The Pharmacological Potential of Marantodes pumilum: A Comprehensive Review of Its Medicinal Properties
Abstract
1. Introduction
2. Methods
3. The Botany of Marantodes pumilum
4. Traditional Use of Marantodes pumilum
5. Phytochemistry of Marantodes pumilum
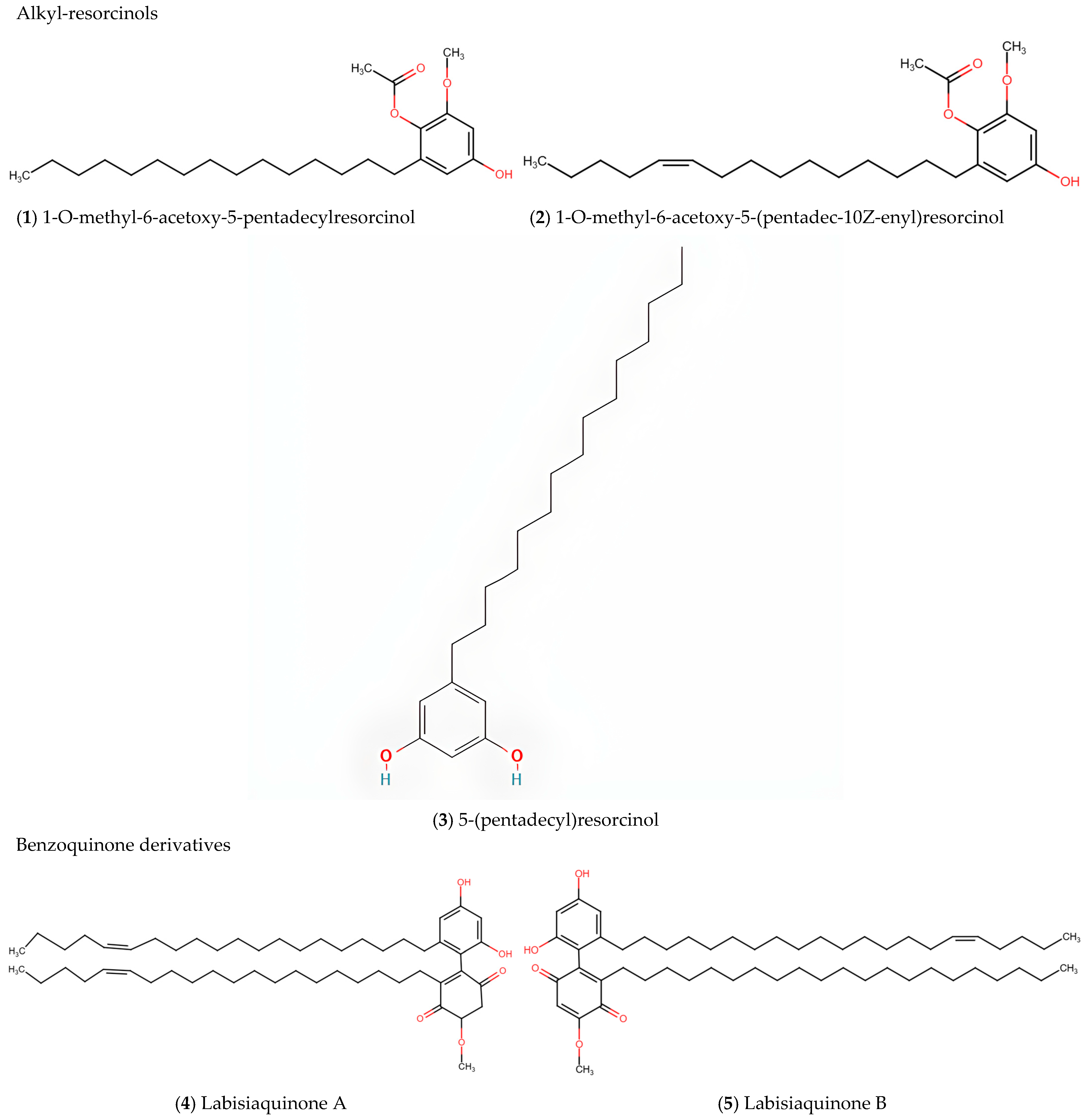
5.1. Alkyl-Resorcinols
5.2. Labisiaquinones and Benzoquinone Derivatives
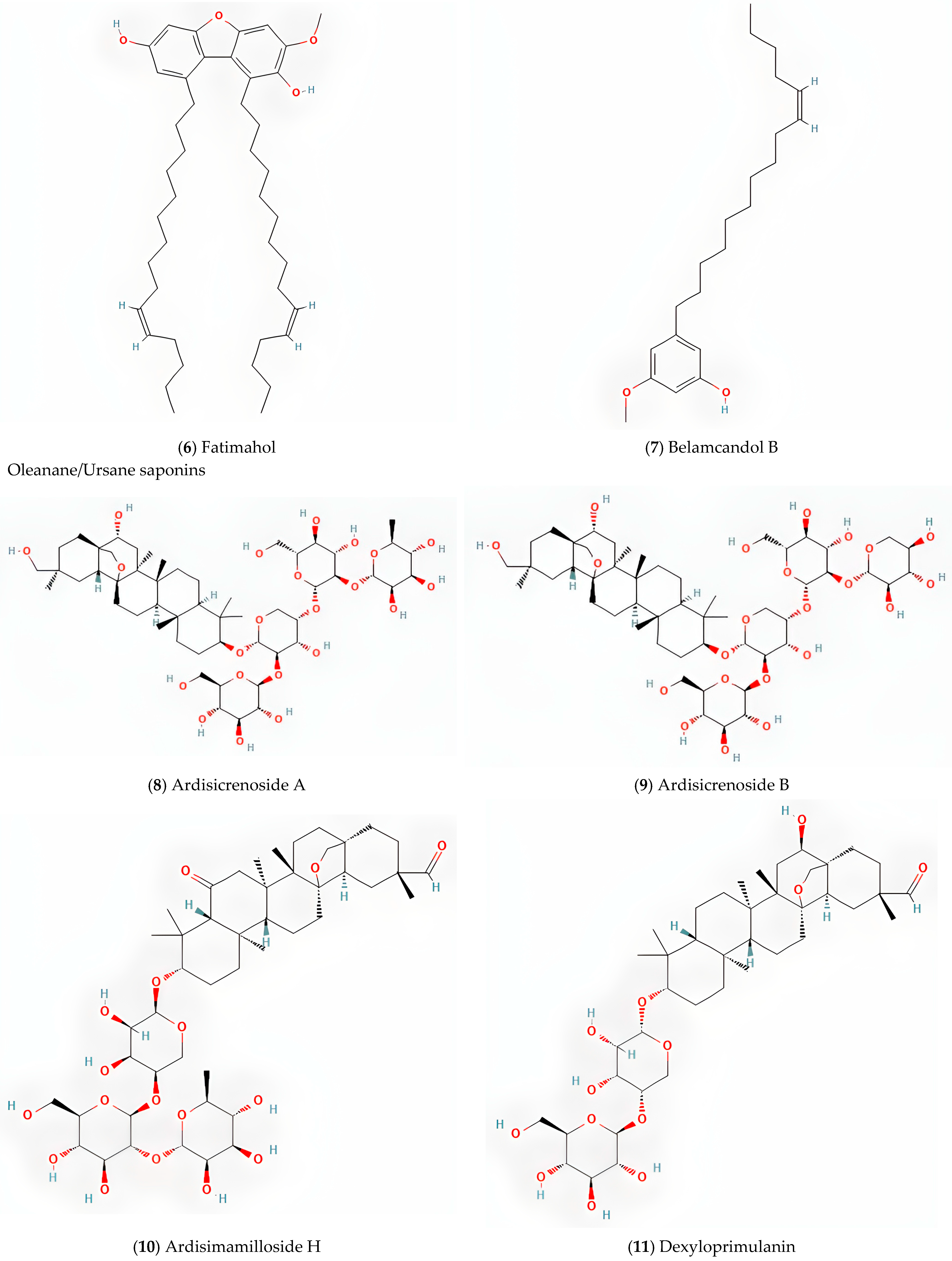
5.3. Oleanane/Ursane-Type Saponins
5.4. Flavonoids
5.5. Phenolic Compounds
5.6. Varietal Differences in Phytochemical Distribution
| Chemotype | Representative Compounds | References |
|---|---|---|
| Alkyl-resorcinols | 5-(pentadec-10Z-enyl)resorcinol, 5-(pentadecyl)resorcinol; 1-O-methyl-6-acetoxy-5-(pentadec-10Z-enyl)resorcinol; 1-O-methyl-6-acetoxy-5-pentadecylresorcinol; 5-(pentadec-10Z-enyl)resorcinol; 5-(pentadecyl)resorcinol | [11] |
| 5-Henicosene-1-yl-resorcinol | [17] | |
| Irisresorcinol (detected at concentrations as low as 0.2 μg/mL) | [19] | |
| Benzoquinone derivatives | Demethylbelamcandaquinone B | [10,18] |
| Labisiaquinone A and B | [11] | |
| Fatimahol (di-alkenated dibenzofuran) | [18] | |
| Belamcandol B | [19] | |
| Oleanane/Ursane saponins | Dexyloprimulanin | [18] |
| Ardisicrenoside B, Ardisiacrispin A, Ardisimamilloside H | [19] | |
| Flavonoids | Naringin, Apigenin, Quercetin, Myricetin, Kaempferol | [6] |
| (+)-Catechin; (−)-Epicatechin; Kaempferol-3-O-α-rhamnopyranosyl-7-O-β-glycopyranoside; Kaempferol-4′-O-β-glycopyranoside; Quercetin-3-O-α-rhamnopyranoside; Kaempferol-3-O-α-rhamnopyranoside | [11] | |
| Phenolic acids | Gallic acid, Ellagic acid | [23] |
6. Pharmacological Properties of Marantodes pumilum
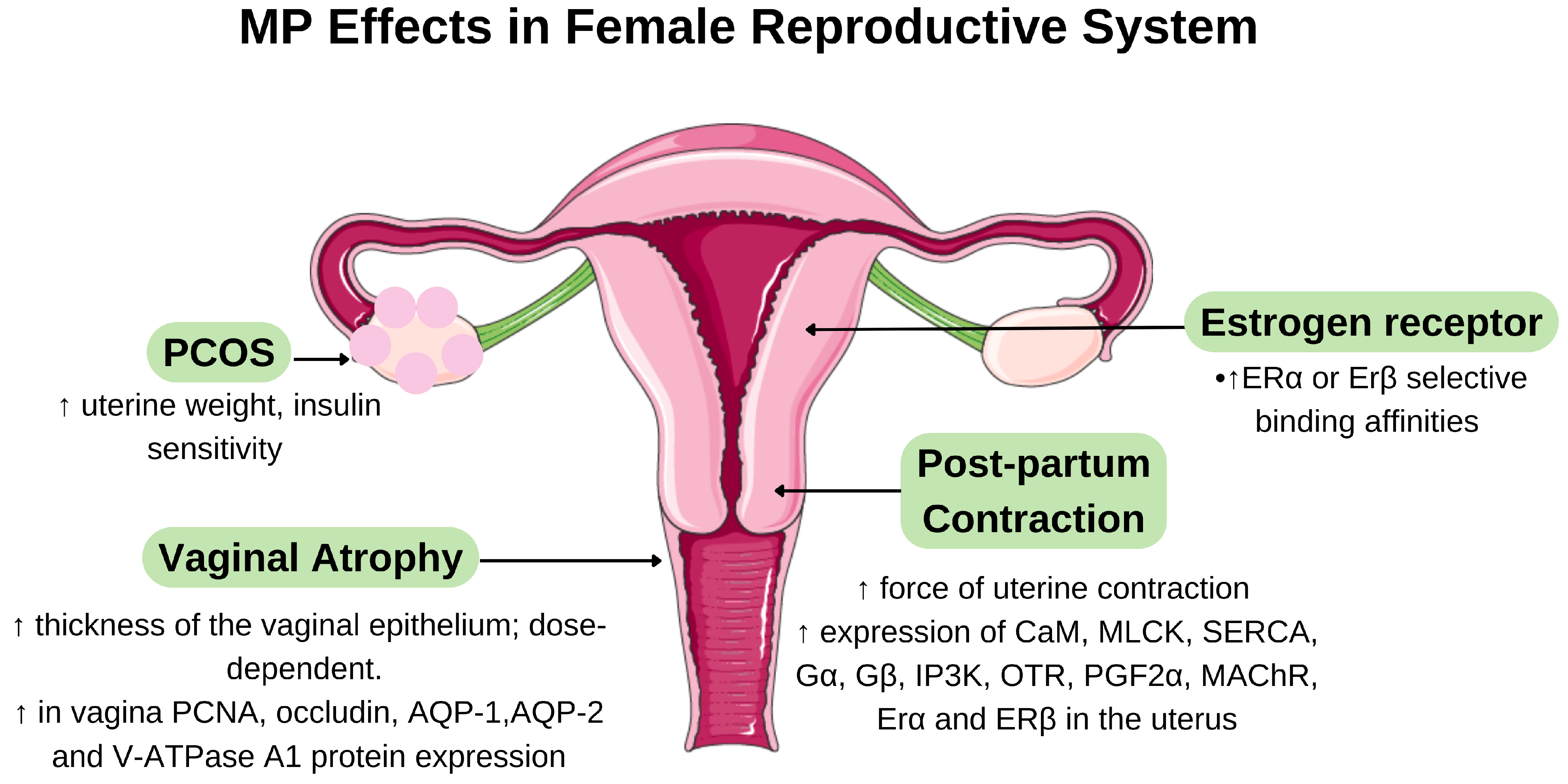
6.1. Phytoestrogenic Effects of Marantodes pumilum
6.2. Effects of MP on Postmenopausal Syndrome
6.3. Effects on MP on Polycystic Ovary Syndrome and Postnatal Period
6.4. Effects of MP on Bone Markers and Bone Density
6.5. Effects of MP on Obesity and Cardiovascular System
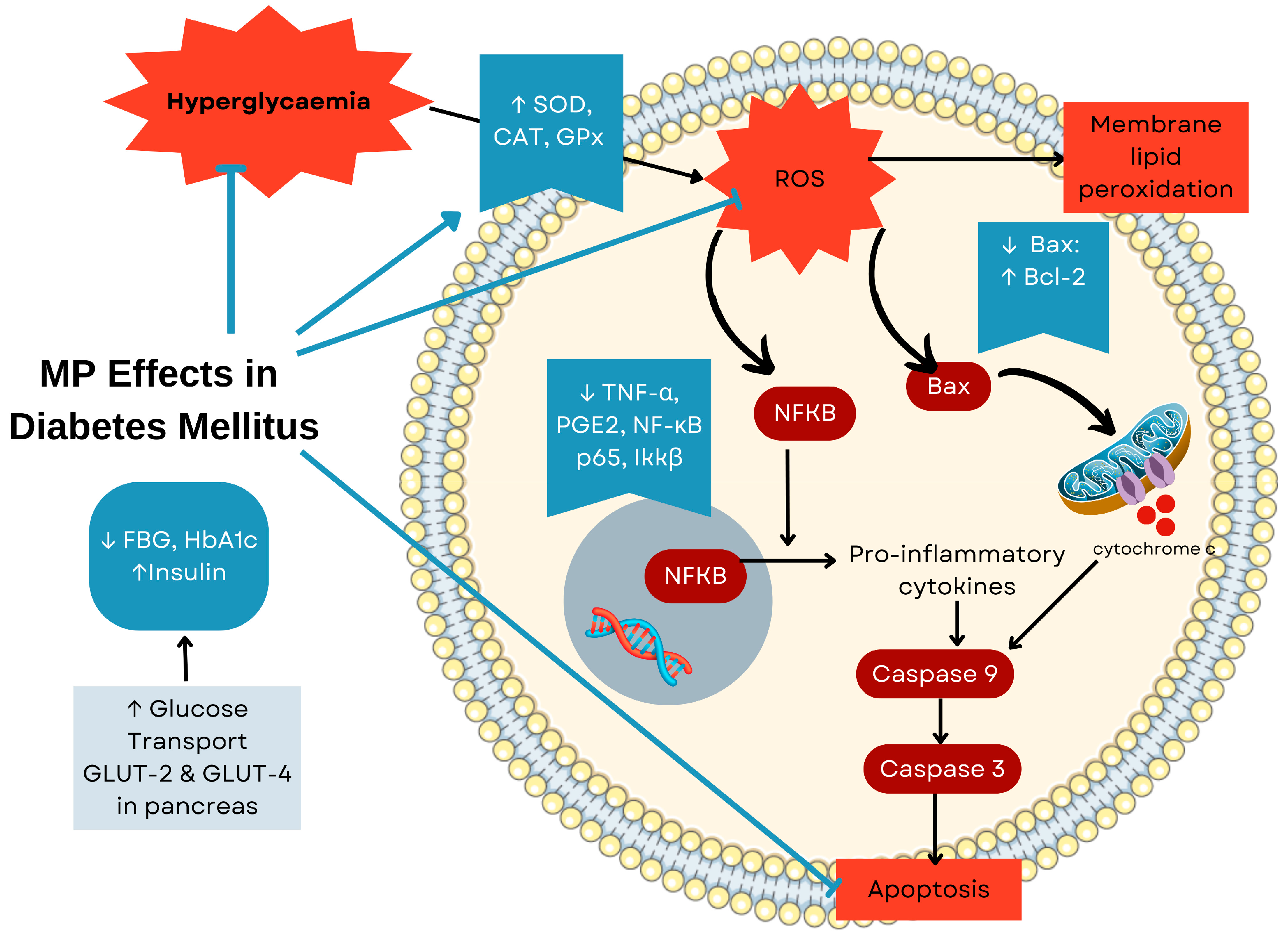
6.6. Effects of MP on Diabetes Mellitus
6.7. Effects of MP on Wound Healing
6.8. Effects of MP on Gout
| Extracts/Compound | Study Design | Treatment Dosage | Findings | References |
|---|---|---|---|---|
| Wound Healing | ||||
| MP var. alata leaf and root aqueous extraction | In vivo: 50 male Sprague-Dawley rats with second-degree burn wounds (80 °C steel rod, 10 s) | 2% MP ointment for 3 weeks | (1) ↑ Hydroxyproline content → ↑ collagen synthesis → improved structural integrity; (2) ↑ fibroblast proliferation → ↑ ECM production → accelerated granulation tissue formation; (3) ↑ neovascularization → ↑ oxygen/nutrient delivery → ↑tissue regeneration→ significant reduction in burn wound size | [67] |
| MP var. alata and var. pumila leaf and root extract aqueous extraction | In vivo: 126 male Sprague-Dawley rats with excisional wounds (6 mm diameter, 2 mm thickness) | 1% and 2% of MP ext for 13 days | (1) ↑ Collagen-III to collagen-I transformation → enhanced tensile strength; (2) ↑ fibronectin → improved scaffold for cellular migration and attachment; (3) high phenolics→ ↑ antioxidant enzyme activities + ↓ lipid peroxidation → reduced oxidative damage→ accelerated re-epithelialisation and improved wound closure rate | [23] |
| MP var. alata dicholoromethane extraction Isolated compounds: naringin (NAR), eicosan (EIC), and octacosan (OCT) | In vitro HDF in normal and insulin-resistant conditions In vivo 36 diabetic SD rats with excisional wounds (3 wounds with 5 mm thickness) | 10 g of each compound for 30 days | (1) High radical scavenging against DPPH, NO, OH•, and O2• → reduced oxidative stress; (2) NAR → ↑fibroblast migration in both normal and insulin-resistant conditions; (3) NAR → regulated ECM degradation/remodelling; (4) NAR> EIC/OCT for epithelialisation, fibroblast proliferation, ↓ inflammation | [69] |
| MP var. pumila and alata root and leaf extraction | In vivo: 99 ovariectomized Sprague-Dawley rats with full-thickness excisional wounds (6 mm diameter) | 1% and 2% of MP ext for 13 days | (1) ↑ collagen content and fibroblast cells → enhanced structural regeneration; (2) ↓ inflammatory cells in granulation tissues → ↓scarring and scar width →↑ healing rate | [68] |
| Gout | ||||
| MP leaves and roots of dichloromethane and methanol extraction | In vitro xanthin oxidase (XO) assay | 400 μg/mL for extracts and 100 μg/mL for isolated compound | 3,7-dihydroxy-5-methoxy-4,8-dimethyl-isocoumarin → extremely potent XO inhibition (IC50 = 0.66 ± 0.01 μg/mL) → potential specific biochemical target | [4,70] |
