Synthesis of Novel Podophyllotoxin–Benzothiazole Congeners and Their Biological Evaluation as Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
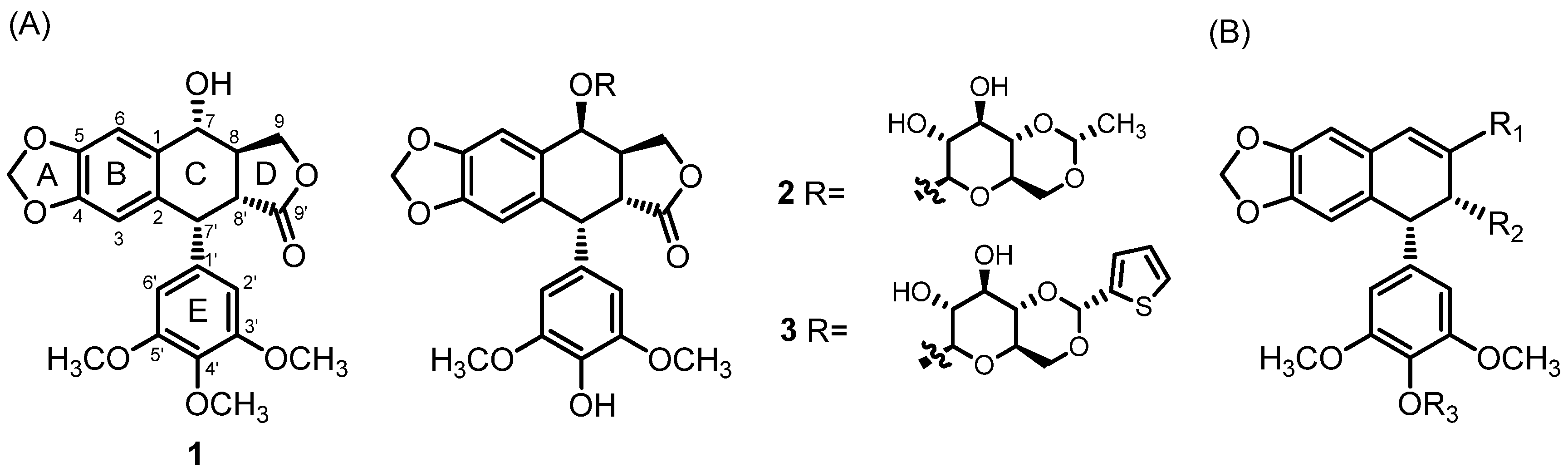
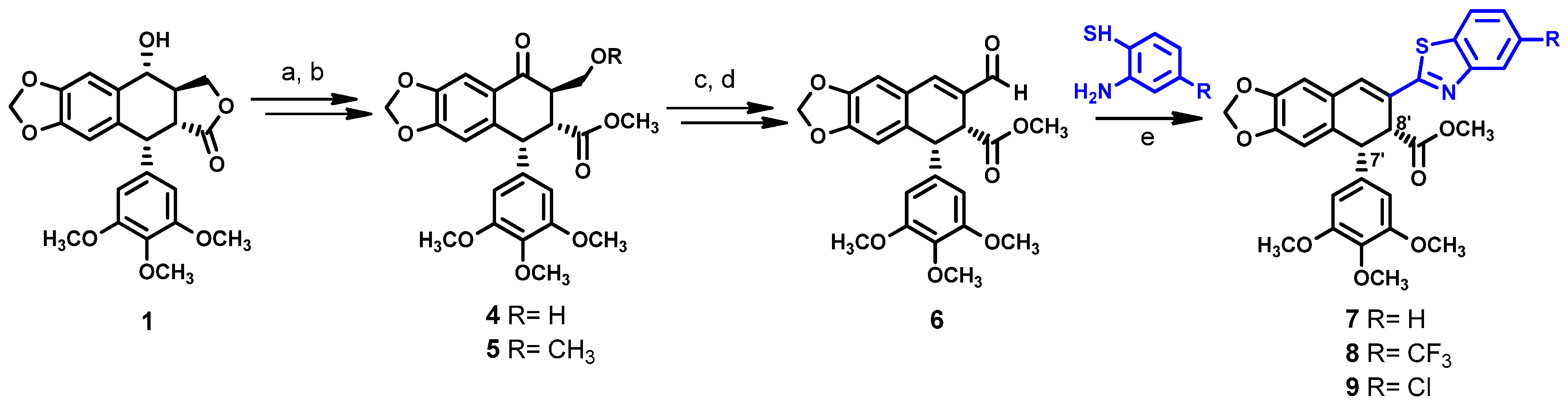
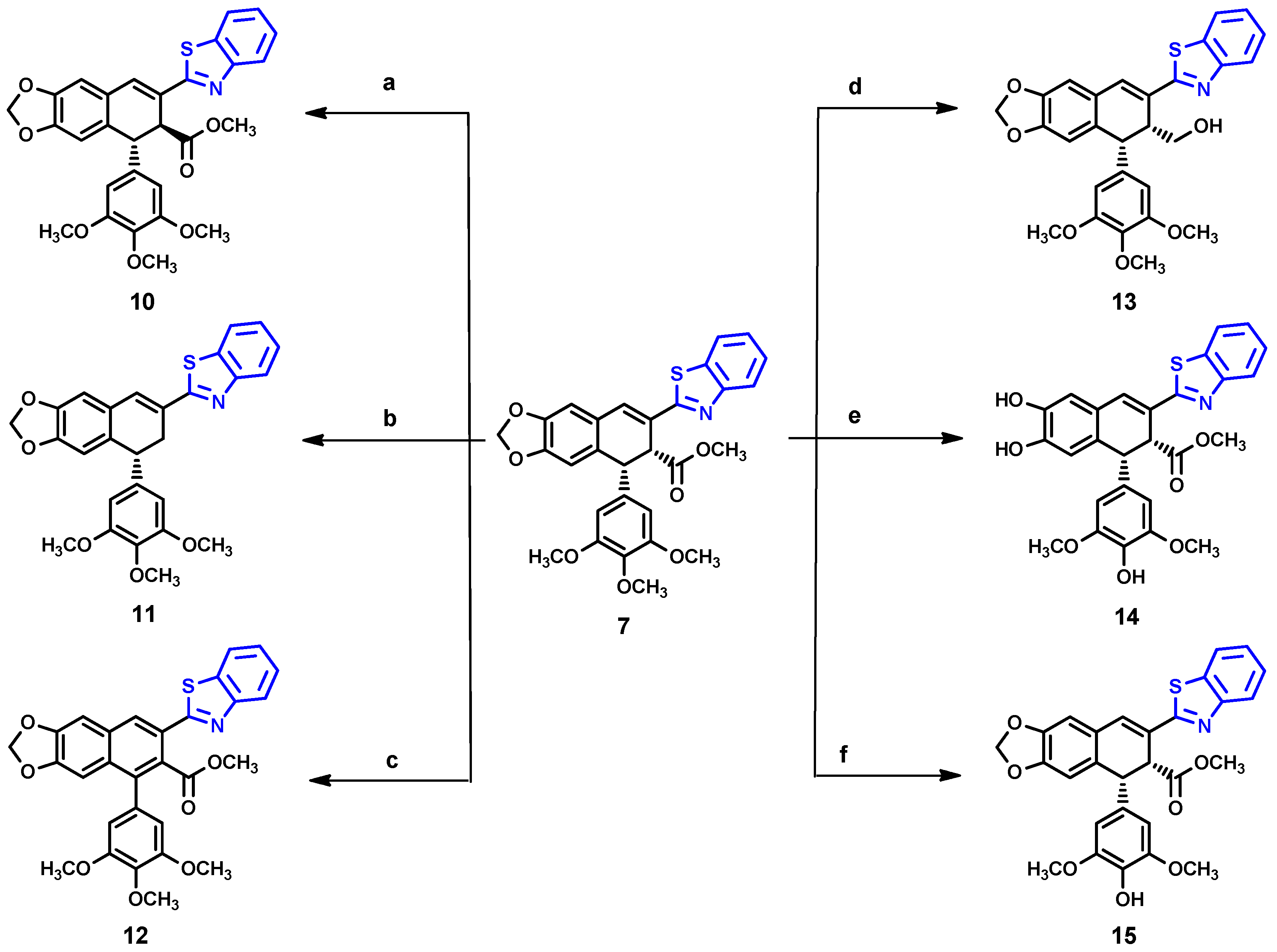
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. The In Vitro Cytotoxicity Assay
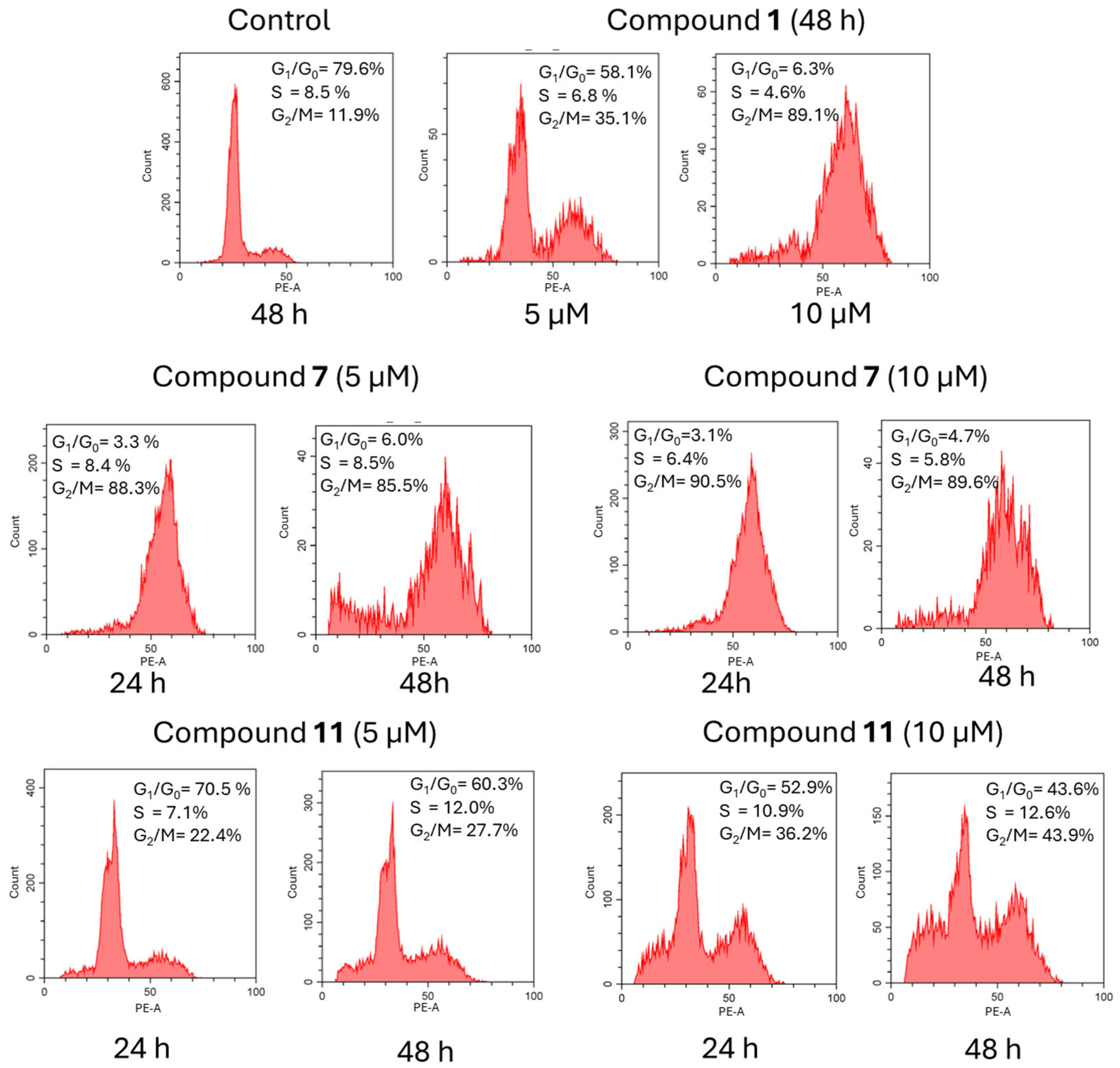
2.2.2. Cell Cycle Analysis
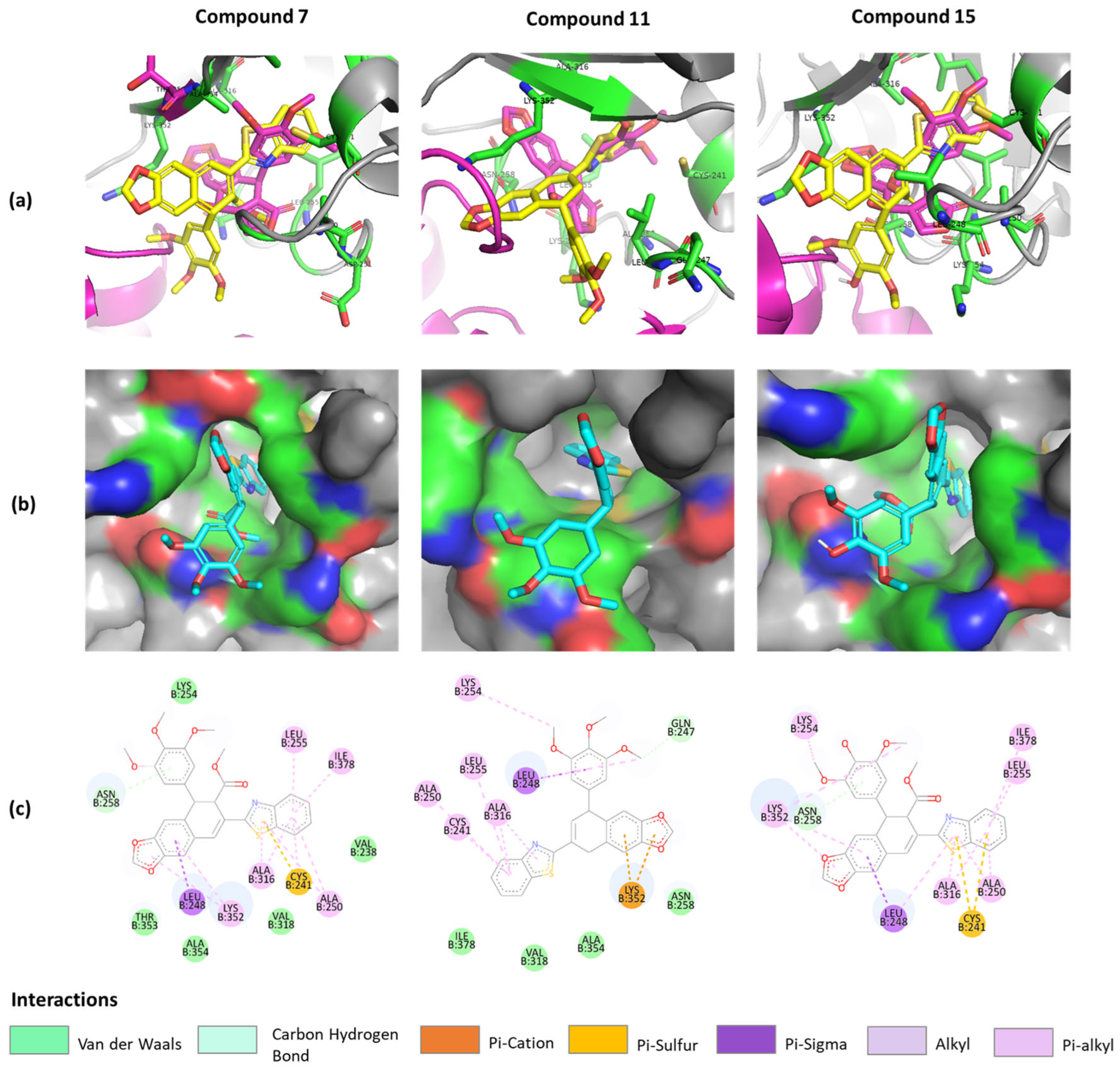
2.3. Molecular Docking Studies
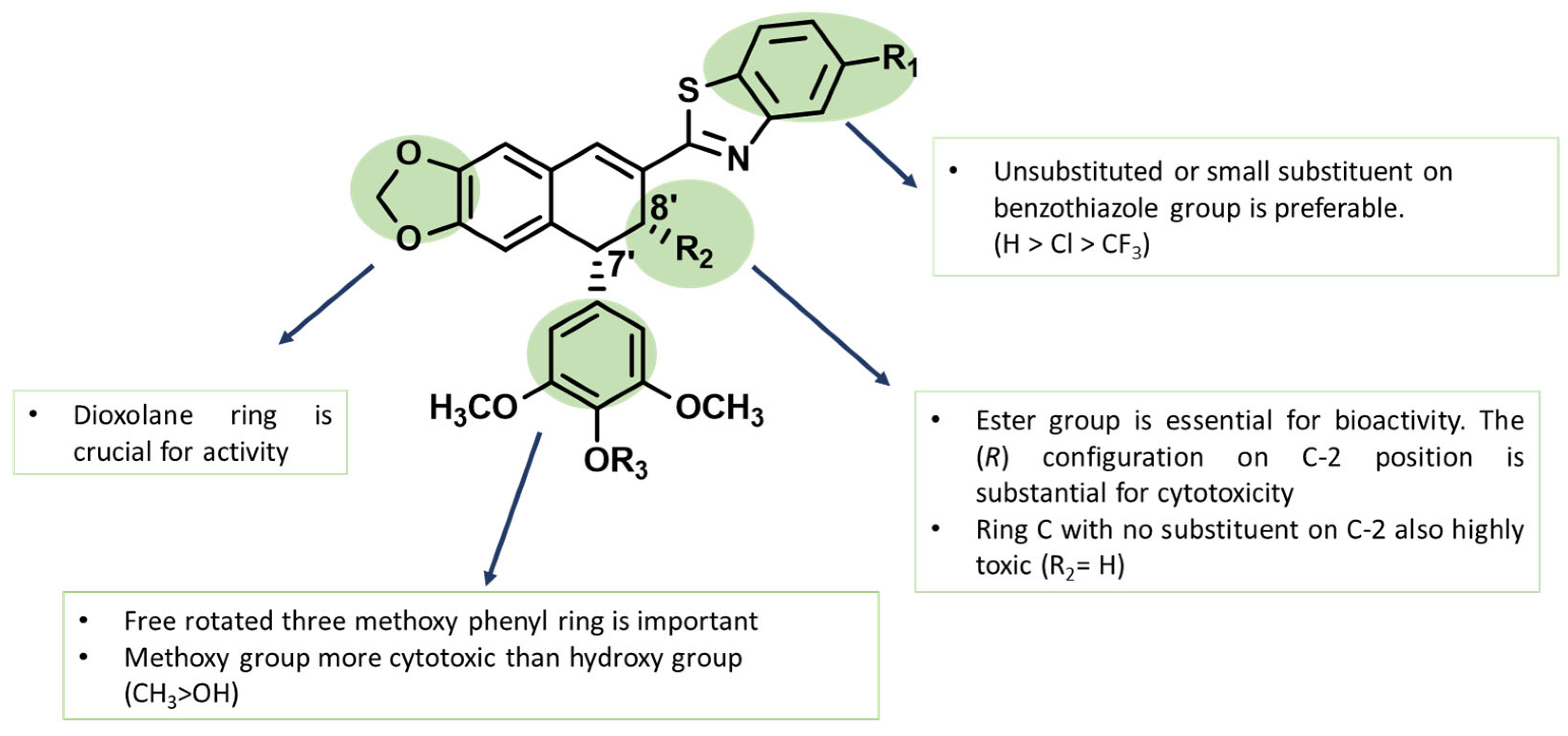
2.4. The Structure–Activity Relationships
2.5. Prediction of ADMET Parameter with SwissADME
3. Materials and Methods
3.1. Chemistry
3.1.1. General Method of Synthesis
Compound 4
Compound 6
General Procedure for the Synthesis of Compounds 7–9
Compound 10
Compound 11
Compound 12
Compound 13
Compound 14
Compound 15
3.1.2. X-Ray Crystallography Analysis of 7, 9 and 10
3.2. Biological Evaluation
3.2.1. Cell Culture
3.2.2. The Crystal Violet Assay
3.2.3. The PrestoBlue Assay
3.2.4. Cell Cycle Analysis by Flow Cytometry
3.2.5. Statistical Analysis
3.3. In Silico Studies
3.4. ADME Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Che, Z.; Xu, H. Recent Advances in the Chemistry and Biology of Podophyllotoxins. Chem. Eur. J. 2017, 23, 4467–4526. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and Microparticles for Skin Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Canel, C.; Moraes, R.M.; Dayan, F.E.; Ferreira, D. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef]
