Antimicrobial Activity of Carboxymethyl Cellulose Films Containing Plantaricin W and Enterocin F4-9 for Meat Preservation
Abstract
1. Introduction
2. Results
2.1. Antilisterial Activity of CMC Film Activated with Plantaricin W
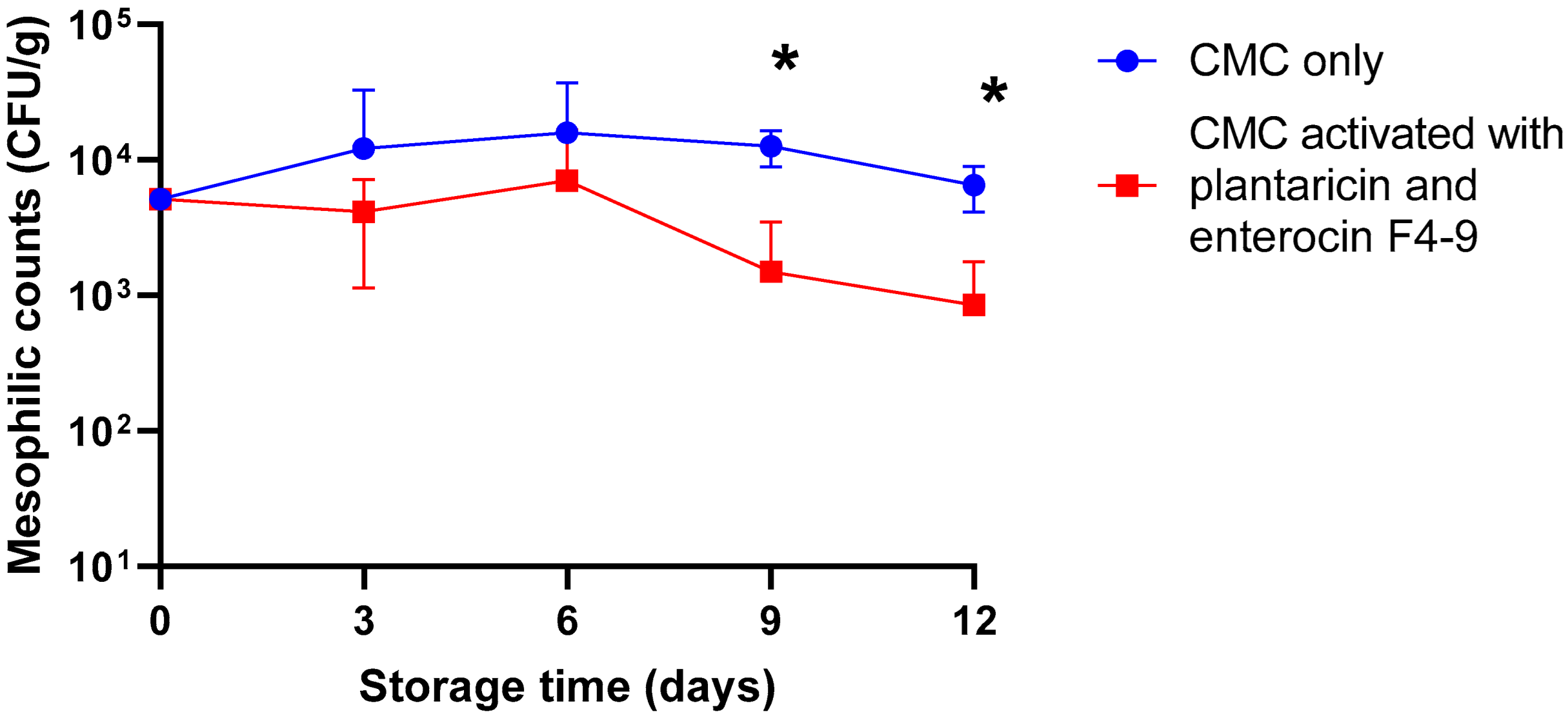
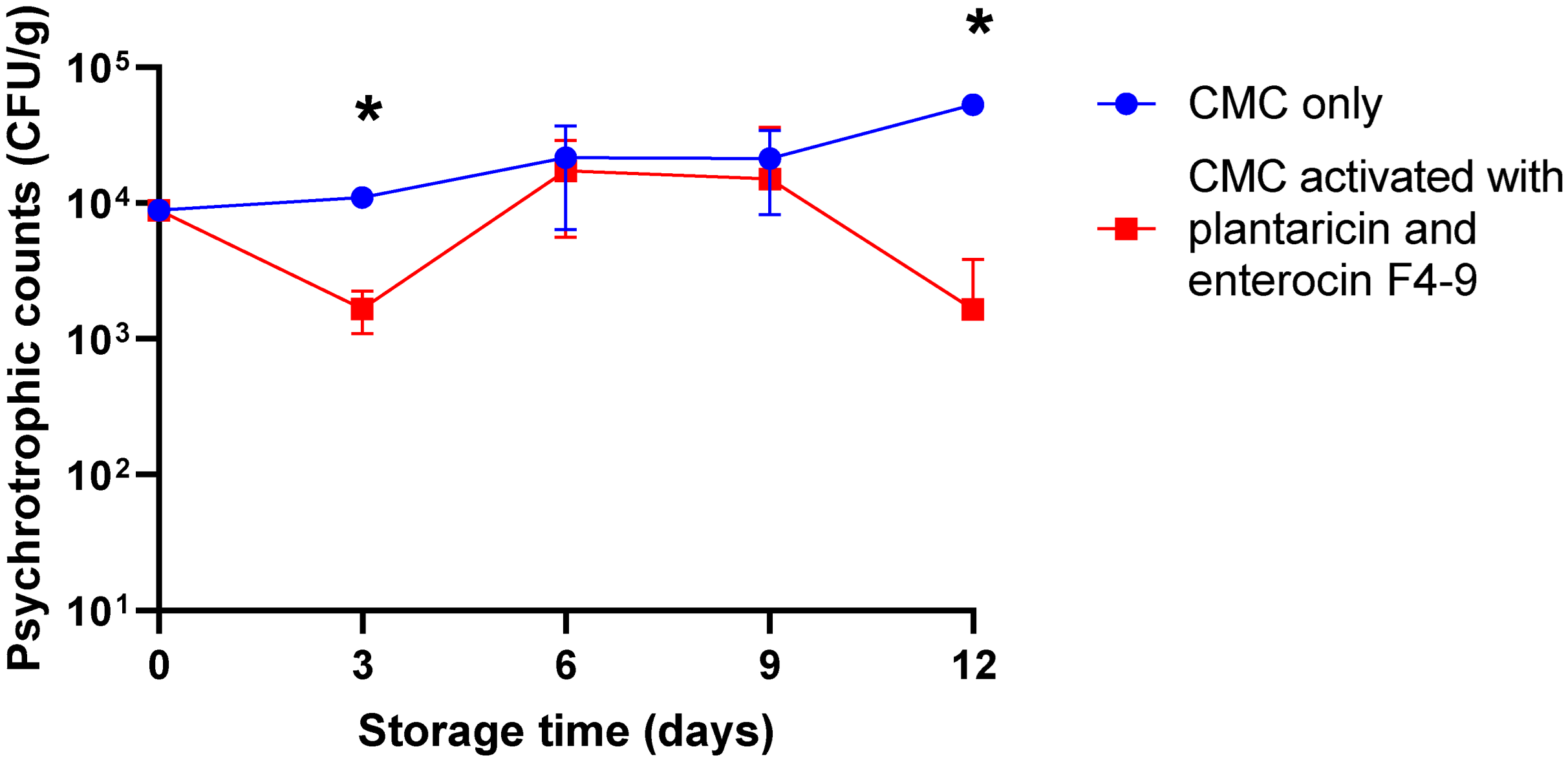
2.2. Antimicrobial Activity of CMC Film Incorporated with Plantaricin W and Enterocin F4-9 Against Meat Microflora
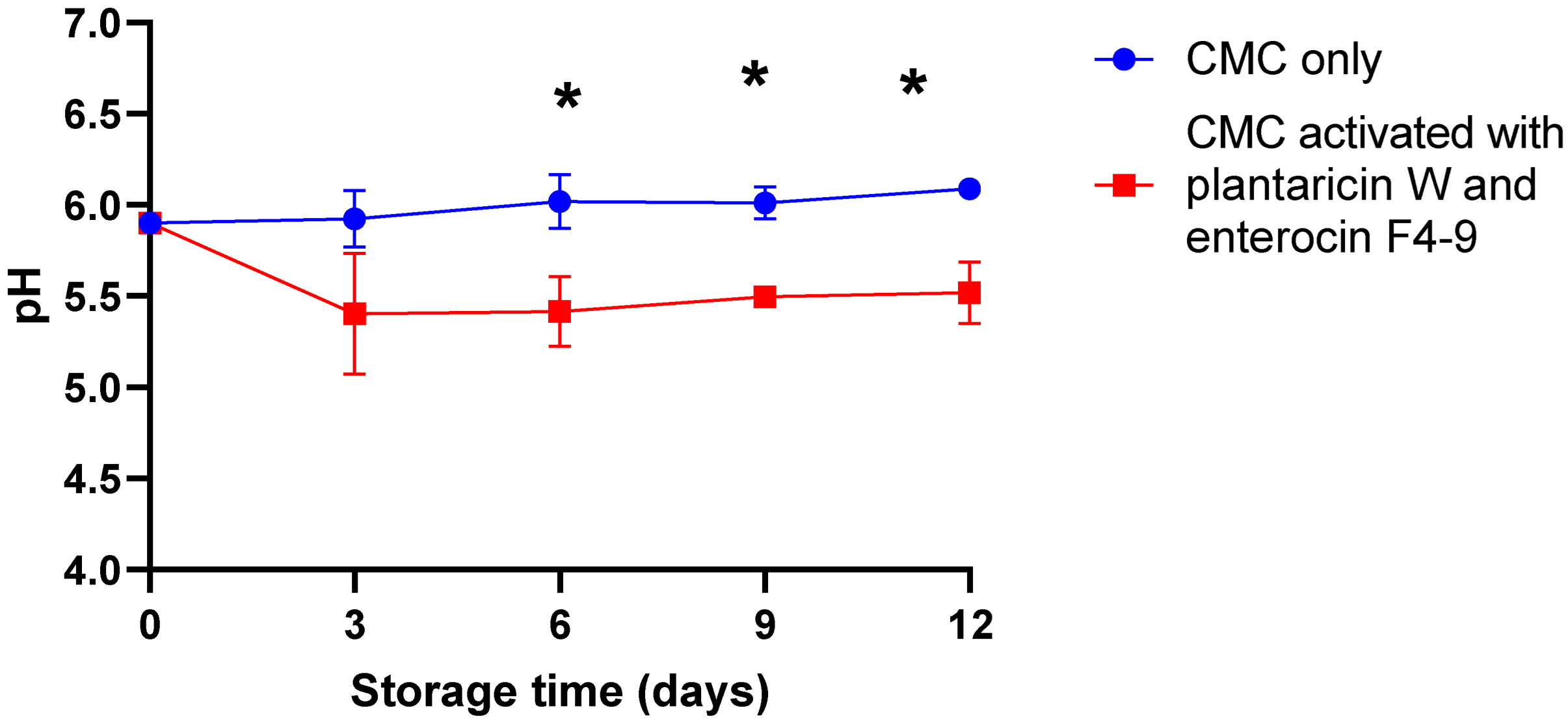
2.3. Effect of CMC Film Incorporated with Plantaricin W and Enterocin F4-9 on the pH of Meat
2.4. Effect of CMC Film Incorporated with Plantaricin W on Listeria During the Storage of Beef Minced Meat
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Media
4.2. Purification of Plantaricin W and Enterocin F4-9
4.3. Production of Two Kinds of Active Films
4.4. Antilisterial Activity of the Plantaricin W-Activated CMC Films
4.5. Activity of CMC Films Incorporated with Plantaricin W and Enterocin F4-9 During the Storage of Beef Minced Meat
4.6. Antilisterial Activity of CMC Film Incorporated with Plantaricin W During the Storage of Beef Minced Meat
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antolinos, V.; Muñoz, M.; Ros-Chumillas, M.; Aznar, A.; Periago, P.M.; Fernández, P.S. Combined Effect of Lysozyme and Nisin at Different Incubation Temperature and Mild Heat Treatment on the Probability of Time to Growth of Bacillus cereus. Food Microbiol. 2011, 28, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, D.; Dernburg, A.; Vernozy-Rozand, C. An Updated Review of Listeria monocytogenes in the Pork Meat Industry and Its Products. J. Appl. Microbiol. 