Effect of Calcination Temperatures on Crystallite Size, Particle Size, and Antimicrobial Activity of Synthesized MgO and Its Cytotoxicity
Abstract
1. Introduction
2. Results and Discussion
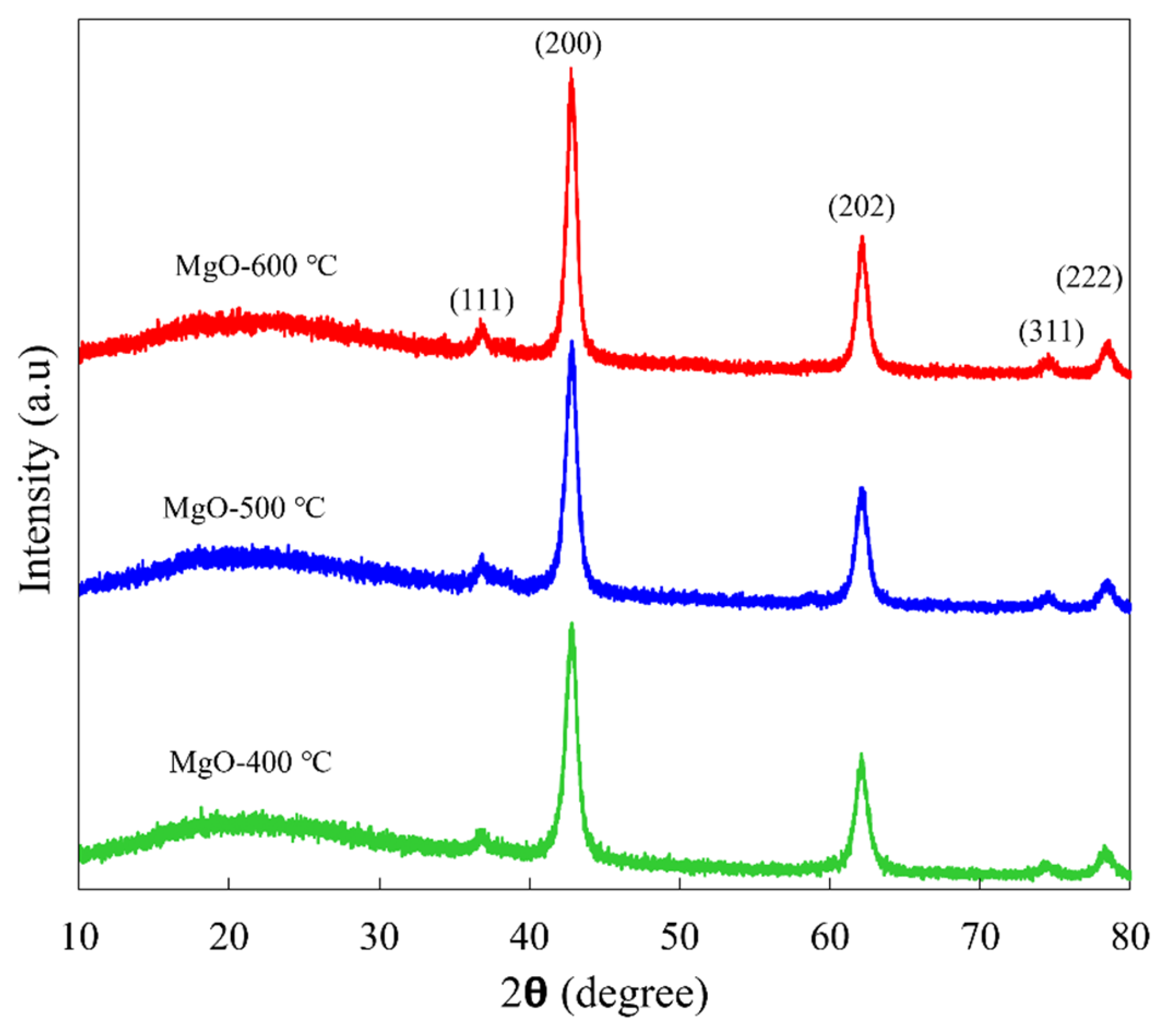
2.1. Crystallographic Structure and Crystallite Size
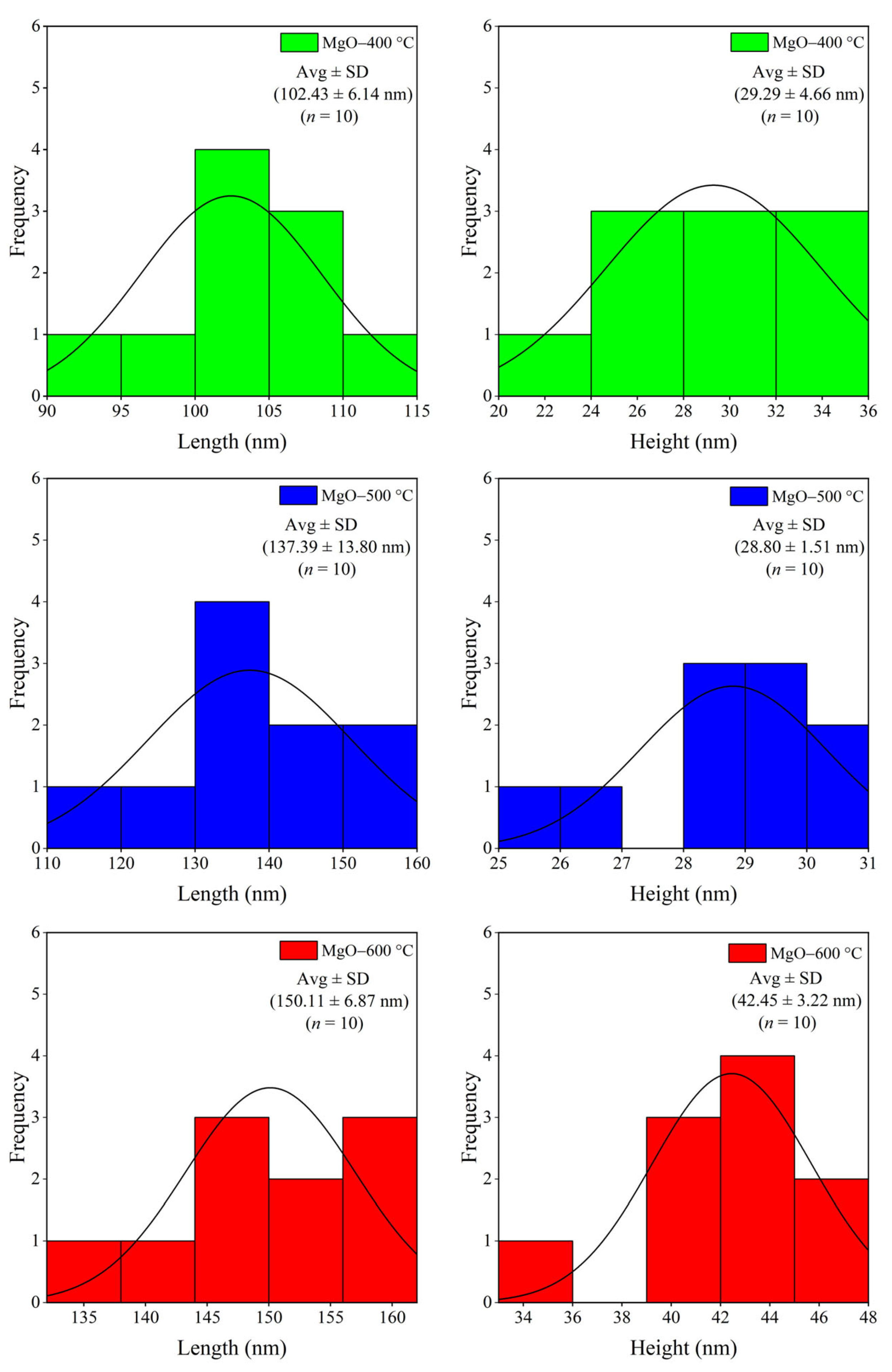
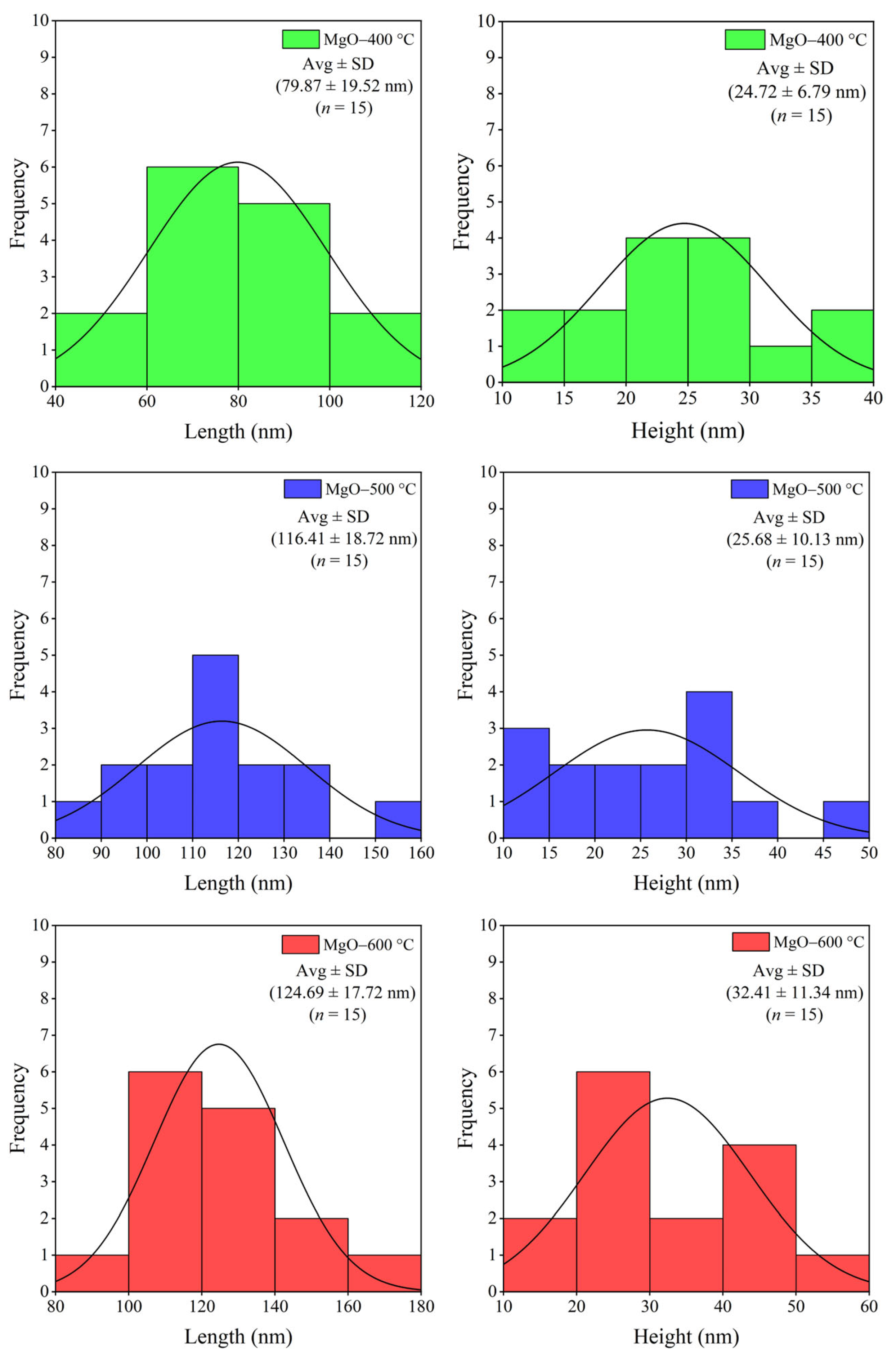
2.2. Morphology and Element Composition
| Sample | Average Crystal Size (nm) | BET Surface Area (m2/g) |
|---|---|---|
| MgO-400 °C | 8.80 | 127.88 |
| MgO-500 °C | 8.88 | 88.06 |
| MgO-600 °C | 10.97 | 86.45 |
2.3. Surface Area of MgO
