Abstract
Mitochondrial DNA (mtDNA) damage in trabecular meshwork (TM) cells occurs in open-angle glaucoma (OAG). However, current in vitro models for OAG-like changes in TM cells do not explicitly incorporate mtDNA damage. This work validated two methods of mtDNA damage in immortalized TM cells and assessed OAG-associated expression changes. mtDNA was depleted in TM-1 cells via both ethidium bromide (EtBr) treatment and doxycycline (Dox) induction of a mutant (Y147A) version of Uracil DNA Glycosylase 1 (UNG1) in TM-1 cells (TM-1rtTAadv-TRE-UNG1Y147A). Levels of mitochondrial proteins (ATP5F1A, COXII, and COXIV) were measured via western blot. mtDNA levels and mRNA for OAG-associated transcripts (CTGF, FN1, PAI1, and SFRP1) were measured by qPCR. There was a statistically significant decrease in mtDNA levels per cell at all treatment times in both EtBr-treated TM-1 cells and induced TM-1rtTAadv-TRE-UNG1Y147A cells. Protein levels of ATP5F1A were not significantly changed; COXII and COXIV showed significant decreases after both EtBr and Dox induction. Both models resulted in upregulation of CTGF, FN1, and PAI1; additionally, EtBr treatment but not Dox induction resulted in SFRP1 upregulation. In conclusion, two models of mitochondrial depletion were demonstrated in immortalized TM cells; damage was associated with increases in OAG-associated transcripts, supporting a link between mitochondrial damage and glaucoma phenotypes.
1. Introduction
Open-angle glaucoma (OAG) is the second leading cause of irreversible blindness worldwide [1]; major subtypes include Pseudo-Exfoliative Glaucoma (PEXG) and Primary OAG (POAG) [2,3]. In both PEXG and POAG, changes in the trabecular meshwork (TM) restrict the outflow of aqueous humor, leading to increases in intraocular pressure (IOP) and disease progression [4]. Substantial research has been dedicated to identifying OAG-associated changes to the TM; of note, fibrosis [4,5], TM cell viability [6,7], and oxidative stress [8,9] are associated with the disease. However, this progress has faced challenges in clinical translation, with only one approved therapy targeting the TM [10]. There is a need to elucidate additional OAG-associated mechanisms in the TM to identify novel therapeutic targets. Prior research has identified mitochondrial damage and dysregulation in TM aging and OAG. Most notably, mitochondrial DNA (mtDNA) has been shown to exhibit oxidative damage (demonstrated by the presence of 8-oxo-7,8-dihydro-2′-deoxyguanosine), the mitochondrial common deletion, mtDNA mutations, and changes in mtDNA per cell [11,12,13,14]. Further, there is evidence of disruption of the electron transport chain in POAG, especially Complex I [15]; importantly, disruption of Complex I itself can lead to upregulation of reactive oxygen species (ROS), further damaging the cell. Despite these promising findings, the role of mitochondrial dysfunction in OAG remains poorly elucidated.
Mitochondria perform many functions in the cell. Most prominently, they generate ATP through electron transport chain-mediated membrane potential but have numerous other regulatory roles, including regulating cellular ROS [16]. The proteins required for mitochondrial function are encoded in part by nuclear DNA and in part by mitochondrial DNA (mtDNA). All of the protein complexes involved in the electron transport chain have at least one subunit encoded by mtDNA and several proteins encoded by nuclear DNA. While a complete description of the electron transport chain is outside the scope of this paper, three gene products are especially relevant for this study. ATP synthase F1 subunit alpha (ATP5F1A), a nuclear-encoded subunit of ATP synthase, catalyzes the synthesis of ATP. Cytochrome c oxidase subunit II (COXII), an mtDNA encoded gene, is involved in the transfer of electrons in the final stages of the electron transport chain. Cytochrome c oxidase subunit IV (COXIV) is also a catalyst in the electron transport chain, playing a similar role to COXII; however, it is encoded by nuclear DNA.
Replication of the mitochondrial genome and synthesis of additional mitochondrial proteins is primarily regulated by two transcription factors. One is Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α (PGC-1α), often referred to as the master regulator of mitochondrial biogenesis [17,18,19,20,21]. This activates a series of other factors, including nuclear respiratory factors, which trigger the synthesis of mitochondrial proteins [22,23]. One of these factors, mitochondrial transcription factor A (TFAM), activates transcription of the mitochondrial genome [24,25]. In addition, TFAM plays a role in packaging [26,27,28] and degrading [29] mtRNA. PGC-1α is also known to respond to damage by ROS via the expression of antioxidant enzymes [30]. One such antioxidant is superoxide dismutase, which catalyzes the conversion of superoxide anions (O2−) to hydrogen peroxide (H2O2) [31]. Another related antioxidant enzyme involved is catalase, which catalyzes the degradation of H2O2 into water and oxygen [32]. Catalase has been known to be active in the calf trabecular meshwork [33], and single-nucleotide polymorphisms in the catalase gene have been linked to primary open-angle glaucoma in Chinese populations [34].
The above data highly suggest that there is a link between glaucoma and mitochondrial dysfunction, but the mechanisms (and their directionality) of this link remain poorly understood. Prior work has demonstrated that mitochondrial function, specifically ATP generation, is dysregulated in TM cells treated with dexamethasone in vitro, demonstrating that glaucoma stimuli can disrupt mitochondrial function [35,36,37]. However, it remains unknown if mitochondrial damage can contribute to glaucoma progression. Here, we turn to prior work in other cell and tissue types to identify potential models. One potential model is treatment with doses of ethidium bromide (EtBr) between 10 ng/mL and 1 µg/mL, which can be an effective way of inducing mitochondrial DNA damage and depletion in several cell types [38,39,40,41,42,43]. It is believed that EtBr limits mtDNA synthesis and can trigger damage through excessive supercoiling and dsDNA breaks in the circular mtDNA [40,43], leading to the degradation of mtDNA [44]. Importantly, the effects of EtBr on nuclear DNA are more limited [40,41,45]. EtBr has minor influences on nuclear DNA levels at higher concentrations between 1 µg/mL and 5 µg/mL, while mtDNA is significantly inhibited at lower concentrations [40,45]. Another potential model is the disruption of the mtDNA repair system. Human Uracil DNA Glycosylase is a base excision DNA repair enzyme that removes uracil that has been incorporated into DNA; human Uracil DNA Glycosylase 1 (UNG1) operates specifically in the mitochondria. A mutant version of this enzyme with tyrosine substituted by alanine at amino acid position 147 (UNG1Y147A) removes thymine in addition to uracil, resulting in damage to mtDNA [46]. This has been engineered into a tetracycline-inducible expression vector with an MYC tag, providing a useful tool for inducible mtDNA damage [47]. When induced by tetracycline or doxycycline (Dox) treatment, expressing cells produce UNG1Y147A, leading to temporally controlled mtDNA depletion. Importantly, mtDNA depletion occurs within hours, but defects in mitochondrial function can take up to 6 d to establish [47]. The mechanism(s) by which this mtDNA depletion occurs is still unclear, but there is strong evidence to suggest that the depletion observed with this model is the result of active degradation rather than a simple inhibition of replication [47]. In particular, it is believed that the mtDNA is degraded via the formation of abasic sites, which are known to lead to mtDNA degradation by TFAM [29]. In summary, EtBr and UNG1Y147A may provide accessible in vitro approaches for modeling mitochondrial damage in OAG.
Glaucoma is a disease with a complex etiology. While several genetic links have been identified, most notably myocilin (MYOC), none are fully predictive of disease onset. For example, mutations in MYOC are associated with approximately 5% of adult-onset OAG cases [48]. However, several phenotypes are well preserved. As mentioned above, fibrotic changes in the TM are broadly associated with disease progression in POAG and PEXG [4,5]. While not exhaustive, several markers are associated with this phenotype. These include Connective Tissue Growth Factor (CTGF), Fibronectin (FN1), and Plasminogen Activator Inhibitor 1 (PAI1). CTGF is a signaling protein that is involved in the regulation of extracellular matrix proteins [49]. Elevated levels of CTGF have been found in the aqueous humor (AH) of patients with PEXG [50], and CTGF has been directly linked to the expression of glaucoma-related ECM proteins [50,51]. Further, elevation of CTGF to the anterior chamber of the mouse eye leads to increased IOP and optic nerve damage [51]. FN1 is a major extracellular matrix (ECM) protein and is significantly increased in the TM in both normal aging and POAG [52]. Furthermore, even though FN1 has yet to be causatively linked to increased IOP, an in vitro study has shown that HTM monolayers have reduced permeability when treated with FN1 [53]. Although increased ECM production is involved in OAG, including FN1, decreased ECM removal may also play a role. Inhibition of matrix metalloproteases (MMPs) has been shown to inhibit aqueous humor outflow through the TM in ex vivo experiments [54,55]. Elevated levels of PAI1, a protease inhibitor, have been found in the aqueous humor of patients with glaucoma, including POAG [56]. Further, PAI1 has been mechanistically linked to MMP inhibition in TGF-β2 induced TM cells in vitro [57].