2006, 101, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Corsetti, A. Application of Bacteriocins in Vegetable Food Biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Zendo, T. Screening and Characterization of Novel Bacteriocins from Lactic Acid Bacteria. Biosci. Biotechnol. Biochem. 2013, 77, 893–899. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel Bacteriocins from Lactic Acid Bacteria (LAB): Various Structures and Applications. Microb. Cell Fact. 2014, 13, S3. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, G.; Ercolini, D.; La Storia, A.; Casaburi, A.; Villani, F. Development of Polythene Films for Food Packaging Activated with an Antilisterial Bacteriocin from Lactobacillus curvatus 32Y. J. Appl. Microbiol. 2004, 97, 314–322. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial Food Packaging. In Novel Food Packaging Techniques; Woodhead Publishing: London, UK, 2003; pp. 50–70. [Google Scholar] [CrossRef]
- Pérez-Pérez, C.; Regalado-González, C.; Rodríguez-Rodríguez, C.; Barbosa-Rodríguez, J.R.; Villaseñor-Ortega, F.; Guevara-González, R.; Torres-Pacheco, I. Incorporation of Antimicrobial Agents in Food Packaging Films and Coatings; Research Signpost: Trivandrum, India, 2006. [Google Scholar]
- Gennadios, A.; Hanna, M.A.; Kurth, L.B. Application of Edible Coatings on Meats, Poultry and Seafoods: A Review. Lwt—Food Sci. Technol. 1997, 30, 337–350. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial Food Packaging in Meat Industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Coma, V.; Sebti, I.; Pardon, P.; Deschamps, A.; Pichavant, F.H. Antimicrobial Edible Packaging Based on Cellulosic Ethers, Fatty Acids, and Nisin Incorporation to Inhibit Listeria innocua and Staphylococcus aureus. J. Food Prot. 2001, 64, 470–475. [Google Scholar] [CrossRef]
