Anthozoan Chemical Defenses: Integrating Compounds, Enzymatic Activities, and Omics-Based Discoveries
Abstract
1. Introduction
Defense System in Anthozoans
2. Compounds and Toxins
2.1. Hexacorallia
2.1.1. Zoanthids/Zoantharian
2.1.2. Scleractinia/Stony Corals
Associated Microbiota
2.1.3. Sea Anemones/Actinarians
2.2. Octocorallia
2.2.1. Soft Corals
2.2.2. Gorgonian Corals
3. Defensive Enzymes
3.1. Antioxidant Enzymes in Anthozoans
3.2. Enzymes in Symbionts
3.3. Species-Specific Enzymatic Response
4. Molecular Resources Available: Genomes and Transcriptomes
4.1. Genomes and Transcriptomes in the Deep Sea
4.2. Genomes and Transcriptomes in Response to Abiotic and Biotic Stressors
4.3. Genomes and Transcriptomes to Study Toxins and Bleaching Events
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, C.; Scharff, N.L.; Bsen, D.E.-J. Cladistic Analyses of the Animal Kingdom. Biol. J. Linn. Soc. 1996, 57, 385–410. [Google Scholar] [CrossRef]
- Cartwright, P.; Halgedahl, S.L.; Hendricks, J.R.; Jarrard, R.D.; Marques, A.C.; Collins, A.G.; Lieberman, B.S. Exceptionally Preserved Jellyfishes from the Middle Cambrian. PLoS ONE 2007, 2, e1121. [Google Scholar] [CrossRef]
- Rachamim, T.; Morgenstern, D.; Aharonovich, D.; Brekhman, V.; Lotan, T.; Sher, D. The Dynamically Evolving Nematocyst Content of an Anthozoan, a Scyphozoan, and a Hydrozoan. Mol. Biol. Evol. 2015, 32, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.; Zlotkin, E. A Hydra with Many Heads: Protein and Polypeptide Toxins from Hydra and Their Biological Roles. Toxicon 2009, 54, 1148–1161. [Google Scholar] [CrossRef]
- Tardent, P. The Cnidarian Cnidocyte, a Hightech Cellular Weaponry. BioEssays 1995, 17, 351–362. [Google Scholar] [CrossRef]
- David, C.N.; Özbek, S.; Adamczyk, P.; Meier, S.; Pauly, B.; Chapman, J.; Hwang, J.S.; Gojobori, T.; Holstein, T.W. Evolution of Complex Structures: Minicollagens Shape the Cnidarian Nematocyst. Trends Genet. 2008, 24, 431–438. [Google Scholar] [CrossRef]
- Sunagar, K.; Columbus-Shenkar, Y.Y.; Fridrich, A.; Gutkovich, N.; Aharoni, R.; Moran, Y. Cell Type-Specific Expression Profiling Unravels the Development and Evolution of Stinging Cells in Sea Anemone. BMC Biol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.; Knebel, A.; Bsor, T.; Nesher, N.; Tal, T.; Morgenstern, D.; Cohen, E.; Fishman, Y.; Zlotkin, E. Toxic Polypeptides of the Hydra—A Bioinformatic Approach to Cnidarian Allomones. Toxicon 2005, 45, 865–879. [Google Scholar] [CrossRef]
- Technau, U.; Steele, R.E. Evolutionary Crossroads in Developmental Biology: Cnidaria. Development 2011, 138, 1447–1458. [Google Scholar] [CrossRef]
- Tarrant, A.M. Hormonal Signaling in Cnidarians: Do We Understand the Pathways Well Enough to Know Whether They Are Being Disrupted? Ecotoxicology 2007, 16, 5–13. [Google Scholar] [CrossRef]
- McFadden, C.S.; Quattrini, A.M.; Brugler, M.R.; Cowman, P.F.; Dueñas, L.F.; Kitahara, M.V.; Paz-García, D.A.; Reimer, J.D.; Rodríguez, E. Phylogenomics, Origin, and Diversification of Anthozoans (Phylum Cnidaria). Syst. Biol. 2021, 70, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.D. Deep-Water Corals: An Overview with Special Reference to Diversity and Distribution of Deep-Water Scleractinian Corals. Bull. Mar. Sci. 2007, 81, 311–322. [Google Scholar]
- Daly, M.; Chaudhuri, A.; Gusmão, L.; Rodríguez, E. Phylogenetic Relationships among Sea Anemones (Cnidaria: Anthozoa: Actiniaria). Mol. Phylogenetics Evol. 2008, 48, 292–301. [Google Scholar] [CrossRef]
- Rogers, A. Cnidarians (Cnidaria). In The Timetree of Life; Oxford University Press: Oxford, UK, 2009; pp. 233–238. [Google Scholar]
- Parisi, M.G.; Parrinello, D.; Stabili, L.; Cammarata, M. Cnidarian Immunity and the Repertoire of Defense Mechanisms in Anthozoans. Biology 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Sunagar, K.; Moran, Y. The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals. PLOS Genet. 2015, 11, e1005596. [Google Scholar] [CrossRef]
- Moran, Y.; Praher, D.; Schlesinger, A.; Ayalon, A.; Tal, Y.; Technau, U. Analysis of Soluble Protein Contents from the Nematocysts of a Model Sea Anemone Sheds Light on Venom Evolution. Mar. Biotechnol. 2013, 15, 329–339. [Google Scholar] [CrossRef]
- Hines, D.E.; Pawlik, J.R. Assessing the Antipredatory Defensive Strategies of Caribbean Non-Scleractinian Zoantharians (Cnidaria): Is the Sting the Only Thing? Mar. Biol. 2012, 159, 389–398. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Lindquist, N. Hydroid Defenses against Predators: The Importance of Secondary Metabolites versus Nematocysts. Oecologia 2000, 124, 280–288. [Google Scholar] [CrossRef]
- Ashwood, L.M.; Undheim, E.A.B.; Madio, B.; Hamilton, B.R.; Daly, M.; Hurwood, D.A.; King, G.F.; Prentis, P.J. Venoms for All Occasions: The Functional Toxin Profiles of Different Anatomical Regions in Sea Anemones Are Related to Their Ecological Function. Mol. Ecol. 2022, 31, 866–883. [Google Scholar] [CrossRef]
- Durán-Fuentes, J.A.; Mendes, F.; Costa, R.C.d.; Pescinelli, R.A.; Floeter, S.R.; Stampar, S.N. Ecological Notes on Actinostella Flosculifera (Le Sueur, 1817) (Cnidaria: Actiniaria: Actiniidae) in the South-Western Atlantic, Brazil. J. Mar. Biol. Assoc. 2023, 103, e20. [Google Scholar] [CrossRef]
- Gilbert, P.U.P.A.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating Mechanism and Evolutionary History. Sci. Adv. 2022, 8, eabl9653. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, K.; Li, J.; Shi, J.; Wei, J.; Zheng, X.; He, W.; Zhang, X. The Molecular Basis of Octocoral Calcification Revealed by Genome and Skeletal Proteome Analyses. GigaScience 2025, 14, giaf031. [Google Scholar] [CrossRef]
- Frazão, B.; Vasconcelos, V.; Antunes, A. Sea Anemone (Cnidaria, Anthozoa, Actiniaria) Toxins: An Overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef]
- Jaimes-Becerra, A.; Chung, R.; Morandini, A.C.; Weston, A.J.; Padilla, G.; Gacesa, R.; Ward, M.; Long, P.F.; Marques, A.C. Comparative Proteomics Reveals Recruitment Patterns of Some Protein Families in the Venoms of Cnidaria. Toxicon 2017, 137, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fautin, D.G.; Malarky, L.; Soberón, J. Latitudinal Diversity of Sea Anemones (Cnidaria: Actiniaria). Biol. Bull. 2013, 224, 89–98. [Google Scholar] [CrossRef]
- Sunagar, K.; Morgenstern, D.; Reitzel, A.M.; Moran, Y. Ecological Venomics: How Genomics, Transcriptomics and Proteomics Can Shed New Light on the Ecology and Evolution of Venom. J. Proteom. 2016, 135, 62–72. [Google Scholar] [CrossRef]
- O’Hara, E.P.; Caldwell, G.S.; Bythell, J. Equistatin and Equinatoxin Gene Expression Is Influenced by Environmental Temperature in the Sea Anemone Actinia equina. Toxicon 2018, 153, 12–16. [Google Scholar] [CrossRef]
- Sachkova, M.Y.; Macrander, J.; Surm, J.M.; Aharoni, R.; Menard-Harvey, S.S.; Klock, A.; Leach, W.B.; Reitzel, A.M.; Moran, Y. Some like It Hot: Population-Specific Adaptations in Venom Production to Abiotic Stressors in a Widely Distributed Cnidarian. BMC Biol. 2020, 18, 121. [Google Scholar] [CrossRef]
- Surm, J.M.; Smith, H.L.; Madio, B.; Undheim, E.A.B.; King, G.F.; Hamilton, B.R.; van der Burg, C.A.; Pavasovic, A.; Prentis, P.J. A Process of Convergent Amplification and Tissue-Specific Expression Dominates the Evolution of Toxin and Toxin-like Genes in Sea Anemones. Mol. Ecol. 2019, 28, 2272–2289. [Google Scholar] [CrossRef]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an Ancient Venom: Recognition of a Novel Family of Cnidarian Toxins and the Common Evolutionary Origin of Sodium and Potassium Neurotoxins in Sea Anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Macrander, J.; Broe, M.; Daly, M. Tissue-Specific Venom Composition and Differential Gene Expression in Sea Anemones. Genome Biol. Evol. 2016, 8, 2358–2375. [Google Scholar] [CrossRef]
- Macrander, J.; Brugler, M.R.; Daly, M. A RNA-Seq Approach to Identify Putative Toxins from Acrorhagi in Aggressive and Non-Aggressive Anthopleura Elegantissima Polyps. BMC Genom. 2015, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Williamson, J.A. Worldwide Deaths and Severe Envenomation from Jellyfish Stings. Med. J. Aust. 1996, 165, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, L.M.; Norton, R.S.; Undheim, E.A.B.; Hurwood, D.A.; Prentis, P.J. Characterising Functional Venom Profiles of Anthozoans and Medusozoans within Their Ecological Context. Mar. Drugs 2020, 18, 202. [Google Scholar] [CrossRef]
- Schmidt, C.; Daly, N.; Wilson, D. Coral Venom Toxins. Front. Ecol. Evol. 2019, 7, 320. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Rocha, C. Bioactive Compounds from Zoanthids (Cnidaria:Anthozoa): A Brief Review with Emphasis on Alkaloids. Int. Res. J. Biochem. Bioinform. 2013, 3, 1–6. [Google Scholar]
- Sangappellai, T.; Subramanian, B.; Gokula, V. Sea Anemones as Potential Source for Bioactive Metabolites. Int. J. Pept. Res. Ther. 2019, 25, 1–14. [Google Scholar] [CrossRef]
- Madio, B.; King, G.F.; Undheim, E.A.B. Sea Anemone Toxins: A Structural Overview. Mar. Drugs 2019, 17, 325. [Google Scholar] [CrossRef]
- Anderluh, G.; Maček, P. Cytolytic Peptide and Protein Toxins from Sea Anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef]
- Technau, U.; Schwaiger, M. Recent Advances in Genomics and Transcriptomics of Cnidarians. Mar. Genom. 2015, 24, 131–138. [Google Scholar] [CrossRef]
- Bank, R.P.D. 3D View: 4Z7P. X-Ray Structure of Racemic ShK Q16K Toxin. Available online: https://www.rcsb.org/structure/4Z7P (accessed on 23 May 2025).
- Prentis, P.J.; Pavasovic, A.; Norton, R.S. Sea Anemones: Quiet Achievers in the Field of Peptide Toxins. Toxins 2018, 10, 36. [Google Scholar] [CrossRef]
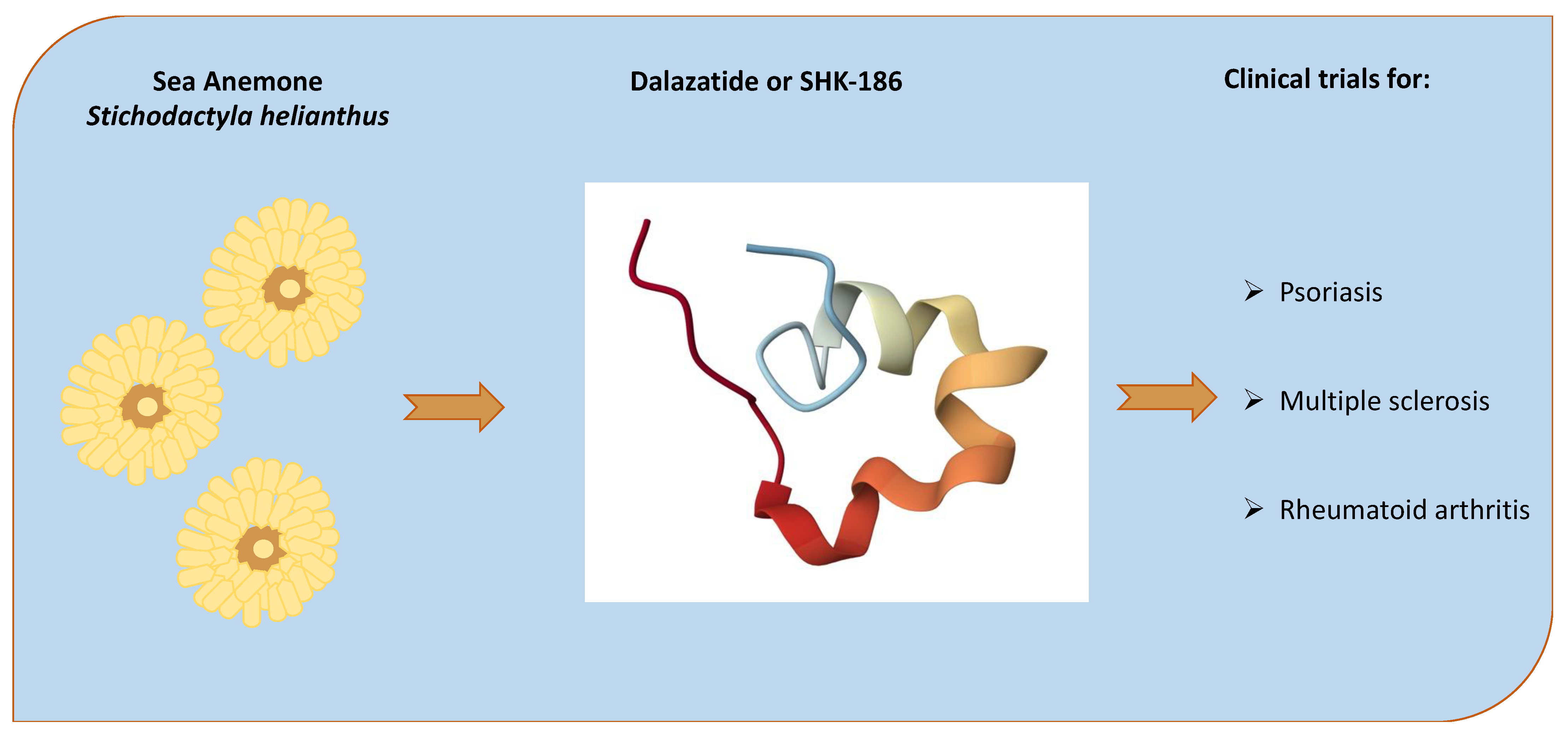
- Pennington, M.W.; Chang, S.C.; Chauhan, S.; Huq, R.; Tajhya, R.B.; Chhabra, S.; Norton, R.S.; Beeton, C. Development of Highly Selective Kv1.3-Blocking Peptides Based on the Sea Anemone Peptide ShK. Mar. Drugs 2015, 13, 529–542. [Google Scholar] [CrossRef]
- Lintermans, L.L.; Stegeman, C.A.; Muñoz-Elías, E.J.; Tarcha, E.J.; Iadonato, S.P.; Rutgers, A.; Heeringa, P.; Abdulahad, W.H. Kv1.3 Blockade by ShK186 Modulates CD4+ Effector Memory T-Cell Activity of Patients with Granulomatosis with Polyangiitis. Rheumatology 2023, 63, 198–208. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Olsen, C.M.; Probst, P.; Peckham, D.; Muñoz-Elías, E.J.; Kruger, J.G.; Iadonato, S.P. Safety and Pharmacodynamics of Dalazatide, a Kv1.3 Channel Inhibitor, in the Treatment of Plaque Psoriasis: A Randomized Phase 1b Trial. PLoS ONE 2017, 12, e0180762. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; David, C.N. Immunocompetence in Hydra: Epithelial cells recognize self-nonself and react against it. J. Exp. Zool. 1986, 238, 225–234. [Google Scholar] [CrossRef]
- Hildemann, W.H.; Raison, R.L.; Cheung, G.; Hull, C.J.; Akaka, L.; Okamoto, J. Immunological Specificity and Memory in a Scleractinian Coral. Nature 1977, 270, 219–223. [Google Scholar] [CrossRef]
- Francis, L. Clone Specific Segregation in the Sea Anemone Anthopleura-Elegantissima. Biol. Bull. 1973, 144, 64–72. [Google Scholar] [CrossRef]
- Lang, J.C.; Chornesky, E.A. Competition between Scleractinian Reef Corals—A Review of Mechanisms and Effects. Ecosyst. World 1990, 25, 209–252. [Google Scholar]
- Rinkevich, B. Links between Alloresponses and Their Genetic Background in Colonial Urochordates and Cnidarians: Evidence for the Recognition of “Nonself” as Opposed to “Self.”. In Modulators of Immune Responses; Stolen, J.S., Fletcher, T.C., Bayne, C.J., Secombes, C.J., Zelikoff, J.T., Twerdok, L., Anderson, D.P., Eds.; Breckenridge series; SOS Publications: Fair Haven, NJ, USA, 1996; pp. 1–13. ISBN 978-1-887052-00-9. [Google Scholar]