- Motyka, S.; Jafernik, K.; Ekiert, H.; Sharifi-Rad, J.; Calina, D.; Al-Omari, B.; Szopa, A.; Cho, W.C. Podophyllotoxin and Its Derivatives: Potential Anticancer Agents of Natural Origin in Cancer Chemotherapy. Biomed. Pharmacother. 2023, 158, 114145. [Google Scholar] [CrossRef]
- Fan, H.; Zhu, Z.; Xian, H.; Wang, H.; Chen, B.; Tang, Y.-J.; Tang, Y.; Liang, X. Insight Into the Molecular Mechanism of Podophyllotoxin Derivatives as Anticancer Drugs. Front. Cell Dev. Biol. 2021, 9, 709075. [Google Scholar] [CrossRef]
- Strus, P.; Sadowski, K.; Ploch, W.; Jazdzewska, A.; Oknianska, P.; Raniszewska, O.; Mlynarczuk-Bialy, I. The Effects of Podophyllotoxin Derivatives on Noncancerous Diseases: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 958. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Gao, P.; Guan, X.; Wang, B.; Zhang, L.; Yao, Y.; Guo, Y.; Wang, Y.; Jiang, S.; et al. Global, Regional, and National Burden of Breast, Cervical, Uterine, and Ovarian Cancer and Their Risk Factors among Women from 1990 to 2021, and Projections to 2050: Findings from the Global Burden of Disease Study 2021. BMC Cancer 2025, 25, 330. [Google Scholar] [CrossRef]
