Disparate Roles of Cell–Cell Contact and Cytokine Secretion in an In Vitro Model of the Seminoma Microenvironment
Abstract
1. Introduction
2. Results
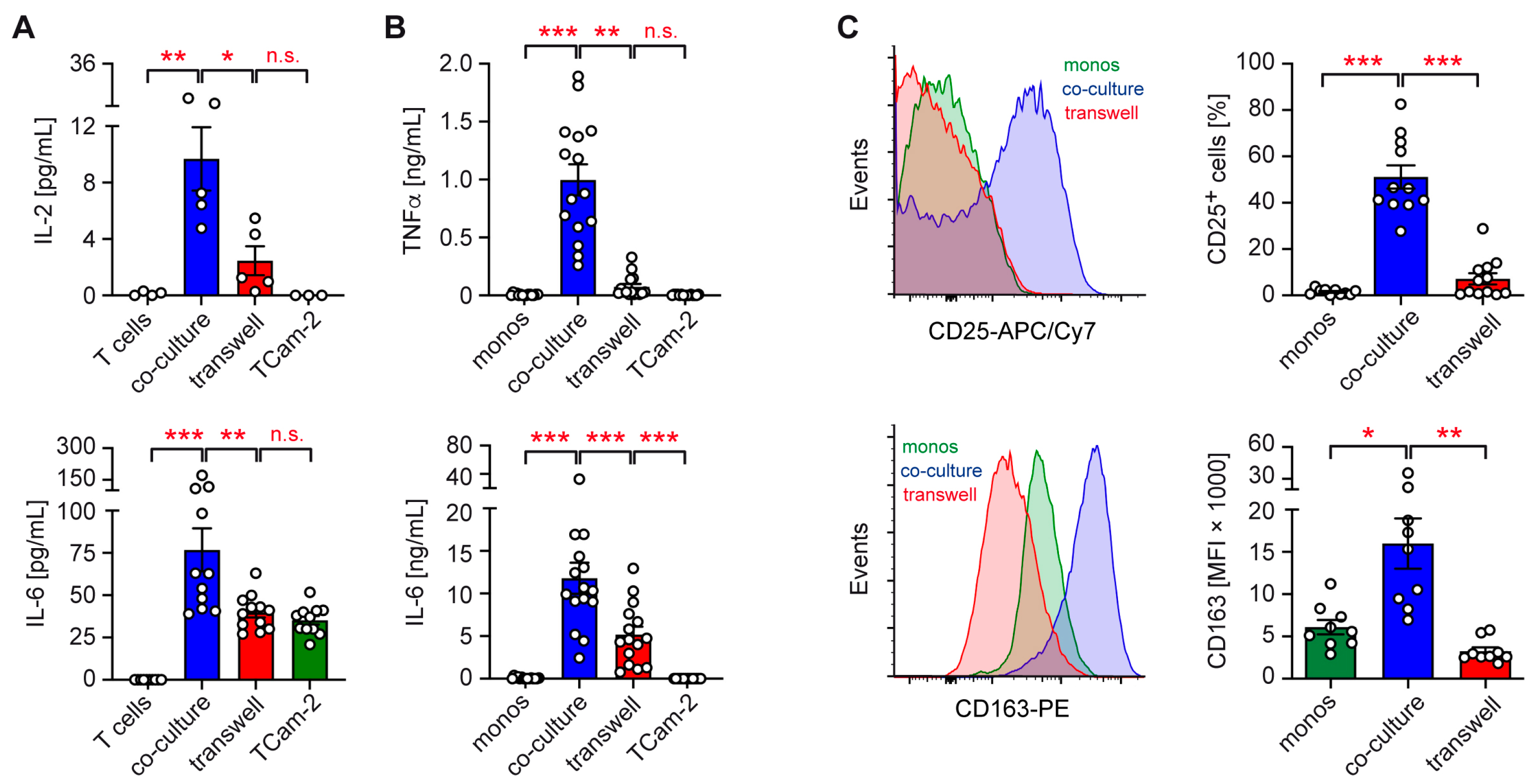
2.1. Role of Cell–Cell Contact in TCam-2 Co-Cultures with Immune Cells
2.2. Control of Pro-Inflammatory Gene Expression by TCam-2 Cells
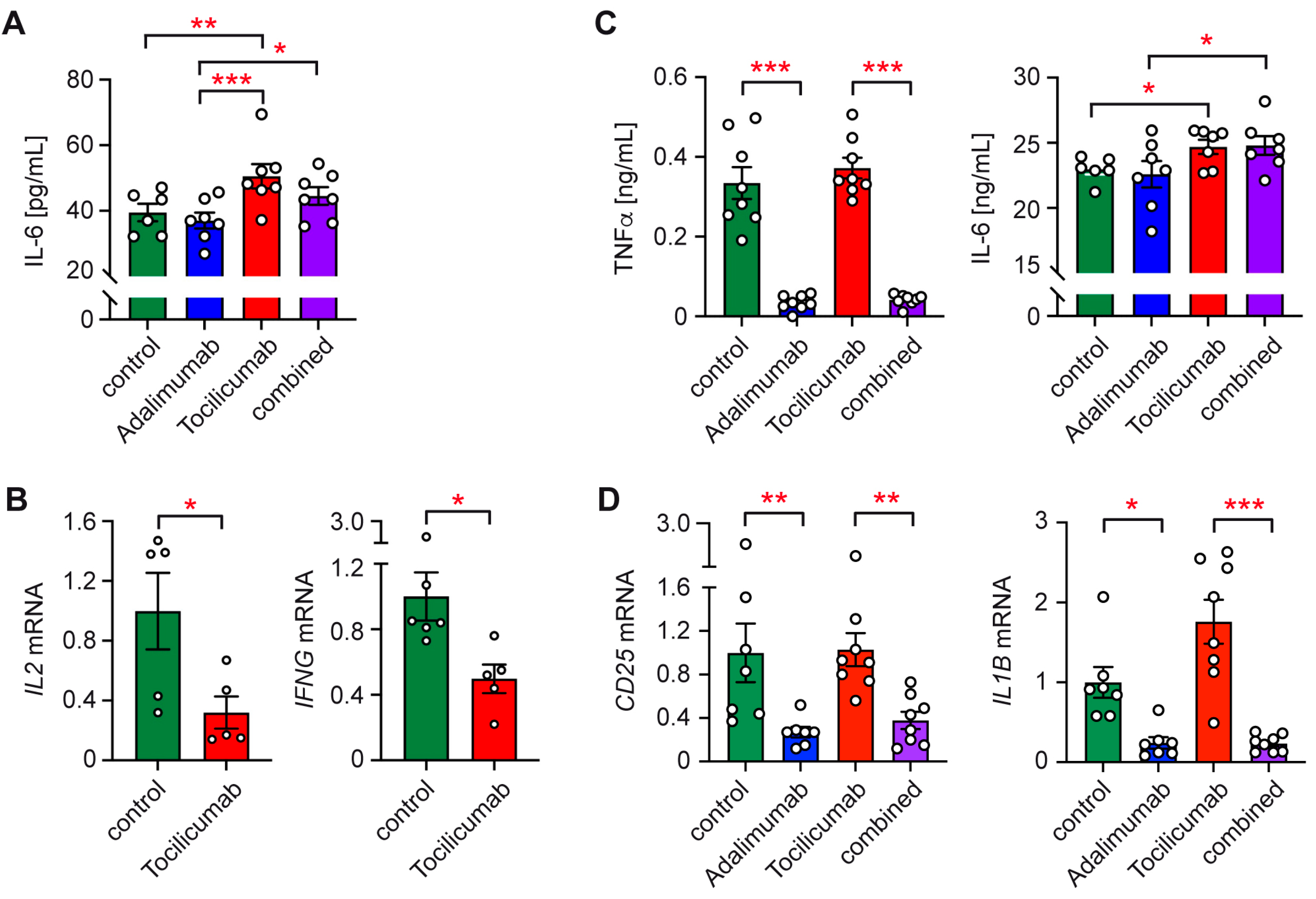
2.3. Involvement of IL-6 and TNFα in Immune Cell Activation by TCam-2 Cells
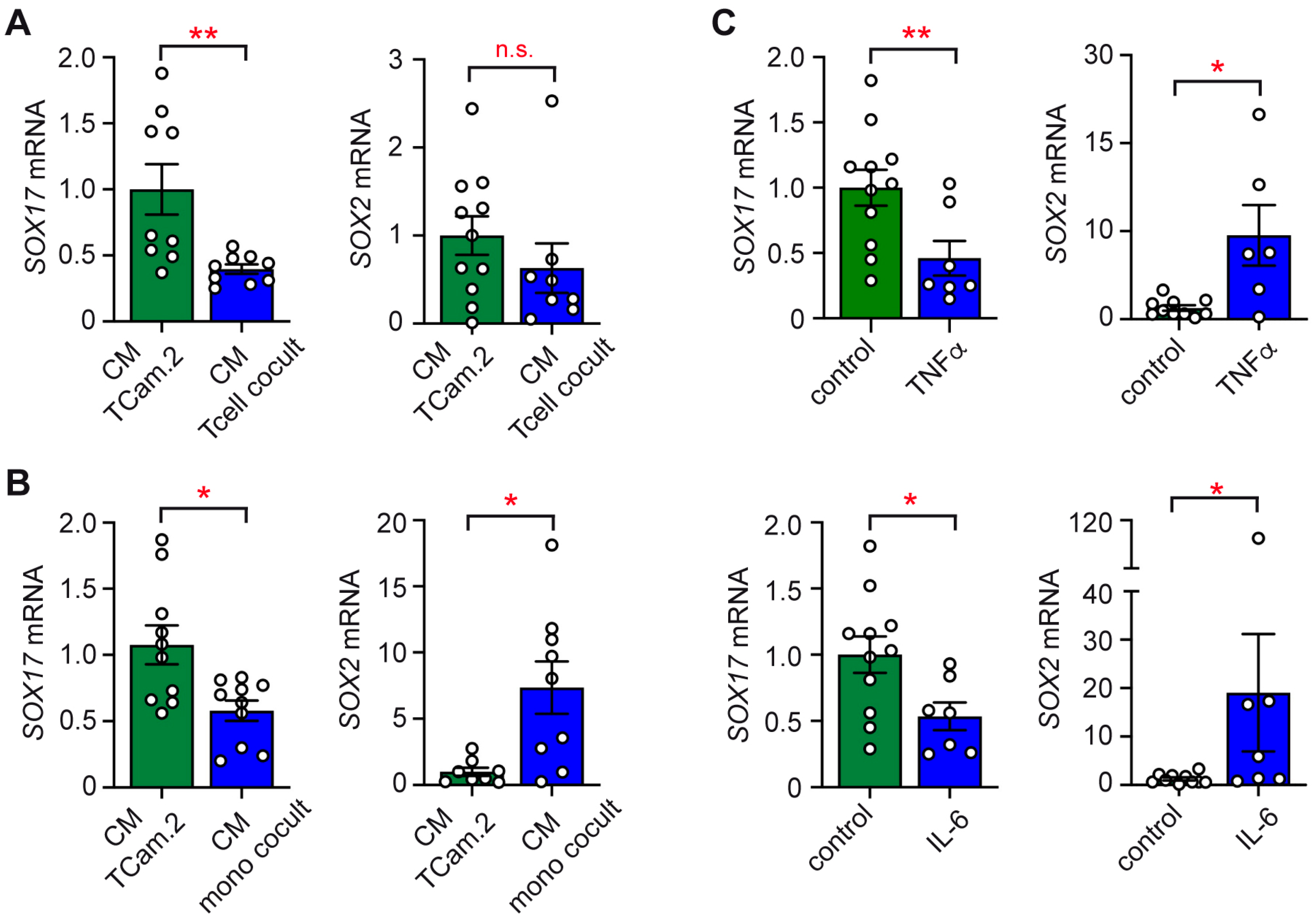
2.4. Impact of Conditioned Media on the Subtype Identity of TCam-2 Cells
2.5. Phenotypic Switch of TCam-2 Cells in Response to Cytokines
3. Discussion
4. Materials and Methods
4.1. Leukocyte Isolation
4.2. Cell Culture
4.3. Quantitative RT-PCR (RT-qPCR)
4.4. Flow Cytometry (FACS)
4.5. Enzyme-Linkened Immunosorbent Assay (ELISA)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singla, N.; Bagrodia, A.; Baraban, E.; Fankhauser, C.D.; Ged, Y.M.A. Testicular Germ Cell Tumors: A Review. JAMA 2025, 333, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, A.A.; Kelly, S.P.; Altekruse, S.F.; Rosenberg, P.S.; McGlynn, K.A. Future of Testicular Germ Cell Tumor Incidence in the United States: Forecast through 2026. Cancer 2017, 123, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Tateo, V.; Thompson, Z.J.; Gilbert, S.M.; Cortessis, V.K.; Daneshmand, S.; Masterson, T.A.; Feldman, D.R.; Pierorazio, P.M.; Prakash, G.; Heidenreich, A.; et al. Epidemiology and Risk Factors for Testicular Cancer: A Systematic Review. Eur. Urol. 2025, 87, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Williamson, S.R.; Delahunt, B.; Magi-Galluzzi, C.; Algaba, F.; Egevad, L.; Ulbright, T.M.; Tickoo, S.K.; Srigley, J.R.; Epstein, J.I.; Berney, D.M. The World Health Organization 2016 Classification of Testicular Germ Cell Tumours: A Review and Update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017, 70, 335–346. [Google Scholar] [CrossRef]
- Dieckmann, K.P.; Skakkebaek, N.E. Carcinoma in Situ of the Testis: Review of Biological and Clinical Features. Int. J. Cancer 1999, 83, 815–822. [Google Scholar] [CrossRef]