- Rinkevich, B. The ‘Immunology Trap’ of Anthozoans. Invertebr. Surviv. J. 2011, 8, 153–161. [Google Scholar]
- Rosa, T.M.; Giovanna, P.M.; Maria, M.; Angela, M.; Matteo, C. Old Weapons for New Wars: Bioactive Molecules From Cnidarian Internal Defense Systems. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem.–Cent. Nerv. Syst. Agents) 2016, 16, 183–196. [Google Scholar] [CrossRef]
- McFadden, C.; Daly, M.; Brugler, M.; Cartwright, P.; Collins, A.; Dawson, M.; Fautin, D.; France, S.; Opresko, D.; Rodriguez, E.; et al. The Phylum Cnidaria: A Review of Phylogenetic Patterns and Diversity 300 Years After Linnaeus. In Linnaeus Tercentenary: Progress in Invertebrate Taxonomy; Magnolia Press: Auckland, New Zealand, 2007; pp. 127–182. [Google Scholar]
- Deeds, J.R.; Schwartz, M.D. Human Risk Associated with Palytoxin Exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.R.; Handy, S.M.; White, K.D.; Reimer, J.D. Palytoxin Found in Palythoa Sp. Zoanthids (Anthozoa, Hexacorallia) Sold in the Home Aquarium Trade. PLoS ONE 2011, 6, e18235. [Google Scholar] [CrossRef]
- Riobó, P.; Paz, B.; Franco, J.M.; Vázquez, J.A.; Murado, M.A.; Cacho, E. Mouse Bioassay for Palytoxin. Specific Symptoms and Dose-Response against Dose–Death Time Relationships. Food Chem. Toxicol. 2008, 46, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Sawelew, L.; Gault, F.; Nuccio, C.; Perez, Y.; Lorquin, J. Characterisation of Palytoxin from an Undescribed Palythoa (Anthozoa: Zoantharia: Sphenopidae) with Significant in Vitro Cytotoxic Effects on Cancer Cells at Picomolar Dose. BioRxiv 2018. [CrossRef]
- Wattenberg, E.V. Palytoxin: Exploiting a Novel Skin Tumor Promoter to Explore Signal Transduction and Carcinogenesis. Am. J. Physiol.-Cell Physiol. 2007, 292, C24–C32. [Google Scholar] [CrossRef]
- Mascuch, S.; Kubanek, J. A Marine Chemical Defense Partnership. Science 2019, 364, 1034–1035. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, N.; Zhao, C.; He, Y.; Kam, S.H.T.; Wu, X.; Huang, P.; Yang, M.; Wong, C.T.T.; Radis-Baptista, G.; et al. A New Invertebrate NPY-like Polypeptide, ZoaNPY, from the Zoanthus Sociatus, as a Novel Ligand of Human NPY Y2 Receptor Rescues Vascular Insufficiency via PLC/PKC and Src- FAK-Dependent Signaling Pathways. Pharmacol. Res. 2024, 203, 107173. [Google Scholar] [CrossRef]
- Liao, Q.; Gong, G.; Poon, T.C.W.; Ang, I.L.; Lei, K.M.K.; Siu, S.W.I.; Wong, C.T.T.; Rádis-Baptista, G.; Lee, S.M.-Y. Combined Transcriptomic and Proteomic Analysis Reveals a Diversity of Venom-Related and Toxin-like Peptides Expressed in the Mat Anemone Zoanthus Natalensis (Cnidaria, Hexacorallia). Arch. Toxicol. 2019, 93, 1745–1767. [Google Scholar] [CrossRef]
- Liao, Q.; Li, S.; Siu, S.W.I.; Yang, B.; Huang, C.; Chan, J.Y.-W.; Morlighem, J.-É.R.L.; Wong, C.T.T.; Rádis-Baptista, G.; Lee, S.M.-Y. Novel Kunitz-like Peptides Discovered in the Zoanthid Palythoa Caribaeorum through Transcriptome Sequencing. J. Proteome Res. 2018, 17, 891–902. [Google Scholar] [CrossRef]
- Stabili, L.; Piraino, S.; Rizzo, L. The Mediterranean Zoanthid Parazoanthus Axinellae as a Novel Source of Antimicrobial Compounds. J. Mar. Sci. Eng. 2024, 12, 354. [Google Scholar] [CrossRef]
- Igbinosa, E.O. Detection and Antimicrobial Resistance of Vibrio Isolates in Aquaculture Environments: Implications for Public Health. Microb. Drug Resist. 2016, 22, 238–245. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-Saari, N.; Mohamad, A.; Mursidi, F.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Trinanes, J.; Martinez-Urtaza, J. Future Scenarios of Risk of Vibrio Infections in a Warming Planet: A Global Mapping Study. Lancet Planet. Health 2021, 5, e426–e435. [Google Scholar] [CrossRef]
- Miselli, F.; Frabboni, I.; Di Martino, M.; Zinani, I.; Buttera, M.; Insalaco, A.; Stefanelli, F.; Lugli, L.; Berardi, A. Transmission of Group B Streptococcus in Late-Onset Neonatal Disease: A Narrative Review of Current Evidence. Ther. Adv. Infect. Dis. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- George, C.R.R.; Jeffery, H.E.; Lahra, M.M. Infection of Mother and Baby. In Keeling’s Fetal and Neonatal Pathology; Khong, T.Y., Malcomson, R.D.G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 207–245. ISBN 978-3-030-84168-3. [Google Scholar]
- Schindler, Y.; Rahav, G.; Nissan, I.; Madar-Shapiro, L.; Abtibol, J.; Ravid, M.; Maor, Y. Group B Streptococcus Serotypes Associated with Different Clinical Syndromes: Asymptomatic Carriage in Pregnant Women, Intrauterine Fetal Death, and Early Onset Disease in the Newborn. PLoS ONE 2020, 15, e0244450. [Google Scholar] [CrossRef]
- Adu-Afari, G. Streptococcus Agalactiae Infection Among Parturients and Their Neonates at the Cape Coast Teaching Hospital: An Evaluation of Different Diagnostic Methods, Prevalence and Risk Factors. Master’s Thesis, University of Cape Coast, Cape Coast, Ghana, 2021. [Google Scholar]
- Konuklugil, B.; Uras, I.S.; Karsli, B.; Demirbas, A. Parazoanthus Axinellae Extract Incorporated Hybrid Nanostructure and Its Potential Antimicrobial Activity. Chem. Biodivers. 2023, 20, e202300744. [Google Scholar] [CrossRef]
- Alencar, D.B.; Melo, A.A.; Silva, G.C.; Lima, R.L.; Pires-Cavalcante, K.M.S.; Carneiro, R.F.; Rabelo, A.S.; Sousa, O.V.; Vieira, R.H.S.F.; Viana, F.A.; et al. Antioxidant, Hemolytic, Antimicrobial, and Cytotoxic Activities of the Tropical Atlantic Marine Zoanthid Palythoa Caribaeorum. An. Acad. Bras. Cienc. 2015, 87, 1113–1123. [Google Scholar] [CrossRef]
- Cordie, D.R.; Budd, A.F. Histological Data in a Combined Phylogenetic Analysis of Scleractinian Reef Corals. J. Morphol. 2016, 277, 494–511. [Google Scholar] [CrossRef]
- García-Arredondo, A.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Lazcano-Pérez, F.; Arreguín-Espinosa, R.; Sánchez-Rodríguez, J. Composition and Biological Activities of the Aqueous Extracts of Three Scleractinian Corals from the Mexican Caribbean: Pseudodiploria strigosa, Porites astreoides and Siderastrea siderea. J. Venom. Anim. Toxins incl. Trop. Dis. 2016, 22, 32. [Google Scholar] [CrossRef]
- García-Arredondo, A.; Rojas-Molina, A.; Bah, M.; Ibarra-Alvarado, C.; Gallegos-Corona, M.A.; García-Servín, M. Systemic Toxic Effects Induced by the Aqueous Extract of the Fire Coral Millepora Complanata and Partial Purification of Thermostable Neurotoxins with Lethal Effects in Mice. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2015, 169, 55–64. [Google Scholar] [CrossRef]
- Burnett, J.W. Treatment of Atlantic Cnidarian Envenomations. Toxicon 2009, 54, 1201–1205. [Google Scholar] [CrossRef]
- Zahran, E.M.; Albohy, A.; Khalil, A.; Ibrahim, A.H.; Ahmed, H.A.; El-Hossary, E.M.; Bringmann, G.; Abdelmohsen, U.R. Bioactivity Potential of Marine Natural Products from Scleractinia-Associated Microbes and In Silico Anti-SARS-CoV-2 Evaluation. Mar. Drugs 2020, 18, 645. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.G.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and Sesquiterpene Derivatives from a Marine-Derived Fungus Scopulariopsis Sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef]
- Bara, R.; Aly, A.H.; Wray, V.; Lin, W.; Proksch, P.; Debbab, A. Talaromins A and B, New Cyclic Peptides from the Endophytic Fungus Talaromyces Wortmannii. Tetrahedron Lett. 2013, 54, 1686–1689. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Singab, A.; Lin, W.; Liu, Z.; Proksch, P. Two New Triterpenoids and a New Naphthoquinone Derivative Isolated from a Hard Coral-Derived Fungus Scopulariopsis sp. Fitoterapia 2017, 116, 126–130. [Google Scholar] [CrossRef]
- Sasaki, K.; Minowa, N.; Kuzuhara, H.; Nishiyama, S.; Omoto, S. Synthesis and Hepatoprotective Effects of Soyasapogenol B Derivatives. Bioorg. Med. Chem. Lett. 1997, 7, 85–88. [Google Scholar] [CrossRef]
- Setiyono, E.; Heriyanto; Pringgenies, D.; Shioi, Y.; Kanesaki, Y.; Awai, K.; Brotosudarmo, T.H.P. Sulfur-Containing Carotenoids from A Marine Coral Symbiont Erythrobacter Flavus Strain KJ5. Mar. Drugs 2019, 17, 349. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; Souza, M.D.F.V. de Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef]
- Mourão, P.A.S. Perspective on the Use of Sulfated Polysaccharides from Marine Organisms as a Source of New Antithrombotic Drugs. Mar. Drugs 2015, 13, 2770–2784. [Google Scholar] [CrossRef]
- Carlson, J.C.; Li, S.; Burr, D.A.; Sherman, D.H. Isolation and Characterization of Tirandamycins from a Marine-Derived Streptomyces sp. J. Nat. Prod. 2009, 72, 2076–2079. [Google Scholar] [CrossRef]
- Withers, N.W.; Kokke, W.C.M.C.; Fenical, W.; Djerassi, C. Sterol Patterns of Cultured Zooxanthellae Isolated from Marine Invertebrates: Synthesis of Gorgosterol and 23-Desmethylgorgosterol by Aposymbiotic Algae. Proc. Natl. Acad. Sci. USA 1982, 79, 3764–3768. [Google Scholar] [CrossRef]
- Furla, P.; Allemand, D.; Shick, J.M.; Ferrier-Pagès, C.; Richier, S.; Plantivaux, A.; Merle, P.-L.; Tambutté, S. The Symbiotic Anthozoan: A Physiological Chimera between Alga and Animal. Integr. Comp. Biol. 2005, 45, 595–604. [Google Scholar] [CrossRef]
- Stewart, Z.K.; Pavasovic, A.; Hock, D.H.; Prentis, P.J. Transcriptomic Investigation of Wound Healing and Regeneration in the Cnidarian Calliactis Polypus. Sci. Rep. 2017, 7, 41458. [Google Scholar] [CrossRef]
- Menezes, C.; Thakur, N.L. Sea Anemone Venom: Ecological Interactions and Bioactive Potential. Toxicon 2022, 208, 31–46. [Google Scholar] [CrossRef]
- de Oliveira, J.S.; Zaharenko, A.J.; de Freitas, J.C.; Konno, K.; de Andrade, S.A.; Portaro, F.C.V.; Richardson, M.; Sant’Anna, O.A.; Tambourgi, D.V. Caissarolysin I (Bcs I), a New Hemolytic Toxin from the Brazilian Sea Anemone Bunodosoma Caissarum: Purification and Biological Characterization. Biochim. Biophys. Acta (BBA)–Gen. Subj. 2006, 1760, 453–461. [Google Scholar] [CrossRef]
- Alcaide, M.; Moutinho Cabral, I.; Carvalho, L.; Mendes, V.M.; Alves de Matos, A.P.; Manadas, B.; Saúde, L.; D’Ambrosio, M.; Costa, P.M. A Comparative Analysis of the Venom System between Two Morphotypes of the Sea Anemone Actinia Equina. Animals 2024, 14, 981. [Google Scholar] [CrossRef]
- Lin, X.Y.; Ishida, M.; Nagashima, Y.; Shiomi, K. A Polypeptide Toxin in the Sea Anemone Actinia Equina Homologous with Other Sea Anemone Sodium Channel Toxins: Isolation and Amino Acid Sequence. Toxicon 1996, 34, 57–65. [Google Scholar] [CrossRef]
- Bull, A.T.; Ward, A.C.; Goodfellow, M. Search and Discovery Strategies for Biotechnology: The Paradigm Shift. Microbiol. Mol. Biol. Rev. 2000, 64, 573–606. [Google Scholar] [CrossRef]
- Wright, P.C.; Westacott, R.E.; Burja, A.M. Piezotolerance as a Metabolic Engineering Tool for the Biosynthesis of Natural Products. Biomol. Eng. 2003, 20, 325–331. [Google Scholar] [CrossRef]
- Beliaev, G.M.; Brueggeman, P.L. Deep Sea Ocean Trenches and Their Fauna; Nauka Publishing House: Moscow, Russia, 1989. [Google Scholar]
- Kvetkina, A.; Kostina, E.; Gladkikh, I.; Chausova, V.; Yurchenko, E.; Bakunina, I.; Pivkin, M.; Anastyuk, S.; Popov, R.; Monastyrnaya, M.; et al. Deep-Sea Anemones Are Prospective Source of New Antimicrobial and Cytotoxic Compounds. Mar. Drugs 2021, 19, 654. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Glycosidase Inhibitors: Update and Perspectives on Practical Use. Glycobiology 2003, 13, 93R–104R. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, I.; Silva, S.G.; Costa, R.; Keller-Costa, T. Bioactive Secondary Metabolites from Octocoral-Associated Microbes—New Chances for Blue Growth. Mar. Drugs 2018, 16, 485. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a Source of New Marine Bioactive Compounds—An Overview of the Last Decade and Future Steps for Bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Ospina, C.A.; Rodríguez, A.D.; Zhao, H.; Raptis, R.G. Bipinnapterolide B, a Bioactive Oxapolycyclic Diterpene from the Colombian Gorgonian Coral Pseudopterogorgia bipinnata. Tetrahedron Lett. 2007, 48, 7520–7523. [Google Scholar] [CrossRef]