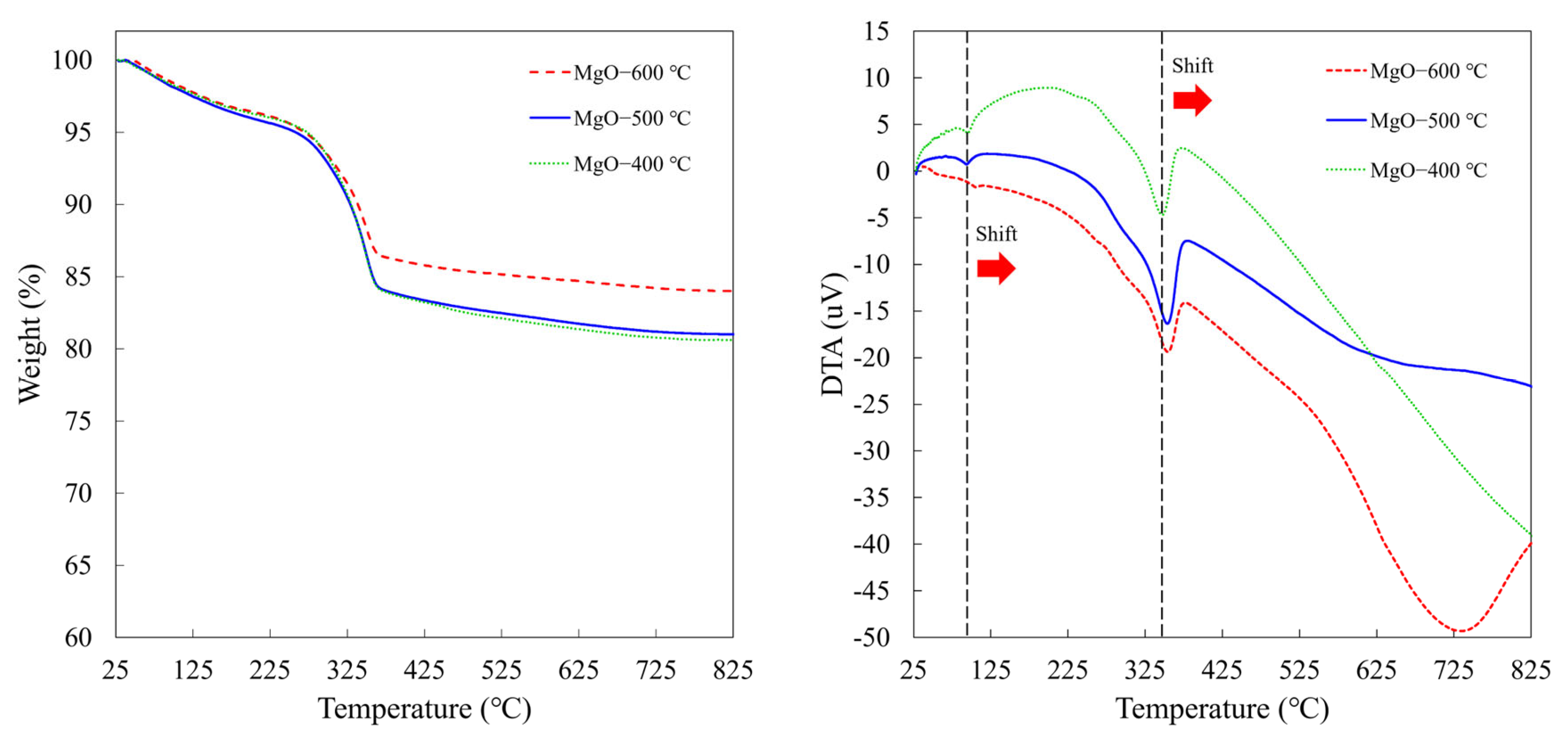
2.4. Thermal Stability
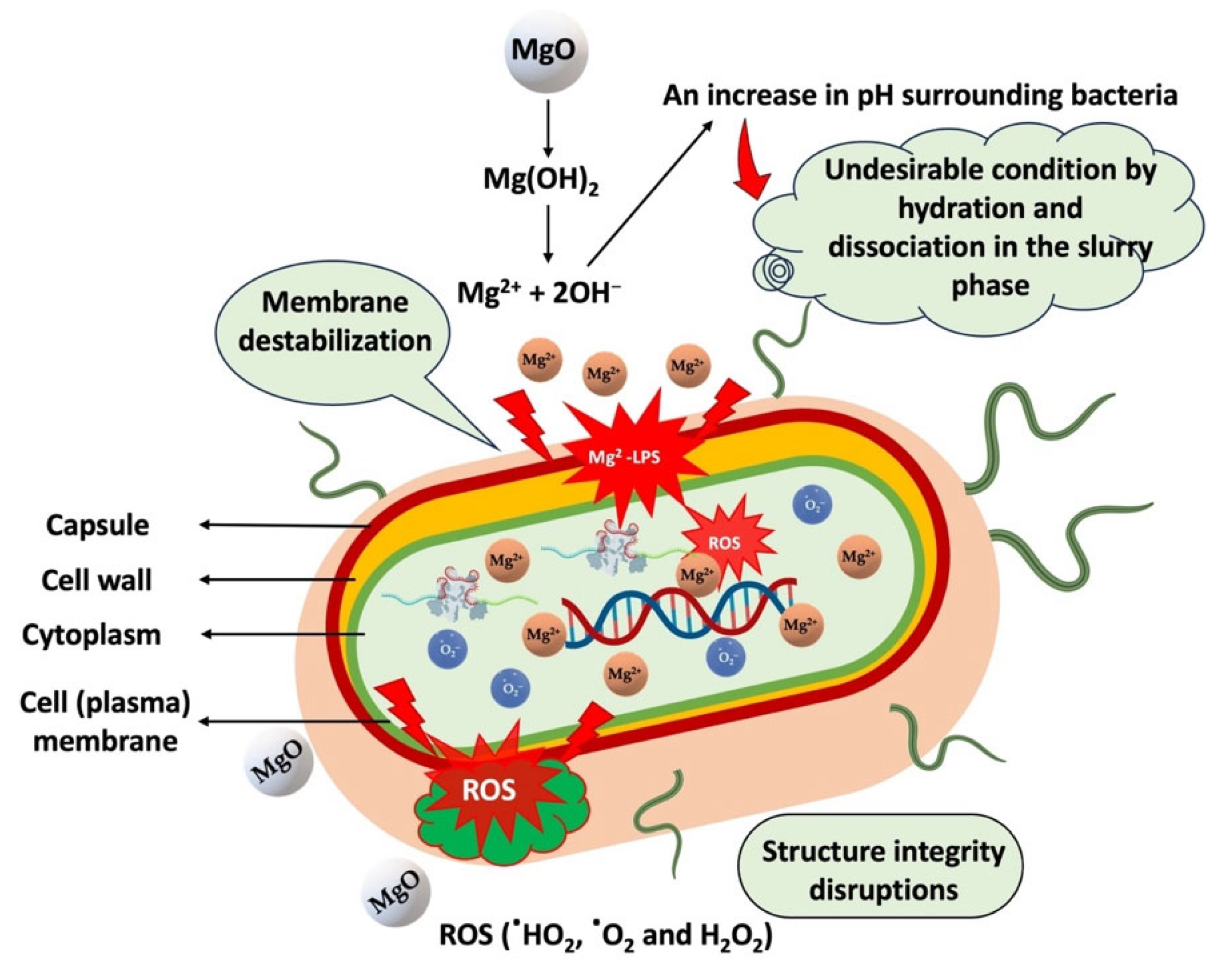
2.5. Antimicrobial Activity
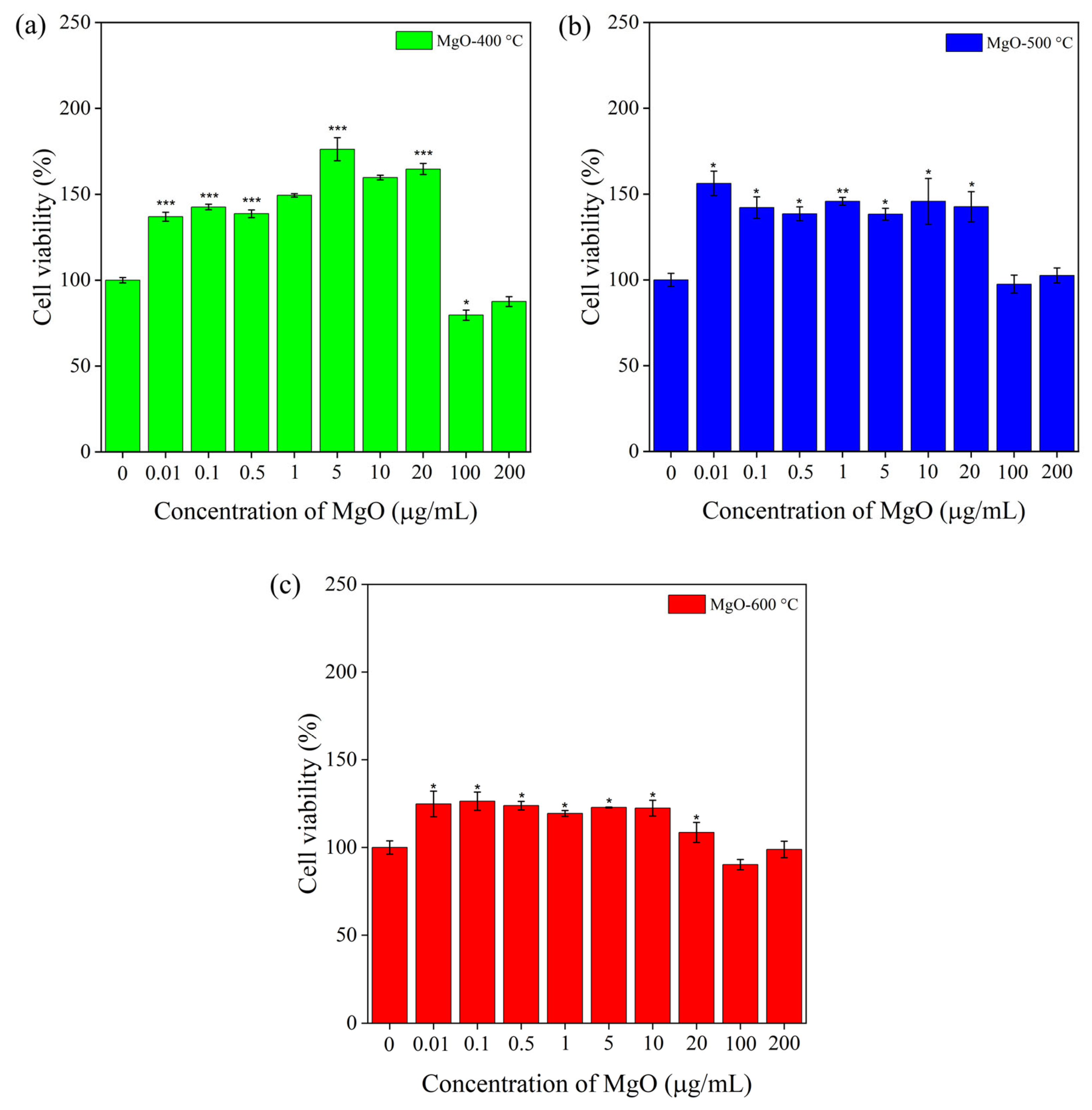
2.6. Viability of RAW 264.7 Macrophage Cells
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MgO
3.3. Characterizations of MgO
3.3.1. XRD Analysis
3.3.2. Morphology and Elemental Composition
3.3.3. Specific Surface Area
3.3.4. Thermal Analysis
3.3.5. Determination of Antimicrobial Activity
3.3.6. Cytotoxicity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Gálvez, F.; Gómez, P.A.; Artés, F.; Artés-Hernández, F.; Aguayo, E. Interactions