- Mantovani, A.; Locati, M. Tumor-Associated Macrophages as a Paradigm of Macrophage Plasticity, Diversity, and Polarization: Lessons and Open Questions. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1478–1483. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Hadrup, S.R.; Brændstrup, O.; Jacobsen, G.K.; Mortensen, S.; Pedersen, L.Ø.; Seremet, T.; Andersen, M.H.; Becker, J.C.; Straten, P. thor Tumor Infiltrating Lymphocytes in Seminoma Lesions Comprise Clonally Expanded Cytotoxic T Cells. Int. J. Cancer 2006, 119, 831–838. [Google Scholar] [CrossRef]
- Hvarness, T.; Nielsen, J.E.; Almstrup, K.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Claesson, M.H. Phenotypic Characterisation of Immune Cell Infiltrates in Testicular Germ Cell Neoplasia. J. Reprod. Immunol. 2013, 100, 135–145. [Google Scholar] [CrossRef]
- Siska, P.J.; Johnpulle, R.A.N.; Zhou, A.; Bordeaux, J.; Kim, J.Y.; Dabbas, B.; Dakappagari, N.; Rathmell, J.C.; Rathmell, W.K.; Morgans, A.K.; et al. Deep Exploration of the Immune Infiltrate and Outcome Prediction in Testicular Cancer by Quantitative Multiplexed Immunohistochemistry and Gene Expression Profiling. Oncoimmunology 2017, 6, e1305535. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Rodrigues, Â.; Guimarães, R.; Cantante, M.; Lopes, P.; Maurício, J.; Oliveira, J.; Jerónimo, C.; Henrique, R. Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers. Cancers 2019, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; Sittig, S.P.; van Dijk, T.; Figdor, C.G.; de Vries, I.J.M. The Nature of Activatory and Tolerogenic Dendritic Cell-Derived Signal II. Front. Immunol. 2013, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Balta, E.; Wabnitz, G.H.; Samstag, Y. Hijacked Immune Cells in the Tumor Microenvironment: Molecular Mechanisms of Immunosuppression and Cues to Improve T Cell-Based Immunotherapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5736. [Google Scholar] [CrossRef]
