Occurrence, Biosynthesis, and Health Benefits of Anthocyanins in Rice and Barley
Abstract
1. Introduction
2. Analysis of Anthocyanins Compositional Diversity, e.g., Cyanidin-3-Glucoside in Black Rice vs. Acylated Forms in Barley
2.1. Extraction and Quantification Methods
2.2. Anthocyanin Composition in Rice and Barley
2.2.1. Genetic Factors (Biosynthetic Pathway Regulation)
Differential Expressions of Anthocyanin Biosynthesis Genes
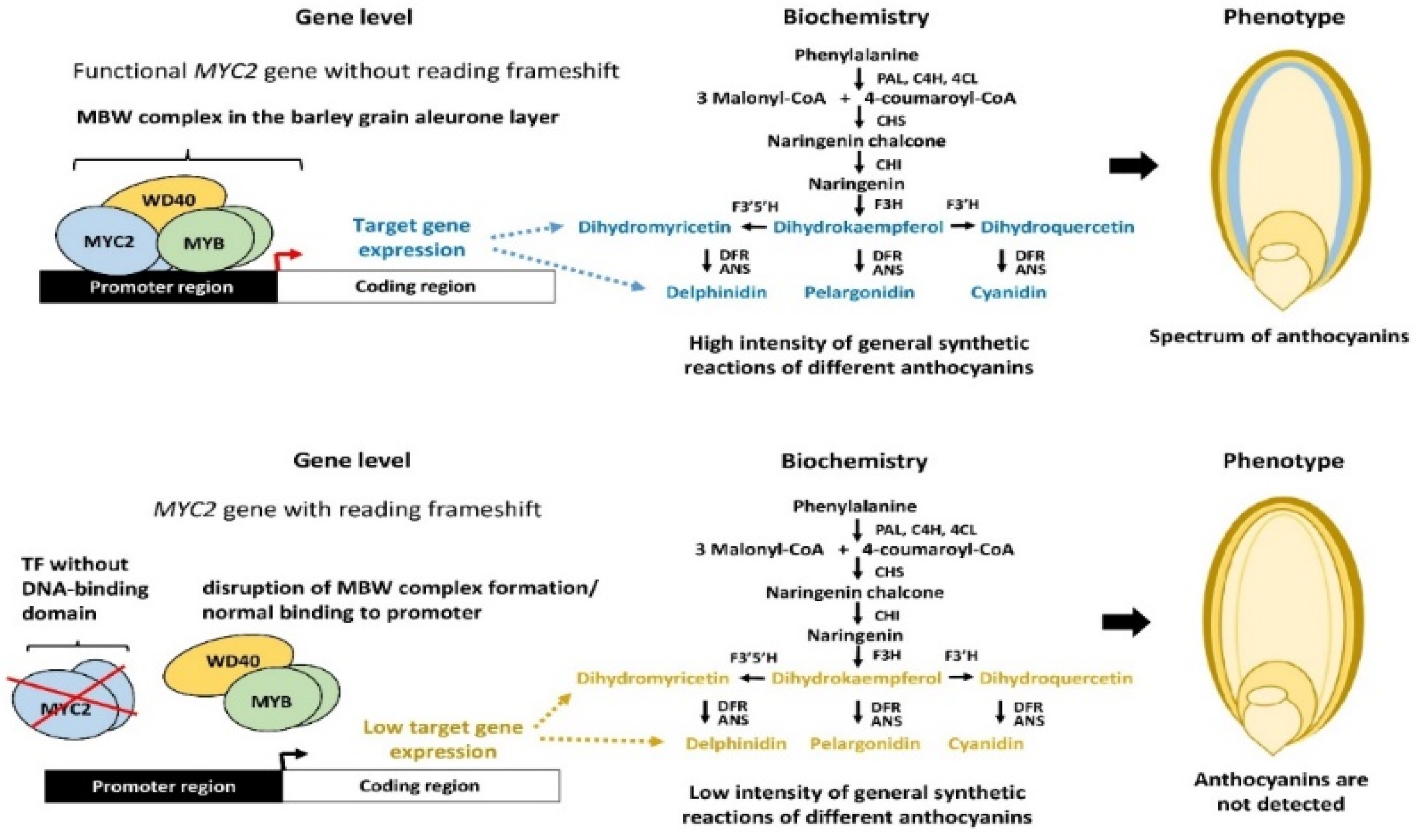
Presence or Absence of Specific Transcription Factors
Mutations and Epigenetic Modifications
Utilizing Genetic Engineering to Enhance Anthocyanin Content in Rice and Barley
Strategies for Enhancing Anthocyanin Production via Genetic Engineering
Over Expression of Key Anthocyanin Biosynthesis Genes
Challenges in Engineering Anthocyanin Biosynthesis
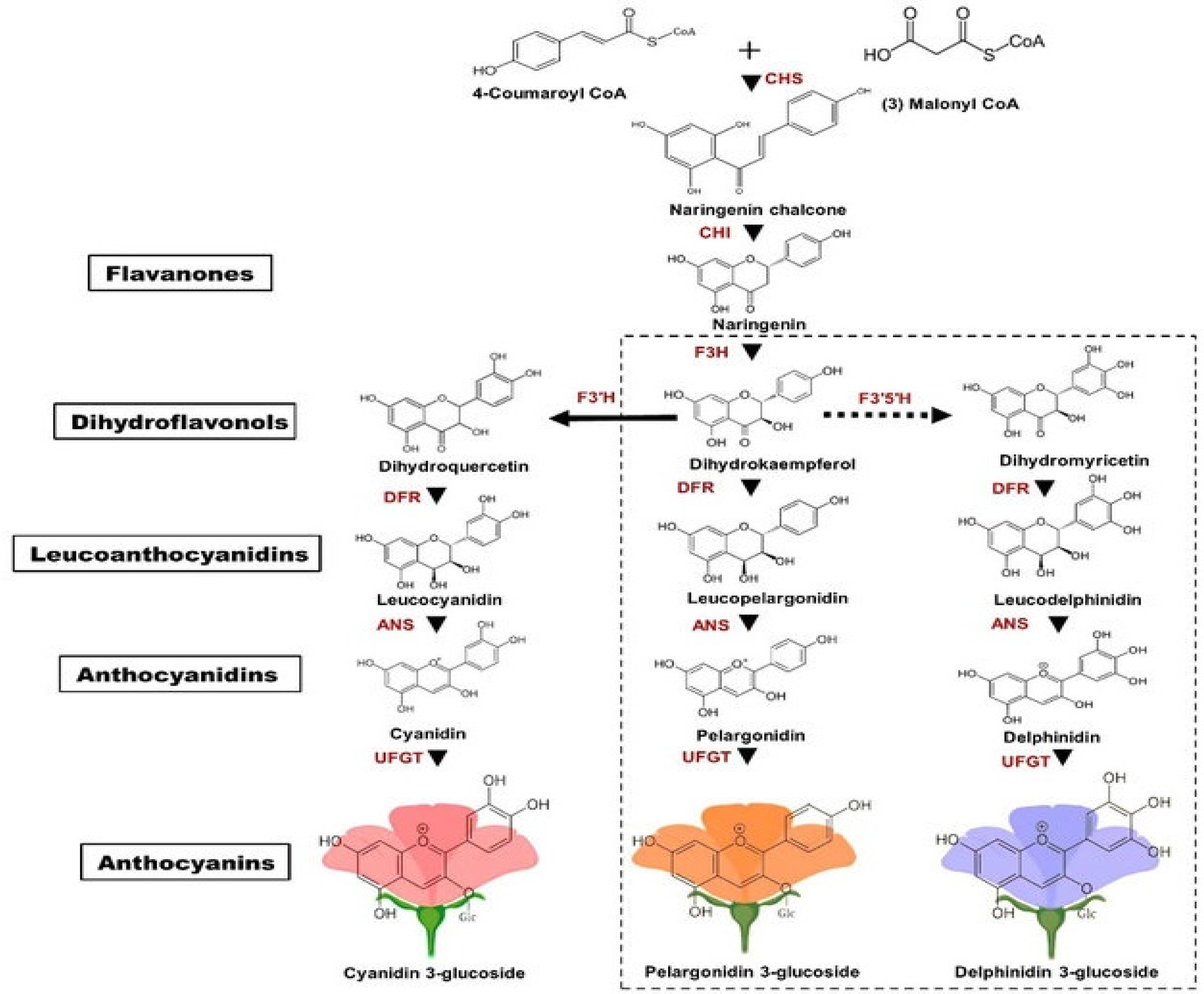
2.2.2. Biochemical Composition of Anthocyanins
Type of Anthocyanidins
Glycosylation and Acylation Patterns
Co-Pigmentation with Other Flavonoids
2.2.3. Environmental and Agronomic Influences
Light Exposure (UV Radiation)
Temperature Stress
Soil Nutrients, pH, and Post-Harvest Processing
2.2.4. Evolutionary and Ecological Adaptations
3. Analysis of Anthocyanins Chemical Stability Under Different Environmental and Processing Conditions
3.1. pH
3.2. Temperature
3.3. Light and Oxygen Exposure
3.4. Enzymatic and Non-Enzymatic Degradation
3.5. Water Stress (Drought vs. Waterlogging)
3.6. Soil Nutrients (Nitrogen, Phosphorus, Metals)
3.7. Strategies to Enhance Anthocyanin Stability
- Acidic processing: using citric or ascorbic acid in food formulations can stabilize anthocyanins [132].
- Encapsulation: microencapsulation techniques protect anthocyanins from degradation [171].
- Cold storage: refrigeration slows down anthocyanin degradation in rice and barley products [172].
