Therapeutic Prospects of αv Integrins Inhibition in Fibrotic Lung Diseases and Carcinogenesis
Abstract
1. Introduction
2. General Information About Integrins
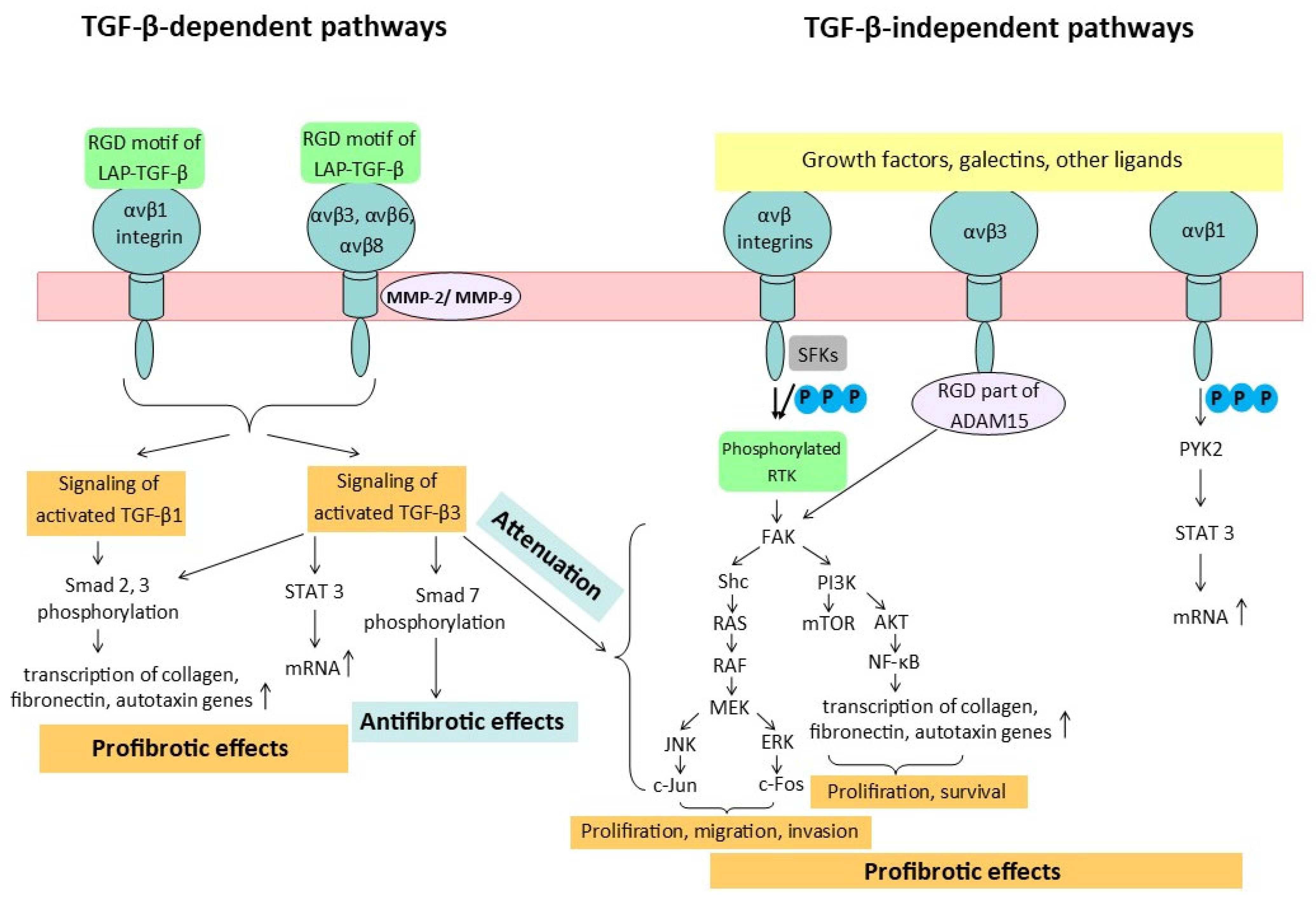
3. Interaction Between Integrins and TGF-β in Fibrotic Diseases and Cancer
4. Integrin-Mediated Fibrosis and Carcinogenesis Mechanisms
5. Drug Strategies for Inhibiting αv Integrins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPF | Idiopathic lung fibrosis |
| COPD | Chronic obstructive pulmonary disease |
| TGF-β | Transforming growth factor-β |
| ECM | Extracellular matrix |
| WHO | World Health Organization |
| RGD | Arginyl–glycyl–aspartic acid |
| GFFKR | Glu-Phe-Phe-Lys-Arg |
| MIDAS | Metal ion-dependent adhesion site |
| Hyb | Hybrid domain of β subunit |
| PSI | Plexin–semaphorin–integrin |
| EGF | Epidermal growth factor |
| ADMIDAS | Adjacent sites to metal ion-dependent adhesion site |
| SyMBS | Synergistic metal ion-binding site |
| NPxY | Asn-Pro-x-Tyr |
| NSCLC | Non-small-cell lung cancer |
| LAP | Latency-associated peptide |
| MMP2 | Matrix metalloproteinase 2 |
| MMP9 | Matrix metalloproteinases 9 |
| ALK 5 | Activin-like kinase 5 |
| CCL2 | C-C motif chemokine ligand 2 |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| FAK | Focal adhesion kinase |
| AKT | Protein kinase B |
| MMP12 | Matrix metalloproteinase 12 |
| PYK2 | Proline-rich tyrosine kinase 2 |
| STAT3 | Activator of transcription 3 |
| LARP6 | La-related protein 6 |
| ADAM15 | Metalloproteinase 15 |
| SFKs | SRC family kinases |
| ERK | Extracellular signal-regulated kinase |
| PI3K | Phosphatidylinositol 3-kinase |
| YAS | Yes-associated protein |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| RTK | Receptor tyrosine kinase |
| FGFR | Fibroblast growth factor receptor |
| FGFs | Fibroblast growth factors |
| mTOR | Mammalian target of rapamycin |
| PDGF β | Platelet-derived growth factor β |
| LIBS | Ligand-induced binding site |
References
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Murărescu, E.D.; Mitrofan, E.C.; Mihailovici, M.S. Chronic obstructive pulmonary disease in a new concept. Rom. J. Morphol. Embryol. 2007, 48, 207–214. [Google Scholar] [PubMed]
- Kim, B.N.; Ahn, D.H.; Kang, N.; Yeo, C.D.; Kim, Y.K.; Lee, K.Y.; Kim, T.; Lee, S.H.; Park, M.S.; Yim, H.W.; et al. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci. Rep. 2020, 10, 10597. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Pu, S.; Wang, X.; Guo, L.; Zhang, L.; Wang, Z. Ameliorative Effects of Arctigenin on Pulmonary Fibrosis Induced by Bleomycin via the Antioxidant Activity. Oxid. Med. Cell. Longev. 2022, 2022, 3541731. [Google Scholar] [CrossRef]
- Cottin, V. Treatment of progressive fibrosing interstitial lung diseases: A milestone in the management of interstitial lung diseases. Eur. Respir. Rev. 2019, 28, 190109. [Google Scholar] [CrossRef]
- Cottin, V.; Valenzuela, C. Evidence from recent clinical trials in fibrotic interstitial lung diseases. Curr. Opin. Pulm. Med. 2024, 30, 484–493. [Google Scholar] [CrossRef]