- Wunderlich, K.; Suppa, M.; Gandini, S.; Lipski, J.; White, J.M.; Del Marmol, V. Risk Factors and Innovations in Risk Assessment for Melanoma, Basal Cell Carcinoma, and Squamous Cell Carcinoma. Cancers 2024, 16, 1016. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.; Sun, Z.; Diao, Q.; Wang, P.; Gao, F. Recent Advances of Podophyllotoxin/Epipodophyllotoxin Hybrids in Anticancer Activity, Mode of Action, and Structure-Activity Relationship: An Update (2010–2020). Eur. J. Med. Chem. 2020, 208, 112830. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, T.; Jin, X.; Lin, K.; Dai, X.; Gao, T.; Huang, G.; Fan, M.; Ma, L.; Liu, Z.; et al. Synthesis and Biological Evaluation of Cytotoxic Activity of Novel Podophyllotoxin Derivatives Incorporating Piperazinyl-Cinnamic Amide Moieties. Bioorganic Chem. 2022, 123, 105761. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.Á.; Miguel Del Corral, J.M.; García, P.A.; Rojo, M.V.; De La Iglesia-Vicente, J.; Mollinedo, F.; Cuevas, C.; San Feliciano, A. Synthesis and Biological Evaluation of New Podophyllic Aldehyde Derivatives with Cytotoxic and Apoptosis-Inducing Activities. J. Med. Chem. 2010, 53, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rakesh, K.P.; Shantharam, C.S.; Manukumar, H.M.; Asiri, A.M.; Marwani, H.M.; Qin, H.-L. Podophyllotoxin Derivatives as an Excellent Anticancer Aspirant for Future Chemotherapy: A Key Current Imminent Needs. Bioorg. Med. Chem. 2018, 26, 340–355. [Google Scholar] [CrossRef]