| MP leaves and roots ethanol extraction | In vitro Xanthine Oxidase Inhibition Assay In vivo 36 SD rats with induced hyperuricemia and MSU crystal-induced inflammation | 200 mg/kg/day orally for 14 days | (1)↓ serum uric acid levels and hepatic XO activity; (2) Inhibition of MSU crystal-induced inflammatory cytokines (IL-1α, IL-1β, IL-8, TNF-α, PGE2) → ↓ inflammatory mediators in synovial fluid | [4] |
| MP leaves and roots dichloromethane methanol and water extraction | In vitro cytokine and PGE2 assay | 50 µg/mL | Inhibited LPS-induced pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, TNF-α) while ↑ PGE2 secretion → differential regulation of inflammatory response pathways | [71] |
6.9. Effects of MP on Cancer
6.10. Antimicrobial Properties of MP
6.10.1. Antibacterial Activity
6.10.2. Antifungal and Antiviral
6.11. Clinical Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bungãu, S.G.; Popa, V.C. Between religion and science some aspects concerning illness and healing in antiquity. Transylv. Rev. 2015, 24, 3–18. [Google Scholar]
- Chua, L.S.; Lee, S.Y.; Abdullah, N.; Sarmidi, M.R. Review on Labisia pumila (Kacip Fatimah): Bioactive phytochemicals and skin collagen synthesis promoting herb. Fitoterapia 2012, 83, 1322–1335. [Google Scholar] [CrossRef]
- Ibrahim, I.S.; Said, M.M.; Zainoor, N.M.; Jamal, J.A. Authentication of Marantodes pumilum (Blume) Kuntze: A Systematic Review. Front. Pharmacol. 2022, 13, 855384. [Google Scholar] [CrossRef]
- Rahmi, E.P.; Kumolosasi, E.; Jalil, J.; Husain, K.; Buang, F.; Razak, A.F.A.; Jamal, J.A. Anti-hyperuricemic and Anti-inflammatory Effects of Marantodes pumilum as Potential Treatment for Gout. Front. Pharmacol. 2020, 11, 289. [Google Scholar] [CrossRef]
- Dianita, R.; Jantan, I.; Jalil, J.; Amran, A.Z. Effects of Labisia pumila var alata extracts on the lipid profile, serum antioxidant status and abdominal aorta of high-cholesterol diet rats. Phytomedicine 2016, 23, 810–817. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.E.; Ahmad, S. Phytochemical Analysis and Antimicrobial Activities of Methanolic Extracts of Leaf, Stem and Root from Different Varieties of Labisa pumila Benth. Molecules 2011, 16, 4438. [Google Scholar] [CrossRef]
- Pihie, A.H.L.; Zakaria, Z.A.; Othman, F. Antiproliferative and proapoptotic effects of Labisia pumila ethanol extract and its active fraction in human melanoma HM3KO cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 123470. [Google Scholar] [CrossRef]
- Kadir, A.A.; Hussain, N.H.N.; Bebakar, W.M.W.; Mohd, D.M.; Mohammad, W.M.Z.W.; Hassan, I.I.; Shukor, N.; Kamaruddin, N.A.; Mohamud, W.N.W. The effect of Labisia pumila var. alata on postmenopausal women: A pilot study. Evid.-Based Complement. Altern. Med. 2012, 2012, 216525. [Google Scholar] [CrossRef]
- Zakaria, A.A.; Noor, M.H.M.; Ahmad, H.; Hassim, H.A.; Mazlan, M.; Latip, M.Q.A. A Review on Therapeutic Effects of Labisia pumila on Female Reproductive Diseases. BioMed Res. Int. 2021, 2021, 9928199. [Google Scholar] [CrossRef]
- Hairi, H.A.; Jamal, J.A.; Aladdin, N.A.; Husain, K.; Sofi, N.S.M.; Mohamed, N.; Mohamed, I.N.; Shuid, A.N. Demethylbelamcandaquinone B (Dmcq B) is the active compound of Marantodes pumilum var. alata (Blume) Kuntze with osteoanabolic activities. Molecules 2018, 23, 1686. [Google Scholar] [CrossRef]
- Al-Mekhlafi, N.A.; Shaari, K.; Abas, F.; Kneer, R.; Jeyaraj, E.J.; Stanslas, J.; Yamamoto, N.; Honda, T.; Lajis, N.H. Alkenylresorcinols and cytotoxic activity of the constituents isolated from Labisia pumila. Phytochemistry 2012, 80, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Shihab, U.A.; Atiqah, A.; Ahmad, N.S.; Isa, N.M. A Dose Optimization Study of Marantodes pumilum (Blume) Kuntze Extracts for Wound Healing Effect in Animal Model. Bangladesh J. Med. Sci. 2022, 21, 659–668. [Google Scholar] [CrossRef]
- Chua, L.S.; Latiff, N.A.; Lee, S.Y.; Lee, C.T.; Sarmidi, M.R.; Aziz, R.A. Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem. 2011, 127, 1186–1192. [Google Scholar] [CrossRef]
- Abdullah, F.; Ling, S.K.; Man, S.; Tan, A.L.; Tan, H.P.; Abdullah, Z. Characterization and identification of Labisia pumila by multi-steps infrared spectroscopy. Vib. Spectrosc. 2012, 62, 200–206. [Google Scholar] [CrossRef]
- Samad, N.A. Labisia Pumila—A Source of Medicine. Aust. Herb. Insight 2018, 1, E004–E006. [Google Scholar] [CrossRef]
- A Dictionary of the Economic Products of the Malay Peninsula. Nature 1936, 137, 255. [CrossRef]
