The Role of High-Risk Cytogenetics in Acute Kidney Injury of Newly Diagnosed Multiple Myeloma: A Cohort Study
Abstract
1. Introduction
2. Results
2.1. AKI at Diagnosis
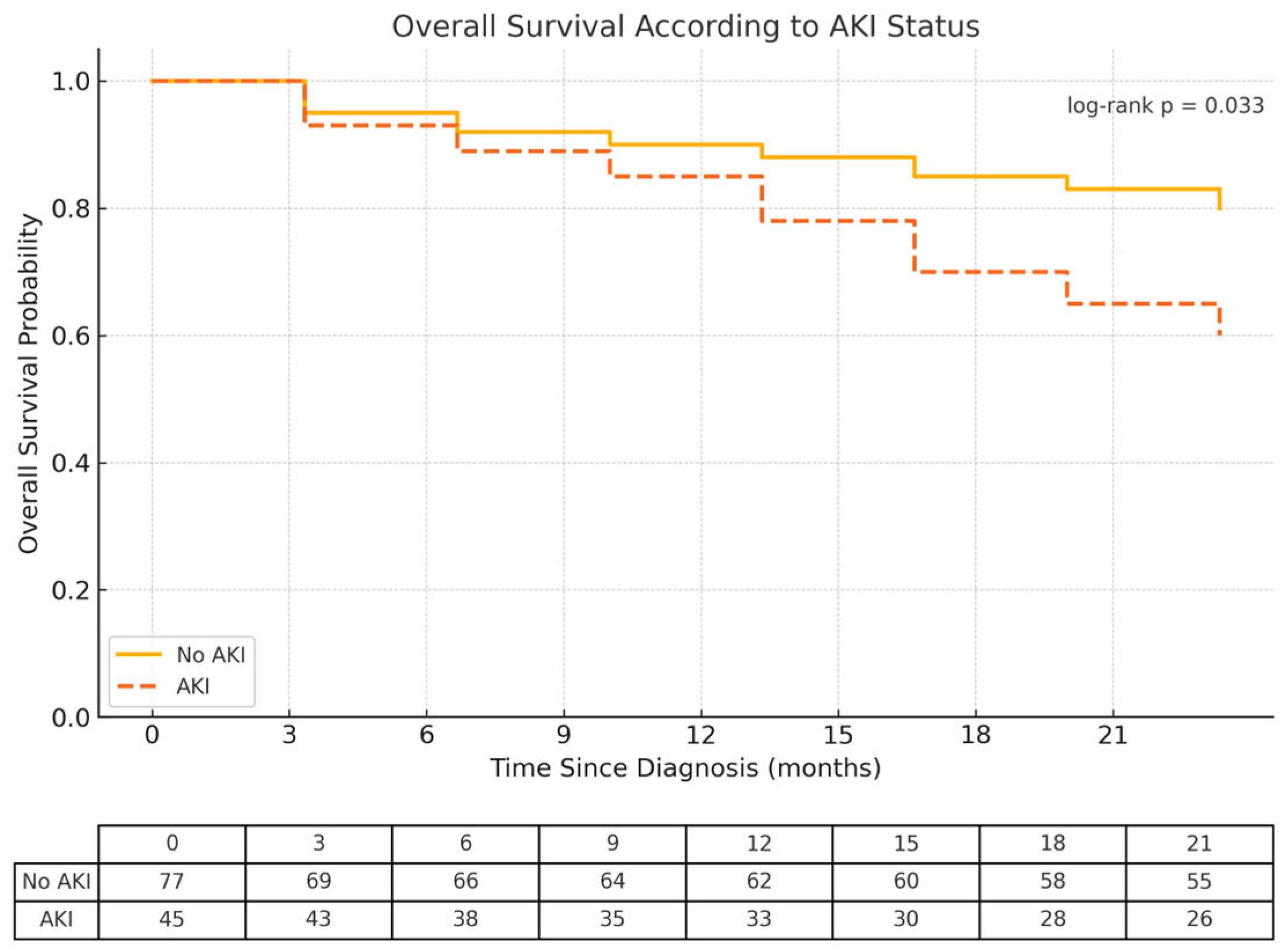
2.2. The Impact of AKI on Overall Survival
2.3. The Impact of AKI on Relapse-Free Survival
2.4. The Impact of AKI on the Progression to CKD
3. Discussion
4. Materials and Methods
4.1. Study Design, Population, and Data Collection
4.2. Definitions
4.3. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castaneda, O.; Baz, R. Multiple Myeloma Genomics—A Concise Review. Acta Med. Acad. 2019, 48, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V. The multiple myelomas—Current concepts in cytogenetic classification and therapy. Nat. Rev. Clin. Oncol. 2018, 15, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic Complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 13–100. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, 48–538. [Google Scholar] [CrossRef]
- Rajan, A.M.; Rajkumar, S.V. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015, 5, e365. [Google Scholar] [CrossRef]
- Chretien, M.L.; Corre, J.; Lauwers-Cances, V.; Ma-grangeas, F.; Cleynen, A.; Yon, E.; Hulin, C.; Leleu, X.; Orsini-Piocelle, F.; Blade, J.-S.; et al. Under-standing the role of hyperdiploidy in myeloma prognosis: Which trisomies really matter? Blood 2015, 12, 2713–2719. [Google Scholar] [CrossRef]
- Binder, M.; Rajkumar, S.V.; Ketterling, R.P.; Greipp, P.T.; Dispenzieri, A.; Lacy, M.Q.; A Gertz, M.; Buadi, F.K.; Hayman, S.R.; Hwa, Y.L.; et al. Prognostic implications of abnormalities of chromosome 13 and the presence of multiple cytogenetic high-risk abnormalities in newly diagnosed multiple myeloma. Blood Cancer J. 2017, 7, e600. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. A high-risk, Double-Hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia 2019, 33, 159–170. [Google Scholar] [CrossRef]
- Hanamura, I. Multiple myeloma with high-risk cytogenetics and its treatment approach. Int. J. Hematol. 2022, 115, 762–777. [Google Scholar] [CrossRef]
- Rees, M.J.; D’Agostino, M.; Leypoldt, L.B.; Kumar, S.; Weisel, K.C.; Gay, F. Navigating High-Risk and Ultrahigh-Risk Multiple Myeloma: Challenges and Emerging Strategies. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e433520. [Google Scholar] [CrossRef]