- Negi, A.S.; Gautam, Y.; Alam, S.; Chanda, D.; Luqman, S.; Sarkar, J.; Khan, F.; Konwar, R. Natural Antitubulin Agents: Importance of 3,4,5-Trimethoxyphenyl Fragment. Bioorg. Med. Chem. 2015, 23, 373–389. [Google Scholar] [CrossRef]
- Kamal, A.; Srinivasa Reddy, T.; Polepalli, S.; Shalini, N.; Reddy, V.G.; Subba Rao, A.V.; Jain, N.; Shankaraiah, N. Synthesis and Biological Evaluation of Podophyllotoxin Congeners as Tubulin Polymerization Inhibitors. Bioorg. Med. Chem. 2014, 22, 5466–5475. [Google Scholar] [CrossRef]
- Nerella, S.; Kankala, S.; Paidakula, S.; Gavaji, B. Synthesis of D-Ring Modified Acid Hydrazide Derivatives of Podophyllotoxin and Their Anticancer Studies as Tubulin Inhibiting Agents. Bioorganic Chem. 2020, 94, 103384. [Google Scholar] [CrossRef] [PubMed]
- Lisiecki, K.; Czarnocki, Z. Flow Photochemistry as a Tool for the Total Synthesis of (+)-Epigalcatin. Org. Lett. 2018, 20, 605–607. [Google Scholar] [CrossRef]
- Strus, P.; Borensztejn, K.; Szczepankiewicz, A.A.; Lisiecki, K.; Czarnocki, Z.; Nieznanska, H.; Wojcik, C.; Bialy, L.P.; Mlynarczuk-Bialy, I. Novel Podophyllotoxin and Benzothiazole Derivative Induces Transitional Morphological and Functional Changes in HaCaT Cells. Toxicol. Vitr. 2021, 73, 105144. [Google Scholar] [CrossRef]
- Lisiecki, K.; Krawczyk, K.K.; Roszkowski, P.; Maurin, J.K.; Czarnocki, Z. Formal Synthesis of (−)-Podophyllotoxin through the Photocyclization of an Axially Chiral 3,4-Bisbenzylidene Succinate Amide Ester–a Flow Photochemistry Approach. Org. Biomol. Chem. 2016, 14, 460–469. [Google Scholar] [CrossRef]
