ABCG2 Transporter: From Structure to Function—Current Insights and Open Questions
Abstract
1. Introduction
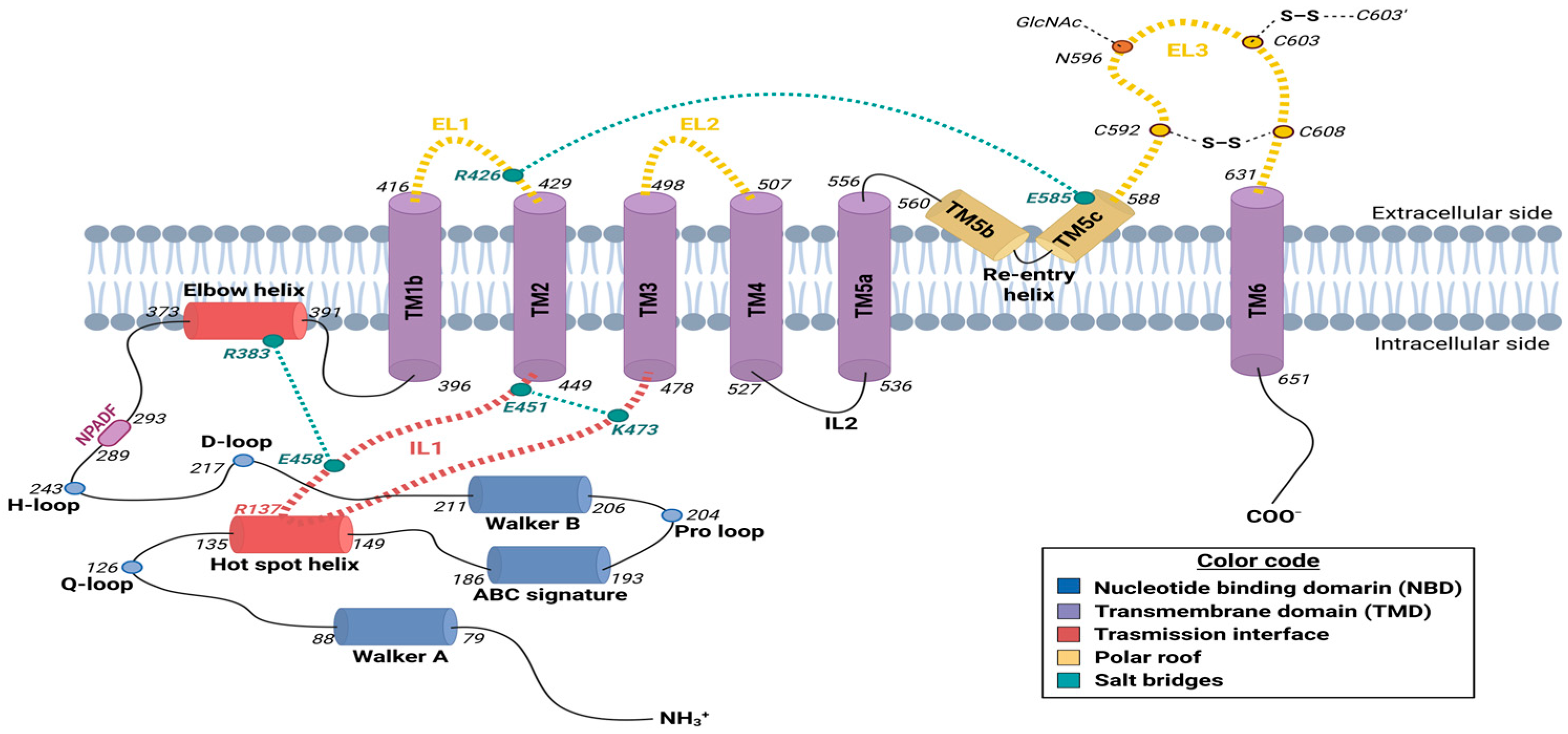