- Hanafi, M.M.M.; Yaakob, H.; Gibbons, S.; Prieto, J.M. In Vitro Pro-Apoptotic and Anti-Migratory Effects of Marantodes pumilum (syn. Labisia pumila) Extracts on Human Prostate Cancer Cell Lines: Bioguided Isolation of 5-Henicosene-1-yl-resorcinol. Plants 2023, 12, 1576. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, I.A. Alkyl phenols and saponins from the roots of Labisia pumila (Kacip Fatimah). Phytochemistry 2011, 72, 2075–2080. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Quantitative determination of triperpene saponins and alkenated-phenolics from Labisia pumila using an LC-UV/ELSD method and confirmation by LC-ESI-TOF. Planta Med. 2011, 77, 1742–1748. [Google Scholar] [CrossRef]
- Wu, H.; Xi, H.; Lai, F.; Ma, J.; Liu, H. Chemical and cellular antioxidant activity of flavone extracts of: Labisia pumila before and after in vitro gastrointestinal digestion. RSC Adv. 2018, 8, 12116–12126. [Google Scholar] [CrossRef]
- Aladdin, N.-A.; Jamal, J.A.; Talip, N.; Hamsani, N.A.M.; Rahman, M.R.A.; Sabandar, C.W.; Muhammad, K.; Husain, K.; Jalil, J. Comparative study of three Marantodes pumilum varieties by microscopy, spectroscopy and chromatography. Rev. Bras. De Farmacogn. 2016, 26, 1–14. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.E.; Ghasemzadeh, A. Chemical composition, antioxidant and anticancer potential of Labisia pumila variety alata under CO2 enrichment. NJAS Wagening. J. Life Sci. 2016, 78, 85–91. [Google Scholar] [CrossRef]
- Ahmad, S.U.; Aladdin, N.A.B.; Jamal, J.A.; Shuid, A.N.; Mohamed, I.N. Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules 2021, 26, 228. [Google Scholar] [CrossRef] [PubMed]
- Norhaiza, M.; Maziah, M.; Hakiman, M. Antioxidative properties of leaf extracts of a popular Malaysian herb, Labisia pumila. J. Med. Plants Res. 2009, 3, 217–223. [Google Scholar]
- Karimi, E.; Jaafar, H.Z.E.; Ahmad, S. Phenolics and flavonoids profiling and antioxidant activity of three varieties of Malaysian indigenous medicinal herb Labisia pumila benth. J. Med. Plants Res. 2011, 5, 1200–1206. [Google Scholar]
- Karimi, E.; Jaafar, H.Z.E.; Ahmad, S. Antifungal, anti-inflammatory and cytotoxicity activities of three varieties of Labisia pumila benth: From microwave obtained extracts. BMC Complement. Altern. Med. 2013, 13, 20. [Google Scholar] [CrossRef]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ. Health Perspect. 2008, 116, 426–433. [Google Scholar] [CrossRef]
- Hussain, N.H.N.; Kadir, A.A. Potential Role of Labisia pumila in the Prevention and Treatment of Chronic Diseases. J. Food Res. 2013, 2, 55. [Google Scholar] [CrossRef]
- Muhamad, M.; Choo, C.Y.; Hasuda, T.; Hitotsuyanagi, Y. Estrogenic phytochemical from Labisia pumila (Myrsinaceae) with selectivity towards estrogen receptor alpha and beta subtypes. Fitoterapia 2019, 137, 104256. [Google Scholar] [CrossRef]
- Jamal, J.A.; Houghton, P.J.; Milligan, S.R.; Jantan, I. The oestrogenic and cytotoxic effects of the extracts of Labasia pumila var. alata and Labasia pumila var pumila in vitro. Malays. J. Health Sci. 2003, 1, 53–60. [Google Scholar]
- Edwards, D.; Panay, N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: How important is vaginal lubricant and moisturizer composition? Climacteric 2015, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.A.; Yusof, W.H.W.; Shuid, A.N.; Mahmoud, W.N.W.; Ali, K.H. Labisia Pumila has Similar Effects to Estrogen on the Reproductive Hormones of Ovariectomised Rats. Internet J. Herb. Plant Med. 2010, 2, 10–5580. [Google Scholar]
- Tan, N.A.S.; Giribabu, N.; Karim, K.; Nyamathulla, S.; Salleh, N. Intravaginal treatment with Marantodes pumilum (Kacip Fatimah) ameliorates vaginal atrophy in rats with post-menopausal condition. J. Ethnopharmacol. 2019, 236, 9–20. [Google Scholar] [CrossRef]
- Yusuf, A.N.M.; Amri, M.F.; Ugusman, A.; Hamid, A.A.; Wahab, N.A.; Mokhtar, M.H. Hyperandrogenism and Its Possible Effects on Endometrial Receptivity: A Review. Int. J. Mol. Sci. 2023, 24, 12026. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Mannerås, L.; Fazliana, M.; Nazaimoon, W.W.; Lönn, M.; Gu, H.; Östenson, C.; Stener-Victorin, E. Beneficial metabolic effects of the Malaysian herb Labisia pumila var. alata in a rat model of polycystic ovary syndrome. J. Ethnopharmacol. 2010, 127, 346–351. [Google Scholar] [CrossRef]
- Mansor, F.; Gu, H.F.; Östenson, C.G.; Mannerås-Holm, L.; Stener-Victorin, E.; Mohamud, W.N.W. Labisia pumila upregulates peroxisome proliferator-activated receptor gamma expression in rat adipose tissues and 3T3-L1 adipocytes. Adv. Pharmacol. Sci. 2013, 2013, 808914. [Google Scholar] [CrossRef]