- Karagöz, Z.; Rijns, L.; Dankers, P.Y.W.; van Griensven, M.; Carlier, A. Towards understanding the messengers of extracellular space: Computational models of outside-in integrin reaction networks. Comput. Struct. Biotechnol. J. 2020, 19, 303–314. [Google Scholar] [CrossRef]
- Zheng, Y.; Leftheris, K. Insights into Protein-Ligand Interactions in Integrin Complexes: Advances in Structure Determinations. J. Med. Chem. 2020, 63, 5675–5696. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Yuki, Y.; Kiyono, H.; Shimaoka, M. Structural basis of blocking integrin activation and deactivation for anti-inflammation. J. Biomed. Sci. 2015, 22, 51. [Google Scholar] [CrossRef]
- Humphries, M.J.; Symonds, E.J.; Mould, A.P. Mapping functional residues onto integrin crystal structures. Curr. Opin. Struct. Biol. 2003, 13, 236–243. [Google Scholar] [CrossRef]
- Coller, B.S. αIIbβ3: Structure and function. J. Thromb. Haemost. 2015, 13, S17–S25. [Google Scholar] [CrossRef]
- Vorup-Jensen, T.; Waldron, T.T.; Astrof, N.; Shimaoka, M.; Springer, T.A. The connection between metal ion affinity and ligand affinity in integrin I domains. Biochim. Biophys. Acta 2007, 1774, 1148–1155. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Salas, A.; Springer, T.A. Bistable regulation of integrin adhesiveness by a bipolar metal ion cluster. Nat. Struct. Biol. 2003, 10, 995–1001. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Iacob, R.E.; Engen, J.R.; Springer, T.A. General structural features that regulate integrin affinity revealed by atypical αVβ8. Nat. Commun. 2019, 10, 5481. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Ramundo, V.; Palazzo, M.L.; Aldieri, E. TGF-β as Predictive Marker and Pharmacological Target in Lung Cancer Approach. Cancers 2023, 15, 2295. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Ask, K.; Bonniaud, P.; Maass, K.; Eickelberg, O.; Margetts, P.J.; Warburton, D.; Groffen, J.; Gauldie, J.; Kolb, M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int. J. Biochem. Cell Biol. 2008, 40, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Honjo, M.; Yamagishi, R.; Igarashi, N.; Nakamura, N.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Aihara, M. Fibrotic Response of Human Trabecular Meshwork Cells to Transforming Growth Factor-Beta 3 and Autotaxin in Aqueous Humor. Biomolecules 2022, 12, 1231. [Google Scholar] [CrossRef]
- Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Kazlauskas, A.; Zieske, J.D. Development of wound healing models to study TGFβ3′s effect on SMA. Exp. Eye Res. 2017, 161, 52–60. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.K.; Zieske, J.D. Molecular insights on the effect of TGF-β1/-β3 in human corneal fibroblasts. Exp. Eye Res. 2016, 146, 233–241. [Google Scholar] [CrossRef]
- Escasany, E.; Lanzón, B.; García-Carrasco, A.; Izquierdo-Lahuerta, A.; Torres, L.; Corrales, P.; Rodríguez Rodríguez, A.E.; Luis-Lima, S.; Martínez Álvarez, C.; Javier Ruperez, F.; et al. Transforming growth factor β3 deficiency promotes defective lipid metabolism and fibrosis in murine kidney. Dis. Model. Mech. 2021, 14, dmm048249. [Google Scholar] [CrossRef]