2. Molecular Structure of the Human ABCG2 Transporter
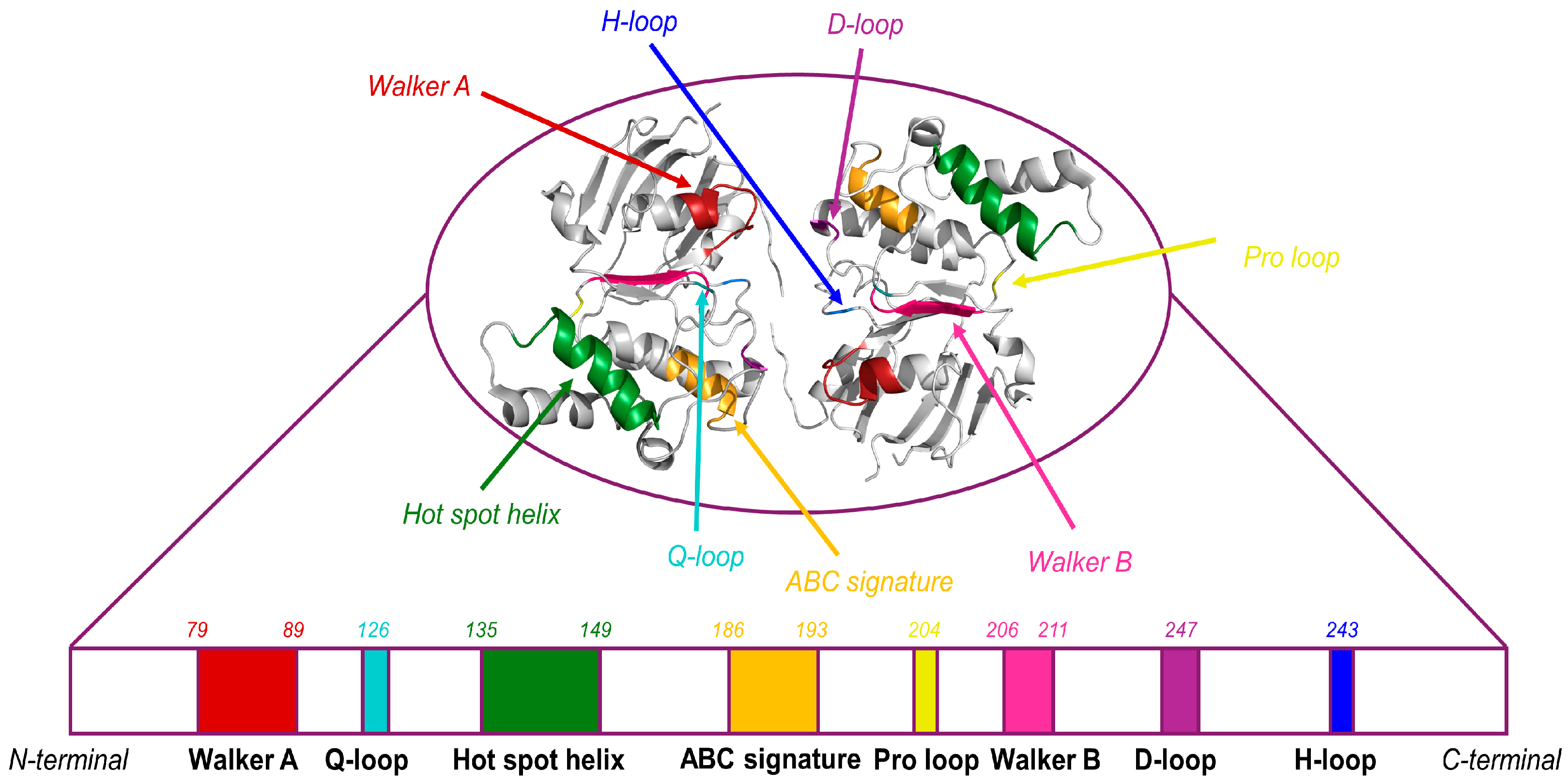
2.1. Nucleotide-Binding Domain (NBD)