- De Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Stephan, A.; Suhrmann, J.-H.; Skowron, M.A.; Che, Y.; Poschmann, G.; Petzsch, P.; Kresbach, C.; Wruck, W.; Pongratanakul, P.; Adjaye, J.; et al. Molecular and Epigenetic Ex Vivo Profiling of Testis Cancer-Associated Fibroblasts and Their Interaction with Germ Cell Tumor Cells and Macrophages. Matrix Biol. 2024, 132, 10–23. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.-Q.; Kariya, Y.; Teshigawara, K.; Uchida, A. Accumulation of γ/δ T Cells in Human Dysgerminoma and Seminoma: Roles in Autologous Tumor Killing and Granuloma Formation. Immunol. Investig. 1995, 24, 607–618. [Google Scholar] [CrossRef]
- Gayer, F.A.; Fichtner, A.; Legler, T.J.; Reichardt, H.M. A Coculture Model Mimicking the Tumor Microenvironment Unveils Mutual Interactions between Immune Cell Subtypes and the Human Seminoma Cell Line TCam-2. Cells 2022, 11, 885. [Google Scholar] [CrossRef]
- Országhová, Z.; Kalavska, K.; Mego, M.; Chovanec, M. Overcoming Chemotherapy Resistance in Germ Cell Tumors. Biomedicines 2022, 10, 972. [Google Scholar] [CrossRef]
- Islam, R.; Heyer, J.; Figura, M.; Wang, X.; Nie, X.; Nathaniel, B.; Indumathy, S.; Hartmann, K.; Pleuger, C.; Fijak, M.; et al. T Cells in Testicular Germ Cell Tumors: New Evidence of Fundamental Contributions by Rare Subsets. Br. J. Cancer 2024, 130, 1893–1903. [Google Scholar] [CrossRef]
- Katsuta, E.; Rashid, O.M.; Takabe, K. Clinical Relevance of Tumor Microenvironment: Immune Cells, Vessels, and Mouse Models. Hum. Cell 2020, 33, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, D.; Heimsoeth, A.; Jostes, S.; Schneider, S.; Fellermeyer, M.; Hofmann, A.; Schorle, H. SOX2 Is Essential for in Vivo Reprogramming of Seminoma-like TCam-2 Cells to an Embryonal Carcinoma-like Fate. Oncotarget 2016, 7, 47095–47110. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Stoop, H.; Gillis, A.J.M.; Hersmus, R.; van Gurp, R.J.H.L.M.; van de Geijn, G.