- Hernández, Á.P.; Díez, P.; García, P.A.; Miguel Del Corral, J.M.; Pérez-Andrés, M.; Díez, D.; San Feliciano, A.; Fuentes, M.; Castro, M.Á. New Hybrids Derived from Podophyllic Aldehyde and Diterpenylhydroquinones with Selectivity toward Osteosarcoma Cells. ACS Med. Chem. Lett. 2018, 9, 328–333. [Google Scholar] [CrossRef]
- Huynh, T.-K.-C.; Nguyen, T.-H.-A.; Tran, N.-H.-S.; Nguyen, T.-D.; Hoang, T.-K.-D. A Facile and Efficient Synthesis of Benzimidazole as Potential Anticancer Agents. J. Chem. Sci. 2020, 132, 84. [Google Scholar] [CrossRef]
- Xi, W.; Sun, H.; Bastow, K.F.; Xiao, Z.; Lee, K.-H. Identification of Novel 4′-O-Demethyl-Epipodophyllotoxin Derivatives as Antitumor Agents Targeting Topoisomerase II. Molecules 2022, 27, 5029. [Google Scholar] [CrossRef]
- Guminski, Y.; Grousseaud, M.; Cugnasse, S.; Imbert, T. Practical Demethylation of Podophyllotoxin and Efficient Preparation of 4-Amino-4-Deoxy-4′-Demethylepipodophyllotoxin. Synth. Commun. 2012, 42, 2780–2789. [Google Scholar] [CrossRef]
- Hao, S.-Y.; Feng, S.-L.; Wang, X.-R.; Wang, Z.; Chen, S.-W.; Hui, L. Novel Conjugates of Podophyllotoxin and Coumarin: Synthesis, Cytotoxicities, Cell Cycle Arrest, Binding CT DNA and Inhibition of Topo IIβ. Bioorg. Med. Chem. Lett. 2019, 29, 2129–2135. [Google Scholar] [CrossRef]