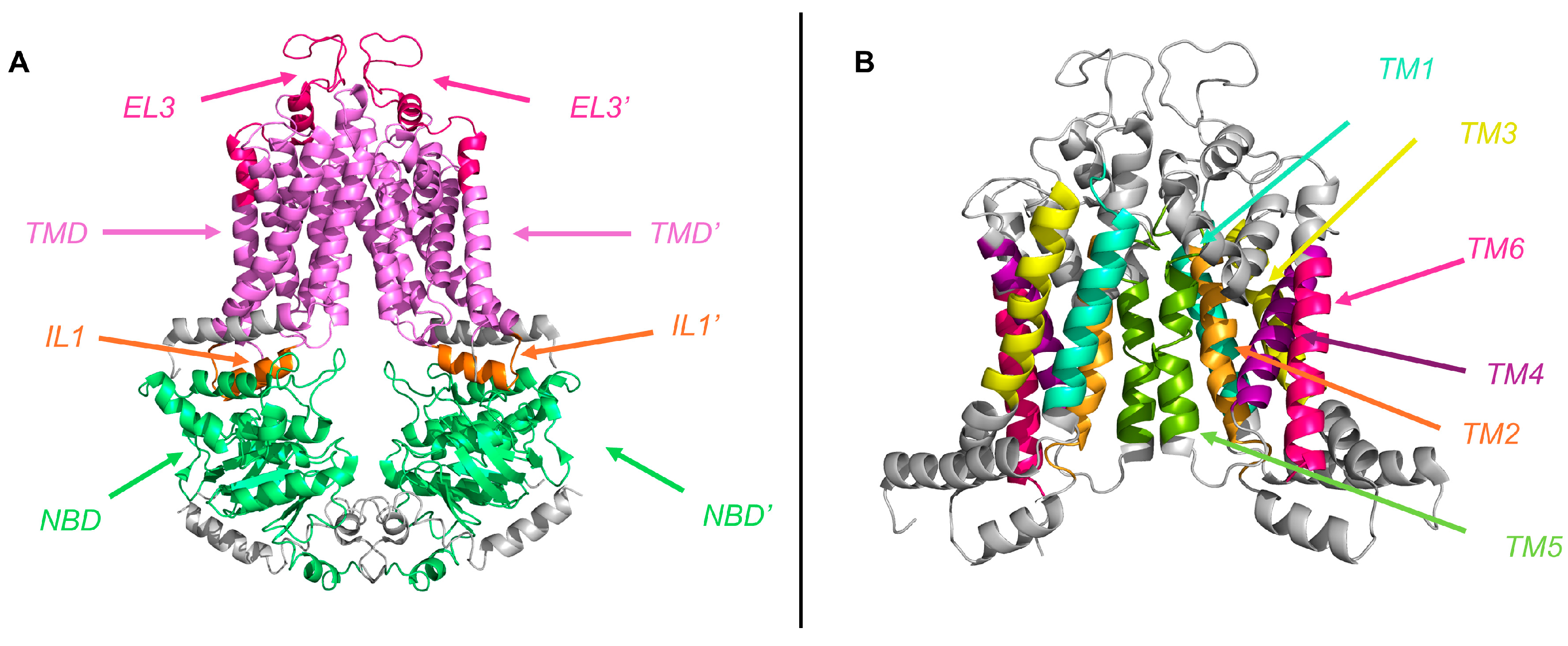
2.2. Transmembrane Domain (TMD)
2.3. Transmission Interface
2.4. Structural Characterization of ABCG2 Homodimer