- D’Agostino, M.; Cairns, D.; Lahuerta, J.; Wester, R.; Bertsch, U.; Waage, A.; Zamagni, E.; Mateos, M.-V.; Dall’OLio, D.; van de Donk, N.W.; et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J. Clin. Oncol. 2022, 40, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, F.; Leung, N.; Nasr, S.H.; Jaccard, A.; Royal, V.; International Kidney and Monoclonal Gammopathy Research Group. Kidney disease in multiple myeloma. Presse Med. 2025, 54, 104264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, J.; Kang, H.; Peng, S.; Tung, T.H.; Shen, B. Prognosis of concurrent renal impairment at diagnosis of multiple myeloma: A systematic review. Ann. Med. 2024, 56, 2380301. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, B. Aetiology and management of acute kidney injury in multiple myeloma. Nephrol. Dial. Transplant. 2018, 33, 722–724. [Google Scholar] [CrossRef]
- Renaghan, A.D.; Costa, J.M.; Esteves, A. Kidney Disease and Hematopoietic Stem Cell Transplantation. Kidney360 2025, 1, 317–330. [Google Scholar] [CrossRef]
- Rodrigues, N.; Costa, C.; Branco, C.; Martins, C.; Lopes, J.A. Acute kidney injury in multiple myeloma patients undergoing autologous hematopoietic stem cell transplant: A cohort study. J. Nephrol. 2024, 37, 419–428. [Google Scholar] [CrossRef]
- Sirohi, B.; Powles, R.; Mehta, J.; Treleaven, J.; Raje, N.; Kulkarni, S.; Rudin, C.; Bhagwati, N.; Horton, C.; Saso, R.; et al. The implication of compromised renal function at presentation in myeloma: Similar outcome in patients who receive high-dose therapy: A single-center study of 251 previously untreated patients. Med. Oncol. 2001, 18, 39–50. [Google Scholar]
- Zhu, W.; Lu, J.; Lu, J.; Hou, J.; Huang, X.; Chen, W. Clinical analysis of newly diagnosed multiple myeloma patients with renal dysfunction. Zhonghua Yi Xue Za Zhi 2015, 17, 741–744. [Google Scholar]
- Courant, M.; Orazio, S.; Monnereau, A.; Preterre, J.; Combe, C.; Rigothier, C. Incidence, prognostic impact and clinical outcomes of renal impairment in patients with multiple myeloma: A population-based registry. Nephrol. Dial. Transplant. 2021, 36, 482–490. [Google Scholar] [CrossRef]
