Novel Tetraene Macrodiolides Are Effective Inducers of Mitochondrial Apoptosis in Jurkat Cells
Abstract
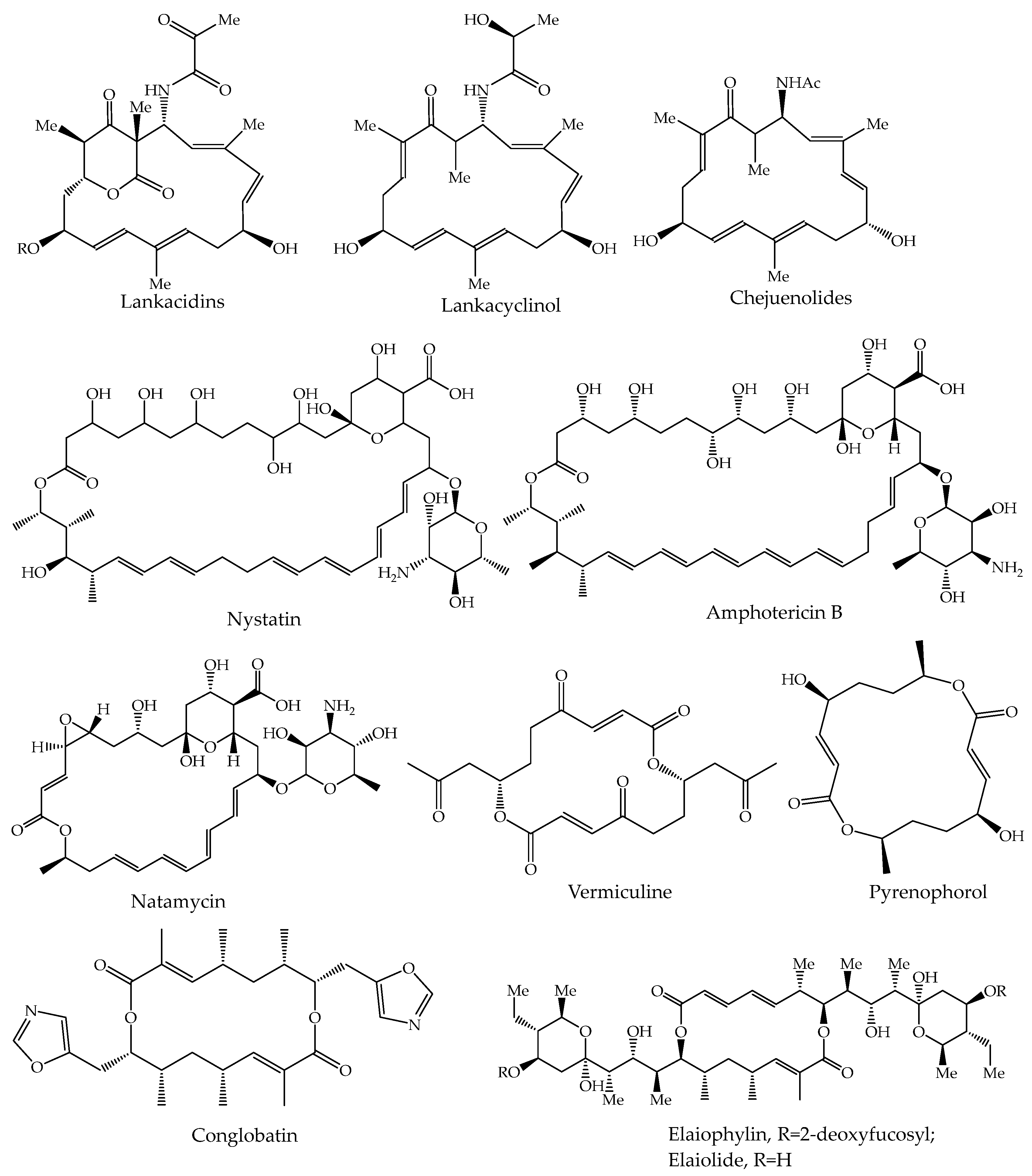
1. Introduction
2. Results and Discussion