- Urban, L.; Čoma, M.; Lacina, L.; Szabo, P.; Sabová, J.; Urban, T.; Šuca, H.; Lukačín, Š.; Zajíček, R.; Smetana, K., Jr.; et al. Heterogeneous response to TGF-β1/3 isoforms in fibroblasts of different origins: Implications for wound healing and tumorigenesis. Histochem. Cell Biol. 2023, 160, 541–554. [Google Scholar] [CrossRef]
- Xue, K.; Zhang, J.; Li, C.; Li, J.; Wang, C.; Zhang, Q.; Chen, X.; Yu, X.; Sun, L.; Yu, X. The role and mechanism of transforming growth factor beta 3 in human myocardial infarction-induced myocardial fibrosis. J. Cell Mol. Med. 2019, 23, 4229–4243. [Google Scholar] [CrossRef]
- Wang, M.Y.; Liu, W.J.; Wu, L.Y.; Wang, G.; Zhang, C.L.; Liu, J. The Research Progress in Transforming Growth Factor-β2. Cells 2023, 12, 2739. [Google Scholar] [CrossRef]
- Reed, N.I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T.D.; DeGrado, W.F.; Sheppard, D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015, 7, 288ra79. [Google Scholar] [CrossRef]
- Plosa, E.J.; Benjamin, J.T.; Sucre, J.M.; Gulleman, P.M.; Gleaves, L.A.; Han, W.; Kook, S.; Polosukhin, V.V.; Haake, S.M.; Guttentag, S.H.; et al. β1 Integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight 2020, 5, e129259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Liu, T.; Tang, J.; Liu, Y.; Gou, T.; Chen, K.; Wang, L.; Zhang, J.; Yang, Y.; et al. Exploring the role of ITGB6: Fibrosis, cancer, and other diseases. Apoptosis 2024, 29, 570–585. [Google Scholar] [CrossRef] [PubMed]
- John, A.E.; Luckett, J.C.; Tatler, A.L.; Awais, R.O.; Desai, A.; Habgood, A.; Ludbrook, S.; Blanchard, A.D.; Perkins, A.C.; Jenkins, R.G.; et al. Preclinical SPECT/CT imaging of αvβ6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J. Nucl. Med. 2013, 54, 2146–2152. [Google Scholar] [CrossRef]
- Sime, P.; Jenkins, G. Goldilocks and the Three Trials: Clinical Trials Targeting the αvβ6 Integrin in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 206, 1062–1063. [Google Scholar] [CrossRef]
- Lo, W.C.Y.; Boas, C.W.V.; Huynh, T.T.; Klaas, A.; Grogan, F.; Strong, L.; Samson, P.; Robinson, C.G.; Rogers, B.; Bergom, C. Using Integrin αvβ6-Targeted Positron Emission Tomography Imaging to Longitudinally Monitor Radiation-Induced Pulmonary Fibrosis In Vivo. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 484–492. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Fei, J.; Li, Z. C2C12 myoblasts differentiate into myofibroblasts via the TGF-β1 signaling pathway mediated by Fibulin2. Gene 2025, 936, 149048. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Xie, H.; Jiao, Y.; Zhou, X.; Liao, X.; Chen, J.; Chen, H.; Chen, L.; Yu, S.; Deng, Q.; Sun, L.; et al. Integrin αvβ6 contributes to the development of intestinal fibrosis via the FAK/AKT signaling pathway. Exp. Cell Res. 2022, 411, 113003. [Google Scholar] [CrossRef]
- Morris, D.G.; Huang, X.; Kaminski, N.; Wang, Y.; Shapiro, S.D.; Dolganov, G.; Glick, A.; Sheppard, D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 2003, 422, 169–173. [Google Scholar] [CrossRef]