- Reduced oxygen packaging: vacuum or nitrogen-flushed packaging minimizes oxidation [173].
| Environmental Conditions | Rice | Barley | References | |
|---|---|---|---|---|
| Water conditions | Drought | Moderate increase | Strong increase | [174,175] |
| Water logging | Sharp decrease | Mild decrease | [174] | |
| Nutrient Condition | Low N | Moderate increase | Strong increase | [174] |
| Low P | Mild increase | Strong increase | [174,175] | |
| Heavy metals | High induction (roots) | Low induction | [175] | |
| Environmental factor | Light dependency | High (UV/blue) | Moderate (blue/red) | [113] |
| Cold response | Moderate (grain/leaf) | Strong (hull/stem) | [113,175] | |
| Heat tolerance | Low (degrades >30 °C) | Moderate (retains pigments) | [113,175,176] | |
| Drought induction | Moderate | Strong | [113,175] | |
| Nutrient stress | N and metal-sensitive | N and P-sensitive | [174] | |
4. Implications of the Nutritional and Health Benefits of Anthocyanins, and Their Role in Disease Prevention
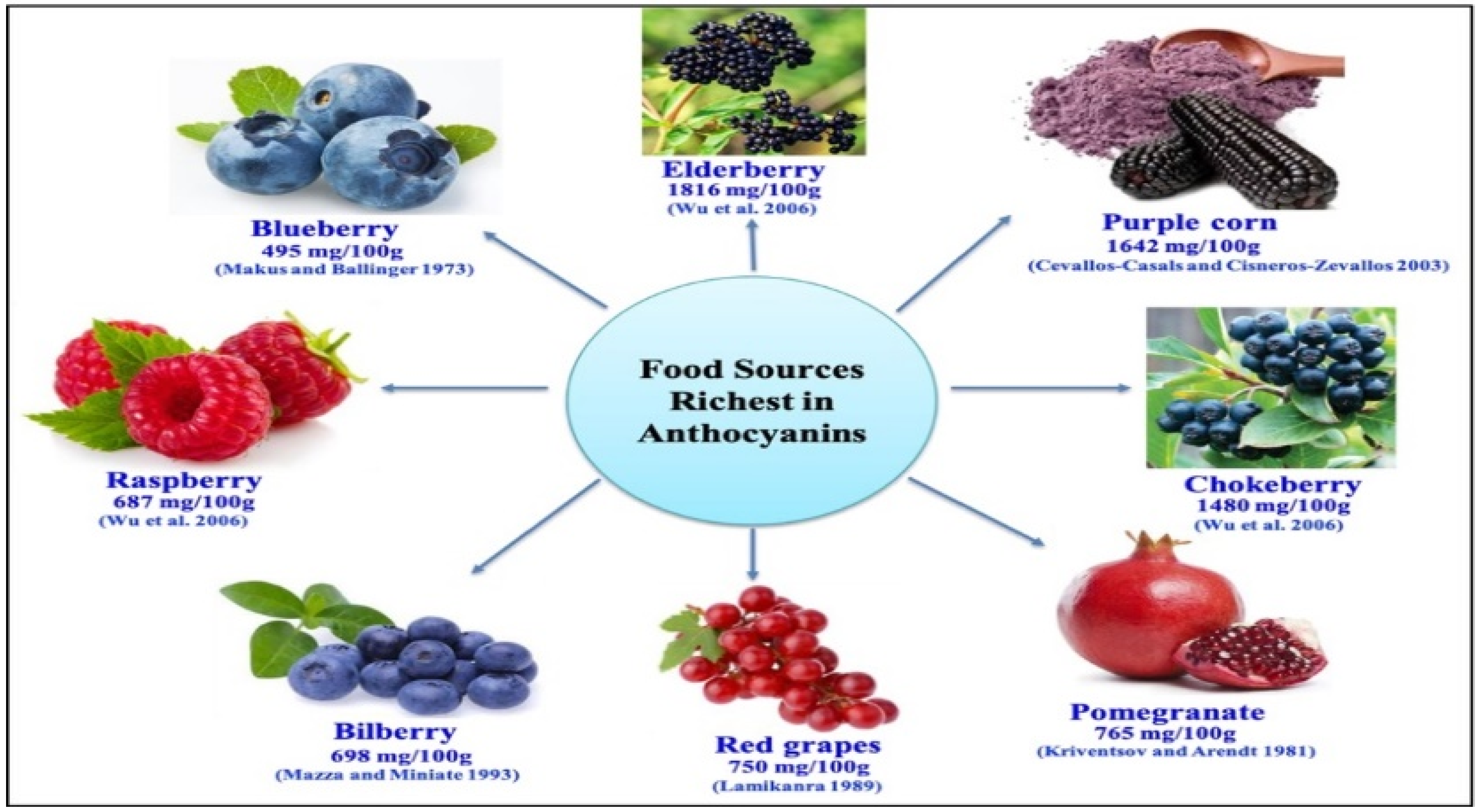
4.1. Dietary Sources of Anthocyanins
- -
- Fruits: berries (blueberries, blackberries, strawberries, raspberries), cherries, grapes, pomegranates, and blackcurrants.
- -
- Vegetables: red cabbage, eggplant, purple sweet potatoes, and red onions.
- -
- Other sources: red wine, tea, and certain grains such as black rice and barley.
4.2. Bioavailability and Metabolism
- -
- -
- -
- -
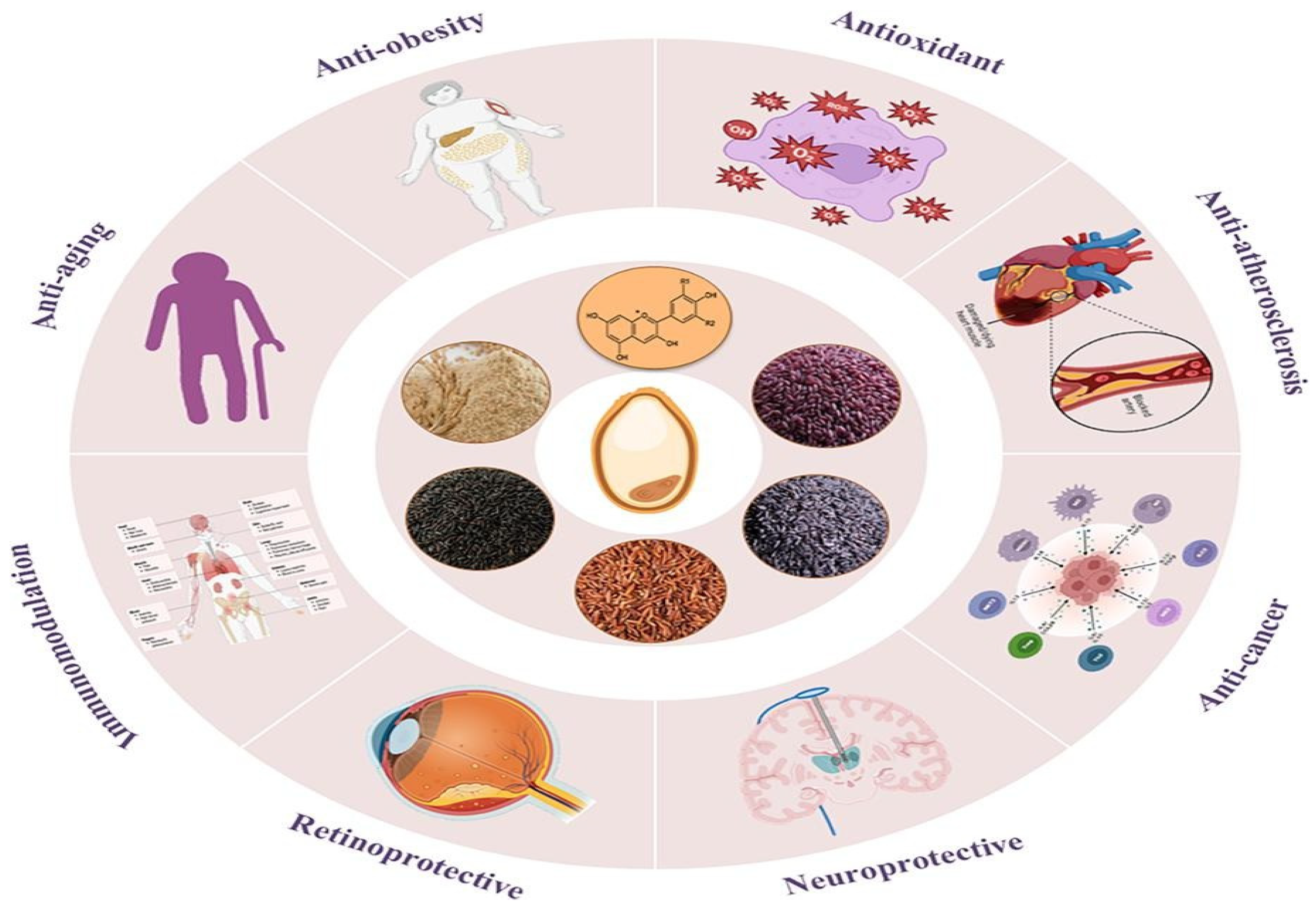
4.3. Health Benefits and Nutritional Implications
- -
- Antioxidant and anti-inflammatory effects
- -
- Cardiovascular protection
- -
- Antidiabetic Effects
- -
- Neuroprotective properties
- -
- Anticancer Potential
- -
- Weight management
- -
- Gut microbiota modulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crozier, A.; Clifford, M.N.; Ashihara, H. Plant secondary metabolites. In Occurrence, Structure and Role in the Human Diet; Blackwell Publishers: Hoboken, NJ, USA, 2006. [Google Scholar]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are key regulators of drought stress tolerance in tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Srikaeo, K. Pigmented and Non-Pigmented Cereals: Comparative Study of Properties; The Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Rusu, A.V.; Socol, C.T.; Bangar, S.P.; Coşier, V.; Trif, M. Colored cereals: Genetics and chemistry of pigments. In Functionality and Application of Colored Cereals; Elsevier: London, UK, 2023; pp. 111–134. [Google Scholar]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Leong, H.Y.; Show, P.L.; Lim, M.H.; Ooi, C.W.; Ling, T.C. Natural red pigments from plants and their health benefits: A review. Food Rev. Int. 2018, 34, 463–482. [Google Scholar] [CrossRef]
- Ashesh, A.; Purohit, N.N.; Paul, R.M.; Kaur, S.; Singh, A.; Kaur, A.; Purewal, S.S.; Kohli, R. Current Scenario and Future Prospects. In Colored Cereals; CRC Press: Boca Raton, FL, USA, 2025; pp. 392–412. [Google Scholar]
- Rai, D.C.; Duary, R.K.; Singh, R.K. Bio-functional Compounds in Pigmented Rice: Recent Updates on Extractions, Delivery Mode, and Application in Food Systems. Food Bioprocess Technol. 2025, 18, 4251–4279. [Google Scholar]
- Rani, N.; Mishra, T.; Thakur, B.; Kaur, R.; Kaur, S. Coloured Cereals: Nutritional Benefits and Therapeutic Properties. Curr. Funct. Foods 2024, 2, E260723219161. [Google Scholar] [CrossRef]
- Kushwaha, U.K.S.; Kushwaha, U. Black Rice; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Glagoleva, A.; Kukoeva, T.; Mursalimov, S.; Khlestkina, E.; Shoeva, O. Effects of combining the genes controlling anthocyanin and melanin synthesis in the barley grain on pigment accumulation and plant development. Agronomy 2022, 12, 112. [Google Scholar] [CrossRef]
- Faris, D.G. Physiology and Genetics of the Kernel Color of Barley; University of British Columbia: Vancouver, BC, Canada, 1955. [Google Scholar]
- Ahmed, R.; Das, S.; Samanta, S.; Banerjee, J.; Ali, K.M.; Dash, S.K.; Kaur, A.; Purewal, S.S. Health Benefits of Colored Cereals. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; Routledge: London, UK, 2025; p. 335. [Google Scholar]
- Ötleş, S.; Nakilcioglu, E. Health Effects of Anthocyanins. In Handbook of Analysis and Extraction Methods of Anthocyanins; CRC Press: Boca Raton, FL, USA, 2025; pp. 114–134. [Google Scholar]
- Kruszka, J.; Martyński, J.; Szewczyk-Golec, K.; Woźniak, A.; Nuszkiewicz, J. The Role of Selected Flavonoids in Modulating Neuroinflammation in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. Brain Sci. 2025, 15, 485. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications—A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef] [PubMed]
- Said, N.S.; Lee, W.Y. Pectin-Based Active and Smart Film Packaging: A Comprehensive Review of Recent Advancements in Antimicrobial, Antioxidant, and Smart Colorimetric Systems for Enhanced Food Preservation. Molecules 2025, 30, 1144. [Google Scholar] [CrossRef]
- Abdullahi, A.; Korumilli, T.; Rao, K.J. Revolutionizing Food Packaging with Biobased Polymers, Active and Intelligent Materials for Enhanced Food Safety and Sustainability. Food Bioprocess Technol. 2025, 1–33. [Google Scholar] [CrossRef]
- Panda, J.; Amrit, R.; Mishra, A.K.; Chakraborty, A.; Rustagi, S.; Nath, P.C.; Sarabandi, K.; Sarma, H.; Wagh, M.S.; Mohanta, Y.K. Sustainable Valorization of Fruit and Vegetable Waste for Bioactive Compounds: Advancing Functional Food and Wellness. Waste Biomass Valorization 2025, 16, 1–30. [Google Scholar] [CrossRef]
- Kamble, M.G.; Singh, A.; Singh, S.V.; Kamble, M.G.; Sagar, N.A.; Rani, N. Nanotechnology for encapsulation of bioactive components: A review. Discov. Food 2025, 5, 116. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The genetic basis and nutritional benefits of pigmented rice grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-M.; Dang, B.; Zhang, W.-G.; Zheng, W.-C.; Yang, X.-J. Polyphenol and anthocyanin composition and activity of highland barley with different colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef]
- Dang, B.; Zhang, W.-G.; Zhang, J.; Yang, X.-J.; Xu, H.-D. Evaluation of nutritional components, phenolic composition, and antioxidant capacity of highland barley with different grain colors on the Qinghai Tibet Plateau. Foods 2022, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Crizel, R.L.; Siebeneichler, T.J.; Zandoná, G.P.; Hoffmann, J.F.; Ferreira, C.D. Phenolic Compound Extraction, Identification, and Health Aspects: Part II. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; Routledge: London, UK, 2025; p. 255. [Google Scholar]
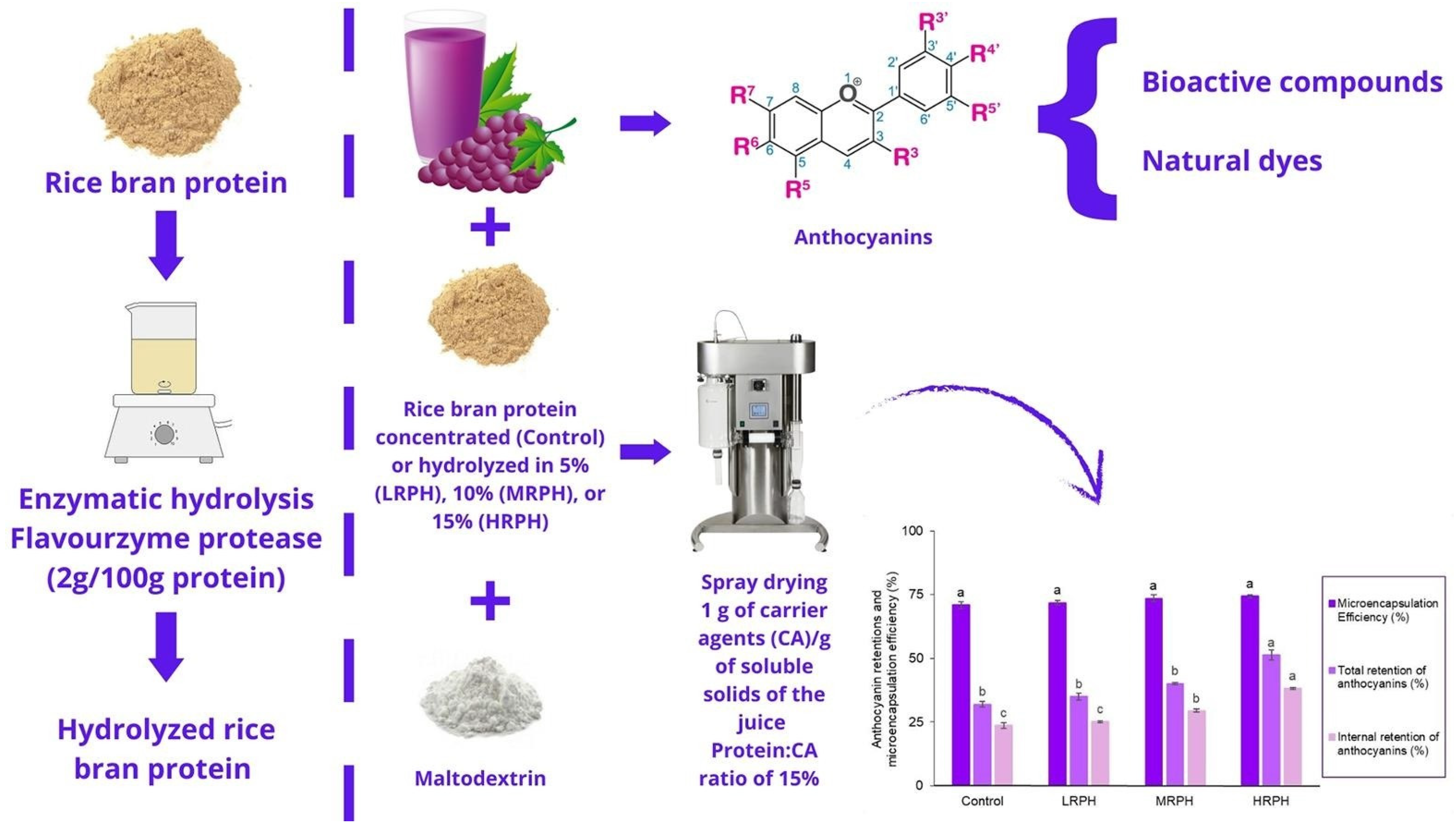
- Almeida, R.F.; Gomes, M.H.G.; Kurozawa, L.E. Enzymatic hydrolysis improves the encapsulation properties of rice bran protein by increasing retention of anthocyanins in microparticles of grape juice. Food Res. Int. 2024, 180, 114090. [Google Scholar] [CrossRef]