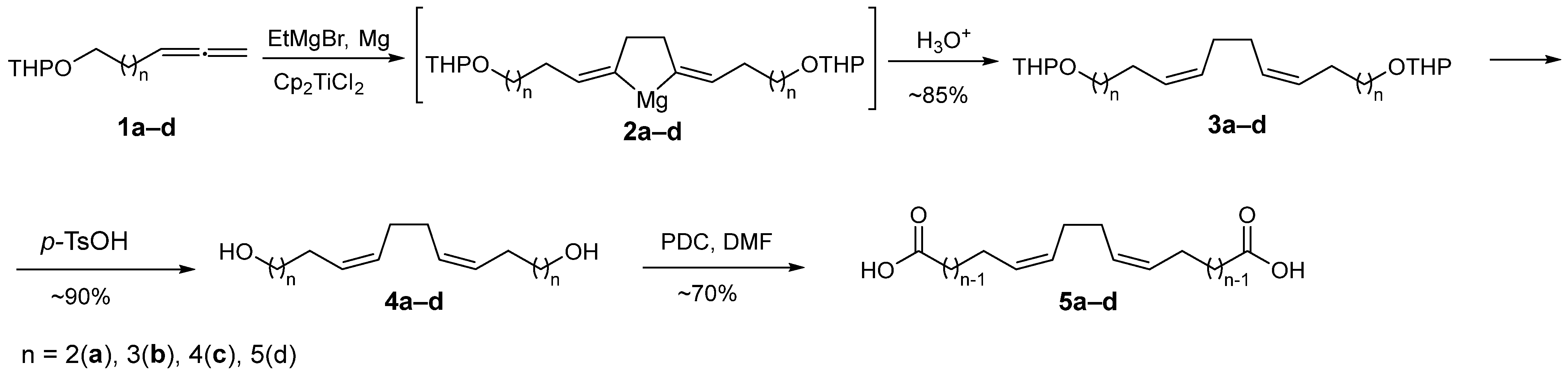
2.1. Chemistry
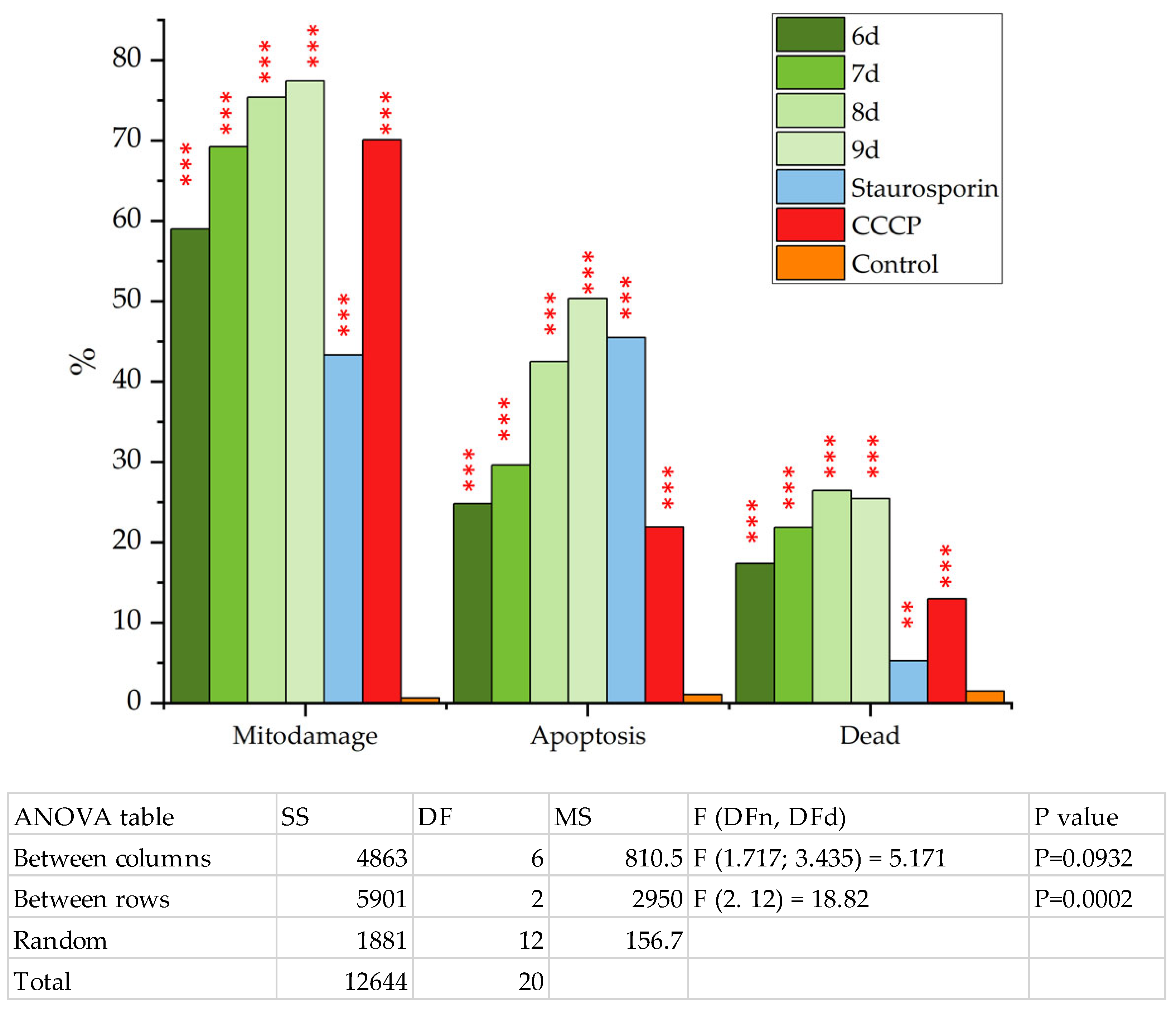
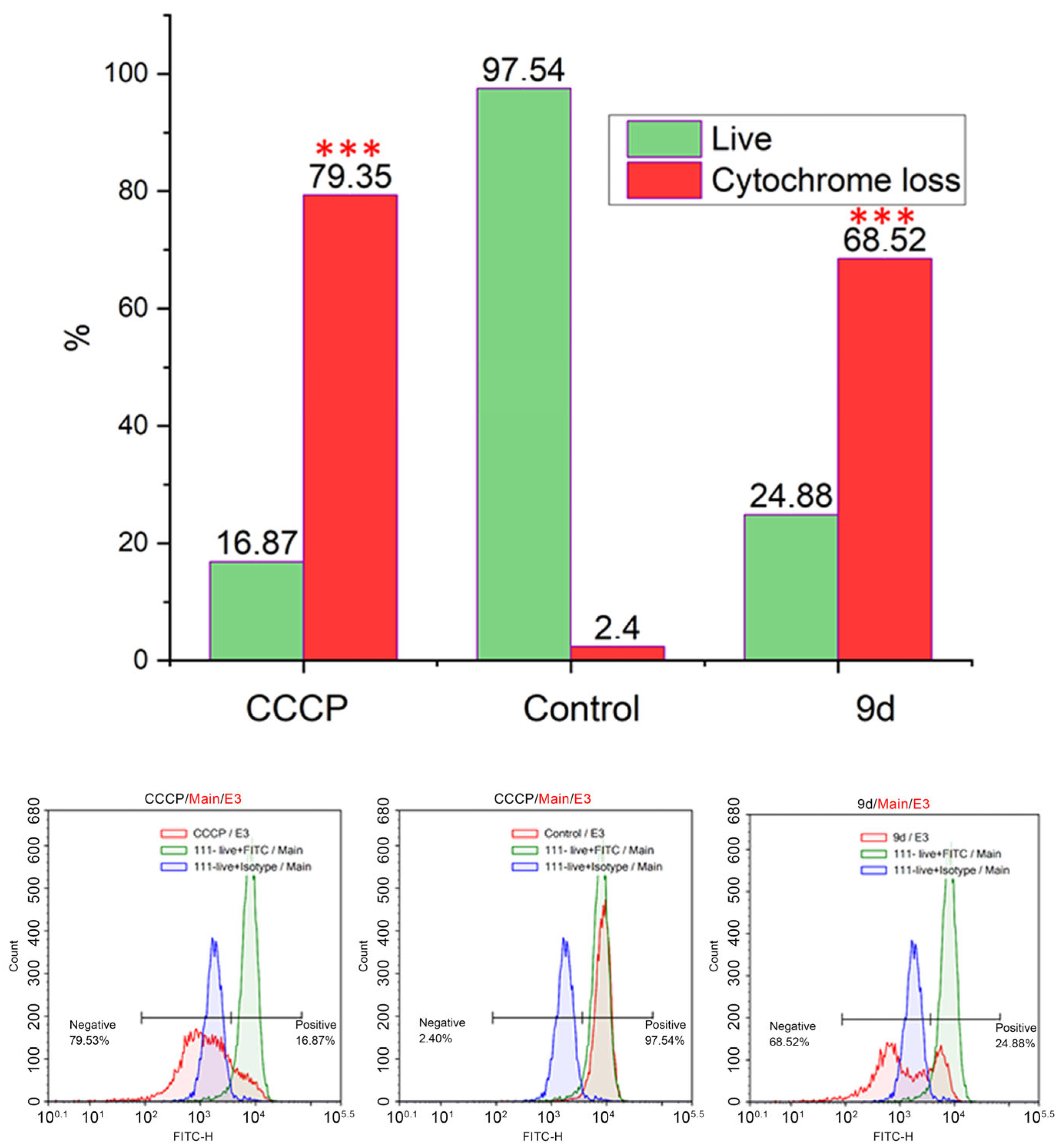
2.2. Biological Studies
- -
- The following significant conclusions can be drawn from the analysis of the obtained data:
- -
- The cytotoxic effect on the studied tumor lines increases in the series: Jurkat < K562 < U937 ≤ HL-60.