- Thomas, S.A.L.; Sanchez, A.; Kee, Y.; Wilson, N.G.; Baker, B.J. Bathyptilones: Terpenoids from an Antarctic Sea Pen, Anthoptilum Grandiflorum (Verrill, 1879). Mar. Drugs 2019, 17, 513. [Google Scholar] [CrossRef]
- Shin, J.; Seo, Y.; Rho, J.-R.; Cho, K.W. Isolation of Polyhydroxysteroids from the Gorgonian Acabaria Undulata. J. Nat. Prod. 1996, 59, 679–682. [Google Scholar] [CrossRef]
- Parisi, M.G.; Trapani, M.R.; Cardinale, L.; Cammarata, M. Evidence of Cytotoxic Activity against Mammalian Red Blood Cell of Na+ Channel Neurotoxin (Ae1) from Sea Anemone (Actinia Equina). Invertebr. Surviv. J. 2016, 13, 309–314. [Google Scholar] [CrossRef]
- Bunc, M.; Bregar, R.; Šuput, D. The Importance of Hemolysis in the Lethal Effects of Equnatoxin II, a Protein from the Sea Anemone Actinia equina (L.). Pflügers Arch. 2000, 440, R151–R152. [Google Scholar] [CrossRef] [PubMed]
- Šuput, D.; Frangež, R.; Bunc, M. Cardiovascular Effects of Equinatoxin III from the Sea Anemone Actinia equina (L.). Toxicon 2001, 39, 1421–1427. [Google Scholar] [CrossRef]
- Shiomi, K.; Ishikawa, M.; Yamanaka, H.; Kikuchi, T. Isolation and Properties of Four Serine Protease Inhibitors in the Sea Anemone Actinia Equina. Nippon Suisan Gakkaishi 1989, 55, 1235–1241. [Google Scholar] [CrossRef]
- Wunderer, G.; Eulitz, M. Amino-Acid Sequence of Toxin I from Anemonia Sulcata. Eur. J. Biochem. 1978, 89, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.-M.; Béress, L.; Lazdunski, M. Kalicludines and Kaliseptine: Two Different Classes of Sea Anemone Toxins for Voltage-Sensitive K+ Channels (*). J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef]
- Moran, Y.; Kahn, R.; Cohen, L.; Gur, M.; Karbat, I.; Gordon, D.; Gurevitz, M. Molecular Analysis of the Sea Anemone Toxin Av3 Reveals Selectivity to Insects and Demonstrates the Heterogeneity of Receptor Site-3 on Voltage-Gated Na+ Channels. Biochem. J. 2007, 406, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Honma, T.; Hasegawa, Y.; Ishida, M.; Nagai, H.; Nagashima, Y.; Shiomi, K. Isolation and Molecular Cloning of Novel Peptide Toxins from the Sea Anemone Antheopsis maculata. Toxicon 2005, 45, 33–41. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaller, C.; Schulze, C.; Sanchez-Rodriguez, J.; Dannmeier, C.; Ravens, U.; Heubach, J.F.; Eckhardt, K.; Schmidtmayer, J.; Schmidt, H.; et al. Isolation and Characterisation of Five Neurotoxic and Cardiotoxic Polypeptides from the Sea Anemone Anthopleura elegantissima. Toxicon 2001, 39, 693–702. [Google Scholar] [CrossRef]
- Kohno, Y.; Satoh, H.; Iguchi, A.; Nagai, H. Characterization of a New Hemolytic Protein Toxin from the Sea Anemone Anthopleura Asiatica. Fish. Sci. 2009, 75, 1049–1054. [Google Scholar] [CrossRef]
- John, S.T.; Velmurugan, S.; Nagaraj, D.S.; Kumaran, S.; Pugazhvendan, S.R. Antimicrobial Activity of Sea Anemone Stichodactyla Haddoni and Anthopleura Elegantissima Extracts against Human Pathogens. Int. J. Adv. Res. Biol. Sci. 2015, 2, 27–35. [Google Scholar]
- Borbón, H.; Váldes, S.; Alvarado-Mesén, J.; Soto, R.; Vega, I. Antimicrobial Properties of Sea Anemone Anthopleura Nigrescens from Pacific Coast of Costa Rica. Asian Pac. J. Trop. Biomed. 2016, 6, 418–421. [Google Scholar] [CrossRef]
- Kelso, G.J.; Blumenthal, K.M. Identification and Characterization of Novel Sodium Channel Toxins from the Sea Anemone Anthopleura Xanthogrammica. Toxicon 1998, 36, 41–51. [Google Scholar] [CrossRef]
- Norton, T.R. Cardiotonic Polypeptides from Anthopleura Xanthogrammica (Brandt) and A. Elegantissima (Brandt). Fed. Proc. 1981, 40, 21–25. [Google Scholar] [PubMed]
- Alarif, W.; Al-Lihaibi, S.; Ayyad, S.-E.; Ghandourah, M.; Orif, M.; Basaif, S.A.; Abdel-Lateff, A. Cytotoxic Ceramides from Antipathes Dichotoma. J. Chem. Soc. Pakistan 2016, 38, 553–557. [Google Scholar]
- Alarif, W.; Abdel-Lateff, A.; Al-Lihaibi, S.; Ayyad, S.-E.; Badria, F.; Alsofyani, A.; Abou-Elnaga, D. Marine Bioactive Steryl Esters from the Red Sea Black Coral Antipathes dichotoma. CLEAN Soil. Air Water 2013, 41, 1116–1121. [Google Scholar] [CrossRef]
- Lin, Y.-C.; El-Razek, M.H.A.; Hwang, T.-L.; Chiang, M.Y.; Kuo, Y.-H.; Dai, C.-F.; Shen, Y.-C. Asterolaurins A−F, Xenicane Diterpenoids from the Taiwanese Soft Coral Asterospicularia laurae. J. Nat. Prod. 2009, 72, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Yu, S.; van Ofwegen, L.; Totzke, F.; Proksch, P.; Lin, W. 9,10-Secosteroids, Protein Kinase Inhibitors from the Chinese Gorgonian Astrogorgia sp. Bioorg. Med. Chem. 2011, 19, 6873–6880. [Google Scholar] [CrossRef]
- Rodríguez, I.I.; Rodríguez, A.D.; Wang, Y.; Franzblau, S.G. Ileabethoxazole: A Novel Benzoxazole Alkaloid with Antimycobacterial Activity. Tetrahedron Lett. 2006, 47, 3229–3232. [Google Scholar] [CrossRef]
- Sung, P.-J.; Lin, M.-R.; Chiang, M.Y.; Hwang, T.-L. Briaexcavatins V–Z, Discovery of New Briaranes from a Cultured Octocoral Briareum Excavatum. Bull. Chem. Soc. Jpn. 2009, 82, 987–996. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chen, Y.-P.; Hwang, T.-L.; Hu, W.-P.; Fang, L.-S.; Wu, Y.-C.; Li, J.-J.; Sheu, J.-H. Briaexcavatins C–F, Four New Briarane-Related Diterpenoids from the Formosan Octocoral Briareum excavatum (Briareidae). Tetrahedron 2006, 62, 5686–5691. [Google Scholar] [CrossRef]
- Ospina, C.A.; Rodríguez, A.D.; Ortega-Barria, E.; Capson, T.L. Briarellins J−P and Polyanthellin A: New Eunicellin-Based Diterpenes from the Gorgonian Coral Briareum Polyanthes and Their Antimalarial Activity. J. Nat. Prod. 2003, 66, 357–363. [Google Scholar] [CrossRef]
- Orts, D.J.B.; Moran, Y.; Cologna, C.T.; Peigneur, S.; Madio, B.; Praher, D.; Quinton, L.; De Pauw, E.; Bicudo, J.E.P.W.; Tytgat, J.; et al. BcsTx3 Is a Founder of a Novel Sea Anemone Toxin Family of Potassium Channel Blocker. FEBS J. 2013, 280, 4839–4852. [Google Scholar] [CrossRef]
- Malpezzi, E.L.A.; de Freitas, J.; Muramoto, K.; Kamiya, H. Characterization of Peptides in Sea Anemone Venom Collected by a Novel Procedure. Toxicon 1993, 31, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.D.; Alves, R.S.; Martins, A.M.C.; Barbosa, P.S.F.; Evangelista, J.S.A.M.; Evangelista, J.J.F.; Ximenes, R.M.; Toyama, M.H.; Toyama, D.O.; Souza, A.J.F.; et al. Purification and Characterization of the Biological Effects of Phospholipase A2 from Sea Anemone Bunodosoma caissarum. Toxicon 2009, 54, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Zaharenko, A.J.; Ferreira, W.A.; De Oliveira, J.S.; Konno, K.; Richardson, M.; Schiavon, E.; Wanke, E.; De Freitas, J.C. Revisiting Cangitoxin, a Sea Anemone Peptide: Purification and Characterization of Cangitoxins II and III from the Venom of Bunodosoma Cangicum. Toxicon 2008, 51, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.N.C.; Leite, A.B.; França, M.S.F.; França, L.; Vale, O.C.; Cunha, R.B.; Ricart, C.a.O.; Sousa, M.V.; Carvalho, K.M. Partial Sequence and Toxic Effects of Granulitoxin, a Neurotoxic Peptide from the Sea Anemone Bunodosoma Granulifera. Braz. J. Med. Biol. Res. 1998, 31, 1335–1338. [Google Scholar] [CrossRef]
- Aneiros, A.; García, I.; Martínez, J.; Harvey, A.L.; Anderson, A.J.; Marshall, D.L.; Engström, Å.; Hellman, U.; Karlsson, E. A Potassium Channel Toxin from the Secretion of the Sea Anemone Bunodosoma Granulifera. Isolation, Amino Acid Sequence and Biological Activity. Biochim. Biophys. Acta (BBA)–Gen. Subj. 1993, 1157, 86–92. [Google Scholar] [CrossRef]
- Cariello, L.; De Santis, A.; Fiore, F.; Piccoli, R.; Spagnuolo, A.; Zanetti, L.; Parente, A. Calitoxin, a Neurotoxic Peptide from the Sea Anemone Calliactis Parasitica: Amino-Acid Sequence and Electrophysiological Properties. Biochemistry 1989, 28, 2484–2489. [Google Scholar] [CrossRef]
- Chang, C.-H.; Wen, Z.-H.; Wang, S.-K.; Duh, C.-Y. Capnellenes from the Formosan Soft Coral Capnella Imbricata. J. Nat. Prod. 2008, 71, 619–621. [Google Scholar] [CrossRef]
- Duh, C.-Y.; El-Gamal, A.A.H.; Wang, S.-K.; Dai, C.-F. Novel Terpenoids from the Formosan Soft Coral Cespitularia Hypotentaculata. J. Nat. Prod. 2002, 65, 1429–1433. [Google Scholar] [CrossRef]
- Iwashima, M.; Nara, K.; Nakamichi, Y.; Iguchi, K. Three New Chlorinated Marine Steroids, Yonarasterols G, H and I, Isolated from the Okinawan Soft Coral, Clavularia Viridis. Steroids 2001, 66, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yabe, T.; Yamada, H.; Shimomura, M.; Miyaoka, H.; Yamada, Y. Induction of Choline Acetyltransferase Activity in Cholinergic Neurons by Stolonidiol: Structure−Activity Relationship. J. Nat. Prod. 2000, 63, 433–435. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Taha Khalil, A.; Chiou, S.-H.; Kuo, Y.-C.; Cheng, Y.-B.; Liaw, C.-C.; Shen, Y.-C. Bioactive Marine Prostanoids from Octocoral Clavularia Viridis. Chem. Biodivers. 2008, 5, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-C.; Chien, C.-L.; Pan, S.-L.; Chen, W.-P.; Teng, C.-M.; Shen, Y.-C.; Guh, J.-H. Induction of Endoplasmic Reticulum Stress and Apoptosis by a Marine Prostanoid in Human Hepatocellular Carcinoma. J. Hepatol. 2005, 43, 679–686. [Google Scholar] [CrossRef]
- Ständker, L.; Béress, L.; Garateix, A.; Christ, T.; Ravens, U.; Salceda, E.; Soto, E.; John, H.; Forssmann, W.-G.; Aneiros, A. A New Toxin from the Sea Anemone Condylactis Gigantea with Effect on Sodium Channel Inactivation. Toxicon 2006, 48, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.; Marcussi, S.; Marchi-Salvador, D.P.; Silva, F.P.; Fuly, A.L.; Stábeli, R.G.; da Silva, S.L.; González, J.; Monte, A.d.; Soares, A.M. Enzymatic and Structural Characterization of a Basic Phospholipase A2 from the Sea Anemone Condylactis Gigantea. Biochimie 2010, 92, 1063–1071. [Google Scholar] [CrossRef]
- Shiomi, K.; Lin, X.-Y.; Nagashima, Y.; Ishida, M. Isolation and Amino Acid Sequences of Polypeptide Toxins in the Caribbean Sea Anemone Condylactis Passiflora. Fish. Sci. 1995, 61, 1016–1021. [Google Scholar] [CrossRef]
- Klyshko, E.V.; Il’ina, A.P.; Monastyrnaya, M.M.; Burtseva, Y.V.; Kostina, E.E.; Zykova, T.A.; Menzorova, N.I.; Kozlovskaya, E.P. Biologically Active Polypeptides and Hydrolytic Enzymes in Sea Anemones of Northern Temperate Waters. Russ. J. Mar. Biol. 2003, 29, 161–166. [Google Scholar] [CrossRef]
- Grote, D.; Hänel, F.; Dahse, H.-M.; Seifert, K. Capnellenes from the Soft Coral Dendronephthya Rubeola. Chem. Biodivers. 2008, 5, 1683–1693. [Google Scholar] [CrossRef]
- Tomono, Y.; Hirota, H.; Fusetani, N. Isogosterones A−D, Antifouling 13,17-Secosteroids from an Octocoral Dendronephthya Sp. J. Org. Chem. 1999, 64, 2272–2275. [Google Scholar] [CrossRef]
- Verbitski, S.M.; Mullally, J.E.; Fitzpatrick, F.A.; Ireland, C.M. Punaglandins, Chlorinated Prostaglandins, Function as Potent Michael Receptors To Inhibit Ubiquitin Isopeptidase Activity. J. Med. Chem. 2004, 47, 2062–2070. [Google Scholar] [CrossRef]
- Shin, J.; Fenical, W. Fuscosides A-D: Anti-Inflammatory Diterpenoid Glycosides of New Structural Classes from the Caribbean Gorgonian Eunicea Fusca. Available online: https://pubs.acs.org/doi/pdf/10.1021/jo00009a042 (accessed on 30 May 2025).