- Sivadurga, K.; Srivastava, R.; Swamy, C.T.; Chelladurai, P.; Pandey, A.; Reddy, M.P.; Kaur, A.; Purewal, S.S. Colored Pigments: Extraction, Identification, and Health Aspects. In Colored Cereals; CRC Press: Boca Raton, FL, USA, 2025; pp. 119–148. [Google Scholar]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Jesus, S.P.; Meireles, M.A.A. Supercritical fluid extraction: A global perspective of the fundamental concepts of this eco-friendly extraction technique. In Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–72. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Churchwell, M.I.; Twaddle, N.C.; Meeker, L.R.; Doerge, D.R. Improving LC–MS sensitivity through increases in chromatographic performance: Comparisons of UPLC–ES/MS/MS to HPLC–ES/MS/MS. J. Chromatogr. B 2005, 825, 134–143. [Google Scholar] [CrossRef]
- Mackon, E.; Jeazet Dongho Epse Mackon, G.C.; Ma, Y.; Haneef Kashif, M.; Ali, N.; Usman, B.; Liu, P. Recent insights into anthocyanin pigmentation, synthesis, trafficking, and regulatory mechanisms in rice (Oryza sativa L.) caryopsis. Biomolecules 2021, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Isola, P.; Zhu, J.-Y.; Zhou, T.; Efros, A.A. Image-to-image translation with conditional adversarial networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 1125–1134. [Google Scholar]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H. Development of “purple endosperm rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Tuia, D.; Mou, L.; Xia, G.-S.; Zhang, L.; Xu, F.; Fraundorfer, F. Deep learning in remote sensing: A comprehensive review and list of resources. IEEE Geosci. Remote Sens. Mag. 2017, 5, 8–36. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, H.-A.; Koh, K.; Kim, H.-S.; Lee, Y.S.; Kim, Y.H. Identification and quantification of anthocyanin pigments in colored rice. Nutr. Res. Pract. 2008, 2, 46–49. [Google Scholar] [CrossRef]
- Kim, M.-J.; Hyun, J.-N.; Kim, J.-A.; Park, J.-C.; Kim, M.-Y.; Kim, J.-G.; Lee, S.-J.; Chun, S.-C.; Chung, I.-M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef]
- Tyagi, A.; Joshi, A.; Joshi, N. Health-promoting effects of anthocyanin in whole grain cereals: Insights from a quantitative perspective. Int. J. Adv. Biochem. Res. 2025, 9, 1008–1017. [Google Scholar] [CrossRef]
- Yang, T.; Jing, X.; Ahmed, H.; Akram, M.; Iqbal, R.; Alghamdi, A.; Al Farraj, D.; Zeng, Y. Assessing flavonoid content in barley genotypes: Genetic contributions and hybrid potential for nutritional improvement. Appl. Ecol. Environ. Res. 2025, 23, 3677–3690. [Google Scholar] [CrossRef]
- Thilavech, T.; Suantawee, T.; Chusak, C.; Suklaew, P.O.; Adisakwattana, S. Black rice (Oryza sativa L.) and its anthocyanins: Mechanisms, food applications, and clinical insights for postprandial glycemic and lipid regulation. Food Prod. Process. Nutr. 2025, 7, 15. [Google Scholar] [CrossRef]
- Gogoi, P.; Sharma, P.; Goudar, G.; Mahajan, A.; Dutta, H.; Sasikumar, R.; Ananthan, R.; Singh, M.; Nagaraju, M.; Longvah, T. Nutritional profile and mineral bioaccessibility of pigmented rice landraces. J. Food Meas. Charact. 2025, 19, 1513–1530. [Google Scholar] [CrossRef]
- Chelladurai, P.; Pandey, A.; Swamy, C.T.; Sivadurga, K.; Anbarasi, K.; Reddy, M.P.; Sen, A.; Kaur, A.; Purewal, S.S.; Dash, S.K. Phenolic Compounds: Extraction, Identification, and Health Aspects, Part I. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; Routledge: London, UK, 2025; p. 220. [Google Scholar]
- Oh, G.; La, I.-J.; Lee, D.-S.; Chae, J.-W.; Im, J.-H.; Park, S.W.; Fu, X.; Lim, J.-S.; Kim, M.-H.; Seong, Y.-S. Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts. Separations 2025, 12, 68. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Su, B.; Zhang, Y.; Wang, Z.; Ma, K.; Lu, B.; Ren, J.; Xue, J. Evaluation of Functional Quality of Maize with Different Grain Colors and Differences in Enzymatic Properties of Anthocyanin Metabolism. Foods 2025, 14, 544. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kang, J.; Wang, S.; Haider, F.U.; Zhong, Y.; Zhang, P. Variations in the End-Use Quality of Whole Grain Flour Are Closely Related to the Metabolites in the Grains of Pigmented Wheat (Triticum aestivum L.). Plants 2025, 14, 171. [Google Scholar] [CrossRef]
- Abdelsalam, K.M.; Shaalan, A.M.; AbouEl-Soud, G.M.; El-Dalil, M.A.; Marei, A.M.; El-Moneim, D.A.; El-Banna, A.A.; Lamlom, S.F.; Abdelghany, A.M. Comprehensive quality profiling and multivariate analysis of rice (Oryza sativa L.) cultivars: Integrating physical, cooking, nutritional, and micronutrient characteristics for enhanced varietal selection. BMC Plant Biol. 2025, 25, 492. [Google Scholar] [CrossRef]
- Lang, H.; Jia, X.; He, B.; Yu, X. Advances and Future Prospects of Pigment Deposition in Pigmented Rice. Plants 2025, 14, 963. [Google Scholar] [CrossRef]
- Wei, M.; Wen, J.; Ren, Y.; Shao, D.; Wang, Y.; Li, J.; Li, Q. Metabolic Pathways and Molecular Regulatory Mechanisms of Fruit Color Change During Greening Stage of Peppers (Capsicum annuum L.). Int. J. Mol. Sci. 2025, 26, 4508. [Google Scholar] [CrossRef]
- Yang, L.; Tian, L.; Shi, J.; Wei, A. Transcriptome and Metabolome Profiling Provide Insights into Flavonoid Biosynthesis and the Mechanism of Color Formation in Zanthoxylum bungeanum Maxim. Plants 2025, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Guangsi, J.; Xinna, K.; Li, Y.; Jiangtao, H.; Chun, Q.; Wang, S. Regulation of Anthocyanins and Quality in Strawberries Based on Light Quality. Horticulturae 2025, 11, 377. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Yin, X.; Wang, T.; Zhang, M.; Zhang, Y.; Irfan, M.; Chen, L.; Zhang, L. Role of core structural genes for flavonoid biosynthesis and transcriptional factors in flower color of plants. Biotechnol. Biotechnol. Equip. 2021, 35, 1214–1229. [Google Scholar] [CrossRef]
- Khusnutdinov, E.; Sukhareva, A.; Panfilova, M.; Mikhaylova, E. Anthocyanin biosynthesis genes as model genes for genome editing in plants. Int. J. Mol. Sci. 2021, 22, 8752. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Zhou, H. Anthocyanins: From biosynthesis regulation to crop improvement. Bot. Lett. 2021, 168, 546–557. [Google Scholar] [CrossRef]
- Upadhyaya, G.; Das, A.; Ray, S. A rice R2R3-MYB (OsC1) transcriptional regulator improves oxidative stress tolerance by modulating anthocyanin biosynthesis. Physiol. Plant. 2021, 173, 2334–2349. [Google Scholar] [CrossRef] [PubMed]
- Zykin, P.A.; Andreeva, E.A.; Tsvetkova, N.V.; Voylokov, A.V. Anatomical and image analysis of grain coloration in rye. Preprints 2020. [Google Scholar] [CrossRef]