Additionally, inhibition of the Wnt signaling pathway through Secreted Frizzled-Related Protein 1 (SFRP1) has been linked to OAG. In particular, one study showed that SFRP1 mRNA is elevated 4.5 fold in glaucomatous cultured human trabecular meshwork cells, coupled with a 50% increase in SFRP1 protein levels [58]. Furthermore, administration of SFPR1 in both an in vivo mouse model and an ex vivo human perfusion model resulted in increased IOP [58]. While the mechanism is unclear, SFRP1 expression in TM cells is elevated in response to ECM stiffness [59] and fibrotic stimuli of TGF-β2 and dexamethasone [60,61]; further, exogenous SFRP1 itself can increase TM stiffness [62].
Here, we show the first demonstration of in vitro mitochondrial depletion in human TM cells using both EtBr and UNG1Y147A in an immortalized cell model. We demonstrate the loss of mtDNA using both methods. This loss of mtDNA is associated with a loss of expression in proteins (COXII and COXIV) associated with mitochondrial function. Importantly, both models led to the upregulation of OAG-associated transcripts FN1, CTGF, and PAI1, while EtBr treatment also resulted in increased SFRP1 expression. These data suggest a mechanistic link between the mtDNA damage in TM and known markers of OAG. These models may provide useful tools for future studies assessing the role of mitochondrial dysfunction in OAG.
2. Results
2.1. Ethidium Bromide Treatment Depletes mtDNA
TM-1 cells were treated with 50 ng/mL EtBr for 6 d or treated for 4 d and allowed to recover for 2 d; data were normalized to the experimental control. Compared to control, both treatment groups showed a significant decrease in mtDNA levels (p < 0.0001, Figure 1). Additionally, the recovery group was significantly increased compared to the full treatment (p = 0.017). We additionally tested shorter time courses, observing very similar results. Moreover, 50 ng/mL EtBr for 4 d is also significantly decreased compared to the control (Figure S1). As a preliminary demonstration of a similar effect in primary cells, we measured mtDNA levels in EtBr-treated primary human TM (HTM) cells. At 6 d, primary HTM cells showed decreased mtDNA levels after both 50 and 75 ng/mL (Figure S2A). Further, 50 ng/mL EtBr induced an apparent reduction in mtDNA, which was apparent at 2 d, 4 d, 6 d, and after 4 d treatment and 2 d recovery (Figure S2B).
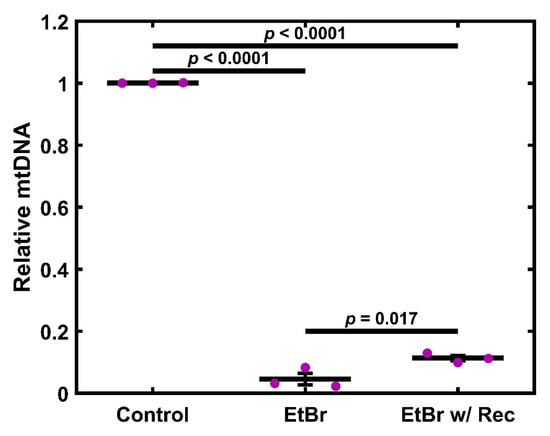
Figure 1.
EtBr depletes mtDNA in TM-1 cells. Treatment with 50 ng/mL EtBr for 6 d, as well as EtBr treatment for 4 d with 2 d recovery, resulted in a significant decrease in mtDNA levels compared to the control. Additionally, although overall levels remained low, the recovery case had significantly increased mtDNA compared to the full 6 d treatment. Magenta ● represent individual experiments; mean and standard deviation error bars are represented by black lines. Control data normalized to 1 is shown for reference. Significance between the two groups is indicated by horizontal lines and the stated p values, assessed by ANOVA followed by Tukey’s post hoc.
2.2. mtDNA Depletion Is Coupled to Loss of Cytochrome C Oxidase but Not ATP Synthase
TM-1 cells were treated with 50 ng/mL EtBr for 6 d. Protein was then isolated and analyzed via Western blot for semi-quantitative expression levels of ATP5A1, COXII, and COXIV (Figure 2). Overall, there was no significant change in ATP5A1, but the Cytochrome C Oxidase subunits COXII and COXIV significantly declined. Similar findings were seen with 4 d treatments (Figure S3). We further extracted mRNA from TM-1 cells treated with 50 ng/mL EtBr for 6 d and assayed the transcript expression of mitochondrial regulators and antioxidant enzymes (Figure S4). PGC1A and TFAM were both significantly reduced (p < 0.0001 and p = 0.017, respectively). SOD2 expression was similarly reduced (p = 0.025), while there was no significant change to CATA expression (p = 0.941).
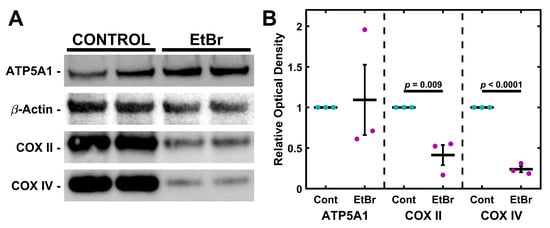
Figure 2.
EtBr depletes subunits of Cytochrome C Oxidase but not ATP Synthase. (A) Treatment with 50 ng/mL EtBr for 6 d results in decreased expression of COXII and COXIV but not ATP5A1. (B) Quantification of triplicate Western blots. Magenta/cyan ● represent individual control/EtBr experiments, respectively; mean and standard deviation error bars are represented by black lines. Control data normalized to 1 is shown for reference. Significance between the two groups is indicated by horizontal lines and the stated p values, assessed by t-test.
2.3. EtBr Treatment Increases Glaucoma-Associated Gene Expression
Having established that EtBr treatment depleted mitochondria and components of the electron transport chain, we wished to establish if this damage was associated with a glaucomatous phenotype. To this end, TM-1 cells were treated with 50 ng/mL EtBr for 6 d and assayed for glaucoma-associated transcripts (CTGF, FN1, PAI1, and SFRP1) via qPCR and compared to controls via paired-sample t-test (Figure 3). There was a significant increase in levels of mRNA coding for CTGF (p = 0.002), FN1 (p = 0.002), PAI1 (p = 0.019), and SFRP1 (p = 0.003). As a preliminary demonstration of a similar effect in primary cells, we measured mRNA levels in EtBr-treated primary HTM cells (Figure S2C). We observed that 50 ng/mL EtBr treatment induced an apparent increase in CTGF, FN1, PAI1, and SFRP1 in primary HTM cells, although this was not as robust as in the immortalized TM-1 (Figure 3).
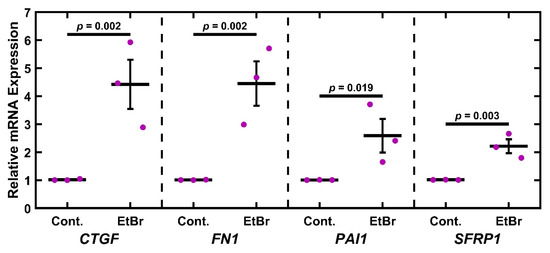
Figure 3.
EtBr treatment upregulates glaucoma-associated genes. Treatment with 50 ng/mL EtBr for 6 d results in significantly increased expression in CTGF, FN1, PAI1, and SFRP1. Magenta ● represent individual experiments; mean and standard deviation error bars are represented by black lines. Control data normalized to 1 is shown for reference. Significance between the two groups is indicated by horizontal lines and the stated p values, assessed by t-test on log-transformed values.
2.4. UNG1Y147A Expression in TM-1 Cells Depletes mtDNA
Having established a chemical model of mitochondrial depletion in TM cells, we wished to establish an alternative genetic method. TM-1 cells were double transduced to express rtTA-Advanced (rtTAadv) and UNG1Y147A under a tetracycline-responsive promoter (TRE-UNGY147A), allowing for Dox-inducible expression of UNG1Y147A. We denote these cells as TM-1rtTAadv-TRE-UNG1Y147A cells; they were treated with 3.5 µg/mL Dox, and the expression of UNG1Y147A was assessed via western blotting for the Myc tag. Expression was detectable at 2, 4, and 6 d of 3.5 µg/mL Dox treatment and was additionally detectable after 4 d Dox and 2 d recovery (Figure 4A). Myc was not readily detectable in a 6 d control culture. When semi-quantitatively assessed using relative optical density compared to the 6 d treatment, both 4 d and 6 d treatments were significantly elevated compared to the 6 d control (p = 0.005 and p = 0.004, respectively; Figure 4B). Further, there was significantly decreased expression in the recovery condition when compared to both 4 d and 6 d (p = 0.040 and p = 0.031, respectively). UNG1Y147A expression was accompanied by loss of mtDNA. TM-1rtTAadv-TRE-UNG1Y147A cells were treated with 3.5 µg/mL Dox for 6 d or treated 4 d and allowed to recover for 2 d; data was normalized to the 6 d control culture of TM-1rtTAadv-TRE-UNG1Y147A. Compared to the control, both treatment groups showed a significant decrease in mtDNA levels (p < 0.001, Figure 4C); however, there was no significant difference between the two treatments. Similar results were observed with the 4 d treatment when compared to the same 6 d control (Figure S5).