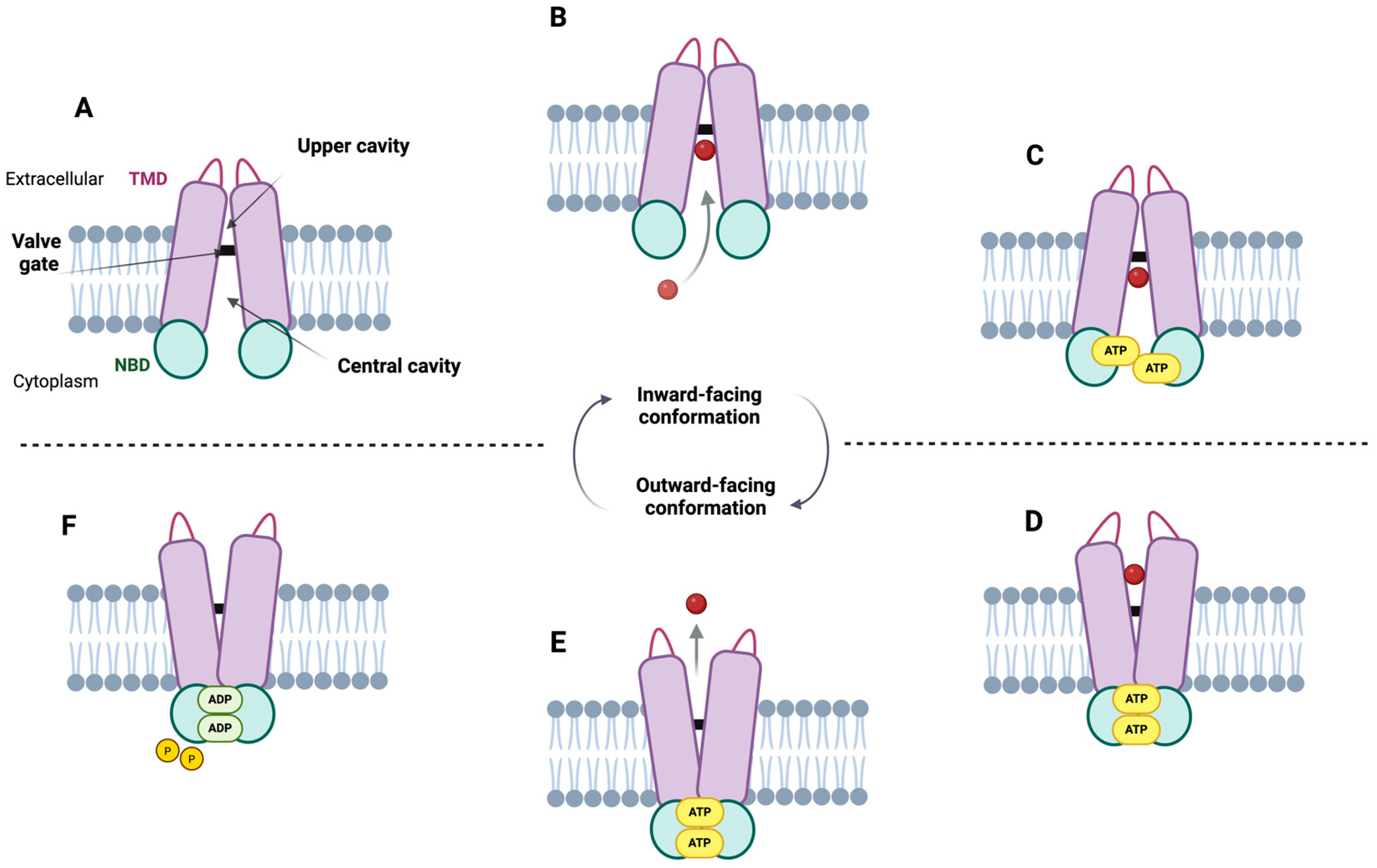
2.4.1. Central Cavity
2.4.2. Upper Cavity
2.4.3. Regulatory Gates
- The “entry” gate
- 2.
- The “valve” gate
- 3.