- Egorova, A.A.; Zykova, T.E.; Hertig, C.W.; Hoffie, I.; Morozov, S.V.; Chernyak, E.I.; Rogachev, A.D.; Korotkova, A.M.; Vikhorev, A.V.; Vasiliev, G.V. Accumulation of Anthocyanin in the Aleurone of Barley Grains by Targeted Restoration of the MYC2 Gene. Int. J. Mol. Sci. 2024, 25, 12705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Du, M.; Chen, H.; Li, G.; Wang, M.; Meng, L. Recent insights into anthocyanin biosynthesis, gene involvement, distribution regulation, and domestication process in rice (Oryza sativa L.). Plant Sci. 2024, 349, 112282. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Shil, P. Black rice developed through interspecific hybridization (O. sativa × O. rufipogon): Origin of black rice gene from Indian wild rice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shiba, H.; Takayama, S. RNA silencing systems and their relevance to allele-specific DNA methylation in plants. Biosci. Biotechnol. Biochem. 2007, 71, 2632–2646. [Google Scholar] [CrossRef]
- Micallef, M.C. Genetic Manipulations in Alfalfa: I. Improvement of Transgenic Alfalfa by Backcrossing. II. Breeding Alfalfa for Increased Anthocyanin Production; The University of Wisconsin-Madison: Madison, WI, USA, 1994. [Google Scholar]
- Md Hatta, M. Host Range Expansion of Wheat Stem Rust Resistance Genes into Barley; University of East Anglia: Norwich, UK, 2017. [Google Scholar]
- Yuenyong, W. Transcriptome of Rice Oryza sativa L. Over-Expressing OsCam1-1 Gene and Characterization of Salt Stress-Responsive Isocitrate Lyase Gene. Doctoral Thesis, Chulalongkorn University, Bangkok, Thailand, 2018. [Google Scholar]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Kreszies, T. Experimental Validation of RNA Interference Efficiency and Off-Target Prediction in Barley; Hochschule Mittweida: Mittweida, Germany, 2013. [Google Scholar]
- Sharma, H.; Sharma, P.; Kumar, A.; Chawla, N.; Dhatt, A.S. Multifaceted regulation of anthocyanin biosynthesis in plants: A comprehensive review. J. Plant Growth Regul. 2024, 43, 3048–3062. [Google Scholar] [CrossRef]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2013, 1829, 1236–1247. [Google Scholar] [CrossRef]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Brockman, A.; Aguirre, L.; Campbell, A.; Bean, A.; Cantero, A.; Gonzalez, A. Advances in the MYB–bHLH–WD repeat (MBW) pigment regulatory model: Addition of a WRKY factor and co-option of an anthocyanin MYB for betalain regulation. Plant Cell Physiol. 2017, 58, 1431–1441. [Google Scholar] [CrossRef]
- Li, S. Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal. Behav. 2014, 9, e27522. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhu, H.; Huang, C.; Liu, C.; Chang, Y.; Kong, Z.; Zhou, Z.; Wang, G.; Lin, Y. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol. 2019, 223, 705–721. [Google Scholar] [CrossRef]
- Ithal, N.; Reddy, A.R. Rice flavonoid pathway genes, OsDfr and OsAns, are induced by dehydration, high salt and ABA, and contain stress responsive promoter elements that interact with the transcription activator, OsC1-MYB. Plant Sci. 2004, 166, 1505–1513. [Google Scholar] [CrossRef]
- Zaidi, S.H.R.; Zakari, S.A.; Zhao, Q.; Khan, A.R.; Shah, J.M.; Cheng, F. Anthocyanin accumulation in black kernel mutant rice and its contribution to ROS detoxification in response to high temperature at the filling stage. Antioxidants 2019, 8, 510. [Google Scholar] [CrossRef]
- Lin, J.; Lai, G.; Guo, A.; He, L.; Yang, F.; Huang, Y.; Che, J.; Lai, C. Overexpression of LAR1 Suppresses Anthocyanin Biosynthesis by Enhancing Catechin Competition Leading to Promotion of Proanthocyanidin Pathway in Spine Grape (Vitis davidii) Cells. Int. J. Mol. Sci. 2024, 25, 12087. [Google Scholar] [CrossRef]
- Zhong, R.; Wei, J.; Liu, B.; Luo, H.; Zhang, Z.; Pang, X.; Fang, F. Metabolite and transcriptome profiles of proanthocyanidin biosynthesis in the development of litchi fruit. Int. J. Mol. Sci. 2022, 24, 532. [Google Scholar] [CrossRef]
- Lei, T.; Huang, J.; Ruan, H.; Qian, W.; Fang, Z.; Gu, C.; Zhang, N.; Liang, Y.; Wang, Z.; Gao, L. Competition between FLS and DFR regulates the distribution of flavonols and proanthocyanidins in Rubus chingii Hu. Front. Plant Sci. 2023, 14, 1134993. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Jung, K.H. Rice tissue-specific promoters and condition-dependent promoters for effective translational application. J. Integr. Plant Biol. 2015, 57, 913–924. [Google Scholar] [CrossRef]
- Qu, L.Q.; Takaiwa, F. Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol. J. 2004, 2, 113–125. [Google Scholar] [CrossRef]
- Liu, B.; Meng, S.; Yang, J.; Wu, J.; Peng, Y.; Zhang, J.; Ye, N. Carbohydrate flow during grain filling: Phytohormonal regulation and genetic control in rice (Oryza sativa). J. Integr. Plant Biol. 2025, 67, 1086–1104. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Khan, R.A.; Abbas, N. Role of epigenetic and post-translational modifications in anthocyanin biosynthesis: A review. Gene 2023, 887, 147694. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Z.; Li, J.; Zhang, H.; Peng, Y.; Li, Z. Uncovering hierarchical regulation among MYB-bHLH-WD40 proteins and manipulating anthocyanin pigmentation in rice. Int. J. Mol. Sci. 2022, 23, 8203. [Google Scholar] [CrossRef]
- Chen, L.; Cui, Y.; Yao, Y.; An, L.; Bai, Y.; Li, X.; Yao, X.; Wu, K. Genome-wide identification of WD40 transcription factors and their regulation of the MYB-bHLH-WD40 (MBW) complex related to anthocyanin synthesis in Qingke (Hordeum vulgare L. var. nudum Hook. f.). BMC Genom. 2023, 24, 166. [Google Scholar] [CrossRef]
- Pandey, A.; Swamy, C.T.; Chelladurai, P.; Sivadurga, K.; Reddy, M.P.; Srivastava, R.; Kaur, A.; Purewal, S.S.; Singh, A. Biotechnological Tools to Improve Colored Cereals: Part II. In Colored Cereals; CRC Press: Boca Raton, FL, USA, 2020; pp. 289–320. [Google Scholar]
- Lakshmikanthan, M.; Muthu, S.; Krishnan, K.; Altemimi, A.B.; Haider, N.N.; Govindan, L.; Selvakumari, J.; Alkanan, Z.T.; Cacciola, F.; Francis, Y.M. A comprehensive review on anthocyanin-rich foods: Insights into extraction, medicinal potential, and sustainable applications. J. Agric. Food Res. 2024, 17, 101245. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Dini, C.; Zaro, M.J.; Viña, S.Z. Bioactivity and functionality of anthocyanins: A review. Curr. Bioact. Compd. 2019, 15, 507–523. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional uses, structural and functional variations, approaches to increase yields and products’ quality, hepatoprotection, liver longevity, and commercial products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef]
- Arunima, S.; Kumar, G.S.; Krishnan, R.; Mohammed, T. Colored grains: Chemistry, health benefits and processing. J. Pharm. Innov. 2021, 10, 317–332. [Google Scholar]