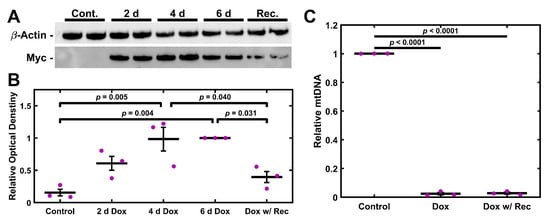
Figure 4.
UNG1Y147A Expression depletes mtDNA in TM-1 cells. (A) Treatment with 3.5 µg/mL Dox results in detectable expression of UNG1Y147A in TM-1rtTAadv-TRE-UNG1Y147A cells at multiple time points, assayed by the protein’s Myc tag. (B) When normalized to the 6 d Dox treatment, UNG1Y147A was significantly elevated at 4 d and 6 d compared to the control. Further, the 4 d Dox with 2 d recovery resulted in significantly decreased UNG1Y147A expression compared to 4 d and 6 d. (C) Treatment with 3.5 µg/mL Dox for 6 d, as well as Dox treatment for 4 d with 2 d recovery, resulted in a significant decrease in mtDNA levels compared to the control. Magenta ● represent individual experiments; mean and standard deviation error bars are represented by black lines. Control data normalized to 1 is shown for reference. Significance between the two groups is indicated by horizontal lines and the stated p values, assessed by ANOVA followed by Tukey’s post hoc.
2.5. UNG1Y147A Meditated mtDNA Depletion Correlates to Loss of Cytochrome C Oxidase but Not ATP Synthase
TM-1rtTAadv-TRE-UNG1Y147A cells were treated with 3.5 µg/mL Dox for 2, 4, and 6 d to induce the expression of UNG1Y147A. In addition, a fourth treatment was carried out whereby cells were treated with 3.5 µg/mL Dox for 4 d and then were left untreated for an additional 2 d (recovery). Untreated 6 d cultures were used as the control. Protein was then isolated and analyzed via Western blot for semi-quantitative expression levels of ATP5A1, COXII, and COXIV (Figure 5). Optical density compared to loading control was used to determine levels of each protein, normalized to 6 d control culture. Relative optical density was assessed using one-way ANOVA followed by Dunnett’s post hoc test. There was a statistically significant decrease in COXII at both 6 d and recovery (p = 0.020 and p = 0.020, respectively; Figure 5B). Further, there was a statistically significant decrease in COXIV at 4 d, 6 d, and recovery (p = 0.018, p = 0.006, and p = 0.007, respectively).
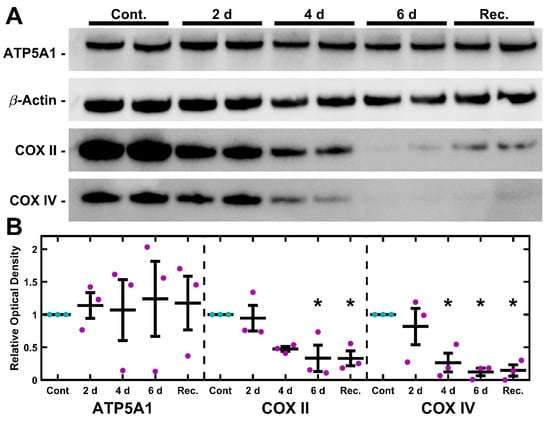
Figure 5.
UNG1Y147A depletes Cytochrome C Oxidase subunits in TM-1 cells. (A) Representative blots of ATP5A1, COXII, and COXIV are shown, and the paired loading control β-actin blot is repeated from Figure 4. Dox induction resulted in a decline in COXII and COXIV but minimal effect in ATP5A1 expression. (B) Quantification of the optical density revealed no significant changes to ATP5A1. When compared to the control, COXII expression was significantly decreased after 6 d Dox treatment, as well as 4 d Dox followed by 2 d recovery. Similarly, COXIV was also significantly reduced at 4 d, 6 d, and the recovery condition. Magenta/cyan ● represent individual control/Dox experiments, respectively; mean and standard deviation error bars are represented by black lines. Control data normalized to 1 is shown for reference. Significance in reference to the 6 d control was assessed by ANOVA followed by Dunnett’s post hoc test. * indicates p < 0.05; p-values provided in the main text.
2.6. UNG1Y147A Alters Glaucoma-Associated Gene Expression
Similar to the EtBr studies, we wished to assess if inducing mitochondrial damage correlates with altered expression of glaucoma-related transcripts. TM-1rtTAadv-TRE-UNG1Y147A cells were treated with 3.5 µg/mL Dox for 4 and 6 d to induce expression of UNG1Y147A. In addition, an additional treatment was carried out whereby cells were treated with 3.5 µg/mL Dox for 4 d and then left untreated for an additional 2 d (recovery). The expression of glaucoma-associated transcripts (CTGF, FN1, PAI1, and SFRP1) was measured via qPCR and assessed using one-way ANOVA followed by Dunnett’s post hoc testing on the log-transformed values (Figure 6). CTGF was significantly upregulated at 6 d and recovery (p = 0.049 and p = 0.024, respectively). Similarly, FN1 and PAI1 were significantly upregulated at the 6 d time point (p = 0.048 and p = 0.014, respectively). No significant changes were observed with SFRP1 expression. As Dox has previously been reported to alter ECM-related gene expression [63,64], we similarly treated untransduced TM-1 cells (e.g., lacking rtTAadv and UNG1Y147A) with Dox and found no significant effects on the expression of CTGF, FN1, PAI1, and SFRP1 (Figure S6).
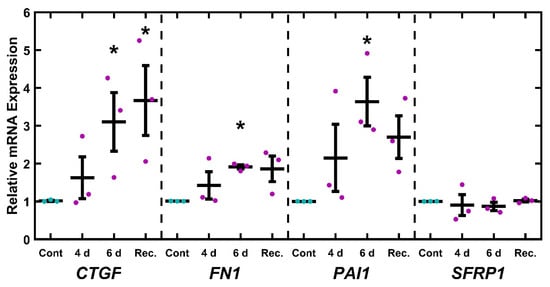
Figure 6.
Glaucoma-related mRNA transcripts response to mtDNA depletion. After 6 d Dox induction, TM-1rtTAadv-TRE-UNG1Y147A cells showed a significant increase in CTGF, FN1, and PAI1 expression. Additionally, CTGF was significantly upregulated after 4 d treatment with 2 d recovery. Magenta/cyan ● represent individual control/Dox experiments, respectively; mean and standard deviation error bars are represented by black lines. Control data normalized to 1 is shown for reference. Significance in reference to the 6 d control was assessed by ANOVA followed by Dunnett’s post hoc test. * indicates p < 0.05; p-values provided in the main text.
3. Discussion
In this paper, we demonstrated two complementary models of mtDNA depletion in TM-1 cells via treatment with EtBr and via Dox induction of TM-1rtTAadv-TRE-UNG1Y147A. We further showed decreases in the level of mitochondrial electron transport chain subunits that are required to maintain mitochondrial membrane polarization and changes in the expression of glaucoma-associated transcripts. To our knowledge, these are the first in vitro TM studies to directly damage mtDNA and assess changes in glaucoma-related gene expression, offering a potential tool to study the mechanistic role of previously reported mitochondrial dysfunction in POAG and PEXG [11,12,13,14].
Both models strongly disrupt mitochondria, shown most evidently by the loss of mtDNA (Figure 1 and Figure 4). Importantly, impacts on electron transport chain subunits were present in both mitochondrial and nuclear-encoded subunits (Figure 2 and Figure 5). While the loss of COXII may be expected due to a loss of mtDNA copies, decreases in COXIV, which is encoded by nuclear DNA, suggest that these depletion models involve crosstalk between mitochondrial activity and nuclear gene expression. The fact that crosstalk can occur between the mitochondria and nuclear gene expression is well described in other systems [65,66,67], but this has not previously been demonstrated in TM cells. By comparison, the nuclear-encoded ATP5A1 did not show a significant change following either treatment. Mitochondrial damage and depletion could directly contribute to glaucoma pathogenesis through cell loss. It has previously been shown in POAG that there is an increase in apoptosis, cell loss, and senescence of TM cells [68,69,70]. Mitochondria are well understood to be mediators of apoptosis and senescence [71]. In particular, mitochondrial DNA damage has been shown to induce mitochondria-mediated apoptosis [72].