- Natrajan, N.; Sheldon, B.W. Efficacy of Nisin-Coated Polymer Films to Inactivate Salmonella Typhimurium on Fresh Broiler Skin. J. Food Prot. 2000, 63, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Kingcha, Y.; Amonphanpokin, P.; Kruenate, J.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Anti-Listeria Activity of Poly(Lactic Acid)/Sawdust Particle Biocomposite Film Impregnated with Pediocin PA-1/AcH and Its Use in Raw Sliced Pork. Int. J. Food Microbiol. 2013, 167, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Antimicrobial Biodegradable Food Packaging Impregnated with Bacteriocin 7293 for Control of Pathogenic Bacteria in Pangasius Fish Fillets. LWT 2018, 89, 427–433. [Google Scholar] [CrossRef]
- Syaputri, Y.; Iwahashi, H. Characteristics of Heterologous Plantaricin from Lactobacillus plantarum and Its Future in Food Preservation. Rev. Agric. Sci. 2020, 8, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xie, Y.; Jin, J.; Liu, H.; Zhang, H. Development and Application of an Active Plastic Multilayer Film by Coating a Plantaricin BM-1 for Chilled Meat Preservation. J. Food Sci. 2019, 84, 1864–1870. [Google Scholar] [CrossRef]
- Aymerich, T.; Garriga, M.; Ylla, J.; Vallier, J.; Monfort, J.M.; Hugas, M. Application of Enterocins as Biopreservatives against Listeria innocua in Meat Products. J. Food Prot. 2000, 63, 721–726. [Google Scholar] [CrossRef]
- Maky, M.A.; Ishibashi, N.; Gong, X.; Sonomoto, K.; Zendo, T. Distribution of Bacteriocin-like Substance-Producing Lactic Acid Bacteria in Egyptian Sources. Appl. Microbiol. 2025, 5, 20. [Google Scholar] [CrossRef]
- Holo, H.; Jeknic, Z.; Daeschel, M.; Stevanovic, S.; Nes, I.F. Plantaricin W from Lactobacillus plantarum Belongs to a New Family of Two-Peptide Lantibiotics. Microbiology 2001, 147, 643–651. [Google Scholar] [CrossRef]