- -
- A well-defined tendency of increasing toxicity of macrocycles with an increasing number of methylene links in dioic acid 5, at a fixed number of carbon atoms in diol 4, in each example for 4a–4d is evident; the mentioned pattern is also observed for both the conditionally normal cell line Hek293 and the normal fibroblasts.
- -
- The maximum cytotoxic effect on the Jurkat and K562 tumor lines was observed for compound 9d (CC50 = 11.04 and 12.11), while for the U937 cell line, compounds 9d and 8d exhibited approximately equal maximum toxicity (CC50 = 12.21 and 12.23). For the HL60 cells, the maximum effect was observed for macrocycles 9c and 9d (CC50 = 12.83 and 13.46).
3. Materials and Methods
3.1. Chemistry
3.2. Synthetic Procedures
3.2.1. The General Procedure for the Synthesis of α,ω-Alka-nZ,(n+4)Z-Dienedioic Acid 5a–d
3.2.2. The General Procedure for the Synthesis of Tetraene Macrodiolides 6a–9d
3.3. Biological Studies
3.3.1. Cell Culturing
3.3.2. Preparation of Effluent and Test Compound Solutions for Biological Testing
3.3.3. Cytotoxicity Assay
3.3.4. Mitodamage Assay
3.3.5. Cytochrome C Assay
3.3.6. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsault, E.; Peterson, M.L. Macrocycles are great cycles: Applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hogan, M.; Muldoon, J.; Evans, P.; Caffrey, P. Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B. Molecules 2024, 29, 1396. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, L.; Sun, B.; Wang, M.; Li, H.; Stoddart, J.F.; Huang, F. Applications of macrocycle-based solid-state host–guest chemistry. Nat. Rev. Chem. 2023, 7, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, L.; Jia, Z.; Loh, T.P. Recent applications of macrocycles in supramolecular catalysis. Chin. Chem. Lett. 2024, 35, 109075. [Google Scholar] [CrossRef]
- Wang, L.; Lu, H.; Jiang, Y. Natural Polyketides Act as Promising Antifungal Agents. Biomolecules 2023, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Dong, Y.; Zhou, H.; Cui, H. Effect of Post–Polyketide Synthase Modification Groups on Property and Activity of Polyene Macrolides. Antibiotics 2023, 12, 119. [Google Scholar] [CrossRef]
- Kricker, J.A.; Page, C.P.; Gardarsson, F.R.; Baldursson, O.; Gudjonsson, T.; Parnham, M.J. Nonantimicrobial actions of macrolides: Overview and perspectives for future development. Pharmacol. Rev. 2021, 73, 1404–1433. [Google Scholar] [CrossRef]
- Lenz, K.D.; Klosterman, K.E.; Mukundan, H.; Kubicek-Sutherland, J.Z. Macrolides: From Toxins to Therapeutics. Toxins 2021, 13, 347. [Google Scholar] [CrossRef]