-J.M.; van Drunen, E.; Beverloo, H.B.; Schneider, D.T.; Sherlock, J.K.; et al. Further Characterization of the First Seminoma Cell Line TCam-2. Genes. Chromosomes Cancer 2008, 47, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.; Nettersheim, D.; Heukamp, L.C.; Kitazawa, S.; Biermann, K.; Schorle, H. TCam-2 but Not JKT-1 Cells Resemble Seminoma in Cell Culture. Cell Tissue Res. 2008, 331, 529–538. [Google Scholar] [CrossRef]
- Gayer, F.A.; Henkel, M.; Luft, J.; Reichardt, S.D.; Fichtner, A.; Legler, T.J.; Reichardt, H.M. The Subtype Identity of Testicular Cancer Cells Determines Their Immunostimulatory Activity in a Coculture Model. Cancers 2023, 15, 2619. [Google Scholar] [CrossRef]
- Nettersheim, D.; Westernströer, B.; Haas, N.; Leinhaas, A.; Brüstle, O.; Schlatt, S.; Schorle, H. Establishment of a Versatile Seminoma Model Indicates Cellular Plasticity of Germ Cell Tumor Cells. Genes Chromosomes Cancer 2012, 51, 717–726. [Google Scholar] [CrossRef]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of Interleukin-6 in Cancer Progression and Therapeutic Resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Nestler, T.; Dalvi, P.; Haidl, F.; Wittersheim, M.; von Brandenstein, M.; Paffenholz, P.; Wagener-Ryczek, S.; Pfister, D.; Koitzsch, U.; Hellmich, M.; et al. Transcriptome Analysis Reveals Upregulation of Immune Response Pathways at the Invasive Tumour Front of Metastatic Seminoma Germ Cell Tumours. Br. J. Cancer 2022, 126, 937–947. [Google Scholar] [CrossRef]
- Jones, S.A.; Scheller, J.; Rose-John, S. Therapeutic Strategies for the Clinical Blockade of IL-6/Gp130 Signaling. J. Clin. Investig. 2011, 121, 3375–3383. [Google Scholar] [CrossRef]
- Torres, A.; Casanova, J.F.; Nistal, M.; Regadera, J. Quantification of Immunocompetent Cells in Testicular Germ Cell Tumours. Histopathology 1997, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Díez-Torre, A.; Silván, U.; Díaz-Núñez, M.; Aréchaga, J. The Role of Microenvironment in Testicular Germ Cell Tumors. Cancer Biol. Ther. 2010, 10, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The