A more indirect mechanism where mitochondrial loss could lead to an increase in glaucoma-related mRNA transcripts is via calcium dysregulation. Mitochondria are heavily involved in calcium regulation [73], and disruption of mitochondria has been linked to disruption in calcium regulation [73,74,75], including in TM cells [15]. Further, dysregulation of transient receptor potential cation channel subfamily V member 4 (TRPV4), a calcium channel, has been implicated in glaucoma [76,77,78,79,80]. For example, one study showed TRPV4 agonists produced a glaucomatous phenotype both in vitro and in vivo, while TRPV4 antagonists were protective [76]; the importance of TRPV4 has been broadly supported by other studies, although the specific mechanisms are unclear [80]. Mitochondrial dysregulation leading to loss of calcium homeostasis could result in similar effects to TRPV4 dysregulation.
OAG can also be characterized by expression changes in the outflow pathway. Both the EtBr and the UNG1Y147A models show a significant increase in transcripts associated with OAG, specifically CTGF, FN1, and PAI1 (Figure 3 and Figure 6). However, SFRP1 was upregulated after EtBr treatment, which was not observed after UNG1Y147A induction. The findings of previously known OAG-associated transcripts in these mtDNA depletion models support further investigation into mitochondrial-mediated OAG mechanisms. While mitochondria do not directly regulate the expression of these genes, we can speculate on potential mechanisms. One promising avenue is the activation of an innate immune response in the cell triggered by damaged mtDNA entering the cytosol of TM cells. A prior study looked at the effects of the presence of mtDNA in the cytosol using mouse embryonic fibroblasts [81]. In this study, cells expressing a mutant TFAM showed a decrease in mtDNA per cell and increasing mtDNA leaking into the cytosol. These were correlated with an increase in the expression of interferon-stimulating genes, typically associated with pathogen response and inflammation [82]. Inflammatory processes are also associated with fibrosis more generally [83,84,85] and increased expression of key OAG-associated transcripts more specifically, including CTGF [86], FN1 [87,88,89], and PAI1 [90].
Future work elucidating these mechanisms in OAG can take advantage of extensive work performed in the context of mitochondrial dysfunction. OAG is an aging-associated disease, and many of the changes in TM cells in OAG are seen in aging more broadly. For example, the electron transport chain is known to be impaired in POAG, specifically respiratory Complex I [15], possibly due to the presence of the common deletion [11,12]. In addition, one report shows that the generation of ROS from Complex I is associated with shorter lifespans when comparing multiple species [91]. Further, the loss of mtDNA per cell is known to occur in TM cells in POAG and normal aging [12]. This is also a hallmark of aging in many tissues and cells, including the pancreas [92], skeletal muscle [93], adipose tissue [94], and fibroblasts [95]. Another report noted that human skin fibroblasts show an increase in the mitochondrial common deletion with age [96], as do buccal squamous epithelial cells [97], heart cells [98], and skeletal muscle cells [99]. Many other kinds of mitochondrial DNA mutations are known to increase in many tissues with aging as well [95,100,101,102,103].
As with all in vitro models, there are limitations to the described approaches. This work was conducted with an immortalized line (TM-1), which limits the physiological relevance. Primary HTM cells are preferred for in vitro studies, and both EtBr and the lentiviral transduction would be feasible in future primary cell studies. As an initial demonstration that these studies can be adapted to primary HTM, we performed limited studies with EtBr and primary HTM cells (Figure S2). While conclusions cannot be drawn without biological replication, the data demonstrate findings similar to the TM-1 studies, with decreased mtDNA and increased expression of OAG-associated transcripts, although the changes are not as robust as shown in TM-1. Future studies pursuing mechanisms would benefit from validation in primary HTM cells and, additionally, in ex vivo models. Additionally, while the application of these models to TM cells provides compelling data linking mitochondrial dysfunction to glaucoma-related transcription, it is essential to note that this mechanism has not been shown in vivo and may not represent a relevant mechanism of disease progression. It is important to note that we only assessed a limited number of glaucoma-associated targets, and other glaucoma-associated genes, such as MYOC, would be valuable to pursue. Further, although both models show a similar pattern in terms of electron transport chain protein complex subunit loss and upregulation of glaucoma-related mRNA transcripts CTGF, FN1, and PAI, there is a notable distinction between the two methods, where SFRP1 is significantly upregulated by EtBr but not by UNG1Y147A expression. Importantly, the mechanism of these changes has not been elucidated and may not be representative of glaucoma pathogenesis in vivo. Further, as mentioned in the Introduction, EtBr can damage nuclear DNA, although the required dose is expected to be much higher [40,45]; testing of other doses would be relevant. Other off-target effects are expected for both models, and with all models, results should be carefully interpreted. When possible, multiple orthogonal methods should be utilized, possibly in combination, to mitigate the nonspecific effects.
In summary, we present here two new models of a glaucomatous state in immortalized trabecular meshwork cells. These models are novel insofar as they incorporate mtDNA damage and depletion, known to be present in TM cells in both aging and OAG. They also show a decrease in levels of mitochondrial electron transport chain proteins, which may produce an effect not unlike the impairment of COXI, which is known to occur in OAG. The increase in fibrotic mRNA transcripts also suggests a relationship between mitochondrial damage and fibrotic glaucoma pathogenesis. For this reason, these models merit additional study in more physiologically relevant models, including primary human TM cells and ex vivo cultures. While this early work is consistent with prior work associating mitochondrial damage with glaucoma, causative mechanistic links remain poorly understood and are deserving of further study.
4. Materials and Methods
4.1. Cell Culture
TM-1 cells (gift of Dr. Paul Russell, University of California, Davis) are SV-40 immortalized human TM cells [104,105]; TM-1 cells have known differences when compared to primary HTM cells, including promotor utilization but have been previously used as an accessible cell model for early studies [104,105,106,107,108]. TM-1 cells were routinely cultured in Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (50:50; DMEM/F12 with L-glutamine and 15 mM HEPES; (Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) [58]. HEK293TN (System Biosciences, San Jose, CA, USA) were cultured in Dulbecco’s modified Eagle medium with 4.5 g/L glucose, L-glutamine, and sodium pyruvate (Corning, Manassas, VA, USA) supplemented with 10% FBS and 1% P/S.
4.2. Plasmid Transfections and Viral Transduction
TM-1 cells were sequentially transduced for the expression of the rtTA-Advanced and UNG1Y147A under a Dox-inducible tetracycline response element (TRE)-containing promotor. The approach is described below and shown schematically in Figure 7. For all plasmids used, plasmid maps are accessible from Addgene using the identifiers provided. pLVX-EF1a-tetOn-IRES-G418 (EtO) was a gift from Fred Gage (Addgene plasmid # 84776), pMA3287 was a gift from Mikhail Alexeyev (Addgene plasmid # 46883), and psPAX2 and pMD2.G were gifts from Didier Trono (Addgene plasmid # 12260 and # 12259). Most relevant to this study, pLVX-EF1a-tetOn-IRES-G418 (EtO) confers G418 resistance and enables the constitutive expression of the transcriptional control activator protein rtTA-Advanced (referred to here as rtTAadv), which binds to a TRE and activates genes in the presence of DOX. Further, pMA3287 confers puromycin resistance and has UNG1Y147A under the TRE-containing promotor PTight (denoted as TRE-UNG1Y147A), allowing rtTA-Advanced-mediated expression of UNG1Y147A. In combination, they provide the Dox-inducible expression of UNG1Y147A. First, to generate an rtTA-Advanced virus, HEK293TN cells were triple transfected with psPAX2, pMD2.G, and the transfer plasmid (pLVX-EF1a-tetOn-IRES-G418 (EtO)) according to manufacturer instructions (TransIT-VirusGEN Transfection Reagent; Mirus Bio LLC, Madison, WI, USA). Media were changed at 24 h, and viral supernatant was harvested at 48 and 72 h. Viral supernatant was centrifuged for 10 min at 1000× g and passed through a 0.45 µm filter prior to use. TM-1 cells were then transduced by incubating them with a 1:5 ratio of the rtTAadv viral supernatant and fresh media for 48 h, followed by 48 h in normal media. Viral titer and MOI were not directly measured. Expressing TM-1 cells were selected with 300 µg/mL G418 for 4 days to generate TM-1rtTAadv cells. A second triple transfection was performed to generate the TRE-UNG1Y147A using pMA3287 as a transfer plasmid. This second virus was used to transduce the previously transduced TM-1rtTAadv cells, with 10 µg/mL puromycin used for selection in addition to 300 µg/mL G418. The double-transduced TM-1 cells were designated TM-1rtTAadv-TRE-UNG1Y147A and were maintained with 10 µg/mL puromycin and 300 µg/mL G418.
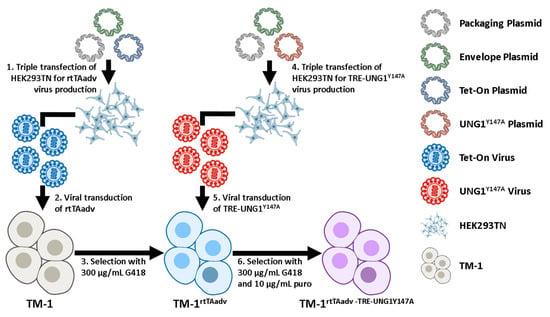
Figure 7.