- The “exit” gate
3. Transport Mechanism
3.1. Transport Model Proposal
3.2. Updating the ABCG2 Transport Mechanism
3.3. ATP Hydrolysis in ABCG2: A Controversial Step
4. Structural Interactions for Substrates and Inhibitors
5. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| BCRP | Breast Cancer Resistance Protein |
| Cryo-EM | Cryo-Electron Microscopy |
| E1S | Estrone-3-Sulfate |
| EL | Extracellular Loop |
| IL | Intracellular Loop |
| P-gp | P-glycoprotein |
| MDR | Multidrug Resistance |
| MRP1 | Multidrug-Resistance-Associated Protein 1 |
| NBD | Nucleotide-Binding Domain |
| THB | Triple Helical Bundle |
| TMD | Transmembrane Domain |
| TM | Transmembrane Helix |
| TO1 | Turnover-1 |
| TO2 | Turnover-2 |
References
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kalwan, G.; Gill, S.S.; Jain, P.K. The ABC transporters and their epigenetic regulation under drought stress in chickpea. Plant Physiol. Biochem. 2025, 223, 109903. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Moon, S.; Jung, K.H. Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J. Plant Physiol. 2014, 171, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, Ü.; Lee, Y.; Martinoia, E.; et al. Plant ABC proteins-a unified nomenclature and updated inventory. Trends Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef]
- Dean, M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005, 400, 409–429. [Google Scholar] [CrossRef]
- Ofori, P.A.; Mizuno, A.; Suzuki, M.; Martinoia, E.; Reuscher, S.; Aoki, K.; Shibata, D.; Otagaki, S.; Matsumoto, S.; Shiratake, K. Genome-wide analysis of ATP binding cassette (ABC) transporters in tomato. PLoS ONE 2018, 13, e0200854. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Benndorf, R.A. 3D structure of the transporter ABCG2—What’s new? Br. J. Pharmacol. 2020, 177, 1485–1496. [Google Scholar] [CrossRef]
- Gerovac, M.; Tampé, R. Control of mRNA translation by versatile ATP-driven machines. Trends Biochem. Sci. 2019, 44, 167–180. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Mutational analysis of ABC proteins. Arch. Biochem. Biophys. 2008, 476, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Xiong, J.; Feng, J.; Yuan, D.; Zhou, J.; Miao, W. Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. Sci. Rep. 2015, 5, 16724. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Tampé, R. Structural and mechanistic principles of ABC transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Jarzyniak, K.M.; Jasiński, M. Membrane transporters and drought resistance-a complex issue. Front. Plant Sci. 2014, 5, 687. [Google Scholar] [CrossRef]
- Mao, Q. BCRP/ABCG2 in the placenta: Expression, function and regulation. Pharm. Res. 2008, 25, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- García-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the mammary gland-contribution to presence of nutrients and drugs into milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef]
- Dudas, B.; Decleves, X.; Cisternino, S.; Perahia, D.; Miteva, M.A. ABCG2/BCRP transport mechanism revealed through kinetically excited targeted molecular dynamics simulations. Comput. Struct. Biotechnol. J. 2022, 20, 4195–4205. [Google Scholar] [CrossRef]
- Kukal, S.; Guin, D.; Rawat, C.; Bora, S.; Mishra, M.K.; Sharma, P.; Paul, P.R.; Kanojia, N.; Grewal, G.K.; Kukreti, S.; et al. Multidrug efflux transporter ABCG2: Expression and regulation. Cell. Mol. Life Sci. 2021, 78, 6887–6939. [Google Scholar] [CrossRef]
- Yu, Q.; Ni, D.; Kowal, J.; Manolaridis, I.; Jackson, S.M.; Stahlberg, H.; Locher, K.P. Structures of ABCG2 under turnover conditions reveal a key step in the drug transport mechanism. Nat. Commun. 2021, 12, 4376. [Google Scholar] [CrossRef]
- Rocchi, E.; Khodjakov, A.; Volk, E.L.; Yang, C.H.; Litman, T.; Bates, S.E.; Schneider, E. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem. Biophys. Res. Commun. 2000, 271, 42–46. [Google Scholar] [CrossRef]