- Panchal, S.K.; John, O.D.; Mathai, M.L.; Brown, L. Anthocyanins in chronic diseases: The power of purple. Nutrients 2022, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Sasahara, H.; Matsushita, K.; Tamura, Y.; Miyaji, M.; Matsuyama, H. Anthocyanin and proanthocyanidin contents, antioxidant activity, and in situ degradability of black and red rice grains. Asian-Australas. J. Anim. Sci. 2018, 31, 1213. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Zhang, M.; Blanchard, C. Phenolics, flavonoids, proanthocyanidin and antioxidant activity of brown rice with different pericarp colors following storage. J. Stored Prod. Res. 2014, 59, 120–125. [Google Scholar] [CrossRef]
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y.-Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef]
- Oren-Shamir, M. Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci. 2009, 177, 310–316. [Google Scholar] [CrossRef]
- Zhou, Y.; Mumtaz, M.A.; Zhang, Y.; Yang, Z.; Hao, Y.; Shu, H.; Zhu, J.; Bao, W.; Cheng, S.; Zhu, G. Response of anthocyanin biosynthesis to light by strand-specific transcriptome and miRNA analysis in Capsicum annuum. BMC Plant Biol. 2022, 22, 79. [Google Scholar] [CrossRef]
- Song, S.; He, A.; Zhao, T.; Yin, Q.; Mu, Y.; Wang, Y.; Liu, H.; Nie, L.; Peng, S. Effects of shading at different growth stages with various shading intensities on the grain yield and anthocyanin content of colored rice (Oryza sativa L.). Field Crops Res. 2022, 283, 108555. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Ding, W.; Wu, C.; Wang, W.; Ye, S.; Cai, C.; Hu, X.; Wang, N.; Bai, W.; Tang, X. Production of purple Ma bamboo (Dendrocalamus latiflorus Munro) with enhanced drought and cold stress tolerance by engineering anthocyanin biosynthesis. Planta 2021, 254, 50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J. Thermal degradation of anthocyanins from purple potato (Cv. Purple Majesty) and impact on antioxidant capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef]
- Liang, J.; He, J. Protective role of anthocyanins in plants under low nitrogen stress. Biochem. Biophys. Res. Commun. 2018, 498, 946–953. [Google Scholar] [CrossRef]
- Kossyvaki, D.; Contardi, M.; Athanassiou, A.; Fragouli, D. Colorimetric indicators based on anthocyanin polymer composites: A review. Polymers 2022, 14, 4129. [Google Scholar] [CrossRef]
- Li, H.; He, K.; Zhang, Z.; Hu, Y. Molecular mechanism of phosphorous signaling inducing anthocyanin accumulation in Arabidopsis. Plant Physiol. Biochem. 2023, 196, 121–129. [Google Scholar] [CrossRef]
- Zeng, Z.; Liao, Y.; Wang, J.; Liang, X.; Duan, L.; Huang, Y.; Han, Z.; Lin, K.; Hu, H.; Ye, K. Combined transcriptomic, metabolomic and physiological analysis reveals the key role of nitrogen, but not phosphate and potassium in regulating anthocyanin biosynthesis induced by nutrient deficiency in Eucalyptus. Int. J. Biol. Macromol. 2024, 283, 137564. [Google Scholar] [CrossRef]
- Ziephru, A.; Singh, J.; Rasane, P.; Kaur, S. Processing of pigmented grains and its effect on their functionality. In Pigmented Grains; Elsevier: London, UK, 2024; pp. 257–279. [Google Scholar]
- Zhao, M.; Xiao, X.; Jin, D.; Zhai, L.; Li, Y.; Yang, Q.; Xing, F.; Qiao, W.; Yan, X.; Tang, Q. Composition and Biological Activity of Colored Rice—A Comprehensive Review. Foods 2025, 14, 1394. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Joye, I.J. Anthocyanins in whole grain cereals and their potential effect on health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef]
- Alseekh, S.; Scossa, F.; Wen, W.; Luo, J.; Yan, J.; Beleggia, R.; Klee, H.J.; Huang, S.; Papa, R.; Fernie, A.R. Domestication of crop metabolomes: Desired and unintended consequences. Trends Plant Sci. 2021, 26, 650–661. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Lin, Z. Convergent loss of anthocyanin pigments is controlled by the same MYB gene in cereals. J. Exp. Bot. 2022, 73, 6089–6102. [Google Scholar] [CrossRef]
- Paul, R.M.; Ashesh, A.; Purohit, N.N.; Kaur, S.; Singh, A.; Purewal, S.S.; Singh, H.; Swamy, C.T. Agrarian Conditions. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; Routledge: London, UK, 2025; p. 33. [Google Scholar]
- del Valle, T. Tucuman biology association. Biocell 2010, 34, A109–A167. [Google Scholar]
- Mohamedshah, Z.Y. Comparative Assessment of Phenolic Bioaccessibility and Bioavailability from 100% Juice and Whole Fruit–a Preclinical Approach; North Carolina State University: Raleigh, NC, USA, 2021. [Google Scholar]
- Nasidi, M.; Agu, R.; Walker, G.; Deeni, Y. Sweet sorghum: Agronomic practice for food, animal feed and fuel production in sub-saharan africa. In Sweet Sorghum: Characteristics, Cultivation and Uses; Nova Science Publishers: Hauppauge, NY, USA, 2019. [Google Scholar]
- Shepherd, L.V.; Fraser, P.; Stewart, D. Metabolomics: A second-generation platform for crop and food analysis. Bioanalysis 2011, 3, 1143–1159. [Google Scholar] [CrossRef]
- Remini, H.; Dahmoune, F.; Sahraoui, Y.; Madani, K.; Kapranov, V.N.; Kiselev, E.F. Recent advances on stability of anthocyanins. J. Agron. 2018, 13, 257–286. [Google Scholar] [CrossRef]
- Oancea, S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Chen, M.; Liu, F.; Zhong, F. Interactions between rice protein and anthocyanin with different pH-cycle: Structural characterization, binding mechanism and stability. Food Hydrocoll. 2025, 165, 111230. [Google Scholar] [CrossRef]
- Macedo, M.J.; Silva, J.D.R.; Júnior, M.R.M. Stability of Anthocyanins during Food Processing. In Handbook of Analysis and Extraction Methods of Anthocyanins; CRC Press: Boca Raton, FL, USA, 2025; pp. 34–64. [Google Scholar]
- Zhu, W.; Ma, Z.; Zhang, L.; Yang, C.; Xu, F.; Yu, J. Natural anthocyanin-based wearable colorimetric pH indicator with high color fastness. Dye. Pigment. 2025, 239, 112789. [Google Scholar] [CrossRef]
- Ghased, S.; Barani, H.; Khaleghi, S. Wool dyeing with purple sweet potato extract by enhancing dye uptake and stability using lecithin during the mordanting process. In Coloration Technology; Wiley Online Library: Hoboken, NJ, USA, 2025. [Google Scholar]
- Dai, W.; Yin, P.; Lin, Q.; Tian, M.; Wu, M.; Ding, J.; Jiang, X.; Fang, Y. Black goji anthocyanin-loaded emulsion film stabilized by rice protein hydrolysate complexes as pH-sensitive indicator for salmon freshness. Food Packag. Shelf Life 2025, 47, 101419. [Google Scholar] [CrossRef]
- Nettey-Oppong, E.E.; Muhammad, R.; Yoo, D.; Hwang, S.-H.; Ali, A.; Mwita, C.S.; Jeong, H.-W.; Kim, S.-W.; Seok, Y.-S.; Choi, S.H. The Use of Biomass-Derived Chitosan for Colorimetric pH Detection. Photonics 2025, 12, 231. [Google Scholar] [CrossRef]
- Markakis, P.; Jurd, L. Anthocyanins and their stability in foods. Crit. Rev. Food Sci. Nutr. 1974, 4, 437–456. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Degradation kinetics of anthocyanins from purple rice bran and effect of hydrocolloids on its stability. J. Food Process Eng. 2020, 43, e13360. [Google Scholar] [CrossRef]