- Maky, M.A.; Ishibashi, N.; Zendo, T.; Perez, R.H.; Doud, J.R.; Karmi, M.; Sonomoto, K. Enterocin F4-9, a Novel O-Linked Glycosylated Bacteriocin. Appl. Environ. Microbiol. 2015, 81, 4819–4826. [Google Scholar] [CrossRef]
- Jeleníková, J.; Pipek, P.; Staruch, L. The Influence of Ante-Mortem Treatment on Relationship between PH and Tenderness of Beef. Meat Sci. 2008, 80, 870–874. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Andishmand, H.; Pilevar, Z.; Hashempour-Baltork, F.; Torbati, M.; Dadgarnejad, M.; Rastegar, H.; Mohammadi, S.A.; Azadmard-Damirchi, S. Innovative Perspectives on Bacteriocins: Advances in Classification, Synthesis, Mode of Action, and Food Industry Applications. J. Appl. Microbiol. 2024, 135, 274. [Google Scholar] [CrossRef] [PubMed]
- Cutter, C.N.; Willett, J.L.; Siragusa, G.R. Improved Antimicrobial Activity of Nisin-Incorporated Polymer Films by Formulation Change and Addition of Food Grade Chelator. Lett. Appl. Microbiol. 2001, 33, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.R.; Cutter, C.N.; Willett, J.L. Incorporation of Bacteriocin in Plastic Retains Activity and Inhibits Surface Growth of Bacteria on Meat. Food Microbiol. 1999, 16, 229–235. [Google Scholar] [CrossRef]
- Paunonen, S.I. Strength and Barrier Enhancements of Cellophane and Cellulose Derivative Films: A Review. BioResources 2013, 8, 3098–3121. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A Comprehensive Review on Recent Advances in Preparation, Physicochemical Characterization, and Bioengineering Applications of Biopolymers. Polym Bull 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Microencapsulation of Thai Rice Grass (O. Sativa Cv. Khao Dawk Mali 105) Extract Incorporated to Form Bioactive Carboxymethyl Cellulose Edible Film. Food Chem. 2018, 242, 239–246. [Google Scholar] [CrossRef]