- Vlaming, M.L.H.; Lagas, J.S.; Schinkel, A.H. Physiological and pharmacological roles of ABCG2 (BCRP): Recent findings in Abcg2 knockout mice. Adv. Drug Deliv. Rev. 2009, 61, 14–25. [Google Scholar] [CrossRef]
- Jonker, J.W.; Merino, G.; Musters, S.; van Herwaarden, A.E.; Bolscher, E.; Wagenaar, E.; Mesman, E.; Dale, T.C.; Schinkel, A.H. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 2005, 11, 127–129. [Google Scholar] [CrossRef]
- Pulido, M.M.; Molina, A.J.; Merino, G.; Mendoza, G.; Prieto, J.G.; Alvarez, A.I. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): Influence of flavonoids and role in milk secretion in sheep. J. Vet. Pharmacol. Ther. 2006, 29, 279–287. [Google Scholar] [CrossRef]
- van Herwaarden, A.E.; Wagenaar, E.; Karnekamp, B.; Merino, G.; Jonker, J.W.; Schinkel, A.H. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 2006, 27, 123–130. [Google Scholar] [CrossRef]
- Wang, L.; Leggas, M.; Goswami, M.; Empey, P.E.; McNamara, P.J. N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) as a chemical ATP-binding cassette transporter family G member 2 (Abcg2) knockout model to study nitrofurantoin transfer into milk. Drug Metab. Dispos. 2008, 36, 2591–2596. [Google Scholar] [CrossRef]
- Sychterz, C.; Shen, H.; Zhang, Y.; Sinz, M.; Rostami-Hodjegan, A.; Schmidt, B.J.; Gaohua, L.; Galetin, A. A close examination of BCRP’s role in lactation and methods for predicting drug distribution into milk. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 1856–1869. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005, 7, E118–E133. [Google Scholar] [CrossRef]
- van Herwaarden, A.E.; Schinkel, A.H. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol. Sci. 2006, 27, 10–16. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X. Contributions of drug transporters to blood-brain barriers. In Drug Transporters in Drug Disposition, Effects and Toxicity; Liu, X., Pan, G., Eds.; Springer: Singapore, 2019; Volume 1141, pp. 407–466. [Google Scholar]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development young. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Teng, Q.X.; Lei, Z.N.; Wang, J.Q.; Yang, Y.; Wu, Z.X.; Acharekar, N.D.; Zhang, W.; Yoganathan, S.; Pan, Y.; Wurpel, J.; et al. Overexpression of ABCC1 and ABCG2 confers resistance to talazoparib, a poly (ADP-Ribose) polymerase inhibitor. Drug Resist. Updat. 2024, 73, 101028. [Google Scholar] [CrossRef]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport-an update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Cohen-Zinder, M.; Peter, C.; Weller, J.I.; Erhardt, G. Short communication: A polymorphism in ABCG2 in Bos indicus and Bos taurus cattle breeds. J. Dairy Sci. 2006, 89, 4921–4923. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Fromm, M.F. Transporter-mediated drug-drug interactions. Pharmacogenomics 2011, 12, 1017–1037. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R.W.; Bates, S.E. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2018, 50, 209–232. [Google Scholar] [CrossRef]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef]
- Robey, R.W.; Massey, P.R.; Amiri-Kordestani, L.; Bates, S.E. ABC transporters: Unvalidated therapeutic targets in cancer and the CNS. Anticancer. Agents Med. Chem. 2010, 10, 625–633. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed]
- Khunweeraphong, N.; Kuchler, K. The human ABCG2 transporter engages three gates to control multidrug extrusion. iScience 2025, 28, 112125. [Google Scholar] [CrossRef]
- László, L.; Sarkadi, B.; Hegedűs, T. Jump into a new fold-a homology based model for the ABCG2/BCRP multidrug transporter. PLoS ONE 2016, 11, e0164426. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC transporters in fish species: A review. Front. Physiol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Khunweeraphong, N.; Kuchler, K. The first intracellular loop is essential for the catalytic cycle of the human ABCG2 multidrug resistance transporter. FEBS Lett. 2020, 594, 4059–4075. [Google Scholar] [CrossRef]