Schematic of the double transduction. (1) We performed triple transfection of HEK293TN cells with plasmids for packaging (psPAX2), envelope (pMD2.G), and transfer of the rtTA-Advanced (rtTAadv) construct (pLVX-EF1a-tetOn-IRES-G418 (EtO)). (2) Viral supernatant was used to transduce TM-1 cells. (3) Transduced cells were selected with G418 to generate a selected population of TM-1rtTAadv. (4) We performed triple transfection as in (1) but with a transfer plasmid for the TRE-UNG1Y147A construct (pMA3287). (5) Viral supernatant of the second triple transfection was used to transduce TM1rtTAadv cells. (6) Cells were selected under both G418 and puromycin (puro) to generate TM-1rtTAadv-TRE-UNG1Y147A cells. Schematic was constructed with graphics from NIH BIOART [109,110,111,112].
4.3. Mitochondrial Depletion Experimental Conditions
For the EtBr model, cells were treated with EtBr (VWR, Radnor, PA, USA) at a dose of 50 ng/mL. Media in both the treated cells and accompanying non-treated controls were changed every two days. We additionally tested 75 ng/mL and found similar but more variable mtDNA depletion (Figure S7). For the UNG1Y147A model, TM-1rtTAadv-TRE-UNG1Y147A cells were treated with 3.5 µg/mL Dox (Doxycycline Hyclate; TCI America, Portland, OR, USA) in water or vehicle control. Untransduced TM-1 cells were treated in parallel as a control for the effects of Dox, which can inhibit mitochondrial protein synthesis [113,114,115]. For both EtBr and UNG1Y147A models, cells were plated at approximately 250,000 cells/well in 6-well plates 24 h prior to treatment initiation and were confluent at treatment. For each experiment and condition, two wells were plated in parallel, one for RNA and DNA and one for protein collection (as described below). All experiments were performed with three biological replicates (three different cell passages plated on separate days). All experiments were performed within 5 passages.
4.4. Isolation of DNA and RNA
Cells were treated as described in 4.3 above. In order to determine mtDNA levels per cell, DNA was isolated according to manufacturer instructions (Monarch Genomic DNA Purification Kit; New England Bio Labs, Ipswich, MA, USA). Total RNA was isolated according to manufacturer instructions (ISOLATE II RNA Mini Kit; Meridian Biosciences, Memphis, TN, USA). DNA and RNA concentrations were determined via absorbance using the Spectramax M2 Microplate Reader (Molecular Devices, San Jose, CA, USA).
4.5. Quantitative Polymerase Chain Reaction
Quantification of DNA and RNA expression was performed using qPCR according to manufacturer instructions (SensiFAST SYBR No-ROX Kit; Meridian Bioscience, Memphis, TN, USA) on a Magnetic Induction Cycler (Biomolecular Systems, Upper Coomera, Queensland, Australia). Total DNA and RNA concentrations were measured as described in Section 4.4 above. For DNA, 5 ng was loaded per reaction. For RNA, 60 ng was loaded per reaction. TATA-box binding protein (TBP) was used as an endogenous control for CTGF, FN1, PAI1, and SFRP1. GAPDH was used as an endogenous control for PGC1A, TFAM, SOD2, and CATA. Microglobin DNA (BGLOB) was used as a nuclear reference gene for total mtDNA (TMDQ); change in mtDNA levels was quantified relative to nuclear DNA. All primers are shown in Table 1. All reactions were performed in technical triplicate for each biological replicate.

Table 1.
Primers used for qPCR.
4.6. Protein Isolation and Western Blotting
Cells were treated as described in 4.3 above. Protein was isolated using RIPA buffer (ThermoFisher Scientific, Waltham, MA, USA) in conjunction with Mammalian Protease Inhibitor Cocktail (VWR, Radnor, PA, USA) and was quantified using the DC Protein Assay (Bio-Rad, Hercules, CA, USA). Equivalent protein amounts were separated on NuPAGE 4 to 12% Bis-Tris gels in MES SDS running buffer (ThermoFisher Scientific, Waltham, MA, USA). Protein was transferred to a 0.45 μm nitrocellulose membrane using the Pierce Power Station (Thermo Fisher Scientific, Waltham, MA, USA). Blots were labeled with mouse anti-β-Actin (3700; Cell Signaling Technology, Danvers, MA, USA), mouse anti-GAPDH (60004-1-Ig; Proteintech, Rosemont, IL, USA), mouse anti-Myc (MA1-21316; ThermoFisher), rabbit anti-COXII (55070-1-AP; Proteintech, Rosemont, IL, USA), mouse anti-COXIV (60251-1-Ig; Proteintech), and rabbit anti-ATP5A1 (14676-1-AP; Proteintech). All primary antibodies were used at a 1:500 dilution overnight at 4 °C with rocking. Secondary staining was conducted with HRP-conjugated goat anti-rabbit or goat anti-mouse, as appropriate (SeraCare, Milford, MA, USA). All secondary antibodies were used at a 1:50,000 dilution with 1 h incubation and rocking at room temperature. Blots were developed using Radiance ECL Chemiluminescent HRP substrate (Azure Biosystems, Dublin, CA, USA) and imaged using the ChemiDoc Imaging System (Bio-Rad, Hercules, CA, USA). The relative optical density of bands was determined using ImageJ 1.53m (NIH ImageJ software) and used as a semi-quantitative measure of expression. Target protein band intensity was normalized to either GAPDH or β-Actin. All samples were run in technical duplicates for each biological replicate.
4.7. Statistical Analysis
All experiments were conducted in biological triplicate. Data were analyzed via paired-sample t-test in the cases where there was only a control and one experimental group. In experiments with more than one treatment, one-way ANOVA was used with either Tukey’s or Dunnett’s post hoc testing for multiple comparisons. The statistical approach for each experimental set is detailed in the appropriate results section. For mRNA expression analysis, all values were log-transformed prior to testing. All statistical analysis was performed in MATLAB 2022a (Mathworks, Natick, MA, USA). All data used to construct the quantitative figures are provided in Supplemental Data.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26136255/s1.