- Castellanos, F.; Amaya-García, F.; Tello, E.; Ramos, F.A.; Umaña, A.; Puyana, M.; Resende, J.A.L.C.; Castellanos, L. Screening of Acetylcholinesterase Inhibitors in Marine Organisms from the Caribbean Sea. Nat. Product. Res. 2019, 33, 3533–3540. [Google Scholar] [CrossRef]
- Garzón, S.P.; Rodríguez, A.D.; Sánchez, J.A.; Ortega-Barria, E. Sesquiterpenoid Metabolites with Antiplasmodial Activity from a Caribbean Gorgonian Coral, Eunicea sp. J. Nat. Prod. 2005, 68, 1354–1359. [Google Scholar] [CrossRef]
- Seo, Y.; Rho, J.-R.; Cho, K.W.; Shin, J. New Farnesylhydroquinone Glycosides from the Gorgonian Euplexaura Anastomosans. Nat. Product. Lett. 2001, 15, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-R.; Li, P.-L.; Tang, X.-L.; Qi, X.; Li, G.-Q. Cytotoxic Tetraprenylated Alkaloids from the South China Sea Gorgonian Euplexaura Robusta. Chem. Biodivers. 2012, 9, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Kulanthaivel, P.; Baker, B.J.; Kalter, K.; Darges, J.; Cofield, D.; Wolff, L.; Adams, L. New Antiproliferative and Antiinflammatory 9,11-Secosterols from the Gorgonian Pseudopterogorgia sp. Tetrahedron 1995, 51, 51–58. [Google Scholar] [CrossRef]
- Ishida, M.; Yokoyama, A.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Halcurin, a Polypeptide Toxin from the Sea Anemone Halcurias sp., with a Structural Resemblance to Type 1 and 2 Toxins. Toxicon 1997, 35, 537–544. [Google Scholar] [CrossRef]
- Kvetkina, A.; Leychenko, E.; Chausova, V.; Zelepuga, E.; Chernysheva, N.; Guzev, K.; Pislyagin, E.; Yurchenko, E.; Menchinskaya, E.; Aminin, D.; et al. A New Multigene HCIQ Subfamily from the Sea Anemone Heteractis Crispa Encodes Kunitz-Peptides Exhibiting Neuroprotective Activity against 6-Hydroxydopamine. Sci. Rep. 2020, 10, 4205. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Osmakov, D.I.; Andreev, Y.A.; Koshelev, S.G.; Gladkikh, I.N.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. A Sea Anemone Polypeptide Toxin Inhibiting the ASIC3 Acid-Sensitive Channel. Russ. J. Bioorg. Chem. 2012, 38, 578–583. [Google Scholar] [CrossRef]
- Kalina, R.; Gladkikh, I.; Dmitrenok, P.; Chernikov, O.; Koshelev, S.; Kvetkina, A.; Kozlov, S.; Kozlovskaya, E.; Monastyrnaya, M. New APETx-like Peptides from Sea Anemone Heteractis Crispa Modulate ASIC1a Channels. Peptides 2018, 104, 41–49. [Google Scholar] [CrossRef]
- Gladkikh, I.; Monastyrnaya, M.; Zelepuga, E.; Sintsova, O.; Tabakmakher, V.; Gnedenko, O.; Ivanov, A.; Hua, K.-F.; Kozlovskaya, E. New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis Crispa. Mar. Drugs 2015, 13, 6038–6063. [Google Scholar] [CrossRef]
- Monastyrnaya, M.; Peigneur, S.; Zelepuga, E.; Sintsova, O.; Gladkikh, I.; Leychenko, E.; Isaeva, M.; Tytgat, J.; Kozlovskaya, E. Kunitz-Type Peptide HCRG21 from the Sea Anemone Heteractis Crispa Is a Full Antagonist of the TRPV1 Receptor. Mar. Drugs 2016, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.A.; Andreev, Y.A.; Murashev, A.N.; Skobtsov, D.I.; D’yachenko, I.A.; Grishin, E.V. New Polypeptide Components from the Heteractis Crispa Sea Anemone with Analgesic Activity. Russ. J. Bioorg. Chem. 2009, 35, 711–719. [Google Scholar] [CrossRef]
- Sintsova, O.V.; Pislyagin, E.A.; Gladkikh, I.N.; Monastyrnaya, M.M.; Menchinskaya, E.S.; Leychenko, E.V.; Aminin, D.L.; Kozlovskaya, E.P. Kunitz-Type Peptides of the Sea Anemone Heteractis Crispa: Potential Anti-Inflammatory Compounds. Russ. J. Bioorg. Chem. 2017, 43, 91–97. [Google Scholar] [CrossRef]
- Sintsova, O.V.; Monastyrnaya, M.M.; Pislyagin, E.A.; Menchinskaya, E.S.; Leychenko, E.V.; Aminin, D.L.; Kozlovskaya, E.P. Anti-Inflammatory Activity of a Polypeptide from the Heteractis Crispa Sea Anemone. Russ. J. Bioorg. Chem. 2015, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chua, K.L.; Khoo, H.E. A New Cytolysin from the Sea Anemone, Heteractis Magnifica: Isolation, cDNA Cloning and Functional Expression1. Biochim. Biophys. Acta (BBA)–Protein Struct. Mol. Enzymol. 2000, 1478, 9–18. [Google Scholar] [CrossRef]
- Kvetkina, A.N.; Leychenko, E.V.; Yurchenko, E.A.; Pislyagin, E.A.; Peigneur, S.; Tytgat, Y.; Isaeva, M.P.; Aminin, D.L.; Kozlovskaya, E.P. A New Iq-Peptide of the Kunitz Type from the Heteractis Magnifica Sea Anemone Exhibits Neuroprotective Activity in a Model of Alzheimer’s Disease. Russ. J. Bioorg. Chem. 2018, 44, 416–423. [Google Scholar] [CrossRef]
- Sintsova, O.; Gladkikh, I.; Kalinovskii, A.; Zelepuga, E.; Monastyrnaya, M.; Kim, N.; Shevchenko, L.; Peigneur, S.; Tytgat, J.; Kozlovskaya, E.; et al. Magnificamide, a β-Defensin-Like Peptide from the Mucus of the Sea Anemone Heteractis Magnifica, Is a Strong Inhibitor of Mammalian α-Amylases. Mar. Drugs 2019, 17, 542. [Google Scholar] [CrossRef]
- Khoo, K.S.; Kam, W.K.; Khoo, H.E.; Gopalakrishnakone, P.; Chung, M.C.M. Purification and Partial Characterization of Two Cytolysins from a Tropical Sea Anemone, Heteractis Magnifica. Toxicon 1993, 31, 1567–1579. [Google Scholar] [CrossRef]
- Gendeh, G.S.; Young, L.C.; de Medeiros, C.L.C.; Jeyaseelan, K.; Harvey, A.L.; Chung, M.C.M. A New Potassium Channel Toxin from the Sea Anemone Heteractis Magnifica: Isolation, cDNA Cloning, and Functional Expression. Biochemistry 1997, 36, 11461–11471. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Hung, K.-C.; Wang, G.-H.; Duh, C.-Y. New Cytotoxic Sesquiterpenes from the Gorgonian Isis Hippuris. J. Nat. Prod. 2000, 63, 1603–1607. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chao, C.-H.; Wang, G.-H.; Hung, K.-C.; Duh, C.-Y.; Chiang, M.Y.; Wu, Y.-C.; Wu, C.-C. The First A-nor-Hippuristanol and Two Novel 4,5-Secosuberosanoids from the Gorgonian Isis Hippuris. Tetrahedron Lett. 2004, 45, 6413–6416. [Google Scholar] [CrossRef]
- Chao, C.-H.; Huang, L.-F.; Yang, Y.-L.; Su, J.-H.; Wang, G.-H.; Chiang, M.Y.; Wu, Y.-C.; Dai, C.-F.; Sheu, J.-H. Polyoxygenated Steroids from the Gorgonian Isis Hippuris. J. Nat. Prod. 2005, 68, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Miao, L.; Gao, C.-H.; Xu, Y.; Zhang, S.; Qian, P.-Y. New Steroids and a New Alkaloid from the Gorgonian Isis Minorbrachyblasta: Structures, Cytotoxicity, and Antilarval Activity. Helv. Chim. Acta 2010, 93, 511–516. [Google Scholar] [CrossRef]
- Chia-Cheng, L.; Su, J.-H.; Wu-Fu, C.; Zhi-Hong, W.; Bo-Rong, P.; Huang, L.-C.; Tsong-Long, H.; Ping-Jyun, S. New 11,20-Epoxybriaranes from the Gorgonian Coral Junceella Fragilis (Ellisellidae). Molecules 2019, 24, 2487. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Chen, Y.-H.; Hwang, T.-L.; Guh, J.-H.; Khalil, A.T. Four New Briarane Diterpenoids from the Gorgonian Coral Junceella Fragilis. Helv. Chim. Acta 2007, 90, 1391–1398. [Google Scholar] [CrossRef]
- Sung, P.-J.; Pai, C.-H.; Su, Y.-D.; Hwang, T.-L.; Kuo, F.-W.; Fan, T.-Y.; Li, J.-J. New 8-Hydroxybriarane Diterpenoids from the Gorgonians Junceella Juncea and Junceella Fragilis (Ellisellidae). Tetrahedron 2008, 64, 4224–4232. [Google Scholar] [CrossRef]
- Qi, S.H.; Zhang, S.; Qian, P.Y.; Xu, H.H. Antifeedant and Antifouling Briaranes from the South China Sea Gorgonian Junceella Juncea. Chem. Nat. Compd. 2009, 45, 49–54. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wu, Y.-C.; Chiang, M.Y.; Su, J.-H.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Eunicellin-Based Diterpenoids from the Cultured Soft Coral Klyxum Simplex. Tetrahedron 2009, 65, 7016–7022. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Chen, B.-W.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Simplexins A−I, Eunicellin-Based Diterpenoids from the Soft Coral Klyxum Simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef]
- Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Yeh, H.-C.; Sheu, J.-H. Cytotoxic and Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum Crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Coval, S.J.; Patton, R.W.; Petrin, J.M.; James, L.; Rothofsky, M.L.; Lin, S.L.; Patel, M.; Reed, J.K.; McPhail, A.T.; Robert Bishop, W. A Cembranolide Diterpene Farnesyl Protein Transferase Inhibitor from the Marine Soft Coral Lobophytum Cristagalli. Bioorg. Med. Chem. Lett. 1996, 6, 909–912. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Hsu, C.-H.; Wang, S.-K.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Durumolides A–E, Anti-Inflammatory and Antibacterial Cembranolides from the Soft Coral Lobophytum Durum. Tetrahedron 2008, 64, 9698–9704. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Unprecedented Hemiketal Cembranolides with Anti-Inflammatory Activity from the Soft Coral Lobophytum Durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-Inhibitory Cembrane Derivatives from a Philippines Collection of the Soft Coral Lobophytum Species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.H.; Van Minh, C.; Van Kiem, P.; Huong, H.T.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Nhiem, N.X.; Hyun, J.-H.; Kang, H.-K.; et al. Chemical Components from the Vietnamese Soft Coral Lobophytum sp. Arch. Pharm. Res. 2010, 33, 503–508. [Google Scholar] [CrossRef]
- Siro, G.; Pipite, A.; Christi, K.; Srinivasan, S.; Subramani, R. Marine Actinomycetes Associated with Stony Corals: A Potential Hotspot for Specialized Metabolites. Microorganisms 2022, 10, 1349. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Mosharova, I.V.; Korolkova, Y.V.; Shelukhina, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Kozlov, S.A.; Stensvåg, K.; et al. Peptide from Sea Anemone Metridium Senile Affects Transient Receptor Potential Ankyrin-Repeat 1 (TRPA1) Function and Produces Analgesic Effect. J. Biol. Chem. 2017, 292, 2992–3004. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Huang, K.-J.; Wang, S.-K.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. New Terpenoids from the Soft Corals Sinularia Capillosa and Nephthea Chabroli. Org. Lett. 2009, 11, 4830–4833. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiang, M.Y.; El-Gamal, A.A.H.; Dai, C.-F.; Duh, C.-Y. Revision of the Absolute Configuration at C(23) of Lanostanoids and Isolation of Secondary Metabolites from Formosan Soft Coral Nephthea Erecta. Chem. Biodivers. 2009, 6, 86–95. [Google Scholar] [CrossRef]
- Il’ina, A.P.; Monastyrnaya, M.M.; Sokotun, I.N.; Egorov, T.A.; Nazarenko, Y.A.; Likhatskaya, G.N.; Kozlovskaya, E.P. Actinoporins from the Sea of Japan Anemone Oulactis Orientalis: Isolation and Partial Characterization. Russ. J. Bioorg. Chem. 2005, 31, 34–42. [Google Scholar] [CrossRef]
- Lazcano-Pérez, F.; Zavala-Moreno, A.; Rufino-González, Y.; Ponce-Macotela, M.; García-Arredondo, A.; Cuevas-Cruz, M.; Gómez-Manzo, S.; Marcial-Quino, J.; Arreguín-Lozano, B.; Arreguín-Espinosa, R. Hemolytic, Anticancer and Antigiardial Activity of Palythoa Caribaeorum Venom. J. Venom. Anim. Toxins incl. Trop. Dis. 2018, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Cruz, M.; Lazcano-Pérez, F.; Hernández-Guzmán, U.; Díaz de la Vega-Castañeda, K.H.; Román-González, S.A.; Valdez-Cruz, N.A.; Velasco-Bejarano, B.; Colín-González, A.L.; Santamaría, A.; Gómez-Manzo, S.; et al. A Novel Phospholipase A2 Isolated from Palythoa Caribaeorum Possesses Neurotoxic Activity. Toxins 2019, 11, 89. [Google Scholar] [CrossRef]
- Reddy, N.S.; Reed, J.K.; Longley, R.E.; Wright, A.E. Two New Cytotoxic Linderazulenes from a Deep-Sea Gorgonian of the Genus Paramuricea. J. Nat. Prod. 2005, 68, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Oshiro, N.; Takuwa-Kuroda, K.; Iwanaga, S.; Nozaki, M.; Nakajima, T. Novel Proteinaceous Toxins from the Nematocyst Venom of the Okinawan Sea Anemone Phyllodiscus Semoni Kwietniewski. Biochem. Biophys. Res. Commun. 2002, 294, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Uechi, G.; Toma, H.; Arakawa, T.; Sato, Y. Molecular Characterization on the Genome Structure of Hemolysin Toxin Isoforms Isolated from Sea Anemone Actineria Villosa and Phyllodiscus Semoni. Toxicon 2010, 56, 1470–1476. [Google Scholar] [CrossRef]
- Mizuno, M.; Nozaki, M.; Morine, N.; Suzuki, N.; Nishikawa, K.; Morgan, B.P.; Matsuo, S. A Protein Toxin from the Sea Anemone Phyllodiscus Semoni Targets the Kidney and Causes a Severe Renal Injury with Predominant Glomerular Endothelial Damage. Am. J. Pathol. 2007, 171, 402–414. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Salceda, E.; Garateix, A.G.; Zaharenko, A.J.; Peigneur, S.; López, O.; Pons, T.; Richardson, M.; Díaz, M.; Hernández, Y.; et al. A Novel Sea Anemone Peptide That Inhibits Acid-Sensing Ion Channels. Peptides 2014, 53, 3–12. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kuo, L.-M.; Su, J.-H.; Hwang, T.-L.; Kuo, Y.-H.; Lin, C.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Pinnigorgiols A–C, 9,11-Secosterols with a Rare Ring Arrangement from a Gorgonian Coral Pinnigorgia sp. Tetrahedron 2016, 72, 999–1004. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, Y.-C.; Chen, W.-F.; Hwang, T.-L.; Fang, L.-S.; Wen, Z.-H.; Chen, Y.-H.; Wu, Y.-C.; Sung, P.-J. Pubinernoid A and Apo-9′-Fucoxanthinone, Secondary Metabolites from a Gorgonian Coral Pinnigorgia sp. Nat. Prod. Commun. 2016, 11, 707–708. [Google Scholar] [CrossRef]
- Nguyen, N.B.A.; El-Shazly, M.; Chen, P.-J.; Peng, B.-R.; Chen, L.-Y.; Hwang, T.-L.; Lai, K.-H. Unlocking the Potential of Octocoral-Derived Secondary Metabolites against Neutrophilic Inflammatory Response. Mar. Drugs 2023, 21, 456. [Google Scholar] [CrossRef]