- Shapiro, S.D. Transgenic and gene-targeted mice as models for chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 29, 375–378. [Google Scholar] [CrossRef]
- Kitamura, H.; Cambier, S.; Somanath, S.; Barker, T.; Minagawa, S.; Markovics, J.; Goodsell, A.; Publicover, J.; Reichardt, L.; Jablons, D.; et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Investig. 2011, 121, 2863–2875. [Google Scholar] [CrossRef] [PubMed]
- Giménez, A.; Duch, P.; Puig, M.; Gabasa, M.; Xaubet, A.; Alcaraz, J. Dysregulated Collagen Homeostasis by Matrix Stiffening and TGF-β1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of FAK/Akt. Int. J. Mol. Sci. 2017, 18, 2431. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pan, L.; Cheng, Y.; Wu, X.; Tang, B.; Zhu, H.; Zhang, M.; Zhang, Y. Nintedanib alleviates pulmonary fibrosis in vitro and in vivo by inhibiting the FAK/ERK/S100A4 signalling pathway. Int. Immunopharmacol. 2022, 113, 109409. [Google Scholar] [CrossRef]
- Wu, Z.; Jiao, M.; Shu, C.; Zhang, S.; Wang, J.; Pu, J.; Zhu, J.; Zeng, Y.; Zhu, Y.; Liu, Z. Integrin αVβ1-activated PYK2 promotes the progression of non-small-cell lung cancer via the STAT3-VGF axis. Cell Commun. Signal. 2024, 22, 313. [Google Scholar] [CrossRef]
- Teoh, C.M.; Tan, S.S.; Tran, T. Integrins as Therapeutic Targets for Respiratory Diseases. Curr. Mol. Med. 2015, 15, 714–734. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, A.; Cai, T.; Li, Y.; Du, W.; Zhang, Y.; Zhang, R.; Zhang, W.; Zhu, J.; Zeng, Y.; et al. Integrin α3/α6 and αV are implicated in ADAM15-activated FAK and EGFR signalling pathway individually and promote non-small-cell lung cancer progression. Cell Death Dis. 2022, 13, 486. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Pulous, F.E.; Carnevale, J.C.; Al-Yafeai, Z.; Pearson, B.H.; Hamilton, J.A.G.; Henry, C.J.; Orr, A.W.; Petrich, B.G. Talin-dependent integrin activation is required for endothelial proliferation and postnatal angiogenesis. Angiogenesis 2021, 24, 177–190. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Lajoie, P.; Goetz, J.G.; Dennis, J.W.; Nabi, I.R. Lattices, rafts, and scaffolds: Domain regulation of receptor signaling at the plasma membrane. Cell Biol. 2009, 185, 381–385. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Tarone, G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003, 19, 173–206. [Google Scholar] [CrossRef] [PubMed]
- Fafilek, B.; Balek, L.; Bosakova, M.K.; Varecha, M.; Nita, A.; Gregor, T.; Gudernova, I.; Krenova, J.; Ghosh, S.; Piskacek, M.; et al. The inositol phosphatase SHIP2 enables sustained ERK activation downstream of FGF receptors by recruiting Src kinases. Sci. Signal. 2018, 11, eaap8608. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2012, 2, 311–319. [Google Scholar] [CrossRef] [PubMed]
- McCarty, J.H. αvβ8 integrin adhesion and signaling pathways in development, physiology and disease. J. Cell Sci. 2020, 133, jcs239434. [Google Scholar] [CrossRef]