- Oldham, M.L.; Davidson, A.L.; Chen, J. Structural insights into ABC transporter mechanism. Curr. Opin. Struct. Biol. 2008, 18, 726–733. [Google Scholar] [CrossRef]
- Robey, R.W.; To, K.K.K.; Polgar, O.; Dohse, M.; Fetsch, P.; Dean, M.; Bates, S.E. ABCG2: A perspective. Adv. Drug Deliv. Rev. 2009, 61, 3–13. [Google Scholar] [CrossRef]
- Kapoor, P.; Horsey, A.J.; Cox, M.H.; Kerr, I.D. ABCG2: Does resolving its structure elucidate the mechanism? Biochem. Soc. Trans. 2018, 46, 1485–1494. [Google Scholar] [CrossRef]
- Al-Shawi, M.K. Catalytic and transport cycles of ABC exporters. Essays Biochem. 2011, 50, 63–83. [Google Scholar] [CrossRef]
- ter Beek, J.; Guskov, A.; Slotboom, D.J. Structural diversity of ABC transporters. J. Gen. Physiol. 2014, 143, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Khunweeraphong, N.; Kuchler, K. Multidrug resistance in mammals and fungi-from MDR to PDR: A rocky road from atomic structures to transport mechanisms. Int. J. Mol. Sci. 2021, 22, 4806. [Google Scholar] [CrossRef] [PubMed]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.I.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563, 426–430. [Google Scholar] [CrossRef]
- Badiee, S.A.; Isu, U.H.; Khodadadi, E.; Moradi, M. The alternating access mechanism in mammalian multidrug resistance transporters and their bacterial homologs. Membranes 2023, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Locher, K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016, 23, 487–493. [Google Scholar] [CrossRef]
- Khunweeraphong, N.; Stockner, T.; Kuchler, K. The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion. Sci. Rep. 2017, 7, 13767. [Google Scholar] [CrossRef]
- Kowal, J.; Ni, D.; Jackson, S.M.; Manolaridis, I.; Stahlberg, H.; Locher, K.P. Structural basis of drug recognition by the multidrug transporter ABCG2. J. Mol. Biol. 2021, 433, 166980. [Google Scholar] [CrossRef]
- Wang, L.; Johnson, Z.L.; Wasserman, M.R.; Levring, J.; Chen, J.; Liu, S. Characterization of the kinetic cycle of an ABC transporter by single-molecule and cryo-EM analyses. eLife 2020, 9, e56451. [Google Scholar] [CrossRef]
- Lewinson, O.; Orelle, C.; Seeger, M.A. Structures of ABC transporters: Handle with care. FEBS Lett. 2020, 594, 3799–3814. [Google Scholar] [CrossRef]
- Linton, K.J.; Higgins, C.F. Structure and function of ABC transporters: The ATP switch provides flexible control. Pflugers Arch. 2007, 453, 555–567. [Google Scholar] [CrossRef]
- Khunweeraphong, N.; Szöllősi, D.; Stockner, T.; Kuchler, K. The ABCG2 multidrug transporter is a pump gated by a valve and an extracellular lid. Nat. Commun. 2019, 10, 5433. [Google Scholar] [CrossRef]
- Li, J.; Jaimes, K.F.; Aller, S.G. Refined structures of mouse P-glycoprotein. Protein Sci. 2014, 23, 34–46. [Google Scholar] [CrossRef]
- Orlando, B.J.; Liao, M. ABCG2 transports anticancer drugs via a closed-to-open switch. Nat. Commun. 2020, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Locher, K.P. Structure and mechanism of human ABC transporters. Annu. Rev. Biophys. 2023, 52, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, N.; Timmermans, R.G.M.; Vieraankivi, M.; Suominen, L.; Vellonen, K.S.; Bhattacharya, M.; Auriola, S.; Kidron, H. Exploring the effect of intracellular loop 1 genetic variants in human ABCG2 on transport activity and protein abundance. Drug Metab. Pharmacokinet. 2025, 62, 101482. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Nakagawa, H.; Tamura, A.; Koshiba, S.; Hoshijima, K.; Komada, M.; Ishikawa, T. Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein. J. Biol. Chem. 2007, 282, 27841–27846. [Google Scholar] [CrossRef] [PubMed]
- Kage, K.; Fujita, T.; Sugimoto, Y. Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2. Cancer Sci. 2005, 96, 866–872. [Google Scholar] [CrossRef]