- Zheng, R.-L.; Ren, T.; Niu, C.-T.; Zheng, F.-Y.; Wang, J.-J.; Liu, C.-F.; Li, Q. Anthocyanins composition and antioxidant activity of purple rice and color degradation under sunlight exposure of purple rice wine. J. Food Meas. Charact. 2022, 16, 1889–1900. [Google Scholar] [CrossRef]
- Nignpense, B.E. Bioaccessibility and Bioavailability of Pigmented Cereal Polyphenols. Doctoral Thesis, Charles Sturt University, Bathurst, Australia, 2024. [Google Scholar]
- Nollet, L.M.; Otles, S. Handbook of Analysis and Extraction Methods of Anthocyanins; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Vega, E.N.; González-Zamorano, L.; Cebadera, E.; Barros, L.; da Silveira, T.F.; Vidal-Diez de Ulzurrun, G.; Tardío, J.; Lázaro, A.; Cámara, M.; Fernández-Ruíz, V. Wild Myrtus communis L. Fruit By-Product as a Promising Source of a New Natural Food Colourant: Optimization of the Extraction Process and Chemical Characterization. Foods 2025, 14, 520. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Akhter, D.; Qin, R.; Nath, U.K.; Eshag, J.; Jin, X.; Shi, C. A rice gene, OsPL, encoding a MYB family transcription factor confers anthocyanin synthesis, heat stress response and hormonal signaling. Gene 2019, 699, 62–72. [Google Scholar] [CrossRef]
- Meng, G.; Rasmussen, S.K.; Christensen, C.S.; Fan, W.; Torp, A.M. Molecular breeding of barley for quality traits and resilience to climate change. Front. Genet. 2023, 13, 1039996. [Google Scholar] [CrossRef]
- Vrábl, D.; Nezval, J.; Pech, R.; Volná, A.; Mašková, P.; Pleva, J.; Kuzniciusová, N.; Provazová, M.; Štroch, M.; Špunda, V. Light drives and temperature modulates: Variation of phenolic compounds profile in relation to photosynthesis in spring barley. Int. J. Mol. Sci. 2023, 24, 2427. [Google Scholar] [CrossRef]
- Surh, J.; Koh, E. Effects of four different cooking methods on anthocyanins, total phenolics and antioxidant activity of black rice. J. Sci. Food Agric. 2014, 94, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, Q.; Sun, Y.; Wu, J.; Sun, Z.; Zuo, M.; Cai, J.; Zhai, X.; Zhou, C.; Shi, J. Comparative study on physicochemical, nutritional and cooking properties of different pigmented dehusked rice varieties influenced by superheated steam treatment. J. Cereal Sci. 2024, 117, 103934. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, P.; Varshney, G.; Singh, P.; Kaur, R. Photocatalytic degradation of crystal violet dye using phytofabricated zinc oxide nanoparticles from anthocyanin extract. Microchem. J. 2025, 213, 113681. [Google Scholar] [CrossRef]
- Ramzan, K.; Zehra, S.H.; Balciunaitiene, A.; Viskelis, P.; Viskelis, J. Valorization of Fruit and Vegetable Waste: An Approach to Focusing on Extraction of Natural Pigments. Foods 2025, 14, 1402. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Pan, S.; Qin, M.; Yuan, Y.; Li, C.; Liu, Y. Effects of Infrared Radiation Parameters on Drying Characteristics and Quality of Rice: A Systematic Review. Food Bioprocess Technol. 2025, 1–23. [Google Scholar] [CrossRef]
- Caroca-Cáceres, R.; Peña-González, M.A.; Lazo-Vélez, M.A. Biotechnological Tools for the Improvement of Colored Cereals: Part I. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; Routledge: London, UK, 2025; p. 1. [Google Scholar]
- Jan, R.; Asif, S.; Asaf, S.; Lubna; Khan, Z.; Kim, K.-M. Unveiling the protective role of anthocyanin in rice: Insights into drought-induced oxidative stress and metabolic regulation. Front. Plant Sci. 2024, 15, 1397817. [Google Scholar] [CrossRef]
- Ding, L.-N.; Liu, R.; Li, T.; Li, M.; Liu, X.-Y.; Wang, W.-J.; Yu, Y.-K.; Cao, J.; Tan, X.-L. Physiological and comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in a rapeseed anthocyanin-more mutant. Biotechnol. Biofuels Bioprod. 2022, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, R. Dual Role of Phenolic Bioactives in Improving Functional Health Benefits and Abiotic Stress Resilience in Barley; North Dakota State University: Fargo, ND, USA, 2021. [Google Scholar]
- Meylani, V.; Fadjarajani, S.; Made Sudiana, I.; Sayyed, R. An Overview of Scientific Approaches Towards the Introduction of Flood-Proof Crops. In Plant Flooding: Sensitivity and Tolerance Mechanisms; Springer: Berlin/Heidelberg, Germany, 2025; pp. 257–275. [Google Scholar]
- Fongfon, S.; Prom-U-Thai, C.; Pusadee, T.; Jamjod, S. Responses of purple rice genotypes to nitrogen and zinc fertilizer application on grain yield, nitrogen, zinc, and anthocyanin concentration. Plants 2021, 10, 1717. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Yun, B.-W.; Lim, S.H.; Kim, C.K. Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front. Plant Sci. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Meng, P.; Yuan, Y.; Li, C.; Zhi, X.; Wang, C. The Impact of Nitrogen and Phosphorus Interaction on Growth, Nutrient Absorption, and Signal Regulation in Woody Plants. Biology 2025, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Juliato, R.A.; Brito, I.P.C.; Silva, E.K. Ultrasound-driven chemical and biochemical changes in jabuticaba juice: Phenolic compounds, volatile profile and inactivation of polyphenol oxidase, peroxidase and pectin methylesterase. Food Chem. 2025, 481, 144037. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.A.; Kheir, A.M.; Hussein, F.A.; Ali, E.F.; Selim, M.E.; Majrashi, A.; El Shamey, E.A. Developing new lines of Japonica rice for higher quality and yield under arid conditions. PeerJ 2021, 9, e11592. [Google Scholar] [CrossRef]
- Islam, M.Z.; Shim, M.-J.; Jeong, S.-Y.; Lee, Y.-T. Effects of soaking and sprouting on bioactive compounds of black and red pigmented rice cultivars. Int. J. Food Sci. Technol. 2022, 57, 201–209. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, O.A.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.E.; Ge, Y.; Fahmy, A.E. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- ElShamey, E.A.; Ali, E.F.; Selim, M.E.; ElSayed, M.A.; Ahmed, M.; Alotaibi, F.M.; Kamara, M.M.; AL-Harbi, M.S.; Kheir, A.M. Water deficit induced physiological and amino acid responses in some rice varieties using NMR-metabolic analysis. Agron. J. 2021, 113, 4690–4704. [Google Scholar] [CrossRef]
- Kassegn, H.H.; Hshe, B.G.; Meresa, B.K.; Berhe, M.H.; Tadesse, H.A. Germination process impact on proximate, minerals, and phytochemicals of malt barley, Abyssinian purple-colored barley and wheat. Discov. Food 2024, 4, 117. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, C.; Wen, G.; Wang, W.; Wang, C.; Sun, Y.; Bai, X. Physiological Mechanism for Anthocyanins to Strengthen the Drought Tolerance of Plants. Agric. Sci. Technol. 2014, 15, 1935. [Google Scholar]