- Schneller, M.; Vuori, K.; Ruoslahti, E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997, 16, 5600–5607. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Raghu, G.; Mouded, M.; Chambers, D.C.; Martinez, F.J.; Richeldi, L.; Lancaster, L.H.; Hamblin, M.J.; Gibson, K.F.; Rosas, I.O.; Prasse, A.; et al. A Phase IIb Randomized Clinical Study of an Anti-αvβ6 Monoclonal Antibody in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 206, 1128–1139. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Saigal, A.; Johnson, J.; Morrisson, J.; Tabrizifard, S.; Hollingsworth, S.A.; Eddins, M.J.; Mao, W.; O’Neill, K.; et al. Discovery of a new class of integrin antibodies for fibrosis. Sci. Rep. 2021, 11, 2118. [Google Scholar] [CrossRef]
- Minagawa, S.; Lou, J.; Seed, R.I.; Cormier, A.; Wu, S.; Cheng, Y.; Murray, L.; Tsui, P.; Connor, J.; Herbst, R.; et al. Selective targeting of TGF-β activation to treat fibroinflammatory airway disease. Sci. Transl. Med. 2014, 6, 241ra79. [Google Scholar] [CrossRef]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef]
- Carreira, V.; Standeven, A.M.; Ma, J.Y.; Hardisty, J.; Cohen, S.M.; Kerns, W.D.; Snook, S. Inhibitors of TGFβR1/ALK4/JNK3/Flt1 Kinases in Cynomolgus Macaques Lead to the Rapid Induction of Renal Epithelial Tumors. Toxicol. Sci. 2021, 180, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.K.; Tian, M.; Schiemann, W.P. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res. 2012, 347, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.A.; Dowling, J.E.; Bursavich, M.G.; Troast, D.M.; Chong, K.M.; Hahn, K.N.; Zhong, C.; Mulvihill, K.M.; Nguyen, H.; Monroy, M.F.; et al. The Discovery of MORF-627, a Highly Selective Conformationally-Biased Zwitterionic Integrin αvβ6 Inhibitor for Fibrosis. J. Med. Chem. 2024, 67, 18656–18681. [Google Scholar] [CrossRef]
- Guffroy, M.; Trela, B.; Kambara, T.; Stawski, L.; Chen, H.; Luus, L.; Montesinos, M.S.; Olson, L.; He, Y.; Maisonave, K.; et al. Selective inhibition of integrin αvβ6 leads to rapid induction of urinary bladder tumors in cynomolgus macaques. Toxicol. Sci. 2023, 191, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Shi, L.; Chang, A.; Fernandez, A.; Kraft, J.C.; Li, J.; Le, V.Q.; Winegar, R.V.; Cherf, G.M.; Slocum, D.; et al. De novo design of highly selective miniprotein inhibitors of integrins αvβ6 and αvβ8. Nat. Commun. 2023, 14, 5660. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; John, A.E.; Barrett, J.W.; Gower, E.; Morrison, V.S.; Man, Y.; Pun, K.T.; Roper, J.A.; Luckett, J.C.; Borthwick, L.A.; et al. Pharmacological characterisation of GSK3335103, an oral αvβ6 integrin small molecule RGD-mimetic inhibitor for the treatment of fibrotic disease. Eur. J. Pharmacol. 2021, 913, 174618. [Google Scholar] [CrossRef]
- Hryczanek, H.F.; Barrett, J.; Barrett, T.N.; Burley, G.A.; Cookson, R.E.; Hatley, R.J.D.; Measom, N.D.; Roper, J.A.; Rowedder, J.E.; Slack, R.J.; et al. Core Modifications of GSK3335103 toward Orally Bioavailable αvβ6 Inhibitors with Improved Synthetic Tractability. J. Med. Chem. 2024, 67, 19689–19715. [Google Scholar] [CrossRef]