Author Contributions
Conceptualization, J.T.M. and L.Z.; methodology, S.P.K. and E.T.; software, J.T.M. and S.P.K.; investigation, S.P.K. and E.T.; resources, J.T.M. and L.Z.; data curation, J.T.M. and S.P.K.; writing—original draft preparation, J.T.M. and S.P.K.; writing—review and editing, J.T.M., S.P.K., E.T. and L.Z.; visualization, J.T.M. and S.P.K.; supervision, J.T.M. and L.Z.; funding acquisition, J.T.M. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the US National Science Foundation (NSF) under Grant No. CAREER 2046093 (to J.T.M.) and the US National Institutes of Health (NIH) Grant No. R35GM128854 (to L.Z.). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF or NIH. This work was partially supported by a Grant-In-Aid from The Glaucoma Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.V.; Quigley, H.A. Risk Factors and Open-Angle Glaucoma: Classification and Application. J. Glaucoma 2007, 16, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef]
- Stamer, W.D.; Acott, T.S. Current Understanding of Conventional Outflow Dysfunction in Glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Izumchenko, E.; Kanherkar, R.R.; Teka, M.; Cantor, C.; Manaye, K.; Sidransky, D.; West, M.D.; Makarev, E.; Csoka, A.B. Pro-Fibrotic Pathway Activation in Trabecular Meshwork and Lamina Cribrosa Is the Main Driving Force of Glaucoma. Cell Cycle 2016, 15, 1643–1652. [Google Scholar] [CrossRef]
- Aktas, Z.; Karaca, E.E.; Gonul, I.I.; Hasanreisoglu, M.; Onol, M. Apoptosis in the Iris and Trabecular Meshwork of Medically Treated and Untreated Primary Open Angle Glaucoma Patients. Int. J. Ophthalmol. 2013, 6, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Yegorov, Y.E. Senescent Phenotype of Trabecular Meshwork Cells Displays Biomarkers in Primary Open-Angle Glaucoma. Curr. Mol. Med. 2011, 11, 528–552. [Google Scholar] [CrossRef]
- Iorga, R.E.; Moraru, A.D.; Costin, D.; Munteanu-Dănulescu, R.S.; Brănișteanu, D.C. Current Trends in Targeting the Oxidative Stress in Glaucoma (Review). Eur. J. Ophthalmol. 2024, 34, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, S.; Zhong, W.; Yang, B.; Sun, L.; Zheng, Y. Oxidative Stress in the Trabecular Meshwork (Review). Int. J. Mol. Med. 2016, 38, 995–1002. [Google Scholar] [CrossRef]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New Glaucoma Medications: Latanoprostene Bunod, Netarsudil, and Fixed Combination Netarsudil-Latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef]
- Izzotti, A.; Longobardi, M.; Cartiglia, C.; Saccà, S.C. Mitochondrial Damage in the Trabecular Meshwork Occurs Only in Primary Open-Angle Glaucoma and in Pseudoexfoliative Glaucoma. PLoS ONE 2011, 6, e14567. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Mitochondrial Damage in the Trabecular Meshwork of Patients with Glaucoma. Arch. Ophthalmol. 2010, 128, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Pulliero, A.; Izzotti, A. The Dysfunction of the Trabecular Meshwork during Glaucoma Course. J. Cell Physiol. 2015, 230, 510–525. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial Abnormalities in Patients with Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Leung, K.W.; Zhang, Y.-H.; Duan, S.; Zhong, X.-F.; Jiang, R.-Z.; Peng, Z.; Tombran-Tink, J.; Ge, J. Mitochondrial Complex I Defect Induces ROS Release and Degeneration in Trabecular Meshwork Cells of POAG Patients: Protection by Antioxidants. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1447–1458. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Puigserver, P.; Adelmant, G.; Wu, Z.; Fan, M.; Xu, J.; O’Malley, B.; Spiegelman, B.M. Activation of PPARgamma Coactivator-1 through Transcription Factor Docking. Science 1999, 286, 1368–1371. [Google Scholar] [CrossRef]
- Martínez-Redondo, V.; Pettersson, A.T.; Ruas, J.L. The Hitchhiker’s Guide to PGC-1α Isoform Structure and Biological Functions. Diabetologia 2015, 58, 1969–1977. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional Integration of Mitochondrial Biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Valero, T. Mitochondrial Biogenesis: Pharmacological Approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Nuclear Control of Respiratory Gene Expression in Mammalian Cells. J. Cell Biochem. 2006, 97, 673–683. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the Human Mitochondrial Transcription Factor A Gene by Nuclear Respiratory Factors: A Potential Regulatory Link between Nuclear and Mitochondrial Gene Expression in Organelle Biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Hock, M.B.; Kralli, A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef]
- Kukat, C.; Davies, K.M.; Wurm, C.A.; Spåhr, H.; Bonekamp, N.A.; Kühl, I.; Joos, F.; Polosa, P.L.; Park, C.B.; Posse, V.; et al. Cross-Strand Binding of TFAM to a Single mtDNA Molecule Forms the Mitochondrial Nucleoid. Proc. Natl. Acad. Sci. USA 2015, 112, 11288–11293. [Google Scholar] [CrossRef]
- Ngo, H.B.; Kaiser, J.T.; Chan, D.C. The Mitochondrial Transcription and Packaging Factor Tfam Imposes a U-Turn on Mitochondrial DNA. Nat. Struct. Mol. Biol. 2011, 18, 1290–1296. [Google Scholar] [CrossRef]
- Ngo, H.B.; Lovely, G.A.; Phillips, R.; Chan, D.C. Distinct Structural Features of TFAM Drive Mitochondrial DNA Packaging versus Transcriptional Activation. Nat. Commun. 2014, 5, 3077. [Google Scholar] [CrossRef]
- Xu, W.; Boyd, R.M.; Tree, M.O.; Samkari, F.; Zhao, L. Mitochondrial Transcription Factor A Promotes DNA Strand Cleavage at Abasic Sites. Proc. Natl. Acad. Sci. USA 2019, 116, 17792–17799. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular Mechanism of the Catalase Reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.P.; Chung, M.L.; Anderson, P.J.; Johnson, M.; Epstein, D.L. Hydrogen Peroxide Removal by the Calf Aqueous Outflow Pathway. Investig. Ophthalmol. Vis. Sci. 1988, 29, 976–981. [Google Scholar]
- Gong, B.; Shi, Y.; Qu, C.; Ye, Z.; Yin, Y.; Tan, C.; Shuai, P.; Li, J.; Guo, X.; Cheng, Y.; et al. Association of Catalase Polymorphisms with Primary Open-Angle Glaucoma in a Chinese Population. Ophthalmic Genet. 2018, 39, 35–40. [Google Scholar] [CrossRef]
- Graybeal, K.; Sanchez, L.; Zhang, C.; Stiles, L.; Zheng, J.J. Characterizing the Metabolic Profile of Dexamethasone Treated Human Trabecular Meshwork Cells. Exp. Eye Res. 2022, 214, 108888. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Umetsu, A.; Suzuki, S.; Furuhashi, M.; Ida, Y.; Hikage, F.; Ohguro, H. Human Trabecular Meshwork (HTM) Cells Treated with TGF-Β2 or Dexamethasone Respond to Compression Stress in Different Manners. Biomedicines 2022, 10, 1338. [Google Scholar] [CrossRef]
- Kennedy, S.; Williams, C.; Tsaturian, E.; Morgan, J.T. Dexamethasone Impairs ATP Production and Mitochondrial Performance in Human Trabecular Meshwork Cells. Curr. Issues Mol. Biol. 2024, 46, 9867–9880. [Google Scholar] [CrossRef]
- Nacarelli, T.; Azar, A.; Sell, C. Inhibition of mTOR Prevents ROS Production Initiated by Ethidium Bromide-Induced Mitochondrial DNA Depletion. Front. Endocrinol. 2014, 5, 122. [Google Scholar] [CrossRef]
- Desjardins, P.; Frost, E.; Morais, R. Ethidium Bromide-Induced Loss of Mitochondrial DNA from Primary Chicken Embryo Fibroblasts. Mol. Cell Biol. 1985, 5, 1163–1169. [Google Scholar] [CrossRef]
- Nass, M.M. Differential Effects of Ethidium Bromide on Mitochondrial and Nuclear DNA Synthesis in Vivo in Cultured Mammalian Cells. Exp. Cell Res. 1972, 72, 211–222. [Google Scholar] [CrossRef]
- Zylber, E.; Vesco, C.; Penman, S. Selective Inhibition of the Synthesis of Mitochondria-Associated RNA by Ethidium Bromide. J. Mol. Biol. 1969, 44, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zylber, E.; Penman, S. Mitochondrial-Associated 4 S RNA Synthesis Inhibition by Ethidium Bromide. J. Mol. Biol. 1969, 46, 201–204. [Google Scholar] [CrossRef]
- Nass, M.M. Abnormal DNA Patterns in Animal Mitochondria: Ethidium Bromide-Induced Breakdown of Closed Circular DNA and Conditions Leading to Oligomer Accumulation. Proc. Natl. Acad. Sci. USA 1970, 67, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Khozhukhar, N.; Spadafora, D.; Rodriguez, Y.; Alexeyev, M. Elimination of Mitochondrial DNA from Mammalian Cells. Curr. Protoc. Cell Biol. 2018, 78, 20.11.1–20.11.14. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, R.D. The Effect of Ethidium Bromide on Mitochondrial DNA Synthesis and Mitochondrial DNA Structure in HeLa Cells. J. Cell Biol. 1971, 51, 116–122. [Google Scholar] [CrossRef]