- Yuan, S.; Zhang, W.; Zhang, Y. Characterization of PHT Genes in ‘duli’(Pyrus betulifolia Bunge) and Expression Analysis of PbPHTs in Response to Plant Growth Regulators, P, and Salt Stress. Agriculture 2025, 15, 199. [Google Scholar] [CrossRef]
- Chakraborty, S.; Raychaudhuri, S.S. Melatonin improves the lead tolerance in Plantago ovata by modulating ROS homeostasis, phytohormone status and expression of stress-responsive genes. Plant Cell Rep. 2025, 44, 39. [Google Scholar] [CrossRef]
- Mi, Y.; Tong, K.; Zhu, G.; Zhang, X.; Liu, X.; Si, Y. Surface spraying of anthocyanin through antioxidant defense and subcellular sequestration to decrease Cd accumulation in rice (Oryza sativa L.) grains in a lead–zinc mine area. Environ. Geochem. Health 2021, 43, 1855–1866. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa L. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Ramaswamy, H.S. Post-Harvest Technologies of Fruits & Vegetables; Destech Publications, Inc.: Lancaster, PA, USA, 2014. [Google Scholar]
- Jo, M.G.; An, D.S.; Lee, D.S. Antioxidant packaging material supplementary to N2-flushed modified atmosphere packaging to preserve powdered infant formula. Food Packag. Shelf Life 2020, 26, 100580. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Ekici, L.; Simsek, Z.; Ozturk, I.; Sagdic, O.; Yetim, H. Effects of temperature, time, and pH on the stability of anthocyanin extracts: Prediction of total anthocyanin content using nonlinear models. Food Anal. Methods 2014, 7, 1328–1336. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Yang, K.-M.; Chiang, P.-Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef]
- Makus, D.J.; Ballinger, W.E. Characterization of Anthocyanins During Ripening of Fruit of Vaccinium corymbosum, L. Cv. Wolcott1. J. Am. Soc. Hortic. Sci. 1973, 98, 99–101. [Google Scholar] [CrossRef]
- Wu, X.; Pittman, H.E.; Prior, R.L. Fate of anthocyanins and antioxidant capacity in contents of the gastrointestinal tract of weanling pigs following black raspberry consumption. J. Agric. Food Chem. 2006, 54, 583–589. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Kriventsov, V.; Arendt, N. Anthocyanins of pomegranate juice. Tr. Gos. Nikitsk. Bot. Sada 1981, 83, 110–116. [Google Scholar]
- Lamikanra, O. Anthocyanins of Vitis rotundifolia hybrid grapes. Food Chem. 1989, 33, 225–237. [Google Scholar] [CrossRef]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 1993; pp. 149–199. [Google Scholar]
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr. Res. Rev. 2006, 19, 137–146. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef]
- Hribar, U.; Poklar Ulrih, N. The metabolism of anthocyanins. Curr. Drug Metab. 2014, 15, 3–13. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Fernandes, I.; de Freitas, V.; Mateus, N. Anthocyanins and human health: How gastric absorption may influence acute human physiology. Nutr. Aging 2014, 2, 1–14. [Google Scholar] [CrossRef]
- Zhu, J.; Geng, Y.; Huang, Y.; Ma, C.; Dong, L.; Chen, F.; Hu, X.; Ma, L.; Ji, J. Emerging Novel Processing Technologies Towards the Stabilization of Anthocyanins. Food Rev. Int. 2025, 41, 439–468. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, L.; Zheng, Y.; Chen, L.; Zhang, Y.; Zhang, X.; Zeng, Z. Improvement of overall quality of black rice by stabilization combined with solid-state fermentation. Innov. Food Sci. Emerg. Technol. 2025, 99, 103877. [Google Scholar] [CrossRef]
- Fernández-Moreno, P.; del Carmen Villegas-Aguilar, M.; Huertas-Camarasa, F.; Fernández-Ochoa, Á. Metabolization pathways and bioavailability of polyphenols. In Bioactive Polyphenols for Health and Pathology Treatment; Elsevier: London, UK, 2025; pp. 43–80. [Google Scholar]
- Mhawish, R.; Komarnytsky, S. Small Phenolic Metabolites at the Nexus of Nutrient Transport and Energy Metabolism. Molecules 2025, 30, 1026. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Hasso, S.S.; Ahamad, J. Antioxidant Properties of Anthocyanins. In Handbook of Analysis and Extraction Methods of Anthocyanins; CRC Press: Boca Raton, FL, USA, 2025; pp. 98–113. [Google Scholar]
- Xu, Q.; Zheng, B.; Li, T.; Liu, R.H. Black goji berry anthocyanins extend lifespan and enhance the antioxidant defenses in Caenorhabditis elegans via the JNK-1 and DAF-16/FOXO pathways. J. Sci. Food Agric. 2025, 105, 2282–2293. [Google Scholar] [CrossRef]
- Hernández-Ruiz, R.G.; Olivares-Ochoa, X.C.; Salinas-Varela, Y.; Guajardo-Espinoza, D.; Roldán-Flores, L.G.; Rivera-Leon, E.A.; López-Quintero, A. Phenolic Compounds and Anthocyanins in Legumes and Their Impact on Inflammation, Oxidative Stress, and Metabolism: Comprehensive Review. Molecules 2025, 30, 174. [Google Scholar] [CrossRef]
- Singh, N.; Gusain, A.; Nigam, M.; Mishra, A.P. The pharmacological and therapeutic versatility of Allium species: A comprehensive exploration of bioactive constituents and biological activities. Discov. Appl. Sci. 2025, 7, 349. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Pan, J.; Liang, J.; Xue, Z.; Meng, X.; Jia, L. Effect of dietary anthocyanins on the risk factors related to metabolic syndrome: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0315504. [Google Scholar] [CrossRef]
- Teparak, C.; Uriyapongson, J.; Phoemsapthawee, J.; Tunkamnerdthai, O.; Aneknan, P.; Tong-Un, T.; Panthongviriyakul, C.; Leelayuwat, N.; Alkhatib, A. Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 1098. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, E.; Weston-Green, K.; Roodenrys, S.; do Rosario, V.; Kent, K.; Charlton, K. The Effect of Anthocyanins on Cognition: A Systematic Review and Meta-analysis of Randomized Clinical Trial Studies in Cognitively Impaired and Healthy Adults. Curr. Nutr. Rep. 2025, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Randeni, N.; Luo, J.; Xu, B. Critical Review on Anti-Obesity Effects of Anthocyanins Through PI3K/Akt Signaling Pathways. Nutrients 2025, 17, 1126. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.S.; Chorawala, M.R.; Sivamaruthi, B.S.; Prajapati, B.G.; Kumar, A.; Chaiyasut, C. The Role of Dietary Anthocyanins for Managing Diabetes Mellitus-Associated Complications. Curr. Diabetes Rev. 2025, 21, e15733998322754. [Google Scholar] [CrossRef]
- Thalia, O.P.; Bizimana Rukundo, T. Plant-Derived Flavonoids as Natural Inhibitors of Adipogenesis and Glucose Dysregulation in Obese Diabetic Patients. Res. Invent. J. Sci. Exp. Sci. 2025, 5, 36–41. [Google Scholar]
- Kocsis, A.E.; Kucsápszky, N.; Santa-Maria, A.R.; Hunyadi, A.; Deli, M.A.; Walter, F.R. Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood–Brain Barrier in Diseases. Nutrients 2025, 17, 766. [Google Scholar] [CrossRef]