- John, A.E.; Graves, R.H.; Pun, K.T.; Vitulli, G.; Forty, E.J.; Mercer, P.F.; Morrell, J.L.; Barrett, J.W.; Rogers, R.F.; Hafeji, M.; et al. Translational pharmacology of an inhaled small molecule αvβ6 integrin inhibitor for idiopathic pulmonary fibrosis. Nat. Commun. 2020, 11, 4659. [Google Scholar] [CrossRef]
- Maden, C.H.; Fairman, D.; Chalker, M.; Costa, M.J.; Fahy, W.A.; Garman, N.; Lukey, P.T.; Mant, T.; Parry, S.; Simpson, J.K.; et al. Safety, tolerability and pharmacokinetics of GSK3008348, a novel integrin αvβ6 inhibitor, in healthy participants. Eur. J. Clin. Pharmacol. 2018, 74, 701–709. [Google Scholar] [CrossRef]
- Maher, T.M.; Simpson, J.K.; Porter, J.C.; Wilson, F.J.; Chan, R.; Eames, R.; Cui, Y.; Siederer, S.; Parry, S.; Kenny, J.; et al. A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor. Respir. Res. 2020, 21, 75. [Google Scholar] [CrossRef]
- Decaris, M.L.; Schaub, J.R.; Chen, C.; Cha, J.; Lee, G.G.; Rexhepaj, M.; Ho, S.S.; Rao, V.; Marlow, M.M.; Kotak, P.; et al. Dual inhibition of αvβ6 and αvβ1 reduces fibrogenesis in lung tissue explants from patients with IPF. Respir. Res. 2021, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.; Cottin, V.; Ramaswamy, M.; Wuyts, W.A.; Jenkins, R.G.; Scholand, M.B.; Kreuter, M.; Valenzuela, C.; Ryerson, C.J.; Goldin, J.; et al. Bexotegrast in Patients with Idiopathic Pulmonary Fibrosis: The INTEGRIS-IPF Clinical Trial. Am. J. Respir. Crit. Care Med. 2024, 210, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.J.; Jacobs, S.; Lefebvre, É.A.; Cosgrove, G.P.; Clark, A.; Turner, S.M.; Decaris, M.; Barnes, C.N.; Jurek, M.; Williams, B.; et al. Bexotegrast Shows Dose-Dependent Integrin αvβ6 Receptor Occupancy in Lungs of Participants with Idiopathic Pulmonary Fibrosis: A Phase 2, Open-Label Clinical Trial. Ann. Am. Thorac. Soc. 2025, 22, 350–358. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, J.; Wu, Q.; Wang, B.; Hu, T.; Li, Y.; Yan, X.; Ma, L.; Tan, Z. A dual αvβ1/αvβ6 integrin inhibitor Bexotegrast (PLN-74809) ameliorates organ injury and fibrogenesis in fibrotic kidney disease. Eur. J. Pharmacol. 2024, 983, 176983. [Google Scholar] [CrossRef]
- Reichart, F.; Maltsev, O.V.; Kapp, T.G.; Räder, A.F.B.; Weinmüller, M.; Marelli, U.K.; Notni, J.; Wurzer, A.; Beck, R.; Wester, H.J.; et al. Selective Targeting of Integrin αvβ8 by a Highly Active Cyclic Peptide. J. Med. Chem. 2019, 62, 2024–2037. [Google Scholar] [CrossRef]
- Ritzenthaler, J.D.; Zhang, M.; Torres-Gonzalez, E.; Roman, J. The Integrin Inhibitor Cilengitide and Bleomycin-Induced Pulmonary Fibrosis: Cilengitide and Bleomycin-Induced Pulmonary Fibrosis. Lung 2020, 198, 947–955. [Google Scholar] [CrossRef]
- Forder, A.; Zhuang, R.; Souza, V.G.P.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; Stewart, G.L.; Benard, K.; Marshall, E.A.; Reis, P.P.; et al. Mechanisms Contributing to the Comorbidity of COPD and Lung Cancer. Int. J. Mol. Sci. 2023, 24, 2859. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, J.; Huang, X.; Li, Z. When chronic obstructive pulmonary disease meets small cell lung cancer: An unusual case report of rapid progression. BMC Geriatr. 2023, 23, 836. [Google Scholar] [CrossRef]