- Garcia Jimenez, D.; Poongavanam, V.; Kihlberg, J. Macrocycles in Drug Discovery─Learning from the Past for the Future. J. Med. Chem. 2023, 66, 5377–5396. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, K.; Hong, R. Biomimetic Synthesis of Chejuenolides A–C by a Cryptic Lactone-Based Macrocyclization: Stereochemical Implications in Biosynthesis. ACS Cent. Sci. 2023, 9, 84–92. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, B.; Hong, R. Embracing the Imperfectness of Nature using Highly Reactive N-Acyl Azahexatrienes. Synlett 2024, 35, 513. [Google Scholar] [CrossRef]
- Cai, L.; Seiple, I.B.; Li, Q. Modular Chemical Synthesis of Streptogramin and Lankacidin Antibiotics. Acc. Chem. Res. 2021, 54, 1891–1908. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Sohn, J.H.; Lee, D.; Kim, J.K.; Kong, I.S.; Ahn, S.C.; Oh, H. Chejuenolides A and B, new macrocyclic tetraenes from the marine bacterium Hahella chejuensis. Tetrahedron Lett. 2008, 49, 7128–7131. [Google Scholar] [CrossRef]
- Jiang, C.S.; Liang, L.F.; Guo, Y.W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol. Sin. 2012, 33, 1217–1245. [Google Scholar] [CrossRef]
- Kende, A.S.; Liu, K.; Kaldor, I.; Dorey, G.; Koch, K. Total synthesis of the macrolide antitumor antibiotic lankacidin C. J. Am. Chem. Soc. 1995, 117, 8258–8270. [Google Scholar] [CrossRef]
- Harada, S.; Yamazaki, T.; Hatano, K.; Tsuchiya, K.; Kishi, T. Studies on Lankacidin-Group (T-2636) Antibiotics. J. Antibiot. 1973, 26, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.C.; de Lucca, E.C., Jr. Total synthesis of (−)-marinisporolide C. J. Org. Chem. 2017, 82, 3019–3045. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Caffrey, P.; Hogan, M.; Song, Y. New Glycosylated Polyene Macrolides: Refining the Ore from Genome Mining. Antibiotics 2022, 11, 334. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Rai, A.; Misra, S.R.; Panda, S.; Sokolowski, G.; Mishra, L.; Das, R.; Lapinska, B. Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials. Life 2022, 12, 1677. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhao, G.-Q.; Lin, J.; Wang, X.; Hu, L.-T.; Du, Z.-D.; Wang, Q.; Zhu, C.-C. Natamycin in the Treatment of Fungal Keratitis: A Systematic Review and Meta-Analysis. Int. J. Ophthalmol. 2015, 8, 597–602. [Google Scholar] [PubMed]
- Saravolatz, L.D.; Bern, C.; Adler-Moore, J.; Berenguer, J.; Boelaert, M.; den Boer, M.; Davidson, R.N.; Figueras, C.; Gradoni, L.; Kafetzis, D.A. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin. Infect. Dis. 2006, 43, 917–924. [Google Scholar]
- Zhang, S.; Fan, S.; He, H.; Zhu, J.; Murray, L.; Liang, G.; Ran, S.; Zhu, Y.Z.; Cryle, M.J.; He, H.Y.; et al. Cyclic natural product oligomers: Diversity and (bio) synthesis of macrocycles. Chem. Soc. Rev. 2025, 54, 396–464. [Google Scholar] [CrossRef]
- Ishihara, J. Synthesis of Marine C2-Symmetrical Macrodiolide Natural Products. In Marine Natural Products. Topics in Heterocyclic Chemistry; Kiyota, H., Ed.; Springer: Singapore, 2021; Volume 58, pp. 317–360. [Google Scholar] [CrossRef]