- Zakaria, A.A.; Latip, M.Q.A.; Azizan, T.R.T.; Ahmad, H.; Mazlan, M.; Hassim, H.A.; Noor, M.H. The Effect of Different Concentration of Aqueous Extracts of Labisia pumila in Preventing Osteoporosis and Improvement of Dermal Elasticity in Polycystic Ovary Syndrome Rats. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Omar, W.F.N.W.; Giribabu, N.; Karim, K.; Salleh, N. Marantodes pumilum (Blume) Kuntze (Kacip Fatimah) stimulates uterine contraction in rats in post-partum period. J. Ethnopharmacol. 2019, 245, 112175. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK–RANKL–OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Madzuki, I.N.; Lau, S.F.; Shalan, N.A.A.M.; Ishak, N.I.M.; Mohamed, S. Does cartilage ERα overexpression correlate with osteoarthritic chondrosenescence? Indications from Labisia pumila OA mitigation. J. Biosci. 2019, 44, 100. [Google Scholar] [CrossRef]
- Effendy, N.M.; Shuid, A.N. Time and dose-dependent effects of Labisia pumilaon bone oxidative status of postmenopausal osteoporosis rat model. Nutrients 2014, 6, 3288–3302. [Google Scholar] [CrossRef] [PubMed]
- Effendy, N.M.; Khamis, M.F.; Shuid, A.N. The effects of Labisia pumila extracts on bone microarchitecture of ovariectomized-induced osteoporosis rats: A micro-CT analysis. J. X-Ray Sci. Technol. 2017, 25, 101–112. [Google Scholar] [CrossRef]
- Shuid, A.N.; Ping, L.L.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. The effects of Labisia pumila var. alata on bone markers and bone calcium in a rat model of post-menopausal osteoporosis. J. Ethnopharmacol. 2011, 133, 538–542. [Google Scholar] [CrossRef]
- Fathilah, S.N.; Mohamed, N.; Muhammad, N.; Mohamed, I.N.; Soelaiman, I.N.; Shuid, A.N. Labisia pumila regulates bone-related genes expressions in postmenopausal osteoporosis model. BMC Complement. Altern. Med. 2013, 13, 217. [Google Scholar] [CrossRef]
- Karim, K.; Giribabu, N.; Salleh, N. Marantodes pumilum (blume) Kuntze (Kacip Fatimah) leaves aqueous extract prevents downregulation of Wnt/β-catenin pathway and upregulation of apoptosis in osteoblasts of estrogen-deficient, diabetes-induced rats. J. Ethnopharmacol. 2021, 280, 114236. [Google Scholar] [CrossRef]
- Karim, K.; Giribabu, N.; Salleh, N. Marantodes pumilum Var Alata (Kacip Fatimah) ameliorates derangement in RANK/RANKL/OPG pathway and reduces inflammation and oxidative stress in the bone of estrogen-deficient female rats with type-2 diabetes. Phytomedicine 2021, 91, 153677. [Google Scholar] [CrossRef]
- Fathilah, S.N.; Shuid, A.N.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Labisia pumila protects the bone of estrogen-deficient rat model: A histomorphometric study. J. Ethnopharmacol. 2012, 142, 294–299. [Google Scholar] [CrossRef]
- Giaze, T.R.; Shuid, A.N.; Soelaiman, I.N.; Muhammad, N.; Jamal, J.A.; Fauzi, M.B.; Mohamed, N. Comparative anti-osteoporotic properties of the leaves and roots of Marantodes pumilum var. alata in postmenopausal rat model. J. Tradit. Complement. Med. 2019, 9, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Fathilah, S.N.; Abdullah, S.; Mohamed, N.; Shuid, A.N. Labisia pumila prevents complications of osteoporosis by increasing bone strength in a rat model of postmenopausal osteoporosis. Evid.-Based Complement. Altern. Med. 2012, 2012, 948080. [Google Scholar] [CrossRef]
- Effendy, N.M.; Abdullah, S.; Yunoh, M.F.M.; Shuid, A.N. Time and dose-dependent effects of Labisia pumila on the bone strength of postmenopausal osteoporosis rat model. BMC Complement. Altern. Med. 2015, 15, 58. [Google Scholar] [CrossRef][Green Version]
- Effendy, N.M.; Khamis, M.F.; Soelaiman, I.N.; Shuid, A.N. The effects of Labisia pumila on postmenopausal osteoporotic rat model: Dose and time-dependent micro-CT analysis. J. X-Ray Sci. Technol. 2014, 22, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Madzuki, I.N.; Lau, S.F.; Tantowi, N.A.C.A.; Ishak, N.I.M.; Mohamed, S. Labisia pumila prevented osteoarthritis cartilage degeneration by attenuating joint inflammation and collagen breakdown in postmenopausal rat model. Inflammopharmacology 2018, 26, 1207–1217. [Google Scholar] [CrossRef]
- Fazliana, M.; Nazaimoon, W.M.W.; Gu, H.F.; Östenson, C.G. Labisia pumila extract regulates body weight and adipokines in ovariectomized rats. Maturitas 2009, 62, 91–97. [Google Scholar] [CrossRef]
- Fazliana, M.; Gu, H.F.; Östenson, C.G.; Yusoff, M.M.; Nazaimoon, W.M.W. Labisia pumila extract down-regulates hydroxysteroid (11-beta) dehydrogenase 1 expression and corticosterone levels in ovariectomized rats. J. Nat. Med. 2012, 66, 257–264. [Google Scholar] [CrossRef]