- Knudsen, L.M.; Hippe, E.; Hjorth, M.; Holmberg, E.; Westin, J. Renal function in newly diagnosed multiple myeloma—A demographic study of 1353 patients. Eur. J. Haematol. 1994, 53, 207–212. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 Patients with Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003, 78, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Cook, M.; Cockwell, P. Current Trends of Renal Impairment in Multiple Myeloma. Kidney Dis. 2016, 1, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Eleutherakis-Papaiakovou, V.; Bamias, A.; Gika, D.; Simeonidis, A.; Pouli, A.; Anagnostopoulos, A.; Michali, E.; Economopoulos, T.; Zervas, K.; Dimopoulos, M.A.; et al. Renal failure in multiple myeloma: Incidence, correlations, and prognostic significance. Leuk. Lymphoma 2007, 48, 337–341. [Google Scholar] [CrossRef]
- KDIGO Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 124–138. [Google Scholar]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; The Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Abdallah, N.; Rajkumar, S.V.; Greipp, P.; Kapoor, P.; Gertz, M.A.; Dispenzieri, A.; Baughn, L.B.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; et al. Cytogenetic abnormalities in multiple myeloma: Association with disease characteristics and treatment response. Blood Cancer J. 2020, 10, 82. [Google Scholar] [CrossRef]
- Greenberg, A.J.; Rajkumar, S.V.; Therneau, T.M.; Singh, P.P.; Dispenzieri, A.; Kumar, S.K. Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia 2024, 28, 398–403. [Google Scholar] [CrossRef]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Xue, J.L.; Daniels, F.; Star, R.A.; Kimmel, P.L.; Eggers, P.W.; Molitoris, B.A.; Himmelfarb, J.; Collins, A.J. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 2006, 17, 1135–1142. [Google Scholar] [CrossRef]
- James, M.T.; Grams, M.E.; Woodward, M.; Elley, C.R.; Green, J.A.; Wheeler, D.C.; Gansevoort, R.T.; Levey, A.S.; Warnock, D.G.; Sarnak, M.J.; et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension with Acute Kidney Injury. Am. J. Kidney Dis. 2015, 66, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol. Ther. 2016, 12, S41–S48. [Google Scholar] [CrossRef] [PubMed]
- Menè, P.; Moioli, A.; Stoppacciaro, A.; Lai, S.; Festuccia, F. Acute Kidney Injury in Monoclonal Gammopathies. J. Clin. Med. 2021, 28, 3871. [Google Scholar] [CrossRef]