- Huang, B.; Lu, H.; Zhang, Y.; Gan, X.; Wang, X.; Liu, Y.H.; Luo, X. Bioactive Alkaloids from the Beibu Gulf Coral-Associated Fungus Acremonium sclerotigenum GXIMD 02501. Rec. Nat. Prod. 2023, 17, 165–169. [Google Scholar] [CrossRef]
- Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; Moraes, M.O.d.; Araújo, R.M.; Silva, W.M.B.d.; Silveira, E.R.; Pessoa, O.D.L.; Braz-Filho, R.; Lopes, N.P.; et al. Cytotoxic Lipidic α-Amino Acids from the Zoanthid Protopalythoa Variabilis from the Northeastern Coast of Brazil. J. Braz. Chem. Soc. 2009, 20, 1455–1459. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Ramírez, C.; Rodríguez, I.I.; Barnes, C.L. Novel Terpenoids from the West Indian Sea Whip Pseudopterogorgia Elisabethae (Bayer). Elisapterosins A and B: Rearranged Diterpenes Possessing an Unprecedented Cagelike Framework1. J. Org. Chem. 2000, 65, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.I.; Rodríguez, A.D.; Zhao, H. Aberrarone: A Gorgonian-Derived Diterpene from Pseudopterogorgia Elisabethae. J. Org. Chem. 2009, 74, 7581–7584. [Google Scholar] [CrossRef] [PubMed]
- Ata, A.; Win, H.Y.; Holt, D.; Holloway, P.; Segstro, E.P.; Jayatilake, G.S. New Antibacterial Diterpenes from Pseudopterogorgia Elisabethae. Helv. Chim. Acta 2004, 87, 1090–1098. [Google Scholar] [CrossRef]
- Rodríguez, I.I.; Rodríguez, A.D. Homopseudopteroxazole, a New Antimycobacterial Diterpene Alkaloid from Pseudopterogorgia Elisabethae. J. Nat. Prod. 2003, 66, 855–857. [Google Scholar] [CrossRef]
- Kate, A.S.; Aubry, I.; Tremblay, M.L.; Kerr, R.G. Lipidyl Pseudopteranes A−F: Isolation, Biomimetic Synthesis, and PTP1B Inhibitory Activity of a New Class of Pseudopteranoids from the Gorgonian Pseudopterogorgia Acerosa. J. Nat. Prod. 2008, 71, 1977–1982. [Google Scholar] [CrossRef]
- Kate, A.S.; Pearson, J.K.; Ramanathan, B.; Richard, K.; Kerr, R.G. Isolation, Biomimetic Synthesis, and Cytotoxic Activity of Bis(Pseudopterane) Amines. J. Nat. Prod. 2009, 72, 1331–1334. [Google Scholar] [CrossRef]
- Ospina, C.A.; Rodríguez, A.D.; Sánchez, J.A.; Ortega-Barria, E.; Capson, T.L.; Mayer, A.M.S. Caucanolides A−F, Unusual Antiplasmodial Constituents from a Colombian Collection of the Gorgonian Coral Pseudopterogorgia Bipinnata. J. Nat. Prod. 2005, 68, 1519–1526. [Google Scholar] [CrossRef]
- Marrero, J.; Rodríguez, A.D.; Baran, P.; Raptis, R.G.; Sánchez, J.A.; Ortega-Barria, E.; Capson, T.L. Bielschowskysin, a Gorgonian-Derived Biologically Active Diterpene with an Unprecedented Carbon Skeleton. Org. Lett. 2004, 6, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- McEnroe, F.J.; Fenical, W. Structures and Synthesis of Some New Antibacterial Sesquiterpenoids from the Gorgonian Coral Pseudopterogorgia Rigida. Tetrahedron 1978, 34, 1661–1664. [Google Scholar] [CrossRef]
- Abreu, P.A.; Wilke, D.V.; Araujo, A.J.; Marinho-Filho, J.D.B.; Ferreira, E.G.; Ribeiro, C.M.R.; Pinheiro, L.S.; Amorim, J.W.; Valverde, A.L.; Epifanio, R.A.; et al. Perezone, from the Gorgonian Pseudopterogorgia Rigida, Induces Oxidative Stress in Human Leukemia Cells. Rev. Bras. De Farmacogn. 2015, 25, 634–640. [Google Scholar] [CrossRef]
- Shiomi, K.; Lin, X.-Y.; Nagashima, Y.; Ishida, M. Isolation and Amino Acid Sequence of a Polypeptide Toxin from the Sea Anemone Radianthus Crispus. Fish. Sci. 1996, 62, 629–633. [Google Scholar] [CrossRef]
- Klyshko, E.V.; Issaeva, M.P.; Monastyrnaya, M.M.; Il’yna, A.P.; Guzev, K.V.; Vakorina, T.I.; Dmitrenok, P.S.; Zykova, T.A.; Kozlovskaya, E.P. Isolation, Properties and Partial Amino Acid Sequence of a New Actinoporin from the Sea Anemone Radianthus Macrodactylus. Toxicon 2004, 44, 315–324. [Google Scholar] [CrossRef]
- Wang, G.-H.; Huang, H.-C.; Su, J.-H.; Huang, C.-Y.; Hsu, C.-H.; Kuo, Y.-H.; Sheu, J.-H. Crassocolides N-P, Three Cembranoids from the Formosan Soft Coral Sarcophyton Crassocaule. Bioorg. Med. Chem. Lett. 2011, 21, 7201–7204. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chao, C.-H.; Kuo, Y.-H.; Sheu, J.-H. Crassocolides G-M, Cembranoids from the Formosan Soft Coral Sarcophyton Crassocaule. Chem. Biodivers. 2009, 6, 1232–1242. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, C.-H.; Liaw, C.-C.; Chen, Y.-C.; Kuo, Y.-H.; Shen, Y.-C. Cembrane Diterpenoids from the Taiwanese Soft Coral Sinularia Flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Michalek, K.; Bowden, B.F. A Natural Algacide from Soft Coral Sinularia Flexibilis (Coelenterata, Octocorallia, Alcyonacea). J. Chem. Ecol. 1997, 23, 259–273. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Hsieh, Y.-T.; Wen, Z.-H.; Wu, Y.-C.; Sheu, J.-H. Polyoxygenated Sterols from the Formosan Soft Coral Sinularia Gibberosa. J. Nat. Prod. 2006, 69, 1275–1279. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Hou, R.-S. Cytotoxic Cembranoids from the Soft Corals Sinularia Gibberosa and Sarcophyton Trocheliophorum. J. Nat. Prod. 1996, 59, 595–598. [Google Scholar] [CrossRef]
- Eskander, R.; Al-Sofyani, A.A.; El-Sherbiny, M.M.O.; Ba-Akdah, M.A.; Satheesh, S. Chemical Defense of Soft Coral Sinularia Polydactyla from the Red Sea Against Marine Biofilm-Forming Bacteria. J. Ocean. Univ. China 2018, 17, 1451–1457. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, C.-Y.; Lin, Y.-F.; Wen, Z.-H.; Su, J.-H.; Kuo, Y.-H.; Chiang, M.Y.; Sheu, J.-H. Anti-Inflammatory Cembranoids from the Soft Corals Sinularia Querciformis and Sinularia Granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Honma, T.; Ide, M.; Nagashima, Y.; Ishida, M.; Chino, M. An Epidermal Growth Factor-like Toxin and Two Sodium Channel Toxins from the Sea Anemone Stichodactyla Gigantea. Toxicon 2003, 41, 229–236. [Google Scholar] [CrossRef]
- Williams, G.P.; Babu, S.; Ravikumar, S.; Kathiresan, K.; Prathap, S.A.; Chinnapparaj, S.; Marian, M.P.; Alikhan, S.L. Antimicrobial Activity of Tissue and Associated Bacteria from Benthic Sea Anemone Stichodactyla Haddoni against Microbial Pathogens. J. Environ. Biol. 2007, 28, 789–793. [Google Scholar] [PubMed]
- Castañeda, O.; Sotolongo, V.; Amor, A.M.; Stöcklin, R.; Anderson, A.J.; Harvey, A.L.; Engström, Å.; Wernstedt, C.; Karlsson, E. Characterization of a Potassium Channel Toxin from the Caribbean Sea Anemone Stichodactyla Helianthus. Toxicon 1995, 33, 603–613. [Google Scholar] [CrossRef]
- Tysoe, C.; Williams, L.K.; Keyzers, R.; Nguyen, N.T.; Tarling, C.; Wicki, J.; Goddard-Borger, E.D.; Aguda, A.H.; Perry, S.; Foster, L.J.; et al. Potent Human α-Amylase Inhibition by the β-Defensin-like Protein Helianthamide. ACS Cent. Sci. 2016, 2, 154–161. [Google Scholar] [CrossRef]
- Huerta, V.; Morera, V.; Guanche, Y.; Chinea, G.; González, L.J.; Betancourt, L.; Martínez, D.; Alvarez, C.; Lanio, M.E.; Besada, V. Primary Structure of Two Cytolysin Isoforms from Stichodactyla Helianthus Differing in Their Hemolytic Activity. Toxicon 2001, 39, 1253–1256. [Google Scholar] [CrossRef]
- Ben-Ari, H.; Paz, M.; Sher, D. The Chemical Armament of Reef-Building Corals: Inter- and Intra-Specific Variation and the Identification of an Unusual Actinoporin in Stylophora Pistilata. Sci. Rep. 2018, 8, 251. [Google Scholar] [CrossRef]
- Folmer, F.; Jaspars, M.; Solano, G.; Cristofanon, S.; Henry, E.; Tabudravu, J.; Black, K.; Green, D.H.; Küpper, F.C.; Aalbersberg, W.; et al. The Inhibition of TNF-α-Induced NF-κB Activation by Marine Natural Products. Biochem. Pharmacol. 2009, 78, 592–606. [Google Scholar] [CrossRef]
- Carpes, R.d.M.; Corrêa Fernandes, D.; Coelho, M.G.P.; Creed, J.C.; Fleury, B.G.; Garden, S.J.; Felzenszwalb, I. Anti-Inflammatory Potential of Invasive Sun Corals (Scleractinia: Tubastraea Spp.) from Brazil: Alternative Use for Management? J. Pharm. Pharmacol. 2020, 72, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.N.; Kvetkina, A.N.; Kostina, E.E.; Kalina, R.S.; Grebnev, B.B.; Koshelev, S.G.; Kozlov, S.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P. Peptide Modulators of ASIC Channels of the Sea Anemone Urticina Aff. Coriacea (Cuvier, 1798) from the Sea of Okhotsk. Russ. J. Mar. Biol. 2018, 44, 458–464. [Google Scholar] [CrossRef]
- Razpotnik, A.; Križaj, I.; Kem, W.R.; Maček, P.; Turk, T. A New Cytolytic Protein from the Sea Anemone Urticina Crassicornis That Binds to Cholesterol- and Sphingomyelin-Rich Membranes. Toxicon 2009, 53, 762–769. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Kozlov, S.A.; Andreev, Y.A.; Koshelev, S.G.; Sanamyan, N.P.; Sanamyan, K.E.; Dyachenko, I.A.; Bondarenko, D.A.; Murashev, A.N.; Mineev, K.S.; et al. Sea Anemone Peptide with Uncommon β-Hairpin Structure Inhibits Acid-Sensing Ion Channel 3 (ASIC3) and Reveals Analgesic Activity *. J. Biol. Chem. 2013, 288, 23116–23127. [Google Scholar] [CrossRef]
- Cline, E.I.; Wiebe, L.I.; Young, J.D.; Samuel, J. Toxic Effects of the Novel Protein UPI from the Sea Anemone Urticina Piscivora. Pharmacol. Res. 1995, 32, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Schmitz, F.J.; Williams, G.C. Malayenolides A−D, Novel Diterpenes from the Indonesian Sea Pen Veretillum Malayense. J. Nat. Prod. 1999, 62, 584–586. [Google Scholar] [CrossRef]
- Sharifi, S.; Mostafavi, P.G.; Tarasi, R.; Moradi, A.M.; Givianrad, M.H.; Farimani, M.M.; Ebrahimi, S.N.; Hamburger, M.; Niknejad, H. Purified Compounds from Marine Organism Sea Pen Induce Apoptosis in Human Breast Cancer Cell MDA-MB-231 and Cervical Cancer Cell Hela. Eur. J. Pharmacol. 2020, 877, 173075. [Google Scholar] [CrossRef]
- Chen, S.-P.; Sung, P.-J.; Duh, C.-Y.; Dai, C.-F.; Sheu, J.-H. Junceol A, a New Sesquiterpenoid from the Sea Pen Virgularia Juncea. J. Nat. Prod. 2001, 64, 1241–1242. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Chiang, C.-Y.; Huang, S.-H.; Wang, S.-K.; Duh, C.-Y. Xenia Diterpenoids from the Formosan Soft Coral Xenia Blumi. J. Nat. Prod. 2005, 68, 1336–1340. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Goldberg, I.; Benayahu, Y.; Kashman, Y. Novaxenicins A–D and Xeniolides I–K, Seven New Diterpenes from the Soft Coral Xenia Novaebrittanniae. Tetrahedron 2006, 62, 12092–12097. [Google Scholar] [CrossRef]
- Guillen, P.O.; Gegunde, S.; Jaramillo, K.B.; Alfonso, A.; Calabro, K.; Alonso, E.; Rodriguez, J.; Botana, L.M.; Thomas, O.P. Zoanthamine Alkaloids from the Zoantharian Zoanthus Cf. pulchellus and Their Effects in Neuroinflammation. Mar. Drugs 2018, 16, 242. [Google Scholar] [CrossRef]
- Nakao, Y.; Fusetani, N. Enzyme Inhibitors from Marine Invertebrates. J. Nat. Prod. 2007, 70, 689–710. [Google Scholar] [CrossRef]
- Qi, S.-H. Chapter 12—Bioactive Compounds from Marine Gorgonian Corals. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products; Elsevier: Amsterdam, The Netherlands, 2012; Volume 38, pp. 325–351. [Google Scholar]
- Chang, Y.-C.; Hwang, T.-L.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. New Anti-Inflammatory 9,11-Secosterols with a Rare Tricyclo[5,2,1,1]Decane Ring from a Formosan Gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 218. [Google Scholar] [CrossRef]
- Córdova-Isaza, A.; Jiménez-Mármol, S.; Guerra, Y.; Salas-Sarduy, E. Enzyme Inhibitors from Gorgonians and Soft Corals. Mar. Drugs 2023, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.d.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.d.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S. Phospholipase A2 as Targets for Anti-Cancer Drugs. Biochem. Pharmacol. 2007, 74, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Zandi, E.; Rothwarf, D.M.; Delhase, M.; Hayakawa, M.; Karin, M. The IκB Kinase Complex (IKK) Contains Two Kinase Subunits, IKKα and IKKβ, Necessary for IκB Phosphorylation and NF-κB Activation. Cell 1997, 91, 243–252. [Google Scholar] [CrossRef]
- Senegas, A.; Gautheron, J.; Maurin, A.G.D.; Courtois, G. IKK-Related Genetic Diseases: Probing NF-κB Functions in Humans and Other Matters. Cell Mol. Life Sci. 2014, 72, 1275–1287. [Google Scholar] [CrossRef]
- Andersson, M.; Van Nieuwerburgh, L.; Snoeijs, P. Pigment Transfer from Phytoplankton to Zooplankton with Emphasis on Astaxanthin Production in the Baltic Sea Food Web. Mar. Ecol. Prog. Ser. 2003, 254, 213–224. [Google Scholar] [CrossRef]
- Riely, G.J.; Politi, K.A.; Miller, V.A.; Pao, W. Update on Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2006, 12, 7232–7241. [Google Scholar] [CrossRef]
- Geribaldi-Doldán, N.; Gómez-Oliva, R.; Domínguez-García, S.; Nunez-Abades, P.; Castro, C. Protein Kinase C: Targets to Regenerate Brain Injuries? Front. Cell Dev. Biol. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, V.; Bogomolovas, J.; Ehler, E.; dos Remedios, C.G.; Yu, J.; Gao, C.; Lange, S. PKC and PKN in Heart Disease. J. Mol. Cell. Cardiol. 2019, 128, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein Kinase C: Perfectly Balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ramezanzade, L.; McClements, D.J. Recent Advances in Nanoencapsulation of Hydrophobic Marine Bioactives: Bioavailability, Safety, and Sensory Attributes of Nano-Fortified Functional Foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
- Abdo, A.A.A.; Al-Dalali, S.; Hou, Y.; Aleryani, H.; Shehzad, Q.; Asawmahi, O.; AL-Farga, A.; Mohammed, B.; Liu, X.; Sang, Y. Modification of Marine Bioactive Peptides: Strategy to Improve the Biological Activity, Stability, and Taste Properties. Food Bioprocess. Technol. 2024, 17, 1412–1433. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Fogliano, V. Food Matrix Interaction and Bioavailability of Bioactive Peptides: Two Faces of the Same Coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M. Effect of Chitosan Coating on the Properties of Nanoliposomes Loaded with Flaxseed-Peptide Fractions: Stability during Spray-Drying. Food Chem. 2020, 310, 125951. [Google Scholar] [CrossRef]