- Daley, L.; Meresse, P.; Bertounesque, E.; Monneret, C. A One-Pot, Efficient Synthesis of the Potent Cytotoxic Podophyllotoxin Derivative NPF. Tetrahedron Lett. 1997, 38, 2673–2676. [Google Scholar] [CrossRef]
- Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Reguera, R.M.; Castro, M.Á.; Rojo, M.V.; Santiago, C.; Abad, A.; García, P.A.; López-Pérez, J.L.; San Feliciano, A.; et al. Antileishmanial Activity and Tubulin Polymerization Inhibition of Podophyllotoxin Derivatives on Leishmania Infantum. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 272–285. [Google Scholar] [CrossRef] [PubMed]
- García, P.A.; Hernández, Á.-P.; Gómez-Zurita, M.A.; Miguel Del Corral, J.M.; Gordaliza, M.; Francesch, A.; San Feliciano, A.; Castro, M.Á. Cytotoxic Cyclolignans Obtained by the Enlargement of the Cyclolignan Skeleton of Podophyllic Aldehyde, a Selective Podophyllotoxin-Derived Cyclolignan. Molecules 2024, 29, 1442. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated Flavonoids with Selective Toxicity against Human Cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef]
- Desbene, S.; Giorgi-Renault, S. Drugs That Inhibit Tubulin Polymerization: The Particular Case of Podophyllotoxin and Analogues. Curr. Med. Chem.-Anti-Cancer Agents 2012, 2, 71–90. [Google Scholar] [CrossRef]