- Kavli, B.; Slupphaug, G.; Mol, C.D.; Arvai, A.S.; Peterson, S.B.; Tainer, J.A.; Krokan, H.E. Excision of Cytosine and Thymine from DNA by Mutants of Human Uracil-DNA Glycosylase. EMBO J. 1996, 15, 3442–3447. [Google Scholar] [CrossRef]
- Shokolenko, I.N.; Wilson, G.L.; Alexeyev, M.F. Persistent Damage Induces Mitochondrial DNA Degradation. DNA Repair 2013, 12, 488–499. [Google Scholar] [CrossRef]
- Sharma, R.; Grover, A. Myocilin-Associated Glaucoma: A Historical Perspective and Recent Research Progress. Mol. Vis. 2021, 27, 480–493. [Google Scholar]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.-K.; Zhang, G.; Zhang, B.-T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef]
- Browne, J.G.; Ho, S.L.; Kane, R.; Oliver, N.; Clark, A.F.; O’Brien, C.J.; Crean, J.K. Connective Tissue Growth Factor Is Increased in Pseudoexfoliation Glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3660–3666. [Google Scholar] [CrossRef]
- Junglas, B.; Kuespert, S.; Seleem, A.A.; Struller, T.; Ullmann, S.; Bösl, M.; Bosserhoff, A.; Köstler, J.; Wagner, R.; Tamm, E.R.; et al. Connective Tissue Growth Factor Causes Glaucoma by Modifying the Actin Cytoskeleton of the Trabecular Meshwork. Am. J. Pathol. 2012, 180, 2386–2403. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Brodskaya, M.W. Fibronectin Detection in Drainage Outflow System of Human Eyes in Ageing and Progression of Open-Angle Glaucoma. Mech. Ageing Dev. 1989, 47, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-F.; Tane, N.; Roy, S. Fibronectin Overexpression Inhibits Trabecular Meshwork Cell Monolayer Permeability. Mol. Vis. 2004, 10, 750–757. [Google Scholar] [PubMed]
- Bradley, J.M.; Vranka, J.; Colvis, C.M.; Conger, D.M.; Alexander, J.P.; Fisk, A.S.; Samples, J.R.; Acott, T.S. Effect of Matrix Metalloproteinases Activity on Outflow in Perfused Human Organ Culture. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2649–2658. [Google Scholar]
- Yang, Y.-F.; Sun, Y.Y.; Acott, T.S.; Keller, K.E. Effects of Induction and Inhibition of Matrix Cross-Linking on Remodeling of the Aqueous Outflow Resistance by Ocular Trabecular Meshwork Cells. Sci. Rep. 2016, 6, 30505. [Google Scholar] [CrossRef]
- Dan, J.; Belyea, D.; Gertner, G.; Leshem, I.; Lusky, M.; Miskin, R. Plasminogen Activator Inhibitor-1 in the Aqueous Humor of Patients with and without Glaucoma. Arch. Ophthalmol. 2005, 123, 220–224. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Welge-Lussen, U.; Lütjen-Drecoll, E. The Effect of TGF-Beta2 on Human Trabecular Meshwork Extracellular Proteolytic System. Exp. Eye Res. 2003, 77, 757–765. [Google Scholar] [CrossRef]
- Wang, W.-H.; McNatt, L.G.; Pang, I.-H.; Millar, J.C.; Hellberg, P.E.; Hellberg, M.H.; Steely, H.T.; Rubin, J.S.; Fingert, J.H.; Sheffield, V.C.; et al. Increased Expression of the WNT Antagonist sFRP-1 in Glaucoma Elevates Intraocular Pressure. J. Clin. Investig. 2008, 118, 1056–1064. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Dreier, B.; Reilly, C.M.; Thomasy, S.M.; Wood, J.A.; Ly, I.; Tuyen, B.C.; Hughbanks, M.; Murphy, C.J.; et al. Role of Substratum Stiffness in Modulating Genes Associated with Extracellular Matrix and Mechanotransducers YAP and TAZ. Investig. Ophthalmol. Vis. Sci. 2013, 54, 378–386. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Chang, Y.-R.; Weber, D.; Phinney, B.; Murphy, C.J.; Russell, P. Transforming Growth Factor Beta 3 Modifies Mechanics and Composition of Extracellular Matrix Deposited by Human Trabecular Meshwork Cells. ACS Biomater. Sci. Eng. 2015, 1, 110–118. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef]
- Morgan, J.T.; Raghunathan, V.K.; Chang, Y.-R.; Murphy, C.J.; Russell, P. Wnt Inhibition Induces Persistent Increases in Intrinsic Stiffness of Human Trabecular Meshwork Cells. Exp. Eye Res. 2015, 132, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-M.; Park, J.-H.; Park, I.-H.; Lee, H.-M. Doxycycline Inhibits TGF-Β1-Induced Extracellular Matrix Production in Nasal Polyp-Derived Fibroblasts. Int. Forum Allergy Rhinol. 2016, 6, 256–263. [Google Scholar] [CrossRef]
- Fujita, H.; Sakamoto, N.; Ishimatsu, Y.; Kakugawa, T.; Hara, S.; Hara, A.; Amenomori, M.; Ishimoto, H.; Nagata, T.; Mukae, H.; et al. Effects of Doxycycline on Production of Growth Factors and Matrix Metalloproteinases in Pulmonary Fibrosis. Respiration 2011, 81, 420–430. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, Y.; Jin, K.; Lu, Z.; Zeng, Z.; Xiong, W. Communication between Mitochondria and Other Organelles: A Brand-New Perspective on Mitochondria in Cancer. Cell Biosci. 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Tigano, M.; Vargas, D.C.; Tremblay-Belzile, S.; Fu, Y.; Sfeir, A. Nuclear Sensing of Breaks in Mitochondrial DNA Enhances Immune Surveillance. Nature 2021, 591, 477–481. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Wang, P.; Wang, G. Non-Coding RNA Regulated Cross-Talk Between Mitochondria and Other Cellular Compartments. Front. Cell Dev. Biol. 2021, 9, 688523. [Google Scholar] [CrossRef] [PubMed]
- Baleriola, J.; García-Feijoo, J.; Martínez-de-la-Casa, J.M.; Fernández-Cruz, A.; de la Rosa, E.J.; Fernández-Durango, R. Apoptosis in the Trabecular Meshwork of Glaucomatous Patients. Mol. Vis. 2008, 14, 1513–1516. [Google Scholar]
- Alvarado, J.; Murphy, C.; Juster, R. Trabecular Meshwork Cellularity in Primary Open-Angle Glaucoma and Nonglaucomatous Normals. Ophthalmology 1984, 91, 564–579. [Google Scholar] [CrossRef]
- Liton, P.B.; Challa, P.; Stinnett, S.; Luna, C.; Epstein, D.L.; Gonzalez, P. Cellular Senescence in the Glaucomatous Outflow Pathway. Exp. Gerontol. 2005, 40, 745–748. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as Playmakers of Apoptosis, Autophagy and Senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Ruchko, M.; Gorodnya, O.; LeDoux, S.P.; Alexeyev, M.F.; Al-Mehdi, A.-B.; Gillespie, M.N. Mitochondrial DNA Damage Triggers Mitochondrial Dysfunction and Apoptosis in Oxidant-Challenged Lung Endothelial Cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L530–L535. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sagua, R.; Parra, V.; López-Crisosto, C.; Díaz, P.; Quest, A.F.G.; Lavandero, S. Calcium Transport and Signaling in Mitochondria. Compr. Physiol. 2017, 7, 623–634. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, J.; Zhang, X.; Zhao, X.; An, P.; Luo, Y.; Luo, J. The Regulatory Roles of Mitochondrial Calcium and the Mitochondrial Calcium Uniporter in Tumor Cells. Int. J. Mol. Sci. 2022, 23, 6667. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Frye, A.M.; Phuong, T.T.T.; Yarishkin, O.; Jo, A.O.; Xu, Y.; Lakk, M.; Iuso, A.; Redmon, S.N.; Ambati, B.; et al. TRPV4 Regulates Calcium Homeostasis, Cytoskeletal Remodeling, Conventional Outflow and Intraocular Pressure in the Mammalian Eye. Sci. Rep. 2016, 6, 30583. [Google Scholar] [CrossRef] [PubMed]
- Guarino, B.D.; Paruchuri, S.; Thodeti, C.K. The Role of TRPV4 Channels in Ocular Function and Pathologies. Exp. Eye Res. 2020, 201, 108257. [Google Scholar] [CrossRef]
- Lapajne, L.; Rudzitis, C.N.; Cullimore, B.; Ryskamp, D.; Lakk, M.; Redmon, S.N.; Yarishkin, O.; Krizaj, D. TRPV4: Cell Type-Specific Activation, Regulation and Function in the Vertebrate Eye. Curr. Top. Membr. 2022, 89, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D.; Chen, Y.-L.; Kasetti, R.B.; Maddineni, P.; Mayhew, W.; Millar, J.C.; Ellis, D.Z.; Sonkusare, S.K.; Zode, G.S. Impaired TRPV4-eNOS Signaling in Trabecular Meshwork Elevates Intraocular Pressure in Glaucoma. Proc. Natl. Acad. Sci. USA 2021, 118, e2022461118. [Google Scholar] [CrossRef]
- Luo, N.; Conwell, M.D.; Chen, X.; Kettenhofen, C.I.; Westlake, C.J.; Cantor, L.B.; Wells, C.D.; Weinreb, R.N.; Corson, T.W.; Spandau, D.F.; et al. Primary Cilia Signaling Mediates Intraocular Pressure Sensation. Proc. Natl. Acad. Sci. USA 2014, 111, 12871–12876. [Google Scholar] [CrossRef]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA Stress Primes the Antiviral Innate Immune Response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Wm, S.; Md, C.; Cm, R. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and Fibrosis. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Zaykov, V.; Chaqour, B. The CCN2/CTGF Interactome: An Approach to Understanding the Versatility of CCN2/CTGF Molecular Activities. J. Cell Commun. Signal 2021, 15, 567–580. [Google Scholar] [CrossRef]