- Vrettou, M.; Gray, A.A.; Brewer, A.R.; Barrett, A.G. Strategies for the synthesis of C2 symmetric natural products—A review. Tetrahedron 2007, 63, 1487–1536. [Google Scholar] [CrossRef]
- Anusha, B.; Kothapalli, R.B.; Victor Prem Sagar, M.; Surendra, B.; Subba Reddy, U.V. Concise review on isolation, biological activity, structure elucidation, and total synthetic approaches of 16-membered C2-symmetric macrolide pyrenophorol. Synth. Commun. 2024, 54, 323–347. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Egold, H.; Draeger, S.; Schulz, B. Diversity of Antimicrobial Pyrenophorol Derivatives from an Endophytic Fungus, Phoma sp. Eur. J. Org. Chem. 2008, 25, 4320–4328. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, U.V.; Subba Reddy, B.V. Stereoselective total synthesis of (−)-pyrenophorol. Tetrahedron Lett. 2009, 50, 5984–5986. [Google Scholar] [CrossRef]
- Okujo, N.; Iinuma, H.; George, A.; Eim, K.S.; Li, T.L.; Ting, N.S.; Jye, T.C.; Hotta, K.; Hatsu, M.; Fukagawa, Y.; et al. Bispolides, novel 20-membered ring macrodiolide antibiotics from microbispora. J. Antibiot. 2007, 60, 216–219. [Google Scholar] [CrossRef][Green Version]
- Steib, P.; Breit, B. Concise Total Synthesis of (-)-Vermiculine through a Rhodium-Catalyzed C2 -Symmetric Dimerization Strategy. Chem. Eur. J. 2019, 25, 3532–3535. [Google Scholar] [CrossRef]
- Zhou, Y.; Murphy, A.C.; Samborskyy, M.; Prediger, P.; Dias, L.C.; Leadlay, P.F. Iterative Mechanism of Macrodiolide Formation in the Anticancer Compound Conglobatin. Chem. Biol. 2015, 22, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Zhang, M.-X.; Wu, W.-H.; Sun, P. Natural Occurrence, Bioactivity and Biosynthesis of Elaiophylin Analogues. Molecules 2019, 24, 3840. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, K.; Meng, X.; Zhong, H.; Li, X.; Zhao, H.; Xie, G.; Xie, Y.; Wang, X.; Zhu, X. Elaiophylin Inhibits Tumorigenesis of Human Lung Adenocarcinoma by Inhibiting Mitophagy via Suppression of SIRT1/Nrf2 Signaling. Cancers 2022, 14, 5812. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural compounds with bis-methylene-interrupted Z-double bonds: Plant sources, strategies of total synthesis, biological activity, and perspectives. Phytochem. Rev. 2021, 20, 325–342. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; D’yakonov, V.A. New catalytic organometallic reactions, that changed the strategy of organic synthesis. Russ. Chem. Rev. 2025, 94, RCR5172. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Khusainova, E.M.; Yunusbaeva, M.; Dzhemilev, U.M. Targeted synthesis of macrodiolides containing bis-methylene-separated Z-double bonds and their antitumor activity in vitro. Tetrahedron 2018, 74, 4606–4612. [Google Scholar] [CrossRef]