- Ming, X.; Weber, G.H.; Ayres, J.W.; Sandine, W.E. Bacteriocins Applied to Food Packaging Materials to Inhibit Listeria monocytogenes on Meats. J. Food Sci. 1997, 62, 413–415. [Google Scholar] [CrossRef]
- Inoue, S.; Nakama, A.; Arai, Y.; Kokubo, Y.; Maruyama, T.; Saito, A.; Yoshida, T.; Terao, M.; Yamamoto, S.; Kumagai, S. Prevalence and Contamination Levels of Listeria monocytogenes in Retail Foods in Japan. Int. J. Food Microbiol. 2000, 59, 73–77. [Google Scholar] [CrossRef]
- Maky, M.A.; Ishibashi, N.; Nakayama, J.; Zendo, T. Characterization of the Biosynthetic Gene Cluster of Enterocin F4-9, a Glycosylated Bacteriocin. Microorganisms 2021, 9, 2276. [Google Scholar] [CrossRef]
- Bulet, P.; Urge, L.; Ohresser, S.; Hetru, C.; Otvos, L. Enlarged Scale Chemical Synthesis and Range of Activity of Drosocin, an O-Glycosylated Antibacterial Peptide of Drosophila. Eur. J. Biochem. 1996, 238, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Fangio, M.F.; Fritz, R. Potential Use of a Bacteriocin-like Substance in Meat and Vegetable Food Biopreservation. Int. Food Res. J. 2020, 21, 677–683. [Google Scholar]
- Guerra, N.P.; Macías, C.L.; Agrasar, A.T.; Castro, L.P. Development of a Bioactive Packaging Cellophane Using Nisaplin as Biopreservative Agent. Lett. Appl. Microbiol. 2005, 40, 106–110. [Google Scholar] [CrossRef]
- Dawson, P.L.; Harmon, L.; Sotthibandhu, A.; Han, I.Y. Antimicrobial Activity of Nisin-Adsorbed Silica and Corn Starch Powders. Food Microbiol. 2005, 22, 93–99. [Google Scholar] [CrossRef]