- Vasskog, T.; Andersen, J.; Hansen, E.; Svenson, J. Characterization and Cytotoxicity Studies of the Rare 21:4 n-7 Acid and Other Polyunsaturated Fatty Acids from the Marine Opisthobranch Scaphander Lignarius, Isolated Using Bioassay Guided Fractionation. Mar. Drugs 2012, 10, 2676–2690. [Google Scholar] [CrossRef]
- Martis, E.A.; Doshi, G.M.; Aggarwal, G.V.; Shanbhag, P.P. Novel Antibiotics from Marine Sources. PCI- Approv.-IJPSN 2011, 4, 1446–1461. [Google Scholar] [CrossRef]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871748-5. [Google Scholar]
- Hawkridge, J.M.; Pipe, R.K.; Brown, B.E. Localisation of Antioxidant Enzymes in the Cnidarians Anemonia Viridis and Goniopora Stokesi. Mar. Biology 2000, 137, 1–9. [Google Scholar] [CrossRef]
- Palmer, C.V.; Modi, C.K.; Mydlarz, L.D. Coral Fluorescent Proteins as Antioxidants. PLoS ONE 2009, 4, e7298. [Google Scholar] [CrossRef]
- Clarke, D.N.; Rose, N.H.; De Meulenaere, E.; Rosental, B.; Pearse, J.S.; Pearse, V.B.; Deheyn, D.D. Fluorescent Proteins Generate a Genetic Color Polymorphism and Counteract Oxidative Stress in Intertidal Sea Anemones. Proc. Natl. Acad. Sci. USA 2024, 121, e2317017121. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Shick, J.M. Oxygen Production by Endosymbiotic Algae Controls Superoxide Dismutase Activity in Their Animal Host. Nature 1982, 297, 579–580. [Google Scholar] [CrossRef]
- Dykens, J.A.; Shick, J.M. Photobiology of the Symbiotic Sea Anemone, Anthopleura Elegantissima: Defenses against Photodynamic Effects, and Seasonal Photoacclimatization. Biol. Bull. 1984, 167, 683–697. [Google Scholar] [CrossRef]
- Richier, S.; Merle, P.-L.; Furla, P.; Pigozzi, D.; Sola, F.; Allemand, D. Characterization of Superoxide Dismutases in Anoxia- and Hyperoxia-Tolerant Symbiotic Cnidarians. Biochim. Biophys. Acta (BBA)–Gen. Subj. 2003, 1621, 84–91. [Google Scholar] [CrossRef]
- Plantivaux, A.; Furla, P.; Zoccola, D.; Garello, G.; Forcioli, D.; Richier, S.; Merle, P.-L.; Tambutté, É.; Tambutté, S.; Allemand, D. Molecular Characterization of Two CuZn-Superoxide Dismutases in a Sea Anemone. Free. Radic. Biol. Med. 2004, 37, 1170–1181. [Google Scholar] [CrossRef]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular Superoxide Dismutase in Biology and Medicine. Free. Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef]
- Matés, J.M. Effects of Antioxidant Enzymes in the Molecular Control of Reactive Oxygen Species Toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Higuchi, T.; Fujimura, H.; Arakaki, T.; Oomori, T. Activities of Antioxidant Enzymes (SOD and CAT) in the Coral Galaxea fascicularis against Increased Hydrogen Peroxide Concentrations in Seawater. In Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008. [Google Scholar]
- Ramos, R.; García, E. Induction of Mixed-Function Oxygenase System and Antioxidant Enzymes in the Coral Montastraea Faveolata on Acute Exposure to Benzo(a)Pyrene. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2007, 144, 348–355. [Google Scholar] [CrossRef]
- Liñán-Cabello, M.A.; Flores-Ramírez, L.A.; Cobo-Díaz, J.F.; Zenteno-Savin, T.; Olguín-Monroy, N.O.; Olivos-Ortiz, A.; Tintos-Gómez, A. Response to Short Term Ultraviolet Stress in the Reef-Building Coral Pocillopora Capitata (Anthozoa: Scleractinia). Rev. Biol. Trop. 2010, 58, 103–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, A.P.L.; Silva, D.A.M.; Rodrigues, A.C.M.; Marques, C.R.; Soares, A.M.V.M.; Rocha, R.J.M. Species-Specific Oxidative Stress Responses and Cellular Energy Allocation after Coral Shipping. Aquac. Rep. 2021, 19, 100623. [Google Scholar] [CrossRef]
- Coll, A.; Rufino-Palomares, E.E.; Ramos-Barbero, M.; Ortiz-Maldonado, A.E.; Pantoja-Echevarría, L.M.; González-Ordóñez, I.; Pérez-Jiménez, A.; Trenzado, C.E. Effects of Environmental Factors on the Oxidative Status of Anemonia Viridis in Aquaculture Systems. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2025, 275, 111042. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, A.V.F.; Haran, N.S.; Pereira, N.A.; Acuña, F.H. First Report of Phenoloxidase and Peroxidase Activities in Two Intertidal Sea Anemone Species of Argentina. Invertebr. Surviv. J. 2014, 11, 192–196. [Google Scholar]
- Palmer, C.V.; Bythell, J.C.; Willis, B.L. Enzyme Activity Demonstrates Multiple Pathways of Innate Immunity in Indo-Pacific Anthozoans. Proc. Biol. Sci. 2012, 279, 3879–3887. [Google Scholar] [CrossRef]
- Trapani, M.R.; Parisi, M.G.; Parrinello, D.; Sanfratello, M.A.; Benenati, G.; Palla, F.; Cammarata, M. Specific Inflammatory Response of Anemonia Sulcata (Cnidaria) after Bacterial Injection Causes Tissue Reaction and Enzymatic Activity Alteration. J. Invertebr. Pathol. 2016, 135, 15–21. [Google Scholar] [CrossRef]
- Popkova, D.; Otstavnykh, N.; Sintsova, O.; Baldaev, S.; Kalina, R.; Gladkikh, I.; Isaeva, M.; Leychenko, E. Bioprospecting of Sea Anemones (Cnidaria, Anthozoa, Actiniaria) for β-Defensin-like α-Amylase Inhibitors. Biomedicines 2023, 11, 2682. [Google Scholar] [CrossRef]
- Law, S.T.S.; Yu, Y.; Nong, W.; So, W.L.; Li, Y.; Swale, T.; Ferrier, D.E.K.; Qiu, J.; Qian, P.; Hui, J.H.L. The Genome of the Deep-Sea Anemone Actinernus Sp. Contains a Mega-Array of ANTP-Class Homeobox Genes. Proc. R. Soc. B Biol. Sci. 2023, 290, 20231563. [Google Scholar] [CrossRef]
- Shum, C.W.Y.; Nong, W.; So, W.L.; Li, Y.; Qu, Z.; Yip, H.Y.; Swale, T.; Ang, P.O.; Chan, K.M.; Chan, T.F.; et al. Genome of the Sea Anemone Exaiptasia pallida and Transcriptome Profiles during Tentacle Regeneration. Front. Cell Dev. Biol. 2022, 10, 900321. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Z.; Zhang, H. Adaptation and Evolution of the Sea Anemone Alvinactis Sp. to Deep-Sea Hydrothermal Vents: A Comparison Using Transcriptomes. Ecol. Evol. 2022, 12, e9309. [Google Scholar] [CrossRef]
- Fu, J.; He, Y.; Peng, C.; Tang, T.; Jin, A.; Liao, Y.; Shi, Q.; Gao, B. Transcriptome Sequencing of the Pale Anemones (Exaiptasia diaphana) Revealed Functional Peptide Gene Resources of Sea Anemone. Front. Mar. Sci. 2022, 9, 856501. [Google Scholar] [CrossRef]
- Goldstone, J.V. Environmental Sensing and Response Genes in Cnidaria: The Chemical Defensome in the Sea Anemone Nematostella Vectensis. Cell Biol. Toxicol. 2008, 24, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Seneca, F.; Davtian, D.; Boyer, L.; Czerucka, D. Gene Expression Kinetics of Exaiptasia Pallida Innate Immune Response to Vibrio Parahaemolyticus Infection. BMC Genom. 2020, 21, 768. [Google Scholar] [CrossRef]
- Delgado, A.; Benedict, C.; Macrander, J.; Daly, M. Never, Ever Make an Enemy… Out of an Anemone: Transcriptomic Comparison of Clownfish Hosting Sea Anemone Venoms. Mar. Drugs 2022, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Agrawal, S.; Aranda, M.; Baumgarten, S.; Belcaid, M.; Drake, J.L.; Erwin, D.; Foret, S.; Gates, R.D.; Gruber, D.F.; et al. Comparative Genomics Explains the Evolutionary Success of Reef-Forming Corals. eLife 2016, 5, e13288. [Google Scholar] [CrossRef]
- Cunning, R.; Bay, R.A.; Gillette, P.; Baker, A.C.; Traylor-Knowles, N. Comparative Analysis of the Pocillopora Damicornis Genome Highlights Role of Immune System in Coral Evolution. Sci. Rep. 2018, 8, 16134. [Google Scholar] [CrossRef]
- Pinzón, J.H.; Kamel, B.; Burge, C.A.; Harvell, C.D.; Medina, M.; Weil, E.; Mydlarz, L.D. Whole Transcriptome Analysis Reveals Changes in Expression of Immune-Related Genes during and after Bleaching in a Reef-Building Coral. R. Soc. Open Sci. 2015, 2, 140214. [Google Scholar] [CrossRef]
- Fuess, L.E.; Pinzόn C., J.H.; Weil, E.; Mydlarz, L.D. Associations between Transcriptional Changes and Protein Phenotypes Provide Insights into Immune Regulation in Corals. Dev. Comp. Immunol. 2016, 62, 17–28. [Google Scholar] [CrossRef]
- Takagi, T.; Yoshioka, Y.; Zayasu, Y.; Satoh, N.; Shinzato, C. Transcriptome Analyses of Immune System Behaviors in Primary Polyp of Coral Acropora Digitifera Exposed to the Bacterial Pathogen Vibrio Coralliilyticus under Thermal Loading. Mar. Biotechnol. 2020, 22, 748–759. [Google Scholar] [CrossRef]
- Libro, S.; Kaluziak, S.T.; Vollmer, S.V. RNA-Seq Profiles of Immune Related Genes in the Staghorn Coral Acropora Cervicornis Infected with White Band Disease. PLoS ONE 2013, 8, e81821. [Google Scholar] [CrossRef]
- Huang, C.; Morlighem, J.-É.R.; Zhou, H.; Lima, É.P.; Gomes, P.B.; Cai, J.; Lou, I.; Pérez, C.D.; Lee, S.M.; Rádis-Baptista, G. The Transcriptome of the Zoanthid Protopalythoa Variabilis (Cnidaria, Anthozoa) Predicts a Basal Repertoire of Toxin-like and Venom-Auxiliary Polypeptides. Genome Biol. Evol. 2016, 8, 3045–3064. [Google Scholar] [CrossRef] [PubMed]
- Scesa, P.D.; Lin, Z.; Schmidt, E.W. Ancient Defensive Terpene Biosynthetic Gene Clusters in the Soft Corals. Nat. Chem. Biol. 2022, 18, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Guzman, C.; Shinzato, C.; Lu, T.-M.; Conaco, C. Transcriptome Analysis of the Reef-Building Octocoral, Heliopora Coerulea. Sci. Rep. 2018, 8, 8397. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.A.; Mouchka, M.E.; Harvell, C.D.; Roberts, S. Immune Response of the Caribbean Sea Fan, Gorgonia ventalina, Exposed to an Aplanochytrium Parasite as Revealed by Transcriptome Sequencing. Front. Physiol. 2013, 4, 180. [Google Scholar] [CrossRef]
- Shinzato, C.; Hamada, M.; Shoguchi, E.; Kawashima, T.; Satoh, N. The Repertoire of Chemical Defense Genes in the Coral Acropora Digitifera Genome. jzoo 2012, 29, 510–517. [Google Scholar] [CrossRef]
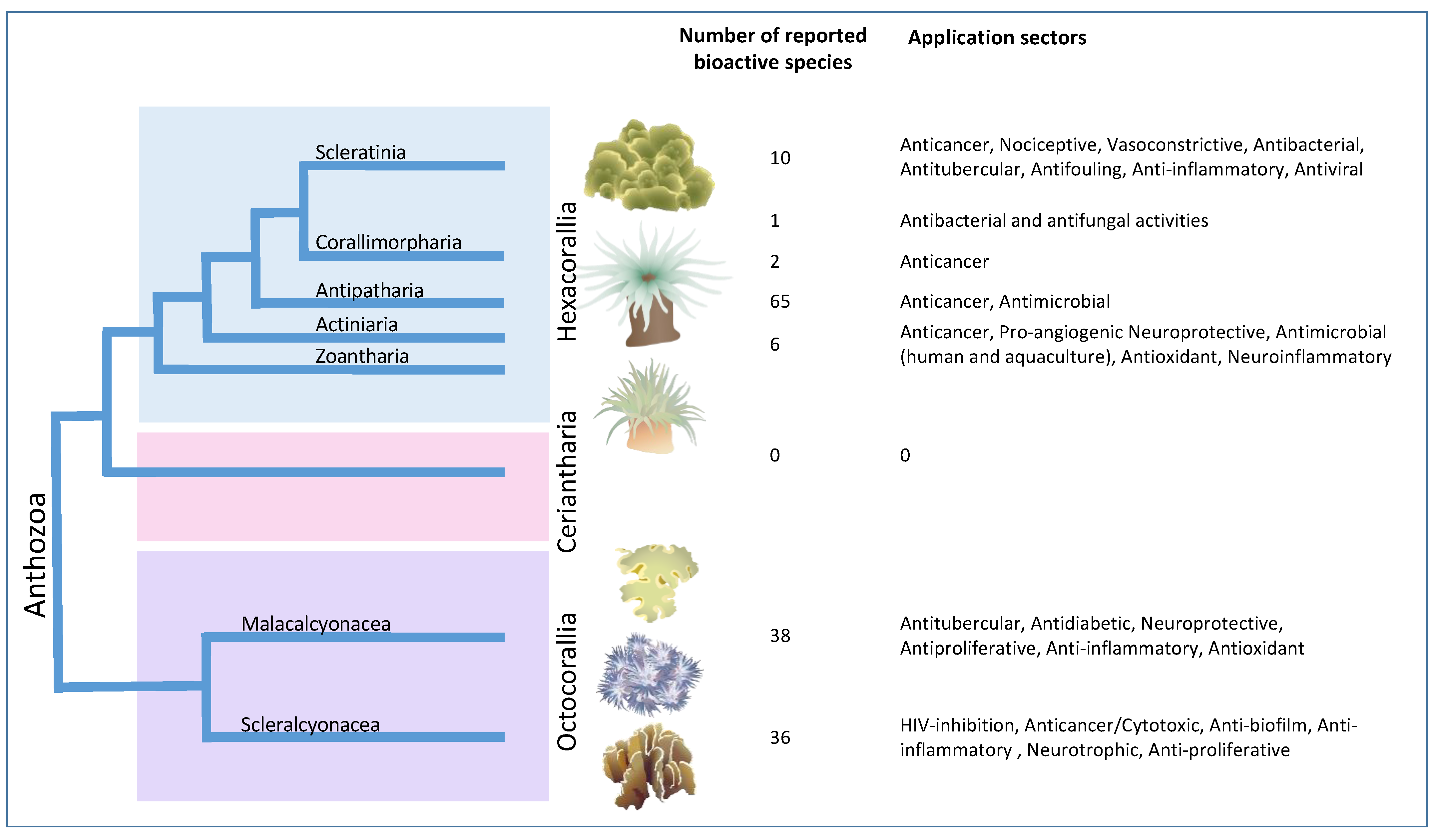
| Organism | Group | Compound/Toxin | Activities | References |
|---|---|---|---|---|
| Anthoptilum grandiflorum (Antarctic Sea Pen) | Pennatulacea (sea pens) | Briarane diterpenes (bathyptilone A) | Cytotoxicity against the pluripotent embryonal carcinoma cell line, NTera-2 (NT2), isolated from lung metastasis of testicular cancer. | [105] |
| Acabaria undulata | Gorgonians (sea fans) | Steroids (7α,8α-epoxy-3β,5α,6α-trihydroxycholestane and 24-methyl-7α,8α-epoxy-3β,5α,6α-trihydroxycholest-22-ene) | Inhibitory effects towards phospholipases A2 (PLA2) | [106] |
| Actinia equina | Sea anemones | Na + channel neurotoxin (AE1) | Cytotoxic activity against mammalian red blood cells | [107] |
| Actinia equina | Sea anemones | Equinatoxin 11 (EqT II) | Hemolytic activity in rats | [108] |
| Actinia equina | Sea anemones | Equinatoxin III | Cardiovascular activity | [109] |
| Actinia equina | Sea anemones | Polypeptide toxin (Ae I) | Lethality against crabs | [96] |
| Actinia equina | Sea anemones | AEPI-I, II, III, and IV | Inhibition of serine proteases, trypsin, and α-chymotrypsin | [110] |
| Actinia equina | Sea anemones | Delta-actitoxin-Aeq2a/Ae 1 (Neurotoxin) | Type-1 sodium channel inhibitory activity | [95] |
| Actinostola faeculenta | Sea anemones | Extract | Cytotoxic effects against murine splenocytes and Ehrlich carcinoma cells | [100] |
| Anemonia sulcata | Sea anemones | Toxin I | Cardiotoxic activity | [111] |
| Anemonia sulcata | Sea anemones | Kalicludines and Kaliseptine | Blockers of voltage-sensitive K+ channels | [112] |
| Anemonia viridis | Sea anemones | Peptide toxin Av3 | Lethality in Crustaceans | [113] |
| Antheopsis maculata | Sea anemones | Am I, II, III | Lethal activity against freshwater crabs (Potamon dehaani) | [114] |
| Anthopleura aff. xanthogrammica | Sea anemones | Kunitz-Type Protease inhibitors (AXPI-I and -II) | Inhibition of Trypsin and inhibition of other serine proteases (α-chymotrypsin and elastase (in case of AXPI-I only)) | [115] |
| Anthopleura asiatica | Sea anemones | Bandaporin | Hemolytic activity in sheep red blood cells, lethal toxicity to crayfish | [116] |
| Anthopleura elegantissima | Sea anemones | Isotoxin APE 2-1 | Cariotoxic effects in isolated right atria (guinea pig) | [115] |
| Anthopleura elegantissima and Anthopleura nigrescens | Sea anemones | Crude venom/extracts | Antimicrobial activities against human pathogens | [117,118] |
| Anthopleura xanthogrammica | Sea anemones | Sodium channel toxins | Enhancing sodium uptake in RT4-B and N1E-115 cells | [119] |
| Anthopleura. xanthogrammica | Sea anemones | Anthopleurin-A (AP-A) and Anthopleurin-B (AP-B) | Cardiotonic activity | [120] |
| Antipathes dichotoma | Black corals | Sphingolipids (ceramides) | Cytotoxicity against HepG2 (Hepatocellular carcinoma), WI 38 (Normal human embryonic lung fibroblasts), VERO (Normal kidney epithelial cells), and MCF-7 (Human breast adenocarcinoma). | [121] |
| Antipathes dichotoma | Black corals | Steryl Esters/steryl hexadecanoates (3β-hexadecanoylcholest-5-en-7-one) and Thymidine | Anticancer activity against VERO and MCF-7 | [122] |
| Asterospicularia laurae | Soft corals | Asterolaurins A−F | Inducing moderate cytotoxicity against HepG2 cells and inhibition of elastase release and superoxide anion generation | [123] |
| Astrogorgia sp. | Gorgonians (sea fans) | Calicoferol A and E, 24-exomethylenecalicoferol E, 9β-hydroxy-9,10-secosteroid astrogorgol F, and 9α-hydroxy-9,10-secosteroid astrogorgi-adiol | Suppression of tumor-associated kinases | [124] |
| Bartholomea annulata | Sea anemones | Polypeptide | Neurotoxic activity in sea crabs (Ocypode quadrata) | [125] |