- Sackett, D.L. Podophyllotoxin, Steganacin and Combretastatin: Natural Products That Bind at the Colchicine Site of Tubulin. Pharmacol. Ther. 1993, 59, 163–228. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major Enhancements for Small-Molecule Docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-W.; Huang, W.-J.; Liu, Y.-H.; Liu, Q.-G.; Song, J.; Hu, T.; Chen, P.; Zhang, S.-Y. Design, Synthesis and Biological Evaluation of 1,2,3-Triazole Benzothiazole Derivatives as Tubulin Polymerization Inhibitors with Potent Anti-Esophageal Cancer Activities. Eur. J. Med. Chem. 2024, 265, 116118. [Google Scholar] [CrossRef]
- Song, J.; Gao, Q.-L.; Wu, B.-W.; Zhu, T.; Cui, X.-X.; Jin, C.-J.; Wang, S.-Y.; Wang, S.-H.; Fu, D.-J.; Liu, H.-M.; et al. Discovery of Tertiary Amide Derivatives Incorporating Benzothiazole Moiety as Anti-Gastric Cancer Agents in Vitro via Inhibiting Tubulin Polymerization and Activating the Hippo Signaling Pathway. Eur. J. Med. Chem. 2020, 203, 112618. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.38.35a; Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. X-Seed 4: Updates to a Program for Small-Molecule Supramolecular Crystallography. J. Appl. Crystallogr. 2020, 53, 1141–1146. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Atwood, J.L.; Barbour, L.J. Molecular Graphics: From Science to Art. Cryst. Growth Des. 2003, 3, 3–8. [Google Scholar] [CrossRef]
- Persistence of Vision Pty. Ltd. Available online: http://www.povray.org/ (accessed on 9 April 2025).