- Dzhemileva, L.; D’yakonov, V.A.; Islamov, I.I.; Yunusbaeva, M.M.; Dzhemilev, U.M. New 1Z,5Z-diene macrodiolides: Catalytic synthesis, anticancer activity, induction of mitochondrial apoptosis, and effect on the cell cycle. Bioorganic Chem. 2020, 99, 103832. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Makarova, E.K.; Dzhemilev, U.M. Direct Synthesis of Polyaromatic Cyclophanes Containing Bis-Methylene-Interrupted Z-Double Bonds and Study of Their Antitumor Activity In Vitro. Int. J. Mol. Sci. 2021, 22, 8787. [Google Scholar] [CrossRef]
- Islamov, I.I.; Dzhemileva, L.U.; Gaisin, I.V.; Dzhemilev, U.M.; D′ yakonov, V.A. New Polyether Macrocycles as Promising Antitumor Agents—Targeted Synthesis and Induction of Mitochondrial Apoptosis. ACS Omega 2024, 9, 19923–19931. [Google Scholar] [CrossRef]
- Islamov, I.I.; Gaisin, I.V.; Dzhemilev, U.M.; D’yakonov, V.A. Synthesis of macrocyclic mono- and diolides based on new ω-hydroxyalkadienoic acids with (Z,Z)-1, 5-diene moiety. Russ. Chem. Bull. 2024, 73, 1623–1630. [Google Scholar] [CrossRef]
- Islamov, I.; Gaisin, I. New 1Z,5Z-Diene Compounds: Stereoselective Synthesis of Tetraenoic Macrodiolides. Chem. Proc. 2024, 16, 33. [Google Scholar] [CrossRef]
- de Léséleuc, M.; Collins, S.K. Direct Macrolactonization of Seco Acids via Hafnium(IV) Catalysis. ACS Catal. 2015, 5, 1462–1467. [Google Scholar] [CrossRef]
- de Léséleuc, M.; Collins, S.K. Direct synthesis of macrodiolides via hafnium(IV) catalysis. Chem. Commun. 2015, 51, 10471–10474. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome С. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Zhou, Z.; Arroum, T.; Luo, X.; Kang, R.; Lee, Y.J.; Tang, D.; Hüttemann, M.; Song, X. Diverse functions of cytochrome c in cell death and disease. Cell Death Differ. 2024, 31, 387–404. [Google Scholar] [CrossRef]
- Corey, E.J.; Schmidt, G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Lett. 1979, 20, 399–402. [Google Scholar] [CrossRef]
- Abercrombie, M. Ross Granville Harrison, 1870–1959. Biogr. Mems Fell. R. Soc. 1961, 7, 7110–7126. [Google Scholar] [CrossRef]
- Vempati, U.D.; Diaz, F.; Barrientos, A.; Narisawa, S.; Mian, A.M.; Millán, J.L.; Boise, L.H.; Moraes, C.T. Role of cytochrome C in apoptosis: Increased sensitivity to tumor necrosis factor alpha is associated with respiratory defects but not with lack of cytochrome C release. Mol. Cell. Biol 2007, 27, 1771–1783. [Google Scholar] [CrossRef]

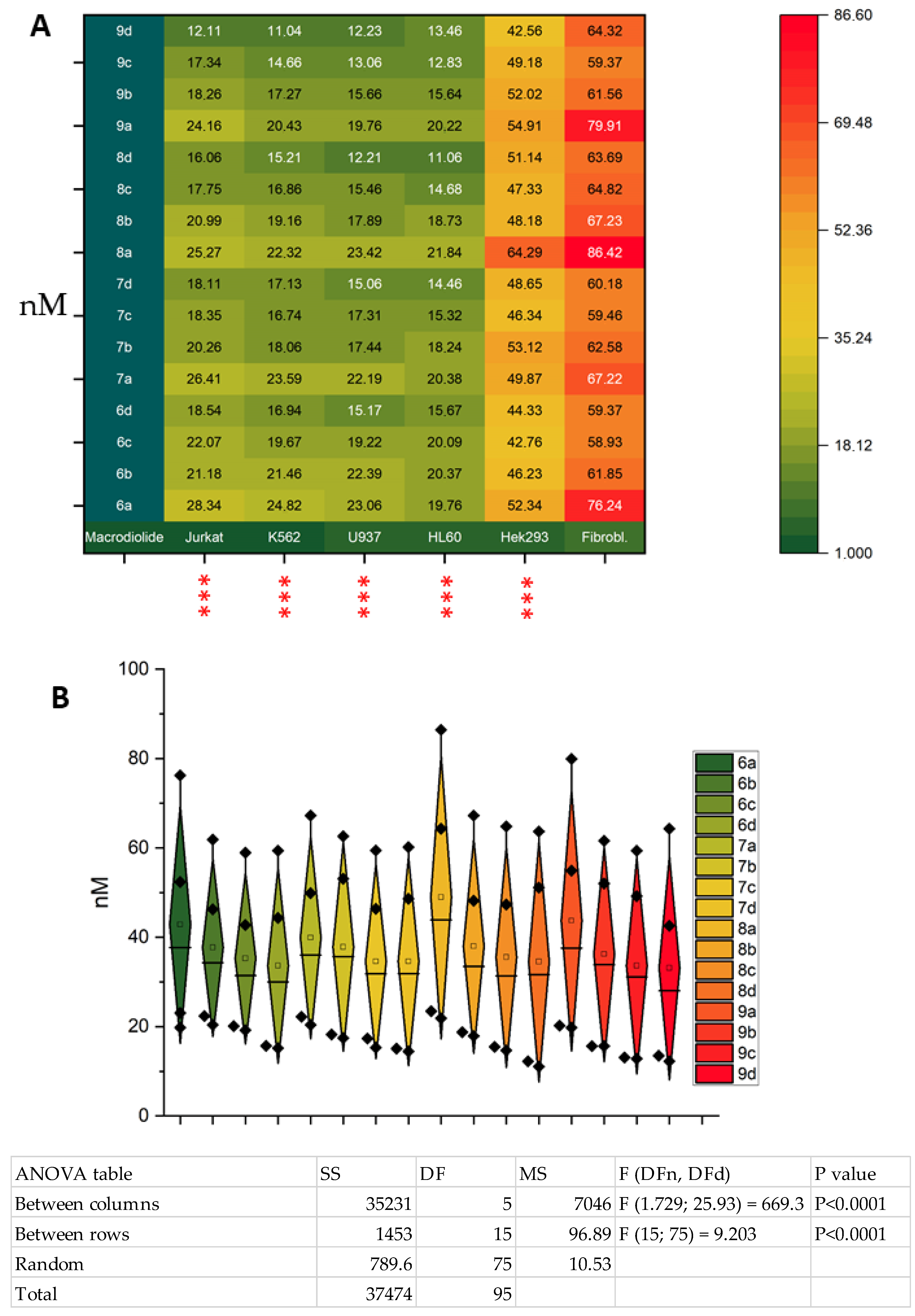
| Macrodiolide | Jurkat (CC50, nM) | SI * | K562 (CC50, nM) | SI | U937 (CC50, nM) | SI | HL60 (CC50, nM) | SI | Hek293 (CC50, nM) | SI | Fibrobl. (CC50, nM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6a | 28.34 ± 2.65 | 2.6 | 24.82 ± 2.37 | 3.0 | 23.06 ± 2.11 | 3.3 | 19.76 ± 1.81 | 3.8 | 52.34 ± 5.17 | 1.4 | 76.24 ± 7.62 |
| 6b | 21.18 ± 1.97 | 2.9 | 21.46 ± 2.01 | 2.8 | 22.39 ± 2.24 | 2.7 | 20.37 ± 2.10 | 3.0 | 46.23 ± 3.75 | 1.3 | 61.85 ± 6.27 |
| 6c | 22.07 ± 2.14 | 2.6 | 19.67 ± 1.63 | 2.9 | 19.22 ± 1.87 | 3.0 | 20.09 ± 1.95 | 2.9 | 42.76 ± 4.39 | 1.3 | 58.93 ± 5.81 |