- Fiorentini, Â.M.; Sant’Anna, E.S.; Porto, A.C.S.; Mazo, J.Z.; Franco, B.D.G.M. Influence of Bacteriocins Produced by Lactobacillus Plantarum BN in the Shelf-Life of Refrigerated Bovine Meat. Braz. J. Microbiol. 2001, 32, 42–46. [Google Scholar] [CrossRef]
- Mach, N.; Bach, A.; Velarde, A.; Devant, M. Association between Animal, Transportation, Slaughterhouse Practices, and Meat PH in Beef. Meat Sci. 2008, 78, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Zendo, T.; Eungruttanagorn, N.; Fujioka, S.; Tashiro, Y.; Nomura, K.; Sera, Y.; Kobayashi, G.; Nakayama, J.; Ishizaki, A.; Sonomoto, K. Identification and Production of a Bacteriocin from Enterococcus Mundtii QU 2 Isolated from Soybean. J. Appl. Microbiol. 2005, 99, 1181–1190. [Google Scholar] [CrossRef]
- Chana-Thaworn, J.; Chanthachum, S.; Wittaya, T. Properties and Antimicrobial Activity of Edible Films Incorporated with Kiam Wood (Cotyleobium Lanceotatum) Extract. LWT—Food Sci. Technol. 2011, 44, 284–292. [Google Scholar] [CrossRef]
- Theinsathid, P.; Visessanguan, W.; Kruenate, J.; Kingcha, Y.; Keeratipibul, S. Antimicrobial Activity of Lauric Arginate-Coated Polylactic Acid Films against Listeria monocytogenes and Salmonella Typhimurium on Cooked Sliced Ham. J. Food Sci. 2012, 77, M142–M149. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods 8: Use of Data for Process Control and Product Acceptance; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
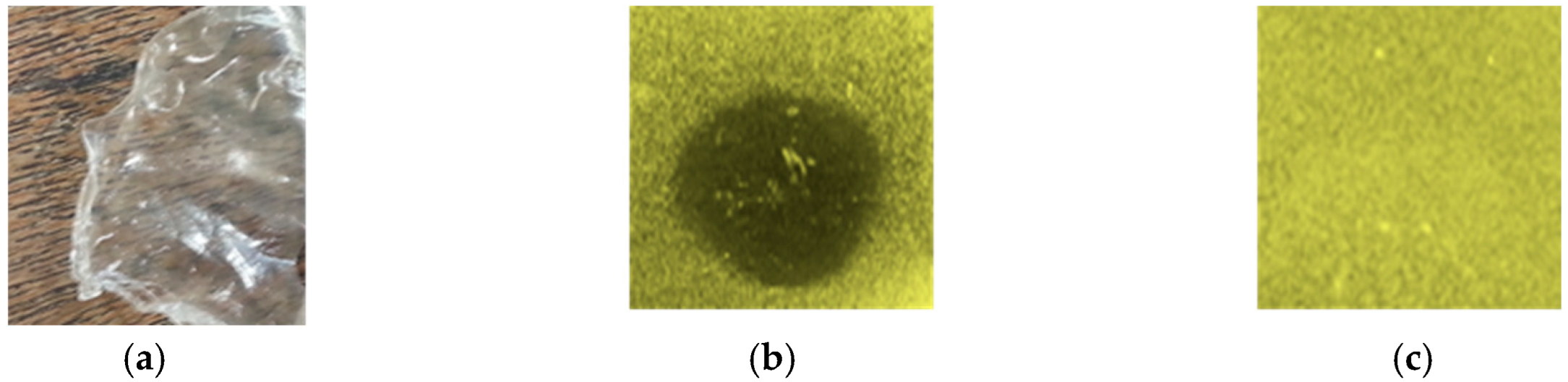

| Mesophilic Bacteria | Psychrotrophic Bacteria | Listeria monocytogenes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | 0 | 1 | 2 | 3 | 4 |