- Domínguez-López, I.; López-Yerena, A.; Vallverdú-Queralt, A.; Pallàs, M.; Lamuela-Raventós, R.M.; Pérez, M. From the gut to the brain: The long journey of phenolic compounds with neurocognitive effects. Nutr. Rev. 2025, 83, e533–e546. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.; Li, T. Natural flavonoids from herbs and nutraceuticals as ferroptosis inhibitors in central nervous system diseases: Current preclinical evidence and future perspectives. Front. Pharmacol. 2025, 16, 1570069. [Google Scholar] [CrossRef]
- Hazards, E.P.o.B.; Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Herman, L.; Koutsoumanis, K.; Lindqvist, R. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2017, 15, e04664. [Google Scholar]
- Roy, S.; Deka, D.; Kondaveeti, S.B.; Ayyadurai, P.; Siripragada, S.; Philip, N.; Pathak, S.; Duttaroy, A.K.; Banerjee, A. An overview of potential of natural compounds to regulate epigenetic modifications in colorectal cancer: A recent update. Epigenetics 2025, 20, 2491316. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, Y. Therapeutic potential of flavonoids in gastrointestinal cancer: Focus on signaling pathways and improvement strategies. Mol. Med. Rep. 2025, 31, 109. [Google Scholar] [CrossRef]
- Laya, A.; Carvalho, E.; Fernandes, I.; Oliveira, J.; Siewe, F.B.; Wangso, H.; Djakba, R. Food Bioactives in the Prevention and Clinical Management of Obesity. In Food Bioactives and Nutraceuticals: Dietary Medicine for Human Health and Well-Beings; Springer: Berlin/Heidelberg, Germany, 2025; pp. 299–327. [Google Scholar]
- Yi, L.; Li, Z.; Xu, H.; Shi, D.; Huang, Y.; Pan, H.; Zhao, Y.; Zhao, H.; Yang, M.; Wei, H. Microbiota-Based Intervention Alleviates High-Fat Diet Consequences Through Host-Microbe Environment Remodeling. Nutrients 2025, 17, 1402. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Cao, J.; Peng, H.; Liu, Y.; Bai, F.; Peng, C.; Cai, H.; Xie, Z.; Li, D. Digestion Characteristics and Probiotic Activity of An Tea Polysaccharides: Promoting Lactobacillus and Bifidobacterium In Vitro and In Vivo. Fermentation 2025, 11, 97. [Google Scholar] [CrossRef]
- Chen, T.; Xie, L.; Wang, G.; Jiao, J.; Zhao, J.; Yu, Q.; Chen, Y.; Shen, M.; Wen, H.; Ou, X. Anthocyanins-natural pigment of colored rice bran: Composition and biological activities. Food Res. Int. 2024, 175, 113722. [Google Scholar] [CrossRef] [PubMed]
- Bulanov, A.; Voylokov, A. Adaptive significance and origin of flavonoid biosynthesis genes in the grain of cultivated cereals. Russ. J. Genet. 2024, 60, 137–151. [Google Scholar] [CrossRef]
- Xia, Q.; Shui, Y.; Zhi, H.; Ali, A.; Yang, Z.; Gao, Z. Exogeneous selenium enhances anthocyanin synthesis during grain development of colored-grain wheat. Plant Physiol. Biochem. 2023, 200, 107742. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Vanniasinkam, T.; Luo, J.; Blanchard, C.L. Chemopreventive potential of cereal polyphenols. Nutr. Cancer 2018, 70, 913–927. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Shetty, K. Functional bioactives from barley for human health benefits. In Functional Foods and Biotechnology; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–85. [Google Scholar]
- Tu, H.; Wen, C.P.; Tsai, S.P.; Chow, W.-H.; Wen, C.; Ye, Y.; Zhao, H.; Tsai, M.K.; Huang, M.; Dinney, C.P. Cancer risk associated with chronic diseases and disease markers: Prospective cohort study. BMJ 2018, 360, k134. [Google Scholar] [CrossRef]

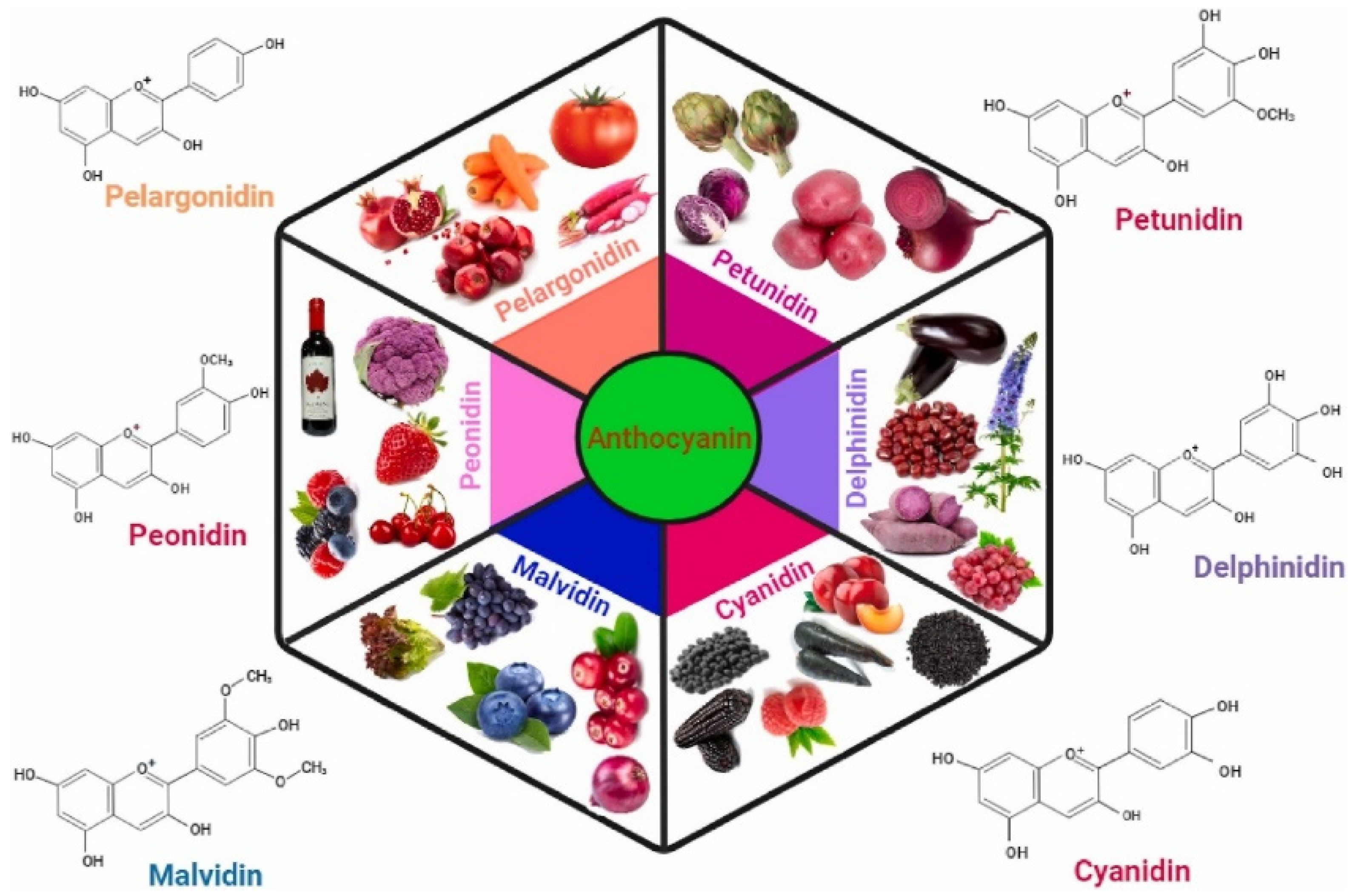
| Feature | Black Rice (Cyanidin-3-Glucoside, C3G) | Barley (Acylated Anthocyanins) | References |
|---|---|---|---|
| Major anthocyanin type | Non-acylated (simple glycoside) | Acylated (complex forms) | [6] |
| Primary anthocyanins | Cyanidin-3-glucoside (dominant) | Cyanidin-3-glucoside acylated with phenolic acids (e.g., sinapic, coumaric, ferulic acids) | [31] |
| Color stability | Less stable (degrades faster under heat/light) | More stable due to acylation (protects against degradation) | [32] |
| Bioavailability | Higher absorption (simpler structure) | Lower initial absorption (complex structure), but slower metabolism | [32,33] |
| Health benefits | Strong antioxidant, anti-inflammatory | Enhanced antioxidant capacity due to acyl groups | [33] |
| Occurrence in grain | Concentrated in the bran layer | Distributed in aleurone/pericarp layers | [31] |
| Genetic control | Controlled by a few key genes (e.g., OsANS, OsDFR) | Complex biosynthesis involving acyltransferases (e.g., HvAT) | [7] |
| Environmental influence | Moderate (affected by soil nutrients) | High (acylation influenced by stress conditions) | [7,31] |
| Processing sensitivity | High (leaching during cooking) | More resistant to processing (stable in baked/fermented products) | [31] |
| Feature | Rice (Black/Purple) | Barley (Purple/Black) | References |
|---|---|---|---|
| Major anthocyanins | Cyanidin-3-glucoside (C3G), Peonidin-3-glucoside (P3G) | Cyanidin-3-glucoside (C3G), Delphinidin-3-glucoside | [31,47] |
| Other compounds | Malvidin, Petunidin (minor) | Pelargonidin, Peonidin (minor) | [47] |
| Pigment location | Primarily in the bran layer (pericarp) | Distributed in the aleurone layer and hull | [32] |
| Color influence | Deep purple to black | Purple to blue-black | [32] |
| Concentration | 100–500 mg/100 g (varies by cultivar) | 50–300 mg/100 g (varies by cultivar) | [31,47] |
| Health benefits | Antioxidant, anti-inflammatory, cardiovascular support | Antioxidant, anti-diabetic, neuroprotective | [48] |
| Genetic control | Regulated by transcription factors like OsC1, Rb | Controlled by Ant2 and Ant13 genes | [48,49] |
| Stability | Sensitive to heat and pH changes | More stable due to matrix interactions in grain | [47] |
| Common uses | Colored rice dishes, supplements, natural dye | Functional foods, brewing, flour fortification | [48,49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElShamey, E.A.; Yang, X.; Yang, J.; Pu, X.; Yang, L.; Ke, C.; Zeng, Y. Occurrence, Biosynthesis, and Health Benefits of Anthocyanins in Rice and Barley. Int. J. Mol. Sci. 2025, 26, 6225. https://doi.org/10.3390/ijms26136225
ElShamey EA, Yang X, Yang J, Pu X, Yang L, Ke C, Zeng Y. Occurrence, Biosynthesis, and Health Benefits of Anthocyanins in Rice and Barley. International Journal of Molecular Sciences. 2025; 26(13):6225. https://doi.org/10.3390/ijms26136225
Chicago/Turabian StyleElShamey, Essam A., Xiaomeng Yang, Jiazhen Yang, Xiaoying Pu, Li’E Yang, Changjiao Ke, and Yawen Zeng. 2025. "Occurrence, Biosynthesis, and Health Benefits of Anthocyanins in Rice and Barley" International Journal of Molecular Sciences 26, no. 13: 6225. https://doi.org/10.3390/ijms26136225
APA StyleElShamey, E. A., Yang, X., Yang, J., Pu, X., Yang, L., Ke, C., & Zeng, Y. (2025). Occurrence, Biosynthesis, and Health Benefits of Anthocyanins in Rice and Barley. International Journal of Molecular Sciences, 26(13), 6225. https://doi.org/10.3390/ijms26136225