- Kashizaki, F.; Yumoto, K.; Tsuchiya, N.; Watanabe, S.; Shonai, S.; Orii, R.; Osada, R.; Kaneko, M.; Kikuchi, A.; Kojima, Y.; et al. Clinical Features of Simultaneous Diagnoses of Lung Cancer and Nontuberculous Mycobacterial Lung Disease: A Systematic Review and Pooled Analysis. J. Clin. Quest. 2025, 2, e59. [Google Scholar] [CrossRef]
- MacIsaac, S.; Somboonviboon, D.; Scallan, C.; Kolb, M. Treatment of idiopathic pulmonary fibrosis: An update on emerging drugs in phase II & III clinical trials. Expert. Opin. Emerg. Drugs 2024, 29, 177–186. [Google Scholar] [CrossRef]
- Calver, J.F.; Parmar, N.R.; Harris, G.; Lithgo, R.M.; Stylianou, P.; Zetterberg, F.R.; Gooptu, B.; Mackinnon, A.C.; Carr, S.B.; Borthwick, L.A.; et al. Defining the mechanism of galectin-3-mediated TGF-β1 activation and its role in lung fibrosis. J. Biol. Chem. 2024, 300, 107300. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Yuan, Y.; Ma, L.; Li, L.; Qin, W.; Wu, B.; Zheng, B.; Liao, X.; Hu, G.; Liu, B. Inhibition of TGFβ1 activation prevents radiation-induced lung fibrosis. Clin. Transl. Med. 2024, 14, e1546. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Li, J.; Xie, Y.; Zhu, J.; Nguyen, T.T.H.; Zhang, Y.; Zhu, J.; Springer, T.A. A general chemical principle for creating closure-stabilizing integrin inhibitors. Cell 2022, 185, 3533–3550.e27. [Google Scholar] [CrossRef] [PubMed]
- Bougie, D.W.; Rasmussen, M.; Zhu, J.; Aster, R.H. Antibodies causing thrombocytopenia in patients treated with RGD-mimetic platelet inhibitors recognize ligand-specific conformers of αIIb/β3 integrin. Blood 2012, 119, 6317–6325. [Google Scholar] [CrossRef]
- Ley, K.; Rivera-Nieves, J.; Sandborn, W.J.; Shattil, S. Integrin-based therapeutics: Biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016, 15, 173–183. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics 2023, 8, 216. [Google Scholar] [CrossRef]
- Li, D.; Zhao, A.; Zhu, J.; Wang, C.; Shen, J.; Zheng, Z.; Pan, F.; Liu, Z.; Chen, Q.; Yang, Y. Inhaled Lipid Nanoparticles Alleviate Established Pulmonary Fibrosis. Small 2023, 19, e2300545. [Google Scholar] [CrossRef]
- Kaur, R.; Shaikh, T.B.; Sripadi, H.P.; Kuncha, M.; Sarathi, U.V.R.V.; Kulhari, H.; Andugulapati, S.B.; Sistla, R. Nintedanib solid lipid nanoparticles improve oral bioavailability and ameliorate pulmonary fibrosis in vitro and in vivo models. Int. J. Pharm. 2024, 649, 123644. [Google Scholar] [CrossRef]
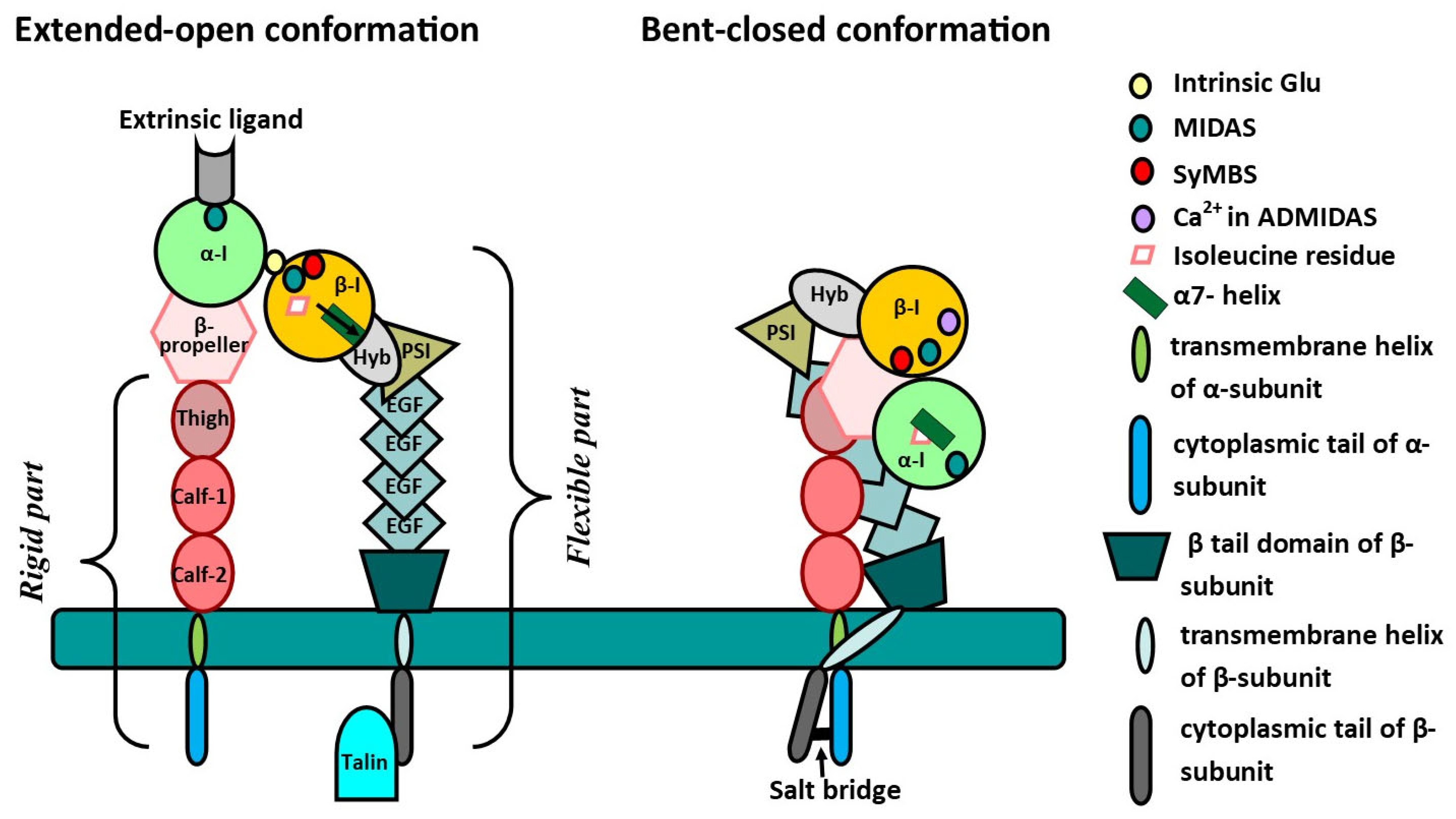

| Subunit | Structural Features | Localization |
|---|---|---|
| β1 | Mg2+ of MIDAS is associated with Ser132, Ser134, and Glu229. | Widely spread |
| β2 | Ca2+ of MIDAS is associated with Ser116, Asp119, Asp120, and Glu325. | Leucocytes |
| β3 | Ca2+ of MIDAS is associated with Ser123, Asp126, Asp127, and Met335. | Platelets and macrophages |
| β4 | The heaviest subunit, as its cytoplasmic domain consists of approximately 1000 amino acid residues. | Epithelial cells |
| β5 | Not found | Brain and blood cells |
| β6 | Mg2+ of MIDAS is associated with Asp140, Ser142, Glu240, and Asp271. | Epithelial cells |