- Manshor, N.M.; Razali, N.; Jusoh, R.R.; Asmawi, M.Z.; Mohamed, N.; Zainol, S.; Altaf, R.; Dewa, A. Vasorelaxant effect of water fraction of Labisia Pumila and its mechanisms in spontaneously hypertensive rats aortic ring preparation. Int. J. Cardiol. Hypertens. 2020, 4, 100024. [Google Scholar] [CrossRef]
- Dianita, R.; Jantan, I.; Amran, A.Z.; Jalil, J. Protective effects of Labisia pumila var. alata on biochemical and histopathological alterations of cardiac muscle cells in isoproterenol-induced myocardial infarction rats. Molecules 2015, 20, 4746–4763. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, A.; Nazaimoon, W.M.W.; Norsyam, W.N.; Farihah, H.S.; Azian, A.L. Effect of Water Extract of Labisia pumila var Alata on Aorta of Ovariectomized Sprague Dawley Rats. Pak. J. Nutr. 2008, 7, 208–213. [Google Scholar] [CrossRef][Green Version]
- Dharmani, M.; Kamarulzaman, K.; Giribabu, N.; Choy, K.; Zuhaida, M.; Aladdin, N.; Jamal, J.; Mustafa, M. Effect of Marantodes pumilum Blume (Kuntze) var. alata on β-cell function and insulin signaling in ovariectomised diabetic rats. Phytomedicine 2019, 65, 153101. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.H.; Giribabu, N.; Bakar, N.M.A.; Salleh, N. Marantodes pumilum (Kacip fatimah) enhances in-vitro glucose uptake in 3T3-L1 adipocyte cells and reduces pancreatic complications in streptozotocin-nicotinamide induced male diabetic rats. Biomed. Pharmacother. 2017, 96, 716–726. [Google Scholar] [CrossRef]
- Nahar, N.; Mohamed, S.; Mustapha, N.M.; Fong, L.S. Protective effects of Labisia pumila against neuropathy in a diabetic rat model. J. Diabetes Metab. Disord. 2022, 21, 1–11. [Google Scholar] [CrossRef]
- Nahar, N.; Mohamed, S.; Mustapha, N.M.; Fong, L.S.; Ishak, N.I.M. Gallic acid and myricetin-rich Labisia pumila extract mitigated multiple diabetic eye disorders in rats. J. Food Biochem. 2021, 45, e13948. [Google Scholar] [CrossRef]
- Ibrahim, I.; Mohamed, I.N.; Mohamed, N.; Ramli, E.S.M.; Shuid, A.N. The effects of aqueous extract of Labisia Pumila (Blume) Fern.-Vill. Var. Alata on wound contraction, hydroxyproline content and histological assessments in superficial partial thickness of second-degree burn model. Front. Pharmacol. 2022, 13, 968664. [Google Scholar] [CrossRef]
- Ahmad, S.U.; Shuid, A.N.; Mohamed, I.N. Labisia pumila improves wound healing process in ovariectomized rat model. Bangladesh J. Pharmacol. 2018, 13, 106–113. [Google Scholar] [CrossRef]
- Balachandran, A.; Choi, S.B.; Beata, M.-M.; Małgorzata, J.; Froemming, G.R.A.; Lavilla, C.A.; Billacura, M.P.; Siyumbwa, S.N.; Okechukwu, P.N. Antioxidant, Wound Healing Potential and In Silico Assessment of Naringin, Eicosane and Octacosane. Molecules 2023, 28, 1043. [Google Scholar] [CrossRef]
- Aladdin, N.A.; Husain, K.; Jalil, J.; Sabandar, C.W.; Jamal, J.A. Xanthine oxidase inhibitory activity of a new isocoumarin obtained from Marantodes pumilum var. pumila leaves. BMC Complement. Med. Ther. 2020, 20, 324. [Google Scholar] [CrossRef]
- Rahmi, E.P.; Jamal, J.A.; Kumolosasi, E.; Jalil, J.; Aladdin, N.A. Marantodes pumilum (Blume) kuntze inhibited secretion of lipopolysaccharide- and monosodium urate crystal-stimulated cytokines and plasma prostaglandin E2. Pharmacogn. Mag. 2017, 13, S578–S586. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Mohd, K.S.; Saeed, M.A.A.; Hassan, L.E.A.; Shafaei, A.; Al-Suede, F.S.R.; Memon, A.H.; Ismail, Z. Anti-uterine fibroid effect of standardized Labisia Pumila Var. Alata extracts in vitro and in human uterine fibroid cancer xenograft model. Asian Pac. J. Cancer Prev. 2020, 21, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Cankaya, I.I.T.; Somuncuoglu, E.I. Potential and Prophylactic Use of Plants Containing Saponin-Type Compounds as Antibiofilm Agents against Respiratory Tract Infections. Evid.-Based Complement. Altern. Med. 2021, 2021, 6814215. [Google Scholar] [CrossRef]
- Farouk, A.; Nawi, M.; Brun, S.H.-S. Antibacterial Peptides from Euycoma longifolia (Tongkat Ali) and Labisia pumila (Kacip Fatimah) Leaves in Malaysia [Online]. Available online: https://www.researchgate.net/profile/Abd-Elaziem-Farouk/publication/233424680_Antibacterial_Peptides_from_Eurycoma_longifolia_Tongkat_Ali_and_Labisia_pumila_Kacip_Fatimah_Leaves_in_Malaysia/links/5727075c08ae586b21e0666f/Antibacterial-Peptides-from-Eurycoma-longifolia-Tongkat-Ali-and-Labisia-pumila-Kacip-Fatimah-Leaves-in-Malaysia.pdf#page=55 (accessed on 31 July 2024).