- Stecher, V.J.; Kaplan, J.E.; Connolly, K.; Mielens, Z.; Saelens, J.K. Fibronectin in Acute and Chronic Inflammation. Arthritis Rheum. 1986, 29, 394–399. [Google Scholar] [CrossRef]
- Kolachala, V.L.; Bajaj, R.; Wang, L.; Yan, Y.; Ritzenthaler, J.D.; Gewirtz, A.T.; Roman, J.; Merlin, D.; Sitaraman, S.V. Epithelial-Derived Fibronectin Expression, Signaling, and Function in Intestinal Inflammation. J. Biol. Chem. 2007, 282, 32965–32973. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Falk, W.; Göke, M.; Schölmerich, J.; Rogler, G. Inflammation Modulates Fibronectin Isoform Expression in Colonic Lamina Propria Fibroblasts (CLPF). Int. J. Color. Dis. 2008, 23, 947–955. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 Trans-Signaling Induces Plasminogen Activator Inhibitor-1 from Vascular Endothelial Cells in Cytokine Release Syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef]
- Lambert, A.J.; Boysen, H.M.; Buckingham, J.A.; Yang, T.; Podlutsky, A.; Austad, S.N.; Kunz, T.H.; Buffenstein, R.; Brand, M.D. Low Rates of Hydrogen Peroxide Production by Isolated Heart Mitochondria Associate with Long Maximum Lifespan in Vertebrate Homeotherms. Aging Cell 2007, 6, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Cree, L.M.; Patel, S.K.; Pyle, A.; Lynn, S.; Turnbull, D.M.; Chinnery, P.F.; Walker, M. Age-Related Decline in Mitochondrial DNA Copy Number in Isolated Human Pancreatic Islets. Diabetologia 2008, 51, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in Skeletal Muscle Mitochondrial Function with Aging in Humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Kaaman, M.; Sparks, L.M.; van Harmelen, V.; Smith, S.R.; Sjölin, E.; Dahlman, I.; Arner, P. Strong Association between Mitochondrial DNA Copy Number and Lipogenesis in Human White Adipose Tissue. Diabetologia 2007, 50, 2526–2533. [Google Scholar] [CrossRef]
- Laderman, K.A.; Penny, J.R.; Mazzucchelli, F.; Bresolin, N.; Scarlato, G.; Attardi, G. Aging-Dependent Functional Alterations of Mitochondrial DNA (mtDNA) from Human Fibroblasts Transferred into mtDNA-Less Cells. J. Biol. Chem. 1996, 271, 15891–15897. [Google Scholar] [CrossRef]
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-Associated Reduction of Cell Spreading Induces Mitochondrial DNA Common Deletion by Oxidative Stress in Human Skin Dermal Fibroblasts: Implication for Human Skin Connective Tissue Aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef]
- Solanky, D.; Fields, J.A.; Iudicello, J.E.; Ellis, R.J.; Franklin, D.; Clifford, D.B.; Gelman, B.B.; Marra, C.M.; Morgello, S.; Rubin, L.H.; et al. Higher Buccal Mitochondrial DNA and Mitochondrial Common Deletion Number Are Associated with Markers of Neurodegeneration and Inflammation in Cerebrospinal Fluid. J. Neurovirol 2022, 28, 281–290. [Google Scholar] [CrossRef]
- Cortopassi, G.A.; Arnheim, N. Detection of a Specific Mitochondrial DNA Deletion in Tissues of Older Humans. Nucleic Acids Res. 1990, 18, 6927–6933. [Google Scholar] [CrossRef]
- Cooper, J.M.; Mann, V.M.; Schapira, A.H. Analyses of Mitochondrial Respiratory Chain Function and Mitochondrial DNA Deletion in Human Skeletal Muscle: Effect of Ageing. J. Neurol. Sci. 1992, 113, 91–98. [Google Scholar] [CrossRef]
- Bender, A.; Krishnan, K.J.; Morris, C.M.; Taylor, G.A.; Reeve, A.K.; Perry, R.H.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; et al. High Levels of Mitochondrial DNA Deletions in Substantia Nigra Neurons in Aging and Parkinson Disease. Nat. Genet. 2006, 38, 515–517. [Google Scholar] [CrossRef]
- Cortopassi, G.A.; Shibata, D.; Soong, N.W.; Arnheim, N. A Pattern of Accumulation of a Somatic Deletion of Mitochondrial DNA in Aging Human Tissues. Proc. Natl. Acad. Sci. USA 1992, 89, 7370–7374. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Pang, C.Y.; You, B.J.; Lee, H.C. Tandem Duplications and Large-Scale Deletions of Mitochondrial DNA Are Early Molecular Events of Human Aging Process. Ann. N. Y. Acad. Sci. 1996, 786, 82–101. [Google Scholar] [CrossRef]
- Zhang, C.; Linnane, A.W.; Nagley, P. Occurrence of a Particular Base Substitution (3243 A to G) in Mitochondrial DNA of Tissues of Ageing Humans. Biochem. Biophys. Res. Commun. 1993, 195, 1104–1110. [Google Scholar] [CrossRef]
- Liu, X.; Huang, C.-Y.; Cai, S.; Polansky, J.R.; Kaufman, P.L.; Brandt, C.R. Transformation of Human Trabecular Meshwork Cells with SV40 TAg Alters Promoter Utilization. Curr. Eye Res. 2002, 25, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Liu, X.; Nguyen, T.D.; Polansky, J.R.; Brandt, C.R.; Kaufman, P.L.; Peters, D.M. In Vitro Localization of TIGR/MYOC in Trabecular Meshwork Extracellular Matrix and Binding to Fibronectin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 151–161. [Google Scholar]
- Doucette, L.P.; Footz, T.; Walter, M.A. FOXC1 Regulates Expression of Prostaglandin Receptors Leading to an Attenuated Response to Latanoprost. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2548–2554. [Google Scholar] [CrossRef]
- Peotter, J.L.; Phillips, J.; Tong, T.; Dimeo, K.; Gonzalez, J.M.; Peters, D.M. Involvement of Tiam1, RhoG and ELMO2/ILK in Rac1-Mediated Phagocytosis in Human Trabecular Meshwork Cells. Exp. Cell Res. 2016, 347, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Filla, M.S.; Faralli, J.A.; Desikan, H.; Peotter, J.L.; Wannow, A.C.; Peters, D.M. Activation of Avβ3 Integrin Alters Fibronectin Fibril Formation in Human Trabecular Meshwork Cells in a ROCK-Independent Manner. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3897–3913. [Google Scholar] [CrossRef]
- Bioart. Available online: https://bioart.niaid.nih.gov/bioart/411 (accessed on 8 May 2025).
- Bioart. Available online: https://bioart.niaid.nih.gov/bioart/171 (accessed on 8 May 2025).
- Bioart. Available online: https://bioart.niaid.nih.gov/bioart/198 (accessed on 8 May 2025).
- Bioart. Available online: https://bioart.niaid.nih.gov/bioart/543 (accessed on 8 May 2025).
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline Antibiotics Impair Mitochondrial Function and Its Experimental Use Confounds Research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef]
- Clark-Walker, G.D.; Linnane, A.W. In Vivo Differentiation of Yeast Cytoplasmic and Mitochondrial Protein Synthesis with Antibiotics. Biochem. Biophys. Res. Commun. 1966, 25, 8–13. [Google Scholar] [CrossRef]
- Wüst, R.C.I.; Coolen, B.F.; Held, N.M.; Daal, M.R.R.; Alizadeh Tazehkandi, V.; Baks-Te Bulte, L.; Wiersma, M.; Kuster, D.W.D.; Brundel, B.J.J.M.; van Weeghel, M.; et al. The Antibiotic Doxycycline Impairs Cardiac Mitochondrial and Contractile Function. Int. J. Mol. Sci. 2021, 22, 4100. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Musrap, N.; Cretu, D.; Karagiannis, G.S.; Batruch, I.; Smith, C.; Drabovich, A.P.; Trudel, D.; van der Kwast, T.; Morrissey, C.; et al. Proteomic Profiling of Androgen-Independent Prostate Cancer Cell Lines Reveals a Role for Protein S during the Development of High Grade and Castration-Resistant Prostate Cancer. J. Biol. Chem. 2012, 287, 34019–34031. [Google Scholar] [CrossRef] [PubMed]
- Fuchshofer, R.; Yu, A.H.L.; Welge-Lüssen, U.; Tamm, E.R. Bone Morphogenetic Protein-7 Is an Antagonist of Transforming Growth Factor-Beta2 in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 715–726. [Google Scholar] [CrossRef]
- Han, H.; Kampik, D.; Grehn, F.; Schlunck, G. TGF-Β2-Induced Invadosomes in Human Trabecular Meshwork Cells. PLoS ONE 2013, 8, e70595. [Google Scholar] [CrossRef]
- Luna, C.; Parker, M.; Challa, P.; Gonzalez, P. Long-Term Decrease of Intraocular Pressure in Rats by Viral Delivery of miR-146a. Transl. Vis. Sci. Technol. 2021, 10, 14. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.G.; Patton, J.T.; Anders, R.A.; Yolken, R.H.; Schwarz, K.B. Rotavirus Infects Human Biliary Epithelial Cells and Stimulates Secretion of Cytokines IL-6 and IL-8 via MAPK Pathway. Biomed. Res. Int. 2015, 2015, 697238. [Google Scholar] [CrossRef]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Di Paola, M.; Dell’aquila, C.; De Mari, M.; et al. Effect of Resveratrol on Mitochondrial Function: Implications in Parkin-Associated Familiar Parkinson’s Disease. Biochim. Biophys. Acta 2014, 1842, 902–915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).