| Briareum excavatum | Soft corals | Briaexcavatins | Mild cytotoxicity toward MDA-MB-231 human breast tumor cells and inhibiting neutrophil elastase release in humans | [126] |
| Briareum excavatum. | Soft corals | Briaexcavatolides | Cytotoxicity towards cancer cell lines | [127] |
| Briareum polyanthes | Soft corals | Briarellins and polyanthellin A | Antimalarial activity against Plasmodium falciparum. | [128] |
| Bunodosoma caissarum | Sea anemones | Caissarolysin I (Bcs I) | Hemolytic activity to human erythrocytes | [94] |
| Bunodosoma caissarum | Sea anemones | BcsTx3 toxin | Potassium channel blocker | [129] |
| Bunodosoma caissarum | Sea anemones | BcI, II, and II | Neurotoxicity and hemolytic activity | [130] |
| Bunodosoma caissarum | Sea anemones | PLA2 proteins (BcPLA21, BcPLA22, and BcPLA23 | Inducing insulin secretion in the presence of high glucose, increasing perfusion pressure, renal vascular resistance, urinary flow, glomerular filtration rate, and sodium, potassium, and chloride levels of excretion in isolated kidney | [131] |
| Bunodosoma cangicum. | Sea anemones | Cangitoxin (CGTX-II and CGTX-III) | Sodium (Nav1.1) channels toxin | [132] |
| Bunodosoma granulifera | Sea anemones | Granulitoxin (GRX) | Severe neurologic effects such as aggressive behavior, dyspnea, circular movements, etc. | [133] |
| Bunodosorna granulifera | Sea anemones | Peptide toxin | Facilitating acetylcholine release at avian neuromuscular junctions, competing with dendrotoxin I for attachment to synaptosomal membranes of rat brain, and suppressing potassium currents in rat dorsal root ganglion neurons in culture. | [134] |
| Calliactis parasitica | Sea anemones | Calitoxin (CLX) | Inducing a strong release of neurotransmitters, which causes high muscle contraction in crustaceans | [135] |
| Capnella imbricata (Formosan Soft Coral) | Soft corals | Capnellenes (sesquiterpenes) | Anti-inflammatory activity | [136] |
| Cespitularia hypotentaculata | Soft corals | Cespitularins | Cytotoxicity against cancer cell lines | [137] |
| Clavularia koellikeri | Soft corals | Marine diterpenoid | Cytotoxic activity against adenocarcinoma cells (DLD-1) and potent growth inhibiting activity against human T lymphocytic leukemia cells (MOLT-4) | [138] |
| Clavularia sp., | Soft corals | Stolonidiol (Diterpenoid) | Producing a neurotrophic factor-like agent on the cholinergic nervous system | [139] |
| Clavularia viridis | Soft corals | Marine prostanoids (claviridic acids A–E) | Inhibitory effect on PHA-induced proliferation of peripheral blood mononuclear cells (PBMC) and cytotoxicity against human gastric cancer cells (AGS) | [140] |
| Clavularia viridis | Soft corals | Bromovulone III and chlorovulone II | cytotoxicity against human prostate (PC-3) and colon (HT29) cancer cells | [141] |
| Clavularia viridis | Soft corals | Marine prostanoid, bromovulone III | Antitumor activity in human hepatocellular carcinoma | [141] |
| Clavularia viridis | Soft corals | Chlorinated marine steroids (Yonarasterols) | Antitumor activity | [138] |
| Condylactis gigantea | Sea anemones | Peptide toxins (type I sea anemone sodium channel toxins) | Paralytic activity on crab | [142] |
| Condylactis gigantea | Sea anemones | Phospholipase A2(CgPLA2) | Hemolytic activity, enzymatic activity | [143] |
| Condylactis gigantea | Sea anemones | Peptide toxin, CgNa | Type I sodium channel toxin, prolonging the duration of cardiac activity, and enhancing contractile force in rats | [142] |
| Condylactis gigantea | Sea anemones | Phospholipase A2 (CgPLA2) | High catalytic activity upon fluorescent phospholipids | [143] |
| Condylactis passiflora | Sea anemones | Polypeptide toxins (Cp I, II, and III) | Lethal activity against crabs | [144] |
| Corallimorphus cf. pilatus | Corallimorphs | Extract | Antibacterial and antifungal activities | [100] |
| Cribrinopsis similis | Sea anemones | Bioactive Polypeptides | Hemolytic activity, Laminarinase activity | [145] |
| Dendronephthya rubeola | Soft corals | Capnellenes (dihydroxycapnellene (capnell-9(12)-ene-8β,10α-diol)) | Antiproliferative (against murine fibroblast (L-929) cell line) and Cytotoxic (against Human cervix carcinoma (HeLa) cell line) activity, Inhibition of the Tax/CREB, Myc/Max, and Myc/Mic-1 interaction | [146] |
| Dendronephthya sp., | Soft corals | Sogosterones A−D | Antifouling activities | [147] |
| Elesto riisei | Gorgonians (sea fans) | Punaglandins | Inhibiting Ubiquitin isopeptidase activity and exhibiting antiproliferative effects | [148] |
| Eunicea fusca | Gorgonians (sea fans) | Fuscosides | Anti-inflammatory activities | [149] |
| Eunicea knight | Gorgonians (sea fans) | Cembranes asperdiol | Inhibitory activity against PTP1B | [150] |
| Eunicea sp., | Gorgonians (sea fans) | Sesquiterpenes (elemane, eudesmane, and germacrane types) | Inhibitory effect on the growth of the malarial parasite Plasmodium falciparum | [151] |
| Euplexaura anastomosans | Gorgonians (sea fans) | Farnesylhydroquinone glycosides (Euplexides), and Euplexide A, B, and G | Inhibition of Phospholipases A2 (PLA2) | [152] |
| Euplexaura robusta | Gorgonians (sea fans) | Tetra-prenylated alkaloid, Malonganenone D | Inhibitory activity against MET Receptor Tyrosine Kinase, or c-Met | [153] |
| Fungus Scopulariopsis sp., isolated from the inner tissue of the coral Stylophora | Stony corals (Host) | Antibiotic AGI-B4, violaceol I, violaceol II, scopularide A | Cytotoxicity against mouse lymphoma cell line (L5178Y) | [81] |
| Fungus Scopulariopsis sp., isolated from the inner tissue of the coral Stylophora | Stony corals (Host) | 3β,7β,15α,24-tetrahydroxyolean-12-ene-11,22-dione and 15α,22β,24-trihydroxyolean-11,13-diene-3-one | Cytotoxicity against mouse lymphoma cell line (L5178Y) | [84] |
| Gorgonian Pseudopterogorgia sp. | Gorgonians (sea fans) | 9,11-secosterol | Inhibitory effects on Protein kinase C (PKC)/Antiproliferative potential | [154] |
| Halcurias sp. | Sea anemones | Halcurin | Lethality against crabs | [155] |
| Heteractis crispa | Sea anemones | Kunitz/BPTI-type peptides | Neuroprotective activity against 6-hydroxydopamine-induced neurotoxicity. | [156] |
| Heteractis crispa | Sea anemones | Polypeptide toxin (π-AnmTX Hcr 1b-1) | Inhibiting the ASIC3 acid-sensitive channel | [157] |
| Heteractis crispa | Sea anemones | ASIC1a inhibitor (Hcr 1b-2) | Antihyperalgesic effects in the acid-induced pain model | [158] |
| Heteractis crispa | Sea anemones | Kunitz-type inhibitors/polypeptides (HCRG1 and HCRG2) | Anti-inflammatory | [159] |
| Heteractis crispa | Sea anemones | HCRG21 | Inhibiting the capsaicin-induced current through the transient receptor potential family member vanilloid 1 (TRPV1) | [160] |
| Heteractis crispa | Sea anemones | Polypeptides (APHC2 and APHC3) | Analgesic effect on mammals | [161] |
| Heteractis crispa | Sea anemones | Recombinant polypeptide (HCGS 1.20) | Anti-inflammatory activity | [162,163] |
| Heteractis magnifica | Sea anemones | Cytolysin, HMgIII | Hemolytic activity | [164] |
| Heteractis magnifica | Sea anemones | HMIQ3c1 recombinant peptide | Neuroprotective activity in a model of Alzheimer’s disease. | [165] |
| Heteractis magnifica | Sea anemones | Magnificamide, a β-Defensin-Like Peptide | Inhibition of α-amylases (treatment of type 2 diabetes mellitus) | [166] |
| Heteractis magnifica (formerly Radianthus ritteri) | Sea anemones | Magnificalysins I and II | Hemolytic activities and lethal effects | [167] |
| Heteractis magnifica. | Sea anemones | Potassium channel toxin, HmK | Inhibiting the binding ofα-dendrotoxin (a ligand for voltage-gated K channels) to rat brain synaptosomal membranes, blocking K+ currents through Kv 1.2 channels expressed in a mammalian cell line, and facilitating acetylcholine release at the avian neuromuscular junction | [168] |
| Isis hippuris | Gorgonians (sea fans) | Suberosane sesquiterpenes (suberosenol B, suberosanone, suberosenol B acetate) | Exhibiting potent cytotoxicity toward P-388, A549, and HT-29 cancer cell lines | [169] |
| Isis hippuris | Gorgonians (sea fans) | Isishippuric acid B | Producing potent cytotoxicity toward a limited panel of cancer cells | [170] |
| Isis hippuris | Gorgonians (sea fans) | Hippuristanols | Cytotoxicity against several cancer cell lines. | [171] |
| Isis minorbrachyblasta | Gorgonians (sea fans) | 22-epihippuristanol and hippuristanol | Cytotoxicity against cancer cell lines (A549, HONE1, HeLa) | [172] |
| Junceella fragilis | Gorgonians (sea fans) | Juncin Z | Anti-inflammatory activity | [173] |
| Junceella fragilis | Gorgonians (sea fans) | Briarane-type diterpenoids (Frajunolides) | Antioxidant activities | [174] |
| Junceella juncea | Gorgonians (sea fans) | Junceoal A | Inhibition of superoxide anions by human neutrophils | [175] |
| Junceella juncea | Gorgonians (sea fans) | Juncin ZII | Antifouling activity against the larval settlement of barnacle Balanus Amphitrite and antifeedant activity against second-instar larvae of Spodoptera litura | [176] |
| Klyxum simplex | Soft corals | Klysimplexins B and H | Cytoxic activity in human cancer cells (liver carcinoma, gingival carcinoma, breast cancer) | [177] |
| Klyxum simplex | Soft corals | Simplexin E | Reduction of iNOS (Inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) proteins in lipo-polysaccharide (LPS)-stimulated macrophage cells | [178] |
| Liponema brevicorne, Actinostola callosa | Sea anemones | Extracts | Hemolytic activity | [100] |
| Lobophytum crassum | Soft corals | Crassumolides | Anti-inflammatory (inhibits the accumulation of the pro-inflammatory proteins iNOS and COX-2) and cytotoxic activities | [179] |
| Lobophytum cristagalli | Soft corals | Cembranolide diterpene | Inhibition of farnesyl protein transferase (FPT) | [180] |
| Lobophytum cristagalli | Soft corals | Cembranolide (diterpene) | Inhibition of farnesyl protein transferase (FPT) | [180] |
| Lobophytum durum | Soft corals | Durumolides | Anti-inflammatory effects and antibacterial activities | [181] |
| Lobophytum durum | Soft corals | Durumhemiketalolides | Anti-inflammatory activities | [182] |
| Lobophytum Species | Soft corals | Cembranoid diterpenes (Lobohedleolide, (7Z) -lobohedleolid, 17-Dimethylaminolobohedleolide) | HIV inhibitory activity | [183] |
| Lobophytum Species | Soft corals | Lobophytene | Cytotoxic activities against lung (A549) and colon (HT-29) cell lines | [184] |
| Marine actinomycetes associated with stony corals | Stony corals (Host) | Bioactive metabolites | Antimalarial, antibacterial, antifungal, antimicrobial, anti-inflammatory, cytotoxic, and antitumor activity | [185] |
| Marine bacterium Erythrobacter flavus strain KJ5 from hard coral Acropora nasuta | Stony corals | Sulfotransferases | Antithrombotic, antifouling, antiviral, and anti-inflammatory activities | [87,88] |
| Marine-derived fungus Gliomastix sp., from Stylophora sp. | Stony corals (Host) | Gliomastins A–D, 9-O-methylgliomastin C, acremonin A 1-O-β-D-glucopyranoside, gliomastin E 1-O-β-D-glucopyranoside, and 6′-O-acetyl-isohomoarbutin. | Cytotoxicity against mouse lymphoma cell line (L5178Y) | [85] |
| Metridium senile | Sea anemones | Peptide, τ-AnmTX Ms 9a-1 (short name: Ms 9a-1) | Anti-inflammatory and analgesic effects in mice | [186] |
| Nephthea chabroli | Soft corals | Chabranol (terpenoid) | Cytotoxicity against mouse lymphocytic leukemia (P-388) cell line | [187] |
| Nephthea erecta | Soft corals | Oxygenated ergostanoids | Anti-inflammatory effects | [188] |
| Oulactis orientalis | Sea anemones | Cytolysins Or-A and Or-G | Hemolytic activity | [189] |
| Palythoa caribaeorum | Zoanthids | Nematocyst Venom | Antitumor activity (human glioblastoma (U251) and (human lung adenocarcinoma (SKLU-1) cell lines), Antigardial activity against the parasite Giardia intestinalis | [190] |
| Palythoa caribaeorum | Zoanthids | Phospholipase (A2-PLTX-Pcb1a) | Neurotoxic activity in rats’ tissues. | [191] |
| Palythoa. caribaeorum | Zoanthids | PcKuz3 isotoxin | Neuroprotective activity | [65] |
| Paramuricea sp., (Deep-sea gorgonian) | Gorgonians (sea fans) | Linderazulene | Moderate cytotoxicity against P388 murine leukemia cell line | [192] |
| Parazoanthus axinellae | Zoanthids | cnidocyst extract | Antimicrobial activities (human and aquaculture) | [66] |
| Phyllodiscus semoni | Sea anemones | PsTX-60A and PsTX-60B | Lethal activity to shrimp Palaemon paucidence, hemolytic activity on sheep red blood cells | [193] |
| Phyllodiscus semoni | Sea anemones | Hemolytic toxins—Pstx20 | Hemolytic activity | [194] |
| Phyllodiscus semoni | Sea anemones | PsTX-T | Nephrotoxic activity (glomerular endothelial damage) | [195] |
| Phyllodiscus semoni | Sea anemones | PsTX-60A and PsTX-60B | Lethal toxicity to shrimp Palaemon paucidence, hemolytic activity towards sheep erythrocytes | [193] |
| Phymanthus crucifer | Sea anemones | Toxin, PhcrTx1 | Inhibiting acid-sensing ion channel (ASIC) | [196] |
| Pinnigorgia sp., | Gorgonians (sea fans) | Pinnigorgiols A-E | Anti-inflammatory activity | [197] |
| Pinnigorgia sp., | Gorgonians (sea fans) | Apo-9′-fucoxanthinone | Inhibition of elastase release by human neutrophils | [198,199] |
| Pocillopora damicornis associated fungus, Acremonium sclerotigenum | Stony corals (host) | 4-hydroxy-2-pyridone alkaloid and phenazine alkaloid | Cytotoxicity against two prostate cancer cell lines, anti-Vibrio activity | [200] |