- Fong, P.; Tong, H.H.Y.; Chao, C.M. In silico prediction of tyrosinase and adenylyl cyclase inhibitors from natural compounds. Nat. Prod. Commun. 2014, 9, 189–194. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Nihei, K.I.; Nihei, A. Antibacterial Activity of Akyl Gallates against Bacillus subtilis. J. Agric. Food Chem. 2004, 52, 1072–1076. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Zięba, S.; Dubis, A.T.; Karolak, M.; Pałkowski, Ł.; Marcinkowska, A.; Skrzypczak, A.; Putowska, A.; Materna, K. Impact of Alkyl Spacer and Side Chain on Antimicrobial Activity of Monocationic and Dicationic Imidazolium Surface-Active Ionic Liquids: Experimental and Theoretical Insights. Molecules 2024, 29, 5743. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Fazliana, M.; Ramos, N.L.; Lüthje, P.; Sekikubo, M.; Holm, Å.; Nazaimoon, W.W.; Brauner, A. Labisia pumila var. alata reduces bacterial load by inducing uroepithelial cell apoptosis. J. Ethnopharmacol. 2011, 136, 111–116. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A.; Ebrahimi, M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol. Res. 2015, 48, 9. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Li, J.; Monje-Galvan, V. In Vitro and In Silico Studies of Antimicrobial Saponins: A Review. Processes 2023, 11, 2856. [Google Scholar] [CrossRef]
- Liberato, I.; A Lino, L.; Souza, J.K.D.; A Neto, J.B.; Sá, L.G.A.V.; Cabral, V.P.F.; Silva, C.R.; Cavalcanti, B.C.; O Moraes, M.; Freire, V.N.; et al. Gallic acid leads to cell death of Candida albicans by the apoptosis mechanism. Future Microbiol. 2022, 17, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Lenard, N.; Henagan, T.M.; Lan, T.; Nguyen, A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Azfaralariff, A.; Farahfaiqah, F.; Shahid, M.; Sanusi, S.A.; Law, D.; Isa, A.R.M.; Muhamad, M.; Tsui, T.T.; Fazry, S. Marantodes pumilum: Systematic computational approach to identify their therapeutic potential and effectiveness. J. Ethnopharmacol. 2022, 283, 114751. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.S.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Norhayati, M.N.; George, A.; Hazlina, N.H.N.; Azidah, A.K.; Idiana, H.I.; Law, K.S.; Bahari, I.S.; Zahiruddin, W.M.W.; Liske, E.; Azreena, A. Efficacy and safety of Labisia pumila var. alata water extract among pre- and postmenopausal women. J. Med. Food 2014, 17, 929–938. [Google Scholar] [CrossRef]
- Nantha, Y.S.; Vijayasingham, S.; Adam, N.L.; Vengadasalam, P.; Ismail, M.; Ali, N.; Chang, L.C.; Ling, L.Y.L.; Tee, T.; Cheah, Y. Labisia pumila standardized extract (SKF7®) reduces percentage of waist circumference and waist-to-height ratio in individuals with obesity. Diabetes Obes. Metab. 2023, 25, 3298–3306. [Google Scholar] [CrossRef]


| Extracts/Compound | Study Design | Dosage | Findings | Refs. |
|---|---|---|---|---|
| Phytoestrogenic Properties | ||||
| MP var. alata aqueous -methanolic extract | In vitro PolarScreen™ ER Alpha and Beta Competitor Assay Kit | 100, 30, 10, 3, 1, 0.3, 0.1 μg/mL | 4 alkylresorcinol derivatives and belamcandol B → varying affinities for ERα (IC50: 4.7–453 μM) and ERβ (IC50: 5.1–86.7 μM) → demonstrating distinct phytoestrogenic activity with receptor subtype preferences | [29] |
| MP var. pumilum root and leaf aqueous extraction | In vitro Ishikawa cells (human endometrial adenocarcinoma cells) | 100 µg/ mL | Tissue-selective oestrogen action → ↑ ALP activity with simultaneous ↓ proliferation of cells → suggesting mixed agonist/antagonist effects on uterine tissue | [30] |
| Postmenopausal Syndrome | ||||
| MP whole plant aqueous extraction | In vivo 30 ovariectomised Sprague-Dawley rats | 17.5, 35.0, and 70.0 mg/kg/day orally for 60-day | Restoration of oestrogen-dependent parameters → ↑ oestrogen and testosterone levels with ↓ FSH and LH levels | [32] |
| MP whole plant aqueous extraction | In vivo 36 ovariectomised Sprague-Dawley rats | 10, 25, and 50 mg intravaginal gel preparation; treated for 7 days | Dose-dependent ↑ in vaginal epithelium thickness → ↑ expression of proliferation marker (PCNA), tight junction protein (occludin), and water/ion transporters (AQP-1, AQP-2, V-ATPase A1) → alleviation of vaginal atrophy symptoms | [33] |
| PCOS | ||||
| MP var. alata aqueous extraction | In vivo 22 female Wistar rats; 7 weeks of DHT exposure, implanted subcutaneously in the neck with a 90-day continuous-release pellet | 50 mg/kg/day: orally for 3.5 weeks | ↑ uterine weight (restored oestrogenic activity) and insulin sensitivity → altered adipokine balance (↑ plasma resistin levels, ↓ mRNA expression of leptin in adipose tissue) → improved lipid profile→ metabolic and reproductive improvements | [38] |
| MP aqueous extraction | In vivo 36 Sprague-Dawley rats; PCOS group fed with HFD for 90 days | 25 and 50 mg/kg/day; stomach gavage for 90 days | Anti-inflammatory and hormonal regulation → ↑ oestradiol levels and insulin sensitivity → ↓ inflammatory markers (C-reactive protein and TNF-α) → improved PCOS parameters | [40] |
| MP var. alata aqueous extraction | In vitro 3T3-L1 adipocyte cell line In vivo 22 female Wistar rats | 50 mg/kg/day: orally for 4 weeks | PPARγ expression (40% mRNA upregulation) and protein levels → ↑ insulin sensitivity (35% improvement) and glucose uptake in adipocytes → improved glucose homeostasis via euglycemic-hyperinsulinemic clamp → metabolic improvements in PCOS | [39] |
| Postpartum | ||||
| MP var. alata aqueous extraction | In vivo 24 day-1 post-delivery Sprague-Dawley female rats | 100, 250, and 500 mg/kg/day; orally for 7 days | Dose-dependent ↑ in uterine contraction force → ↑ key contractility proteins and receptors (CaM, MLCK, SERCA, Gα, Gβ, IP3K, OTR, PGF2α, MAChR, ERα and ERβ) despite ↓ oestradiol levels → improved postpartum uterine tone | [41] |
| Extracts/Compound | Study Design | Treatment Dosage | Findings | References |
|---|---|---|---|---|
| Osteoporosis | ||||
| MP var. alata crude aqueous extract and fractionated with hexane, dichloromethane and methanol solvents. | In vitro cell culture: Murine pre-osteoblastic MC3T3-E1 cells | 10–100 µg/mL | DmcqB activates ERα/ERβ → ↑ osteoblastic gene expression (BMP2, Osx, Ocn) → ↑ collagen synthesis, ALP activity and calcium deposition while modulating RANKL/OPG ratio (↓RANKL, ↑OPG) → inhibit osteoclastogenesis | [10] |
| MP var. alata root aqueous extraction | In vivo 32 ovariectomised Wistar rats | 17.5 mg/kg/day orally for 8 weeks | Stimulates osteoblast activity (↑ObS/BS, OS/BS, OV/BV) and inhibiting osteoclast function (↓OcS/BS, ES/BS) → improves dynamic bone formation parameters and bone microarchitecture | [52] |
| MP var. alata whole plant aqueous, ethanol and methanol extraction | In vivo 48 ovariectomised Sprague-Dawley rats | 20 or 100 mg/kg/day orally for 9 weeks. | Phytoestrogens enhance trabecular microstructure → denser trabecular network with ↑ connectivity density, bone volume (BV/TV), and trabecular number (TbN) with ↓ trabecular separation (TbSp) and structure model index (SMI) → improved bone quality | [47] |
| MP var. alata leaves and roots aqueous extraction | In vivo 30 ovariectomised Sprague-Dawley rats | 20 mg/kg/day orally for 8 weeks | Plant flavonoids and saponins enhance mineral deposition → ↑ trabecular densities, bone volume, cortical thickness (Ct.Th), connectivity density (Conn.D), and bone mineral density (BMD) → improved mechanical properties (↑ maximum force and stress) | [53] |
| MP var. alata root aqueous extraction | In vivo 32 ovariectomised Wistar rats | 17.5 mg/kg/day orally for 8 weeks | Bioactive compounds shift bone turnover balance → ↑ osteocalcin (bone formation marker) with ↓ CTX (bone resorption marker) → net bone formation over resorption | [48] |
| MP var. alata root aqueous extraction | In vivo 32 ovariectomised Wistar rats | 17.5 mg/kg/day orally for 8 weeks | Phytoestrogens → enhanced biomechanical competence with improved stress values, strain values, and Young’s modulus through modulation of collagen cross-linking | [54] |
| MP var. alata whole plant aqueous extraction | In vivo 96 ovariectomised Sprague-Dawley rats | 20 or 100 mg/kg/day orally for 3, 6, or 9 weeks. | Time-dependent enhancement of bone matrix quality → gradual ↑ in maximum load, stress value, stiffness value, and Young’s modulus (elasticity) → reflecting development of mature bone | [55] |