| 6d | 18.54 ± 1.78 | 3.2 | 16.94 ± 1.88 | 3.5 | 15.17 ± 1.56 | 3.9 | 15.67 ± 1.43 | 3.7 | 44.33 ± 4.21 | 1.3 | 59.37 ± 5.46 |
| 7a | 26.41 ± 2.46 | 2.5 | 23.59 ± 2.14 | 2.8 | 22.19 ± 2.34 | 3.0 | 20.38 ± 2.07 | 3.2 | 49.87 ± 4.83 | 1.3 | 67.22 ± 6.39 |
| 7b | 20.26 ± 2.04 | 3.0 | 18.06 ± 1.83 | 3.4 | 17.44 ± 1.71 | 3.5 | 18.24 ± 1.76 | 3.4 | 53.12 ± 4.96 | 1.1 | 62.58 ± 5.94 |
| 7c | 18.35 ± 1.86 | 3.2 | 16.74 ± 1.59 | 3.5 | 17.31 ± 1.62 | 3.4 | 15.32 ± 1.48 | 3.8 | 46.34 ± 3.99 | 1.2 | 59.46 ± 5.37 |
| 7d | 18.11 ± 1.91 | 3.3 | 17.13 ± 1.84 | 3.5 | 15.06 ± 1.43 | 3.9 | 14.46 ± 1.57 | 4.1 | 48.65 ± 4.82 | 1.2 | 60.18 ± 5.21 |
| 8a | 25.27 ± 2.63 | 3.4 | 22.32 ± 2.16 | 3.8 | 23.42 ± 2.27 | 3.6 | 21.84 ± 1.96 | 3.9 | 64.29 ± 5.37 | 1.3 | 86.42 ± 7.11 |
| 8b | 20.99 ± 2.34 | 3.2 | 19.16 ± 1.79 | 3.5 | 17.89 ± 1.83 | 3.7 | 18.73 ± 1.71 | 3.5 | 48.18 ± 4.34 | 1.3 | 67.23 ± 6.24 |
| 8c | 17.75 ± 1.73 | 3.6 | 16.86 ± 1.62 | 3.8 | 15.46 ± 1.49 | 4.1 | 14.68 ± 1.42 | 4.4 | 47.33 ± 4.22 | 1.3 | 64.82 ± 6.35 |
| 8d | 16.06 ± 1.58 | 3.9 | 15.21 ± 1.46 | 4.1 | 12.21 ± 1.37 | 5.2 | 11.06 ± 1.39 | 5.7 | 51.14 ± 5.60 | 1.2 | 63.69 ± 6.07 |
| 9a | 24.16 ± 2.39 | 3.3 | 20.43 ± 1.89 | 3.9 | 19.76 ± 1.97 | 4.0 | 20.22 ± 2.17 | 3.9 | 54.91 ± 5.37 | 1.4 | 79.91 ± 6.90 |
| 9b | 18.26 ± 2.04 | 3.3 | 17.27 ± 1.64 | 3.5 | 15.66 ± 1.53 | 3.9 | 15.64 ± 1.49 | 3.9 | 52.02 ± 5.43 | 1.1 | 61.56 ± 5.92 |
| 9c | 17.34 ± 1.59 | 3.4 | 14.66 ± 1.49 | 4.0 | 13.06 ± 1.31 | 4.5 | 12.83 ± 1.37 | 4.6 | 49.18 ± 4.77 | 1.2 | 59.37 ± 5.29 |
| 9d | 12.11 ± 1.77 | 5.3 | 11.04 ± 1.25 | 5.8 | 12.23 ± 1.41 | 5.2 | 13.46 ± 1.21 | 4.7 | 42.56 ± 4.31 | 1.5 | 64.32 ± 5.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamov, I.I.; Dzhemileva, L.U.; Gaisin, I.V.; Makarov, A.A.; Dzhemilev, U.M.; D’yakonov, V.A. Novel Tetraene Macrodiolides Are Effective Inducers of Mitochondrial Apoptosis in Jurkat Cells. Int. J. Mol. Sci. 2025, 26, 5139. https://doi.org/10.3390/ijms26115139
Islamov II, Dzhemileva LU, Gaisin IV, Makarov AA, Dzhemilev UM, D’yakonov VA. Novel Tetraene Macrodiolides Are Effective Inducers of Mitochondrial Apoptosis in Jurkat Cells. International Journal of Molecular Sciences. 2025; 26(11):5139. https://doi.org/10.3390/ijms26115139
Chicago/Turabian StyleIslamov, Ilgiz I., Lilya U. Dzhemileva, Ilgam V. Gaisin, Alexey A. Makarov, Usein M. Dzhemilev, and Vladimir A. D’yakonov. 2025. "Novel Tetraene Macrodiolides Are Effective Inducers of Mitochondrial Apoptosis in Jurkat Cells" International Journal of Molecular Sciences 26, no. 11: 5139. https://doi.org/10.3390/ijms26115139
APA StyleIslamov, I. I., Dzhemileva, L. U., Gaisin, I. V., Makarov, A. A., Dzhemilev, U. M., & D’yakonov, V. A. (2025). Novel Tetraene Macrodiolides Are Effective Inducers of Mitochondrial Apoptosis in Jurkat Cells. International Journal of Molecular Sciences, 26(11), 5139. https://doi.org/10.3390/ijms26115139