| Porites astreoides | Stony corals | Aqueous extract | Hemolytic activity in humans and rats | [77] |
| Protopalythoa variabilis | Zoanthids | Lipidic α-amino acids/LAAs | Cytotoxic activity against human tumor cell lines: HCT-8 (human colon carcinoma), MDA-MB-435 (melanoma), SF-295 (CNS glioblastoma), and HL-60 (leukemia) | [201] |
| Pseudodiploria strigosa | Stony corals | Aqueous extract | Hemolytic activity in humans and rats | [77] |
| Pseudoplexaura porosa | Gorgonians (sea fans) | 14-acetoxycrassine | Inhibitory activity against PTP1B | [150] |
| Pseudopterogogia elisabethae | Gorgonians (sea fans) | Elisapterosin B | In vitro antituberculosis activity | [202] |
| Pseudopterogogia elisabethae | Gorgonians (sea fans) | Aberrarone | In vitro antimalarial activity against a chloroquine-resistant strain of the protozoan parasite Plasmodium falciparum | [203] |
| Pseudopterogogia elisabethae | Gorgonians (sea fans) | Methanol extract | Antibacterial activity selectively against the Gram-positive bacteria Streptococcus pyogenes, Staphylococcus aureus, and Enterococcus faecalis | [204] |
| Pseudopterogogia elisabethae | Gorgonians (sea fans) | Homopseudopteroxazole | Strong growth inhibitor of Mycobacterium tuberculosis | [205] |
| Pseudopterogorgia acerosa | Gorgonians (sea fans) | lipidyl pseudopteranes A and D | Inhibitory activity against PTP1B (Protein tyrosine phosphatase 1B) | [206] |
| Pseudopterogorgia acerosa | Gorgonians (sea fans) | Bis(pseudopterane) amine | Growth inhibition activity against cancer cell lines (HCT116 and HeLa) | [207] |
| Pseudopterogorgia bipinnata | Gorgonians (sea fans) | Bipinnapterolide B | Antitubercular activity | [103,104] |
| Pseudopterogorgia bipinnata | Gorgonians (sea fans) | Caucanolides A−F | In vitro antiplasmodial activity against the malaria parasite, Plasmodium falciparum | [208] |
| Pseudopterogorgia elisabethae | Gorgonians (sea fans) | Carienol A and B, Elisapterosin B | Antitubercular activity | [103,104] |
| Pseudopterogorgia elisabethae | Gorgonians (sea fans) | Ileabethoxazole | Inhibition of Mycobacterium tuberculosis | [125] |
| Pseudopterogorgia kallos | Gorgonians (sea fans) | Bielschowskysin | Antimalarial activity against Plasmodium falciparum, as well as strong anticancer activity against human cancer cell lines | [209] |
| Pseudopterogorgia rigida | Gorgonians (sea fans) | Curcuphenol | Antibacterial activity | [210] |
| Pseudopterogorgia. elisabethae and its dinoflagellate (Symbiodinium sp.) symbiont | Pennatulacea (Sea fans (Host) | Pseudopterosin X and Y (diterpenes) | Re-epithelialization and enhanced wound healing | [103,204] |
| Pseudoterogorgia rigida | Gorgonians (sea fans) | Perezone | Cytotoxicity against human cancer cell lines | [211] |
| Radianthus crispus | Sea anemones | Polypeptide toxin (Re I) | Lethality against crabs | [212] |
| Radianthus macrodactylus | Sea anemones | Actinoporin RTX-S II | Hemolytic activity and lethal effects | [213] |
| Sarcophyton crassocaule | Soft corals | Polyoxygenated cembranoids ((crassocolides G–M,) Crassocolides N-P) Crassocolide N | Cytotoxicity against human medulloblastoma (Daoy cells), human oral epidermoid carcinoma, and human cervical epithelioid carcinoma cell lines | [214,215] |
| Siderastrea siderea | Stony corals | Aqueous extract | Hemolytic activity in humans and rats | [77] |
| Sinularia flexibilis | Soft corals | Flexilarin D | Exhibiting cytotoxicity against Hep2 tumor cells | [216] |
| Sinularia flexibilis | Soft corals | 11-episinulariolide | Exhibiting strong algacidal properties | [217] |
| Sinularia gibberosa | Soft corals | Gibberoketosterol | Anti-inflammatory effects and cytotoxicity | [218] |
| Sinularia gibberosa and Sarcophyton trocheliophorum | Soft corals | Cembranoids Diterpenes | Cytotoxic effects | [219] |
| Sinularia polydactyla | Soft corals | Methanol and hexane extracts | Inhibition of biofilm-forming bacteria | [220] |
| Sinularia querciformis | Soft corals | Querciformolide C | Anti-inflammatory effects | [221] |
| Stichodactyla gigantea | Sea anemones | Gigantoxins I | Exhibiting human epidermal growth factor (EGF) like activity | [222] |
| Stichodactyla haddoni-associated bacteria | Sea anemones (Host) | Culture extracts | Antimicrobial activity, human bacterial and fungal pathogens | [223] |
| Stichodactyla helianthus | Sea anemones | ShK | Inhibiting voltage-dependent potassium channels, competing with dendrotoxin I and α-dendrotoxin for attachment to synaptosomal membranes of rat brain, and suppressing potassium currents in rat dorsal root ganglion neurons in culture. | [224] |
| Stichodactyla helianthus | Sea anemones | Helianthamide (β-Defensin-like Protein) | Inhibiting glycosidase (controlling blood sugar levels in the management of diabetes) | [225] |
| Stichodactyla helianthus | Sea anemones | Sticholysin I (St-I) and sticholysin II (St-II) | Hemolytic activity | [226] |
| Streptomyces sp. SCSIO 41399, from coral Porites sp. | Stony corals (host) | Isotirandamycin B, tirandamycin A, and tirandamycin B | Bacteriostatic activity against Streptococcus agalactiae | [89] |
| Stylophora sp. | Stony corals | Δ-Pocilopotoxin-Spi1 (Δ-PCTX-Spi1 | Hemolytic activity | [227] |
| Subergorgia sp., | Gorgonians (sea fans) | Astaxanthin | Inhibitory activity towards the IKKbeta kinase | [228] |
| Tubastraea coccinea and Tubastraea tagusensis (Sun corals) | Stony corals | Extracts | Anti-inflammatory activity, Cytotoxicity | [229] |
| Urticina aff. coriacea | Biologically active peptides | Inhibiting currents of mammalian ASIC1a channels | [230] | |
| Urticina crassicornis | Sea anemones | UcI (Cytolysin) | Cytolytic activity | [231] |
| Urticina eques | Sea anemones | Peptide-τ-AnmTx Ueq 12-1 | Antibacterial against Gram-positive bacteria and a potentiating activity on the transient receptor potential ankyrin 1 (TRPA1) | [186] |
| Urticina grebelnyi | Sea anemones | π-AnmTX Ugr 9a-1 (Ugr 9-1) | Anti-inflammatory activity and reversing acid-induced pain using in vivo mice | [232] |
| Urticina piscivora | Sea anemones | UpI protein | Hemolytic activity on erythrocytes of rat, guinea pig, dog, pig, and human | [233] |
| Veretillum malayense | Pennatulacea (sea pens) | Briarane diterpenes (Malayenolides A−D), Malayenolide A | Toxic to brine shrimp | [234] |
| Virgularia gustaviana | Pennatulacea (sea pens) | Cholest,5en,3ol (cholesterol), Hexadecanoic acid, 2-Hexadecanol | Reduced viability of breast cancer cell line MDA-MB-231 and human cervical cancer cell line HeLa, induction of apoptosis. | [235] |
| Virgularia juncea | Pennatulacea (Sea pens) | Sesquiterpenoid (Junceol A) and diterpenoids (Sclerophytin A and Cladiellisin. | Cytotoxicity towards P-388 cancer cells | [236] |
| Xenia blumi | Soft corals | Blumiolide C (diterpenoid) | Cytotoxicity against mouse lymphocytic leukemia cells (P-388) | [237] |
| Xenia novaebrittanniae | Soft corals | Xeniolides I | Antibacterial activity | [238] |
| Xenia novaebrittanniae | Soft corals | Novaxenicins (diterpenoids) | Pro-apoptotic activity in transformed mammalian cells | [238] |
| Zoanthus cf.pulchellus | Zoanthids | Zoanthamine | ROS and NO modulators in Neuroinflammation in microglia BV-2 cells | [239] |
| Zoanthus sociatus | Zoanthids | Y-like polypeptide, ZoaNPY | Proangiogenic activity (In vitro) | [63] |
| Zoanthus. natalensis | Zoanthids | ZoaKuz1 | Neuroprotective activity | [64] |
| Assembly Accession | Organism Name | Assembly Stats: Total Sequence Length | Assembly Release Date | Assembly Sequencing Tech | Annotation Count Gene Total | Annotation Count Gene Protein-coding |
|---|---|---|---|---|---|---|
| GCA_932526225.1 | Nematostella vectensis | 269418438 | 23 March 2022 | PacBio, (Pacific Biosciences, Menlo Park, CA, USA) and Arima2 (Arima Genomics, Inc., San Diego, CA, USA) | 38762 | 19231 |
| GCA_013753865.1 | Acropora millepora | 475381253 | 29 July 2020 | PacBio (Pacific Biosciences, Menlo Park, CA, USA) | 42775 | 30136 |
| GCA_036669905.1 | Acropora muricata | 487333246 | 22 February 2024 | PacBio Sequel II (Pacific Biosciences, Menlo Park, CA, USA) | 44841 | 26093 |
| GCA_036669915.2 | Pocillopora verrucosa | 353416094 | 22 February 2024 | PacBio Sequel II (Pacific Biosciences, Menlo Park, CA, USA) | 32915 | 25867 |
| GCA_036669935.2 | Montipora foliosa | 789253072 | 23 August 2024 | PacBio Sequel II (Pacific Biosciences, Menlo Park, CA, USA) | 46203 | 31642 |
| GCA_036669925.2 | Montipora capricornis | 809264945 | 23 August 2024 | PacBio Sequel II (Pacific Biosciences, Menlo Park, CA, USA) | 47382 | 32425 |
| GCA_001417965.1 | Exaiptasia diaphana | 256132296 | 28 October 2015 | Illumina HiSeq; Illumina MiSeq (Illumina, Inc., San Diego CA, USA) | 26789 | 26042 |
| GCA_001417965.1 | Exaiptasia diaphana | 256132296 | 28 October 2015 | Illumina HiSeq; Illumina MiSeq (Illumina, Inc., San Diego, CA, USA) | 24862 | 22509 |
| GCA_000222465.2 | Acropora digitifera | 447478678 | 15 January 2016 | 454 GS FLX (Roche (via Roche Applied Science), Basel, Switzerland); Illumina Genome Analyzer (Illumina, Inc., San Diego, CA, USA) | 32106 | 26073 |
| GCA_002571385.2 | Stylophora pistillata | 397587675 | 17 October 2017 | Illumina HiSeq (Illumina, Inc., San Diego, CA, USA) | 25506 | 23941 |
| GCA_002571385.2 | Stylophora pistillata | 397587675 | 17 October 2017 | Illumina HiSeq (Illumina, Inc., San Diego, CA, USA) | 28498 | 24473 |
| GCA_002042975.1 | Orbicella faveolata | 485532801 | 20 March 2017 | Illumina HiSeq; Illumina MiSeq (Illumina, Inc., San Diego, CA, USA) | 30178 | 25929 |
| GCA_003704095.1 | Pocillopora damicornis | 234333463 | 31 October 2018 | Illumina (Illumina, Inc., San Diego, CA, USA) | 26075 | 25422 |
| GCA_003704095.1 | Pocillopora damicornis | 234333463 | 31 October 2018 | Illumina (Illumina, Inc., San Diego, CA, USA) | 23077 | 19935 |
| GCA_029204205.1 | Desmophyllum pertusum | 556858542 | 8 March 2023 | PacBio Sequel (Pacific Biosciences, Menlo Park, CA, USA); Illumina HiSeq (Illumina, Inc., San Diego, CA, USA) | 40679 | 37484 |
| GCA_009602425.1 | Actinia tenebrosa | 238179736 | 6 November 2019 | Illumina HiSeq (Illumina, Inc., San Diego, CA, USA) | 22927 | 19980 |
| GCA_942486035.1 | Porites lobata | 646152978 | 15 May 2023 | ONT (Oxford Nanopore Technologies Ltd., Oxford, UK;), Illumina (Illumina, Inc., San Diego, CA, USA) | 42872 | 42872 |
| GCA_004324835.1 | Dendronephthya gigantea | 286131786 | 4 March 2019 | PacBio (Pacific Biosciences, Menlo Park, CA, USA) | 29721 | 22045 |
| GCA_902702795.2 | Paramuricea clavata | 606969498 | 8 November 2022 | Illumina (Illumina, Inc., San Diego, CA, USA); ONT (Oxford Nanopore Technologies Ltd., Oxford, UK). | 1600035 | 62650 |
| GCA_942486045.1 | Pocillopora meandrina | 347233126 | 15 May 2023 | ONT (Oxford Nanopore Technologies Ltd., Oxford, UK); Illumina (Illumina, Inc., San Diego, CA, USA). | 32095 | 32095 |
| GCA_942486025.1 | Porites evermanni | 603805388 | 15 May 2023 | Illumina (Illumina, Inc., San Diego, CA, USA) | 40381 | 40380 |
| GCA_032359415.1 | Acropora cervicornis | 307445771 | 4 October 2023 | Oxford Nanopore MinION (Oxford Nanopore Technologies Ltd., Oxford, UK); Illumina HiSeq (Illumina, Inc., San Diego, CA, USA) | 33794 | 28059 |
| GCA_021976095.1 | Xenia sp. Carnegie-2017 | 222699550 | 4 February 2022 | Illumina (Illumina, Inc., San Diego, CA, USA); Nanopore (Oxford Nanopore Technologies Ltd., Oxford, UK); Hi-C (Arima Genomics, Inc., San Diego, CA, USA) | 26389 | 18425 |
| GCA_033675265.1 | Actinostola sp. cb2023 | 424251646 | 15 November 2023 | PacBio Sequel (Pacific Biosciences, Menlo Park, CA, USA) | 20812 | 20812 |
| GCA_000209225.1 | Nematostella vectensis | 356613585 | 22 August 2007 | - | 27173 | 24773 |
| GCA_000209225.1 | Nematostella vectensis | 356613585 | 22 August 2007 | - | 37751 | 23845 |
| GCA_004143615.1 | Acropora millepora | 386599652 | 6 February 2019 | Illumina HiSeq (Illumina, Inc., San Diego, CA, USA). | 31132 | 23710 |
| GCA_030620025.1 | Pocillopora verrucosa | 356884395 | 3 August 2023 | Oxford Nanopore PromethION (Oxford Nanopore Technologies Ltd., Oxford, UK); Illumina NovaSeq (Illumina, Inc., San Diego, CA, USA) | 32047 | 26915 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakariya, M.; Lincoln, O.J.; D’Ambra, I.; Lauritano, C. Anthozoan Chemical Defenses: Integrating Compounds, Enzymatic Activities, and Omics-Based Discoveries. Int. J. Mol. Sci. 2025, 26, 6109. https://doi.org/10.3390/ijms26136109
Zakariya M, Lincoln OJ, D’Ambra I, Lauritano C. Anthozoan Chemical Defenses: Integrating Compounds, Enzymatic Activities, and Omics-Based Discoveries. International Journal of Molecular Sciences. 2025; 26(13):6109. https://doi.org/10.3390/ijms26136109
Chicago/Turabian StyleZakariya, Muhammad, Oliver J. Lincoln, Isabella D’Ambra, and Chiara Lauritano. 2025. "Anthozoan Chemical Defenses: Integrating Compounds, Enzymatic Activities, and Omics-Based Discoveries" International Journal of Molecular Sciences 26, no. 13: 6109. https://doi.org/10.3390/ijms26136109
APA StyleZakariya, M., Lincoln, O. J., D’Ambra, I., & Lauritano, C. (2025). Anthozoan Chemical Defenses: Integrating Compounds, Enzymatic Activities, and Omics-Based Discoveries. International Journal of Molecular Sciences, 26(13), 6109. https://doi.org/10.3390/ijms26136109