Role of PD-1/PD-L1 and Application of Immune-Checkpoint Inhibitors in Human Cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef] [PubMed]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 Signalling through Macrophage Siglec-10 Is a New Target for Cancer Immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Liu, X.; Bai, X.F.; Wen, J.; Gao, J.X.; Liu, J.; Lu, P.; Wang, Y.; Zheng, P.; Liu, Y. B7H Costimulates Clonal Expansion of, and Cognate Destruction of Tumor Cells by, CD8(+) T Lymphocytes in Vivo. J. Exp. Med. 2001, 194, 1339–1348. [Google Scholar] [CrossRef]
- Kilian, M.; Friedrich, M.J.; Lu, K.H.-N.; Vonhören, D.; Jansky, S.; Michel, J.; Keib, A.; Stange, S.; Hackert, N.; Kehl, N.; et al. The Immunoglobulin Superfamily Ligand B7H6 Subjects T Cell Responses to NK Cell Surveillance. Sci. Immunol. 2024, 9, eadj7970. [Google Scholar] [CrossRef]
- Tirapu, I.; Huarte, E.; Guiducci, C.; Arina, A.; Zaratiegui, M.; Murillo, O.; Gonzalez, A.; Berasain, C.; Berraondo, P.; Fortes, P.; et al. Low Surface Expression of B7-1 (CD80) Is an Immunoescape Mechanism of Colon Carcinoma. Cancer Res. 2006, 66, 2442–2450. [Google Scholar] [CrossRef]
- Hersey, P.; Si, Z.; Smith, M.J.; Thomas, W.D. Expression of the Co-Stimulatory Molecule B7 on Melanoma Cells. Int. J. Cancer 1994, 58, 527–532. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Ji, C.; Liu, X.; Gu, B.; Dong, T. Macrophage Polarization in the Tumor Microenvironment: Emerging Roles and Therapeutic Potentials. Biomed. Pharmacother. 2024, 177, 116930. [Google Scholar] [CrossRef]
- Bellora, F.; Castriconi, R.; Dondero, A.; Pessino, A.; Nencioni, A.; Liggieri, G.; Moretta, L.; Mantovani, A.; Moretta, A.; Bottino, C. TLR Activation of Tumor-Associated Macrophages from Ovarian Cancer Patients Triggers Cytolytic Activity of NK Cells. Eur. J. Immunol. 