| Inactive film | 5 × 103 ± 2 × 103 | 1 × 104 ± 2 × 104 | 1 × 104 ± 2 × 104 | 1 × 104 ± 3 × 103 | 6 × 103 ± 2 × 103 | 8 × 103 ± 7 × 103 | 1 × 104 ± 1 × 103 | 2 × 104 ± 1 × 104 | 1 × 104 ± 1 × 104 | 3 × 104 ± 1 × 104 | 9 × 103 ± 3 × 102 | 2 × 104 ± 9 × 103 | 4 × 104 ± 2 × 104 | 9 × 104 ± 1 × 104 | 6 × 104 ± 3 × 104 |
| Active film | 5 × 103 ± 2 × 103 | 4 × 103 ± 3 × 103 | 7 × 103 ± 9 × 103 | 1 × 103 ± 1 × 103 | 8 × 102 ± 9 × 102 | 8 × 103 ± 7 × 103 | 1 × 103 ± 6 × 102 | 1 × 104 ± 1 × 104 | 1 × 104 ± 2 × 104 | 1 × 103 ± 2 × 103 | 9 × 103 ± 3 × 102 | 2 × 103 ± 1 × 103 | 1 × 104 ± 8 × 103 | 1 × 104 ± 1 × 104 | 1 × 103 ± 1 × 103 |
| Reduction% a | 0 | 60 | 30 | 90 | 86.67 | 0 | 90 | 50 | 0 | 96.67 | 0 | 90 | 75 | 88.89 | 98.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maky, M.A.; Sonomoto, K.; Zendo, T. Antimicrobial Activity of Carboxymethyl Cellulose Films Containing Plantaricin W and Enterocin F4-9 for Meat Preservation. Int. J. Mol. Sci. 2025, 26, 6083. https://doi.org/10.3390/ijms26136083
Maky MA, Sonomoto K, Zendo T. Antimicrobial Activity of Carboxymethyl Cellulose Films Containing Plantaricin W and Enterocin F4-9 for Meat Preservation. International Journal of Molecular Sciences. 2025; 26(13):6083. https://doi.org/10.3390/ijms26136083
Chicago/Turabian StyleMaky, Mohamed Abdelfattah, Kenji Sonomoto, and Takeshi Zendo. 2025. "Antimicrobial Activity of Carboxymethyl Cellulose Films Containing Plantaricin W and Enterocin F4-9 for Meat Preservation" International Journal of Molecular Sciences 26, no. 13: 6083. https://doi.org/10.3390/ijms26136083
APA StyleMaky, M. A., Sonomoto, K., & Zendo, T. (2025). Antimicrobial Activity of Carboxymethyl Cellulose Films Containing Plantaricin W and Enterocin F4-9 for Meat Preservation. International Journal of Molecular Sciences, 26(13), 6083. https://doi.org/10.3390/ijms26136083