- Menè, P.; Stoppacciaro, A.; Lai, S.; Festuccia, F. Light Chain Cast Nephropathy in Multiple Myeloma: Prevalence, Impact and Management Challenges. Int. J. Nephrol. Renovasc Dis. 2022, 13, 173–183. [Google Scholar] [CrossRef]
- Leung, N.; Rajkumar, S.V. Multiple myeloma with acute light chain cast nephropathy. Blood Cancer J. 2023, 29, 46. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Scheid, C.; Sonneveld, P.; Schmidt-Wolf, I.G.; van der Holt, B.; Jarari, L.; Bertsch, U.; Salwender, H.; Zweegman, S.; Blau, I.W.; Vellenga, E.; et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: A subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica 2014, 99, 148–154. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Ross, F.M.; Avet-Loiseau, H.; Ameye, G.; Gutiérrez, N.C.; Liebisch, P.; O’connor, S.; Dalva, K.; Fabris, S.; Testi, A.M.; Jarosova, M.; et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012, 97, 1272–1277. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.E.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and Classification of Chronic Kidney Disease: A Position Statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496. [Google Scholar] [CrossRef]
- Iacobelli, S. Suggestions on the Use of Statistical Methodologies in Studies of the Euro-pean Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013, 48, S1–S37. [Google Scholar] [CrossRef] [PubMed]
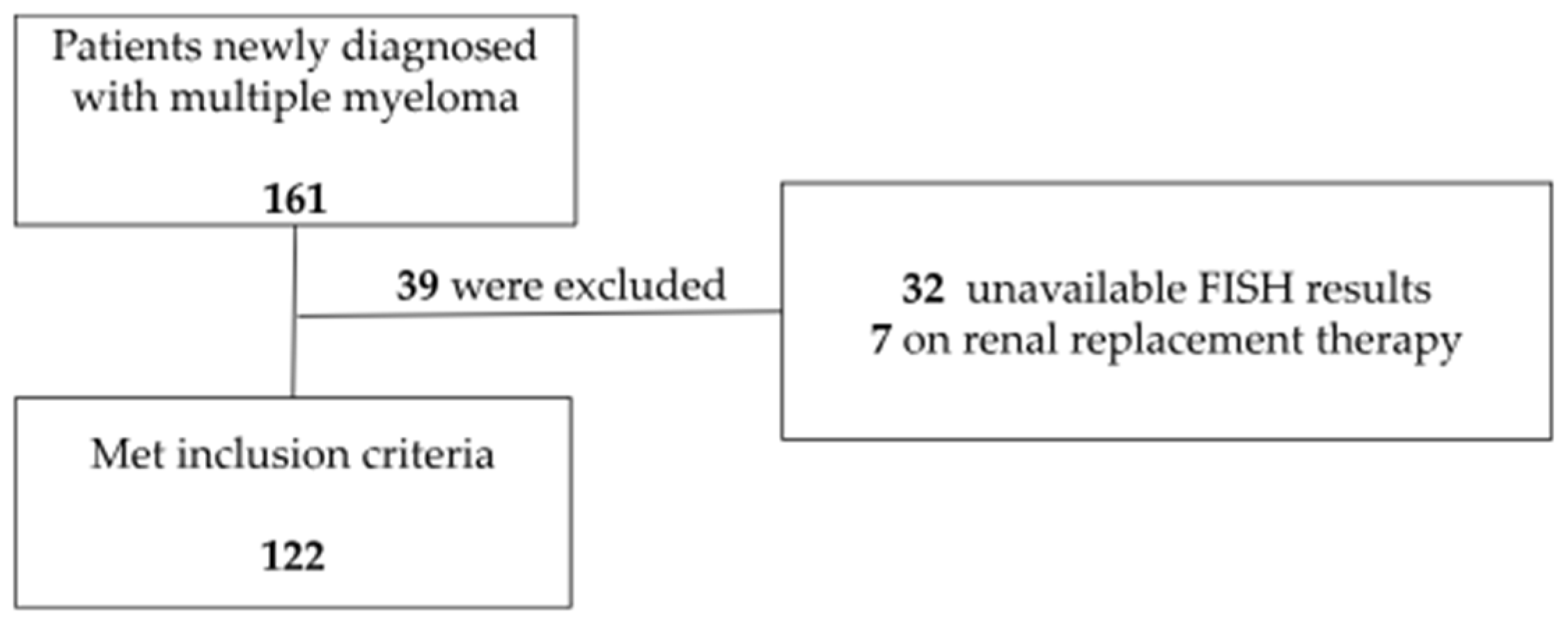
| Patients’ Demographic Characteristics | Category | n (%) or P50 (P25–P75) |
|---|---|---|
| Age (years) | 70.1 (60.8–78.7) | |
| Gender | Male | 64 (52.5) |
| Race | Caucasian | 111 (90.9) |
| Chronic Kidney Disease | 39 (32.0) | |
| Hypertension | 79 (64.8) | |
| Diabetes Mellitus | 28 (23.0) | |
| Heart Disease | 34 (27.9) | |
| MM characteristics at presentation | ||
| R-ISS Staging | I | 31 (25.4) |
| II | 70 (57.4) | |
| III | 21 (17.2) | |
| Secondary Amyloidosis | 4 (3.3) | |
| Extramedullary Disease | 26 (21.3) | |
| Light Chain | L | 55 (45.1) |
| K | 67 (54.9) | |
| Isotype of Myeloma | Light chains | 17 (13.9) |
| IgA | 29 (23.8) | |
| IgD | 1 (0.8) | |
| IgG | 74 (60.7) | |
| IgM | 1 (0.8) | |
| High Risk Cytogenetics | 57 (46.7) | |
| Hypercalcemia | 18 (14.8) | |
| AKI | 45 (36.9) | |
| FLC Ratio | 1.8 (0.05–23.1) | |
| dFLC (mg/L) | 247.8 (48.9–1152.5) | |
| M component (g/dL) | 2.7 (1.1–4.3) | |
| Albumin (g/dL) | 3.7 (3.1–4.1) | |
| β2 Microglobulin (mg/L) | 4.76 (3.0–9.4) | |