2014, 44, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-Karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity Resulting from Functional Mimicry of a Toll-like Receptor Complex Protein. Nature 2009, 457, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent Insights into Targeting the IL-6 Cytokine Family in Inflammatory Diseases and Cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Madeddu, C.; Gramignano, G.; Kotsonis, P.; Coghe, F.; Atzeni, V.; Scartozzi, M.; Macciò, A. Microenvironmental M1 Tumor-Associated Macrophage Polarization Influences Cancer-Related Anemia in Advanced Ovarian Cancer: Key Role of Interleukin-6. Haematologica 2018, 103, e388–e391. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Pan, R.; Zhang, X.; Si, X.; Chen, M.; Wang, M.; Zhang, L. Tocilizumab for Advanced Non-Small-Cell Lung Cancer with Concomitant Cachexia: An Observational Study. J. Cachexia Sarcopenia Muscle 2024, 15, 2815–2825. [Google Scholar] [CrossRef]
- Schiller, J.H.; Storer, B.E.; Witt, P.L.; Alberti, D.; Tombes, M.B.; Arzoomanian, R.; Proctor, R.A.; McCarthy, D.; Brown, R.R.; Voss, S.D. Biological and Clinical Effects of Intravenous Tumor Necrosis Factor-Alpha Administered Three Times Weekly. Cancer Res. 1991, 51, 1651–1658. [Google Scholar]
- Weber, J.; Yang, J.C.; Topalian, S.L.; Parkinson, D.R.; Schwartzentruber, D.S.; Ettinghausen, S.E.; Gunn, H.; Mixon, A.; Kim, H.; Cole, D. Phase I Trial of Subcutaneous Interleukin-6 in Patients with Advanced Malignancies. J. Clin. Oncol. 1993, 11, 499–506. [Google Scholar] [CrossRef]
- Klein, B.; Haggeney, T.; Fietz, D.; Indumathy, S.; Loveland, K.L.; Hedger, M.; Kliesch, S.; Weidner, W.; Bergmann, M.; Schuppe, H.-C. Specific Immune Cell and Cytokine Characteristics of Human Testicular Germ Cell Neoplasia. Hum. Reprod. 2016, 31, 2192–2202. [Google Scholar] [CrossRef]
- Davies, A.; Zoubeidi, A.; Beltran, H.; Selth, L.A. The Transcriptional and Epigenetic Landscape of Cancer Cell Lineage Plasticity. Cancer Discov. 2023, 13, 1771–1788. [Google Scholar] [CrossRef]
