-term potentiation in dentate gyrus: An age-related study in the rat. Neuropharmacology 1997, 36, 973–980. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Yan, H.; Farley, J.A.; Sonntag, W.E.; Freeman, W.M. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J. Neurochem. 2010, 113, 1577–1588. [Google Scholar] [CrossRef]
- Honer, W.G.; Barr, A.M.; Sawada, K.; Thornton, A.E.; Morris, M.C.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl. Psychiatry 2012, 2, e114. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Jones, A.A.; Petyuk, V.A.; Barakauskas, V.E.; Barr, A.M.; Leurgans, S.E.; De Jager, P.L.; Casaletto, K.B.; Schneider, J.A.; Bennett, D.A.; et al. Proteomic identification of select protein variants of the SNARE interactome associated with cognitive reserve in a large community sample. Acta Neuropathol. 2021, 141, 755–770. [Google Scholar] [CrossRef]
- Cropley, V.L.; Fujita, M.; Innis, R.B.; Nathan, P.J. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol. Psychiatry 2006, 59, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Khatik, G.L.; Datusalia, A.K. Strategies for treatment of disease-associated dementia beyond Alzheimer's disease: An update. Curr. Neuropharmacol. 2023, 21, 309–339. [Google Scholar] [CrossRef]
- Vinjavarapu, L.A.; Yadava, S.; Dontiboina, H.R.; Chakravarthi, G.; Kakarla, R. Neuroprotective effects of Indole 3-carbinol against Scopolamine-Induced cognitive and memory impairment in rats: Modulation of oxidative stress, inflammatory and cholinergic pathways. Metab. Brain Dis. 2025, 40, 154. [Google Scholar] [CrossRef]
- Yeh, K.C.; Hung, C.F.; Lee, H.L.; Hsieh, T.Y.; Wang, S.J. Soybean meal extract preserves memory ability by increasing presynaptic function and modulating gut microbiota in rats. Mol. Neurobiol. 2022, 59, 1649–1664. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Gypenoside XVII reduces synaptic glutamate release and protects against excitotoxic injury in rats. Biomolecules 2024, 14, 589. [Google Scholar] [CrossRef]
- Hung, C.F.; Chiu, W.C.; Chen, J.C.; Chuang, W.C.; Wang, S.J. NRICM101 prevents kainic acid-induced seizures in rats by modulating neuroinflammation and the glutamatergic system. Int. Immunopharmacol. 2024, 140, 112842. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Chang, Y.Y.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Albiflorin decreases glutamate release from rat cerebral cortex nerve terminals (synaptosomes) through depressing P/Q-type calcium channels and protein kinase A activity. Int. J. Mol. Sci. 2024, 25, 8846. [Google Scholar] [CrossRef]
- Lu, C.W.; Wu, C.C.; Chiu, K.M.; Lee, M.Y.; Lin, T.Y.; Wang, S.J. Inhibition of synaptic glutamate exocytosis and prevention of glutamate neurotoxicity by eupatilin from Artemisia argyi in the rat cortex. Int. J. Mol. Sci. 2022, 23, 13406. [Google Scholar] [CrossRef]
- Hung, Y.C.; Kuo, Y.H.; Hsieh, P.W.; Hsieh, T.Y.; Kuo, J.R.; Wang, S.J. Chlorogenic acid decreases glutamate release from rat cortical nerve terminals by P/Q-Type Ca2+ channel suppression: A possible neuroprotective mechanism. Int. J. Mol. Sci. 2021, 22, 11447. [Google Scholar] [CrossRef]
- Lu, C.W.; Yeh, K.C.; Chiu, K.M.; Lee, M.Y.; Lin, T.Y.; Wang, S.J. The effect of isosaponarin derived from wasabi leaves on glutamate release in rat synaptosomes and its underlying mechanism. Int. J. Mol. Sci. 2022, 23, 8752. [Google Scholar] [CrossRef]


| Monosaccharides (μmol/g Sample) | |
|---|---|
| myo-inositol | 1.16 ± 0.01 |
| sorbitol | 2.75 ± 0.01 |
| fucose | 628.95 ± 0.70 |
| arabinose | 4.66 ± 0.01 |
| galactose | 28.53 ± 0.01 |
| glucose | 1045.91 ± 0.67 |
| mannose | 53.70 ± 0.10 |
| fructose | 1693.67 ± 9.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-F.; Lin, T.-K.; Chang, C.-C.; Lee, M.-Y.; Lu, C.-Y.; Hung, C.-F.; Wang, S.-J. Birch Sap Preserves Memory Function in Rats by Enhancing Cerebral Blood Flow and Modulating the Presynaptic Glutamatergic System in the Hippocampus. Int. J. Mol. Sci. 2025, 26, 5009. https://doi.org/10.3390/ijms26115009
Huang C-F, Lin T-K, Chang C-C, Lee M-Y, Lu C-Y, Hung C-F, Wang S-J. Birch Sap Preserves Memory Function in Rats by Enhancing Cerebral Blood Flow and Modulating the Presynaptic Glutamatergic System in the Hippocampus. International Journal of Molecular Sciences. 2025; 26(11):5009. https://doi.org/10.3390/ijms26115009
Chicago/Turabian StyleHuang, Chien-Fen, Tzu-Kang Lin, Chia-Chuan Chang, Ming-Yi Lee, Ching-Yi Lu, Chi-Feng Hung, and Su-Jane Wang. 2025. "Birch Sap Preserves Memory Function in Rats by Enhancing Cerebral Blood Flow and Modulating the Presynaptic Glutamatergic System in the Hippocampus" International Journal of Molecular Sciences 26, no. 11: 5009. https://doi.org/10.3390/ijms26115009
APA StyleHuang, C.-F., Lin, T.-K., Chang, C.-C., Lee, M.-Y., Lu, C.-Y., Hung, C.-F., & Wang, S.-J. (2025). Birch Sap Preserves Memory Function in Rats by Enhancing Cerebral Blood Flow and Modulating the Presynaptic Glutamatergic System in the Hippocampus. International Journal of Molecular Sciences, 26(11), 5009. https://doi.org/10.3390/ijms26115009