| LDH (U/L) | 168 (138–211) | |
| Plasmocytes in Bone Marrow (%) | 39 (16–67) | |
| Undergone treatments | ||
| Proteasome Inhibitors | 100 (81.9) | |
| Autologous HSCT | 38 (31.2) | |
| Total | 122 (100.0) |
| Variables | AKI n = 45 | Non AKI n = 77 | p-Value |
|---|---|---|---|
| Age | 69.9 (63.2–76.6) | 71.8 (60.3–79.4) | 0.524 |
| Gender (Male)—n (%) | 27 (60.0) | 37 (48.1) | 0.202 |
| Race (Caucasian)—n (%) | 42 (93.3) | 69 (89.6) | 0.488 |
| Chronic Kidney Disease—n (%) | 25 (55.6) | 14 (18.2) | <0.001 |
| Hypertension—n (%) | 33 (73.3) | 46 (59.7) | 0.129 |
| Diabetes Mellitus—n (%) | 13 (28.9) | 15 (19.5) | 0.233 |
| Heart Disease—n (%) | 23 (51.1) | 11 (14.3) | 0.519 |
| Light Chain (Reference L)—n (%) | 26 (57.8) | 29 (37.7) | 0.031 |
| Hypercalcemia—n (%) | 13 (28.9) | 5 (6.5) | 0.001 |
| Secondary Amyloidosis—n (%) | 2 (4.4) | 2 (2.6) | 0.580 |
| Extramedullary Disease—n (%) | 6 (13.3) | 20 (26.0) | 0.100 |
| High-Risk Cytogenetics—n (%) | 28 (62.2) | 29 (37.7) | 0.009 |
| FLC Ratio | 0.2 (0.01–36) | 2.12 (0.1–20.9) | 0.398 |
| dFLC—(mg/L) | 837.8 (284.6–3501) | 127.6 (22.3–491.8) | <0.001 |
| M component (g/dL) | 2.6 (0.4–4.9) | 2.6 (1.5–3.75) | 0.294 |
| Albumin (g/dL) | 3.4 (3.0–4.1) | 3.8 (3.2–4.1) | 0.433 |
| β2 Microglobulin (mg/L) | 11.3 (6.35–18) | 3.3 (2.6–4.6) | <0.001 |
| LDH (U/L) | 185 (140–223) | 159.5 (136–193) | 0.063 |
| Plasmocytes in Bone Marrow (%) | 51 (35–77) | 30 (12–62) | 0.001 |
| Variables | Odds Ratio Estimate | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Chronic Kidney Disease | 9.14 | 2.92 | 18.65 | <0.001 |
| Light Chain (Reference L) | 3.70 | 1.31 | 6.45 | 0.013 |
| Hypercalcemia | 3.17 | 1.50 | 5.36 | 0.001 |
| High-risk Cytogenetics | 3.32 | 1.17 | 6.40 | 0.024 |
| dFLC | 1.01 | 1.00 | 1.02 | 0.015 |
| Bone Marrow Plasmocyte Percentage | 1.02 | 1.01 | 1.04 | 0.010 |
| Variables | Hazards Ratio Estimate | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age | 1.05 | 1.01 | 1.09 | 0.011 |
| Gender (Reference: Male) | 0.78 | 0.38 | 1.57 | 0.484 |
| Race (Reference: Caucasian) | 1.19 | 0.36 | 3.90 | 0.778 |
| Chronic Kidney Disease | 2.59 | 1.28 | 5.23 | 0.008 |
| Hypertension | 0.83 | 0.40 | 1.71 | 0.613 |
| Diabetes Mellitus | 2.06 | 0.99 | 4.30 | 0.055 |
| Heart Disease | 2.60 | 1.29 | 5.28 | 0.008 |