- Tan, S.Y.X.; Zhang, J.; Tee, W.-W. Epigenetic Regulation of Inflammatory Signaling and Inflammation-Induced Cancer. Front. Cell Dev. Biol. 2022, 10, 931493. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic Regulation in the Tumor Microenvironment: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, M.; Mueller, T.; Weilbacher, A.; Dengler, M.A.; Bedke, J.; Kruck, S.; Oren, M.; Aulitzky, W.E.; van der Kuip, H. Cisplatin Hypersensitivity of Testicular Germ Cell Tumors Is Determined by High Constitutive Noxa Levels Mediated by Oct-4. Cancer Res. 2013, 73, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Wermann, H.; Stoop, H.; Gillis, A.J.M.; Honecker, F.; van Gurp, R.J.H.L.M.; Ammerpohl, O.; Richter, J.; Osterhuis, J.W.; Bokemeyer, C.; Looijenga, L.H.J. Global DNA methylation in fetal human germ cells and germ cell tumours: Association with differentiation and cisplatin resistance. J. Pathol. 2010, 221, 433–442. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruth, P.; Luft, J.; Klaus, L.; Legler, T.J.; Reichardt, H.M.; Gayer, F.A. Disparate Roles of Cell–Cell Contact and Cytokine Secretion in an In Vitro Model of the Seminoma Microenvironment. Int. J. Mol. Sci. 2025, 26, 6173. https://doi.org/10.3390/ijms26136173
Fruth P, Luft J, Klaus L, Legler TJ, Reichardt HM, Gayer FA. Disparate Roles of Cell–Cell Contact and Cytokine Secretion in an In Vitro Model of the Seminoma Microenvironment. International Journal of Molecular Sciences. 2025; 26(13):6173. https://doi.org/10.3390/ijms26136173
Chicago/Turabian StyleFruth, Patrick, Juliane Luft, Lucas Klaus, Tobias J. Legler, Holger M. Reichardt, and Fabian A. Gayer. 2025. "Disparate Roles of Cell–Cell Contact and Cytokine Secretion in an In Vitro Model of the Seminoma Microenvironment" International Journal of Molecular Sciences 26, no. 13: 6173. https://doi.org/10.3390/ijms26136173
APA StyleFruth, P., Luft, J., Klaus, L., Legler, T. J., Reichardt, H. M., & Gayer, F. A. (2025). Disparate Roles of Cell–Cell Contact and Cytokine Secretion in an In Vitro Model of the Seminoma Microenvironment. International Journal of Molecular Sciences, 26(13), 6173. https://doi.org/10.3390/ijms26136173