| MP var. alata root aqueous extraction | In vivo 32 ovariectomised Wistar rats | 17.5 mg/kg/day orally for 2 months | Modulation of RANKL/OPG/BMP-2 signalling axis → prevention of RANKL gene elevation and maintaining OPG and BMP-2 gene expression → transcriptional control of bone remodelling | [49] |
| MP var. alata whole plant aqueous extraction | In vivo 96 ovariectomised Sprague-Dawley rats | 20 or 100 mg/kg/day orally for 3, 6, or 9 weeks. | Phenolic compounds → ↑ SOD and GPx with ↓ LPO (MDA) in femur→ ↓ oxidative stress-induced osteoblast apoptosis | [45] |
| MP var. alata Whole plant aqueous extraction | In vivo 96 ovariectomised Sprague-Dawley rats | 20 or 100 mg/kg/day orally for 3, 6, or 9 weeks. | Dose and time-dependent modulation of bone remodelling → improved trabecular network with ↑ bone volume and trabecular number (TbN) and ↓ trabecular separation (TbSp) → sustained restoration of bone mass | [56] |
| MP var. alata Leaves aqueous extraction | In vivo 42 ovariectomised Sprague-Dawley rats and induction of DM using combination of STZ at 55 mg/kg (i.p.) and nicotinamide at 100 mg/kg | 50 or 100 mg/kg/day orally for 28 days | Dual targeting of glycaemic control and bone metabolism via (1) NF-κB pathway inhibition → ↓ inflammatory markers (NF-κB p65, IKKβ, IL-6, IL-1β) (2) Nrf2 activation → ↑ antioxidant enzymes (NQO-1, HO-1, SOD, CAT) (3) RANKL/OPG modulation and enhancement of osteogenic factors (BMP-2, Type-1 collagen, Runx2) → improved bone quality | [51] |
| MP var. alata Leaves aqueous extraction | In vivo 42 ovariectomised Sprague-Dawley rats and induction of DM using combination of STZ at 55 mg/kg (i.p.) and nicotinamide at 100 mg/kg | 50 or 100 mg/kg/day orally for 28 days | Activation of canonical Wnt/β-catenin signalling → ↑ pathway components (Wnt3a, Frizzled, Dvl, LRP5) → enhanced osteoblast proliferation (↑ PCNA, c-Myc) and ↓ apoptosis (↓ caspase-3/9, Bax) → improved bone regeneration and collagen content | [50] |
| Osteoarthritis | ||||
| MP leaves Ethanol-aqueous extraction | In vivo 40 ovariectomised Sprague-Dawley rats and induction of OA by injecting mono-iodoacetate into the right knee joints | 150 or 300 mg/kg/day, orally for 8 weeks | Suppression of cartilage degradation pathways → ↓ catabolic enzymes (MMP-13) and chondrocyte hypertrophy markers (RUNX2, COL10a1) and ↓ apoptosis (↓ CASP3) → preserved cartilage integrity and improved chondrocyte morphology | [45] |
| MP leaves Ethanol-aqueous extraction | In vivo 40 ovariectomised Sprague-Dawley rats and induction of OA by injecting mono-iodoacetate into the right knee joints | 150 or 300 mg/kg/day, orally for 8 weeks | Multi-target chondroprotection through: (1) ↓ inflammatory mediators (NO, PTGS2) and ↑ IL-10 → anti-inflammatory action (2) ↓ collagenases (MMP-1/3) and proteoglycan release→ matrix preservation (3) ↓ trabecular spacing, porosity, and cartilage fissures → relatively intact cartilage surfaces→ structural maintenance | [57] |
| MP var. alata aqueous extraction | In vivo 42 ovariectomised Sprague-Dawley rats | 10, 20, or 50 mg/kg/day, orally for 30 days | Adipokine modulation → ↑ leptin and ↓ resistin levels → improved metabolic profile with ↓ weight gain and preserved uterus weight ratio→ mitigating obesity-related bone loss | [58] |
| Extracts/Compound | Study Design | Treatment Dosage | Findings | References |
|---|---|---|---|---|
| Cardioprotective Effects | ||||
| MP var. alata aqueous extraction | In vivo 42 ovariectomised Sprague-Dawley rats | 10, 20, or 50 mg/kg/day, orally for 30 days | ↓ HSD11B1 mRNA and protein in liver and adipose tissues → ↓ cortisol activation → ↓visceral adiposity and metabolic risk | [59] |
| MP var. alata aqueous extraction | In vivo 35 ovariectomised Sprague-Dawley rats | 17.5 mg/kg/day, orally for 3 months | maintained the elastic lamellae architecture with significant aortic wall thickness→ enhanced vascular integrity and compliance through balanced elastin-collagen metabolism | [62] |
| MP var. alata hydroalcoholic extraction | In vivo 54 male Wistar rats and induction of myocardial infarction using isoproterenol (10 mg/mL; s.c.) to the rats on day 30 and 31 | 100, 200, or 400 mg/kg/day orally for 28 days | (1) ↑ GPx, CAT, SOD → ↓ free radical damage→ ↓ oxidative stress; (2) membrane stabilisation → improved cell integrity → ↓ cardiac injury markers (cTnI, CK-MB, LDH, ALT, AST) → preserved cardiomyocyte function | [61] |
| MP var. alata Hydroalcoholic extraction | In vivo 54 male Wistar rats fed with 2% cholesterol diet for 8 weeks | 100, 200, or 400 mg/kg/day orally for 4 weeks | (1) Improved lipid metabolism → ↓ TC, TG, LDL; ↑ HDL and atherogenic indices; (2) enhanced antioxidant defence → ↓ oxidative stress and MDA; (3) ↓ AST, ALT, LDH and atheroma lesions in abdominal aorta → vascular and hepatic protection | [5] |
| MP aerial and leaf Multiple solvent extraction | In vivo 36 male spontaneously hypertensive rats (SHRs) | 500 mg/kg/day orally for 28 days | Calcium-dependent vasodilation: (1) calcium channel antagonism → ↓ KCl-induced contraction; (2) inhibition of Ca2+ release from sarcoplasmic reticulum → ↓ intracellular Ca2+; (3) endothelium-independent vasorelaxation → ↓ SBP through direct action on vascular smooth muscle | [60] |
| Extracts/Compound | Study Design | Treatment Dosage | Findings | References |
|---|---|---|---|---|
| Diabetes Mellitus | ||||
| MP 50% aqueous ethanol extraction | In vivo 30 STZ-induced (60 mg/kg) diabetic SD rats with diabetic neuropathy | 150 or 300 mg/kg/day orally for 10 weeks | (1) ↓ NF-κB pathway activation → ↓ TNF-α, PGE2 → reduced neuroinflammation; (2) ↓ oxidative stress → preserved neuronal integrity → improved behavioural outcomes→ these mechanisms collectively led to neuroprotection with histologically confirmed preservation of peripheral nerves | [65] |
| MP 50% aqueous ethanol extraction | In vivo 30 STZ-induced (60 mg/kg) diabetic SD rats with diabetic retinopathy | 150 or 300 mg/kg/day orally for 10 weeks | (1) ↓ Inflammatory cascade → ↓ TNF-α, PGE2 → reduced retinal inflammation; (2) regulation of vascular permeability → ↓ claudin-1, VEGF → preserved blood-retinal barrier; (3) antioxidant effects → ↓ MDA/glutathione ratio → ↓oxidative damage→ maintained retinal structure with reduced vascular leakage | [66] |
| MP var. alata leaves aqueous extraction | In vitro Glucose uptake in 3T3-L1 cell line In vivo 42 male SD rats and DM induction using 55 mg/kg STZ and 110 mg/kg NA | 250 or 500 mg/kg/day for 28 days | (1) Enhanced insulin signalling pathway → ↑ glucose uptake in adipocytes → improved glycaemic control (↓ FBG, HbA1c); (2) ↑ insulin production, ↑ HDL, ↑ antioxidant enzymes; (3) ↓ NF-κB p65, ↓ Ikkβ, ↓ TNF-α → reduced tissue inflammation; (4) ↑ Bcl-2/↓ Bax ratio, ↓ caspase-9 → preserved β-cell mass and function→ pancreas protection effect | [64] |
| MP var. alata aqueous leaf and ethanol (50%) stem-root extracts | In vivo 54 ovariectomised SD rats and DM induction using of 30 mg/kg kg and 110 mg/kg NA | 50 or 100 mg/kg/day for 28 days | Insulin sensitisation pathway → ↑ p-IRS1 → ↑ PI3K → ↑ p-Akt → ↑ GLUT-2/4 expression → enhanced glucose utilisation and improved tolerance; (2) inhibition of inflammatory signalling → ↓ p-IKKβ → ↓ pNF-Kβ → ↓ iNOS, COX2 → reduced inflammatory damage; (3) protection of β-cells → ↓ caspase 3 and cleaved caspase 3 → preserved islet architecture and function | [63] |
| Anti-Cancer | ||||
|---|---|---|---|---|
| Extracts/Compound | Study Design | Treatment Dosage | Findings | References |
| MP whole plant ethanol/water extraction | In vitro Uterine leiomyosarcoma SK-UT-1 cells In vivo 24 uterine fibroid xenograft in athymic mouse model | In vitro 100 and 250 μg/mL In vivo 200 or 400 mg/kg/day for 3 weeks | In vitro induce apoptosis → ↓ viable cell up to 61.64% In vivo ↓ average tumour volume | [64] |
| MP whole plant hexane, ethanol, and water extraction | In vitro Human melanoma HM3KO cells | 0–5 mg/mL | (1) ↑ p53, Bax, Bax/Bcl-2 ratio; ↓ Bcl-2 → mitochondrial membrane destabilisation → cytochrome c release → caspase activation →apoptosis; (2) arrest at G1 phase → prevented DNA replication and proliferation | [7] |