| Hypercalcemia | 1.42 | 0.58 | 3.45 | 0.444 |
| Secondary Amyloidosis | 3.32 | 1.50 | 6.00 | 0.049 |
| Extramedullary Disease | 1.15 | 0.49 | 2.67 | 0.748 |
| Light Chain (Reference L) | 0.90 | 0.44 | 1.84 | 0.777 |
| dFLC (mg/L) | 1.01 | 1.00 | 1.01 | 0.028 |
| M Component (mg/dL) | 0.92 | 0.77 | 1.11 | 0.384 |
| Albumin (g/dL) | 0.96 | 0.57 | 1.59 | 0.865 |
| β2 Microglobulin (mg/L) | 1.06 | 1.02 | 1.10 | 0.003 |
| LDH (U/L) | 1.00 | 1.00 | 1.001 | 0.220 |
| Plasmocytes in Bone Marrow (%) | 1.01 | 1.00 | 1.02 | 0.202 |
| High-risk Cytogenetics | 4.21 | 1.61 | 9.82 | 0.003 |
| AKI | 2.16 | 1.06 | 4.38 | 0.033 |
| Proteasome inhibitors | 0.35 | 0.16 | 0.75 | 0.007 |
| Autologous HSCT | 0.47 | 0.22 | 1.02 | 0.057 |
| Relapse | 1.65 | 0.63 | 4.29 | 0.308 |
| Variables | Hazard Ratio Estimate | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Diabetes Mellitus | 1.99 | 1.59 | 4.44 | 0.026 |
| Heart Disease | 3.70 | 1.75 | 7.83 | 0.001 |
| Autologous HSCT | 0.15 | 0.05 | 0.51 | 0.002 |
| High-Risk Cytogenetics | 3.33 | 1.13 | 9.76 | 0.029 |
| AKI | 2.71 | 1.18 | 6.23 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, C.; Silva, M.; Rodrigues, N.; Vieira, J.; Forjaz Lacerda, J.; Lopes, J.A. The Role of High-Risk Cytogenetics in Acute Kidney Injury of Newly Diagnosed Multiple Myeloma: A Cohort Study. Int. J. Mol. Sci. 2025, 26, 6108. https://doi.org/10.3390/ijms26136108
Branco C, Silva M, Rodrigues N, Vieira J, Forjaz Lacerda J, Lopes JA. The Role of High-Risk Cytogenetics in Acute Kidney Injury of Newly Diagnosed Multiple Myeloma: A Cohort Study. International Journal of Molecular Sciences. 2025; 26(13):6108. https://doi.org/10.3390/ijms26136108
Chicago/Turabian StyleBranco, Carolina, Manuel Silva, Natacha Rodrigues, Joana Vieira, João Forjaz Lacerda, and José António Lopes. 2025. "The Role of High-Risk Cytogenetics in Acute Kidney Injury of Newly Diagnosed Multiple Myeloma: A Cohort Study" International Journal of Molecular Sciences 26, no. 13: 6108. https://doi.org/10.3390/ijms26136108
APA StyleBranco, C., Silva, M., Rodrigues, N., Vieira, J., Forjaz Lacerda, J., & Lopes, J. A. (2025). The Role of High-Risk Cytogenetics in Acute Kidney Injury of Newly Diagnosed Multiple Myeloma: A Cohort Study. International Journal of Molecular Sciences, 26(13), 6108. https://doi.org/10.3390/ijms26136108