| β7 | Two NPxY motifs in cytoplasmic part for binding with talin; Mg2+ in MIDAS is associated with Ser160; two Ca2+ in ADMIDAS and SyMBS associated with Pro257, Asp197, Asp255 and Glu372, Asp166, and Ser162. | Immune cells (NK-cells, B-plasma cells, eosinophils, lymphocytes) |
| β8 | Does not interact with talin; does not have ADMIDAS with Ca2+; Mg2+ of MIDAS is associated with Ser116, Ser114, Asp219, and Glu212; Ca2+ of SyMBS is associated with Asp151, Asn207, Asp209, Pro211, and Glu212. | Genitourinary system (kidneys, placenta, uterus, ovaries) |
| Integrin Inhibitor | Drug Type | Targeted Integrin | Study Status | Reason for Failure |
|---|---|---|---|---|
| BG00011 | Antibody | αvβ6 | IIb (terminated) | Four deaths related to respiratory complications |
| Ab-31 | Antibody | αvβ1, αvβ6 | Preclinical stage | |
| B5 | Antibody | αvβ8 | Preclinical stage | |
| MORF-627 | Small molecule | αvβ6 | Preclinical stage | Bladder epithelium hyperplasia |
| B6_BP_dslf | Peptide | αvβ6 | ||
| GSK3335103 | Small molecule | αvβ6 | Preclinical stage | |
| GSK3008348 | Small molecule | αvβ6 | I (terminated) | The reasons are not clear |
| PLN-74809 | Small molecule | αvβ6, αvβ1 | II (ongoing) | Will end in September 2025 |
| Cyclic octapeptide | Peptide | αvβ8 | ||
| Cilengitide | Peptide | αvβ3, αvβ5, α5β1 | II (terminated) | Did not improve the course of fibrosis in mice with bleomycin-induced lung injury |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovina, E.L.; Kochubey, V.V.; Shabanova, M.A.; Chekhvalova, D.M.; Serebryakova, V.A.; Skurikhin, E.G.; Vaizova, O.E.; Morozov, S.G.; Kubatiev, A.A.; Dygai, A.M. Therapeutic Prospects of αv Integrins Inhibition in Fibrotic Lung Diseases and Carcinogenesis. Int. J. Mol. Sci. 2025, 26, 6202. https://doi.org/10.3390/ijms26136202
Golovina EL, Kochubey VV, Shabanova MA, Chekhvalova DM, Serebryakova VA, Skurikhin EG, Vaizova OE, Morozov SG, Kubatiev AA, Dygai AM. Therapeutic Prospects of αv Integrins Inhibition in Fibrotic Lung Diseases and Carcinogenesis. International Journal of Molecular Sciences. 2025; 26(13):6202. https://doi.org/10.3390/ijms26136202
Chicago/Turabian StyleGolovina, Eugenija Leonidovna, Veronika Vladimirovna Kochubey, Marina Alekseevna Shabanova, Darya Maksimovna Chekhvalova, Valentina Alexandrovna Serebryakova, Evgenii Germanovich Skurikhin, Olga Evgenievna Vaizova, Sergey Georgievich Morozov, Aslan Amirkhanovich Kubatiev, and Alexander Mikhaylovich Dygai. 2025. "Therapeutic Prospects of αv Integrins Inhibition in Fibrotic Lung Diseases and Carcinogenesis" International Journal of Molecular Sciences 26, no. 13: 6202. https://doi.org/10.3390/ijms26136202
APA StyleGolovina, E. L., Kochubey, V. V., Shabanova, M. A., Chekhvalova, D. M., Serebryakova, V. A., Skurikhin, E. G., Vaizova, O. E., Morozov, S. G., Kubatiev, A. A., & Dygai, A. M. (2025). Therapeutic Prospects of αv Integrins Inhibition in Fibrotic Lung Diseases and Carcinogenesis. International Journal of Molecular Sciences, 26(13), 6202. https://doi.org/10.3390/ijms26136202