| MP leaves Methanol/water extraction | In vitro Breast (MCF-7), colon (HCT-116), and prostate (PC-3) cells | 0.1 and 100.0 uM | 1-O-methyl-6-acetoxy-5-(pentadec-10Z-enyl)resorcinol (1) and 1-O-methyl-6-acetoxy-5-pentadecylresorcinol (4) → ↑ submicromolar growth inhibition with selective cytotoxicity against PC-3 and HCT-116→ likely disrupt cell membrane integrity and interfere with mitochondrial function | [11] |
| MP var. pumila methanol extraction | In vitro PC3 and LNCaP prostate cancer cell line | 5-henicosene-1-yl-resorcinol (alkyl-resorcinol) → (1) mitochondrial pathway: ↑Bax, ↓Bcl-2 → mitochondrial membrane depolarisation → ↑caspases 3/7 activation → nuclear DNA fragmentation; (2) anti-migration pathway: ↓ALOX-5 → ↓VEGF-A, ↓CXCL12 → inhibited cell migration/invasion→ induced apoptosis | [17] | |
| Extract/Compound | Study Design | Treatment Dosage | Organisms Tested | Findings | References |
|---|---|---|---|---|---|
| MP leaves acetone, methanol, ethanol, and phosphate buffer pH 7 extraction | In vitro Agar-well diffusion method | 5–100 mg/mL | Staphylococcus aureus | MIC ranging from 25 to 75 mg/mL | [6] |
| Pseudomonas aeruginosa | MIC of 25 mg/mL | ||||
| MP var. pumila, alata, lanceolata leaves, stems, and roots methanol extraction | In vitro Disc diffusion method | 0–500 µg/well | Micrococcus luteus | Zone of inhibition ranging from 0.33 to 0.65 cm; effective but less potent than the standard agent | [6] |
| Bacillus subtilis B145 | Zone of inhibition ranging from 0.79 to 1.15 cm; effective but less potent than the standard agent | ||||
| Bacillus cereus B43 | Zone of inhibition ranging from 0.73 to 1.1 cm; effective but less potent than the standard agent | ||||
| Staphylococcus aureus S1431 | Zone of inhibition ranging from 0.29 to 0.95 cm; effective but less potent than the standard agent | ||||
| Enterobacter aerogenes | Zone of inhibition ranging from 0.71 to 1.23 cm; effective but less potent than the standard agent | ||||
| Klebsiella pneumonia K36 | Zone of inhibition ranging from 0.62 to 1.12 cm; effective but less potent than the standard agent | ||||
| Escherichia coli E256 | Zone of inhibition ranging from 0.45 to 1.32 cm; effective but less potent than the standard agent | ||||
| Pseudomonas aeruginosa PI96 | Zone of inhibition ranging from 0.33 to 0.75 cm; effective but less potent than the standard agent | ||||
| In vitro Agar well diffusion method | 0–500 µg/well | Fusarium sp. | Zone of inhibition ranging from 0.30 to 0.75 cm; weak to moderate antifungal activities compared to the standard agent | ||
| Candida sp. | Zone of inhibition ranging from 0.40 to 0.79 cm; weak to moderate antifungal activities compared to the standard agent | ||||
| Mucor sp. | Zone of inhibition ranging from 0.28 to 0.69 cm; weak to moderate antifungal activities compared to the standard agent | ||||
| MP root methanol extraction | In vitro Broth dilution method | 0–100 μg/mL | Staphylococcus aureus | IC50 of 3.13 μg/mL; mild antibacterial activity of 1,3-dihydroxy-5-[10(Z)-pentadecenyl]benzene compound | [18] |
| Methicillin resistant S. aureus | IC50 ranging from 0.83 to 19.41 μg/mL; effective antibacterial activity of belamcandol B and 1,3-dihydroxy-5-[10(Z)-pentadecenyl]benzene compound but less potent the standard agent | ||||
| Escherichia coli, | No inhibitory activity | ||||
| Pseudomonas aeruginosa | No inhibitory activity | ||||
| Mycobacterium intracellulare | No inhibitory activity | ||||
| Candida albicans | No inhibitory activity | ||||
| Candida glabrata | No inhibitory activity | ||||
| Candida krusei | No inhibitory activity | ||||
| Cryptococcus neoformans | No inhibitory activity | ||||
| Aspergillus fumigatus | No inhibitory activity | ||||
| MP aqueous extraction | In vitro Agar-well diffusion method | 1000 µg/mL | Uropathogenic Escherichia coli (UPEC) strain CFT073 | No inhibitory activity; reduced bacterial load in the bladder epithelial cell (BEC) infection model via BEC apoptosis | [81] |
| Proteus mirabilis | No inhibitory activity | ||||
| Pseudomonas aeruginosa | No inhibitory activity | ||||
| Staphylococcus saprophyticus | No inhibitory activity | ||||
| Candida albicans | No inhibitory activity | ||||
| MP var. pumila, alata, lanceolata leaves, and roots aqueous extraction | In vitro Agar-well diffusion method | 450 µg/well | Fusarium sp. | Zone of inhibition ranging from 0.45 cm to 0.71 cm; weak to moderate antifungal activities compared to the standard agent | [26] |
| Candida sp. | Zone of inhibition ranging from 0.51 cm to 0.82 cm; weak to moderate antifungal activities compared to the standard agent | ||||
| Mucor sp. | Zone of inhibition ranging from 0.33 cm to 0.81 cm; weak to moderate antifungal activities compared to the standard agent | ||||
| MP var. pumila, alata, lanceolata leaves aqueous extract | In vitro Disc diffusion method | 300 µg/disc | Staphylococcus aureus S1431 | Zone of inhibition ranging from 0.55 to 0.70 cm; effective but less potent than the standard agent | [82] |
| Bacillus subtilis B145 | Zone of inhibition ranging from 0.65 to 0.75 cm; effective but less potent than the standard agent | ||||
| Bacillus cereus B43 | Zone of inhibition ranging from 0.60 to 0.75 cm; effective but less potent than the standard agent | ||||
| Enterococcus aerogenes | Zone of inhibition ranging from 0.40 to 0.63 cm; effective but less potent than the standard agent | ||||
| Escherichia coli E256 | Zone of inhibition ranging from 0.78 to 0.90 cm; effective but less potent than the standard agent | ||||
| Pseudomonas aeruginosa PI96 | Zone of inhibition ranging from 0.30 to 0.40 cm; effective but less potent than the standard agent | ||||
| Micrococcus luteus | Zone of inhibition ranging from 0.40 to 0.55 cm; effective but less potent than the standard agent | ||||
| Klebsiella pneumonia K36 | Zone of inhibition ranging from 0.55 to 0.65 cm; effective but less potent than the standard agent |
| Extracts/Compound | Study Design | Treatment Dosage | Findings | References |
|---|---|---|---|---|
| MP water extract | A pilot study: randomised, double-blind, placebo-controlled conducted amongst postmenopausal Malay women (N = 63) | 280 mg/day MP extract for 6 months | Lower fasting glucose, total cholesterol, LDL, HDL, triglycerides, LH, and oestradiol levels; no effect on hormonal profile ↓ adjusted mean triglyceride level (1.4 mmol/L) vs. placebo group (1.9 mmol/L) | [8] |
| MP var. alata water extract | A randomised, double-blind, placebo-controlled, parallel group, 16-week study in healthy pre- and postmenopausal women aged 40–60 years old (N = 197) | 400 mg/day for 16 weeks | Women’s Health Questionnaire: Significant improvements in memory, concentration, vasomotor symptoms, and sleep. No significant changes in FSH, LH, or oestradiol levels ↓ in total cholesterol and LDL-C, especially among participants with elevated triglycerides | [90] |
| SKF7® (a patented standardised extract of MP) | A randomised, double-blind, multicentric, placebo-controlled, phase 2 dose-ranging evaluation of SKF7® in obese (N = 133) (NCT05851599) | 375, 562.5, and 750 mg day for 16 months | Dose dependent reduction in abdominal obesity; ↓ in BW, BMI, WC, and WHtR | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, S.H.; Junaidi, N.S.S.A.; Halim, S.; Ramli, N.F.; Mokhtar, M.H. The Pharmacological Potential of Marantodes pumilum: A Comprehensive Review of Its Medicinal Properties. Int. J. Mol. Sci. 2025, 26, 6155. https://doi.org/10.3390/ijms26136155
Adam SH, Junaidi NSSA, Halim S, Ramli NF, Mokhtar MH. The Pharmacological Potential of Marantodes pumilum: A Comprehensive Review of Its Medicinal Properties. International Journal of Molecular Sciences. 2025; 26(13):6155. https://doi.org/10.3390/ijms26136155
Chicago/Turabian StyleAdam, Siti Hajar, Nor Syaza Syahirah Amat Junaidi, Shariff Halim, Nurul Farisha Ramli, and Mohd Helmy Mokhtar. 2025. "The Pharmacological Potential of Marantodes pumilum: A Comprehensive Review of Its Medicinal Properties" International Journal of Molecular Sciences 26, no. 13: 6155. https://doi.org/10.3390/ijms26136155
APA StyleAdam, S. H., Junaidi, N. S. S. A., Halim, S., Ramli, N. F., & Mokhtar, M. H. (2025). The Pharmacological Potential of Marantodes pumilum: A Comprehensive Review of Its Medicinal Properties. International Journal of Molecular Sciences, 26(13), 6155. https://doi.org/10.3390/ijms26136155