- Henriksen, U.; Fog, J.U.; Litman, T.; Gether, U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J. Biol. Chem. 2005, 280, 36926–36934. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wakabayashi-Nakao, K.; Tamura, A.; Toyoda, Y.; Koshiba, S.; Ishikawa, T. Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J. 2009, 276, 7237–7252. [Google Scholar] [CrossRef]
- Dutra, J.P.; Scheiffer, G.; Kronenberger, T.; Gomes, L.J.C.; Zanzarini, I.; dos Santos, K.K.; Tonduru, A.K.; Poso, A.; Rego, F.G.M.; Picheth, G.; et al. Structural and molecular characterization of lopinavir and ivermectin as breast cancer resistance protein (BCRP/ABCG2) inhibitors. EXCLI J. 2023, 22, 1155–1172. [Google Scholar] [CrossRef]
- Nagy, T.; Tóth, Á.; Telbisz, Á.; Sarkadi, B.; Tordai, H.; Tordai, A.; Hegedűs, T. The transport pathway in the ABCG2 protein and its regulation revealed by molecular dynamics simulations. Cell. Mol. Life Sci. 2021, 78, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Gose, T.; Shafi, T.; Fukuda, Y.; Das, S.; Wang, Y.; Allcock, A.; McHarg, A.G.; Lynch, J.; Chen, T.; Tamai, I.; et al. ABCG2 requires a single aromatic amino acid to “clamp” substrates and inhibitors into the binding pocket. FASEB J. 2020, 34, 4890–4903. [Google Scholar] [CrossRef]
- Tamura, A.; Watanabe, M.; Saito, H.; Nakagawa, H.; Kamachi, T.; Okura, I.; Ishikawa, T. Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: Identification of alleles that are defective in porphyrin transport. Mol. Pharmacol. 2006, 70, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H.; Noguchi, K.; Katayama, K.; Mitsuhashi, J.; Sugimoto, Y. Pharmacological interaction with sunitinib is abolished by a germ-line mutation (1291T>C) of BCRP/ABCG2 gene. Cancer Sci. 2010, 101, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Szöllősi, D.; Rose-Sperling, D.; Hellmich, U.A.; Stockner, T. Comparison of mechanistic transport cycle models of ABC exporters. Biochim. Biophys. Acta Biomembr. 2018, 1860, 818–832. [Google Scholar] [CrossRef]
- Jones, P.M.; George, A.M. The ABC transporter structure and mechanism: Perspectives on recent research. Cell. Mol. Life Sci. 2004, 61, 682–699. [Google Scholar] [CrossRef]
- Cox, M.H.; Kapoor, P.; Briggs, D.A.; Kerr, I.D. Residues contributing to drug transport by ABCG2 are localised to multiple drug-binding pockets. Biochem. J. 2018, 475, 1553–1567. [Google Scholar] [CrossRef]
- Rasouli, A.; Yu, Q.; Dehghani-Ghahnaviyeh, S.; Wen, P.C.; Kowal, J.; Locher, K.P.; Tajkhorshid, E. Differential dynamics and direct interaction of bound ligands with lipids in multidrug transporter ABCG2. Proc. Natl. Acad. Sci. USA 2023, 120, e2213437120. [Google Scholar] [CrossRef]
- Gyöngy, Z.; Mocsár, G.; Hegedűs, É.; Stockner, T.; Ritter, Z.; Homolya, L.; Schamberger, A.; Orbán, T.I.; Remenyik, J.; Szakacs, G.; et al. Nucleotide binding is the critical regulator of ABCG2 conformational transitions. eLife 2023, 12, e83976. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Fernández, L.; Millán-García, A.; Merino, G.; Blanco-Paniagua, E. ABCG2 Transporter: From Structure to Function—Current Insights and Open Questions. Int. J. Mol. Sci. 2025, 26, 6119. https://doi.org/10.3390/ijms26136119
Álvarez-Fernández L, Millán-García A, Merino G, Blanco-Paniagua E. ABCG2 Transporter: From Structure to Function—Current Insights and Open Questions. International Journal of Molecular Sciences. 2025; 26(13):6119. https://doi.org/10.3390/ijms26136119
Chicago/Turabian StyleÁlvarez-Fernández, Laura, Alicia Millán-García, Gracia Merino, and Esther Blanco-Paniagua. 2025. "ABCG2 Transporter: From Structure to Function—Current Insights and Open Questions" International Journal of Molecular Sciences 26, no. 13: 6119. https://doi.org/10.3390/ijms26136119
APA StyleÁlvarez-Fernández, L., Millán-García, A., Merino, G., & Blanco-Paniagua, E. (2025). ABCG2 Transporter: From Structure to Function—Current Insights and Open Questions. International Journal of Molecular Sciences, 26(13), 6119. https://doi.org/10.3390/ijms26136119