- Strus, P.; Sadowski, K.; Kostro, J.; Szczepankiewicz, A.A.; Nieznańska, H.; Niedzielska, M.; Zlobin, A.; Nawar Ra’idah, P.; Molęda, Z.; Szawkało, J.; et al. Cellular Distribution and Ultrastructural Changes in HaCaT Cells, Induced by Podophyllotoxin and Its Novel Fluorescent Derivative, Supported by the Molecular Docking Studies. Int. J. Mol. Sci. 2024, 25, 5948. [Google Scholar] [CrossRef] [PubMed]
- Młynarczuk-Biały, I.; Roeckmann, H.; Kuckelkorn, U.; Schmidt, B.; Umbreen, S.; Gołąb, J.; Ludwig, A.; Montag, C.; Wiebusch, L.; Hagemeier, C.; et al. Combined Effect of Proteasome and Calpain Inhibition on Cisplatin-Resistant Human Melanoma Cells. Cancer Res. 2006, 66, 7598–7605. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into Tubulin Regulation from a Complex with Colchicine and a Stathmin-like Domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]

| Comp. | Cells (IC50 ± SD, µM) | Selectivity Index (SI) | |||||
|---|---|---|---|---|---|---|---|
| B16F10 a | HeLa b | LoVo a | MCF-7 b | SKOV-3 b | HaCaT a | ||
| 7 | 1.78 ± 0.55 | 0.68 ± 0.05 | 0.83 ± 0.08 | 0.59 ± 0.05 | 1.10 ± 0.13 | 0.86 ± 0.05 | 1.26 |
| 8 | >20 | >20 | >20 | >20 | >20 | >20 | - |
| 9 | >20 | 1.81 ± 0.13 | 11.06 ± 2.55 | 2.02 ± 0.11 | 10.38 ± 0.73 | 13.24 ± 2.89 | 7.31 |
| 10 | >20 | >20 | >20 | >20 | >20 | >20 | - |
| 11 | 1.93 ± 0.25 | 1.54 ± 0.24 | 2.88 ± 0.87 | 1.98 ± 0.49 | 1.79 ± 0.28 | 1.90 ± 0.14 | 1.23 |
| 12 | >20 | >20 | >20 | >20 | >20 | >20 | - |
| 13 | >20 | >20 | >20 | >20 | >20 | >20 | - |
| 14 | >20 | >20 | 11.72 ± 1.02 | 11.86 ± 1.75 | 15.30 ± 1.31 | 13.10 ± 1.75 | <1 |
| 15 | 3.76 ± 0.49 | 2.49 ± 0.25 | 10.86 ± 0.95 | 3.44 ± 0.81 | 3.73 ± 0.75 | 7.59 ± 0.76 | 3.05 |
| 1 | 0.092 ± 0.05 | 0.0089 ± 0.0003 | 0.033 ± 0.0077 | 0.0039 ± 0.0005 | 0.017 ± 0.0028 | 0.0046 ± 0.0006 | 0.52 |
| 2 | 6.68 ± 2.32 | 5.73 ± 1.48 | 2.48 ± 0.47 | 0.31 ± 0.017 | 3.15 ± 0.38 | 1.32 ± 0.16 | 0.23 |
| Comp. | MW (g/mol) | M log P | HBA | HBD | TPSA (Å2) | nRB | Pharmacokinetic (GI Absorption) | Drug-Linkeness |
|---|---|---|---|---|---|---|---|---|
| 7 | 531.58 | 2.95 | 8 | 0 | 113.58 | 7 | Low | Yes, nVs: 1 |
| 11 | 473.54 | 3.41 | 6 | 0 | 87.28 | 5 | High | Yes, nVs: 0 |
| 15 | 517.55 | 2.76 | 8 | 1 | 124.58 | 6 | Low | Yes, nVs: 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai’dah, P.N.; Molęda, Z.; Osińska, A.; Budzianowski, A.; Młynarczuk-Biały, I.; Czarnocki, Z. Synthesis of Novel Podophyllotoxin–Benzothiazole Congeners and Their Biological Evaluation as Anticancer Agents. Int. J. Mol. Sci. 2025, 26, 6033. https://doi.org/10.3390/ijms26136033
Rai’dah PN, Molęda Z, Osińska A, Budzianowski A, Młynarczuk-Biały I, Czarnocki Z. Synthesis of Novel Podophyllotoxin–Benzothiazole Congeners and Their Biological Evaluation as Anticancer Agents. International Journal of Molecular Sciences. 2025; 26(13):6033. https://doi.org/10.3390/ijms26136033
Chicago/Turabian StyleRai’dah, Pramukti Nawar, Zuzanna Molęda, Aleksandra Osińska, Armand Budzianowski, Izabela Młynarczuk-Biały, and Zbigniew Czarnocki. 2025. "Synthesis of Novel Podophyllotoxin–Benzothiazole Congeners and Their Biological Evaluation as Anticancer Agents" International Journal of Molecular Sciences 26, no. 13: 6033. https://doi.org/10.3390/ijms26136033
APA StyleRai’dah, P. N., Molęda, Z., Osińska, A., Budzianowski, A., Młynarczuk-Biały, I., & Czarnocki, Z. (2025). Synthesis of Novel Podophyllotoxin–Benzothiazole Congeners and Their Biological Evaluation as Anticancer Agents. International Journal of Molecular Sciences, 26(13), 6033. https://doi.org/10.3390/ijms26136033