between microbial food safety and environmental sustainability in the fresh produce supply chain. Foods 2021, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.; Paudel, M.; Poudel, S.; Koirala, N. Food safety in developing countries: Common foodborne and waterborne illnesses, regulations, organizational structure, and challenges of food safety in the context of Nepal. Food Front. 2025, 6, 86–123. [Google Scholar] [CrossRef]
- Sarno, E.; Pezzutto, D.; Rossi, M.; Liebana, E.; Rizzi, V. A review of significant European foodborne outbreaks in the last decade. J. Food Prot. 2021, 84, 2059–2070. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hatta, T.; Labony, S.S.; Kwofie, K.D.; Kawada, H.; Tsuji, N.; Alim, M.A. Food-and vector-borne parasitic zoonoses: Global burden and impacts. Adv. Parasitol. 2023, 120, 87–136. [Google Scholar]
- Papadochristopoulos, A.; Kerry, J.P.; Fegan, N.; Burgess, C.M.; Duffy, G. Natural anti-microbials for enhanced microbial safety and shelf-life of processed packaged meat. Foods 2021, 10, 1598. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Oliveira-Pinto, P.R.; Oliveira-Fernandes, J.; Pereira-Dias, L.; Sousa, R.M.; Santos, C. Organic Nanoparticles as Delivery Tools for Bio-Based Antimicrobials. In Nanoparticles in Plant Biotic Stress Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 107–179. [Google Scholar]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Sultana, A.; Hezam, A.; Surmeneva, M.A.; Surmenev, R.A.; Nayan, M.; Ramakrishna, S.; Mathur, S. Emerging trends for ZnO nanoparticles and their applications in food packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Garavand, F.; Jafari, S.M. Incorporation of silver nanoparticles into active antimicrobial nanocomposites: Release behavior, analyzing techniques, applications and safety issues. Adv. Colloid Interface Sci. 2021, 293, 102440. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium oxide (MgO) nanoparticles: Synthetic strategies and biomedical applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Khan, M.R.; Hammouda, G.A.; Alam, P.; Meng, L.; Zhang, Z.; Zhang, W. New opportunities and advances in magnesium oxide (MgO) nanoparticles in biopolymeric food packaging films. Sustain. Mater. Technol. 2024, 40, e00976. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Zhang, Y.; Zhuang, P.; Wang, J. Breathable functional aerogel dressings facilitate the healing of diabetic wounds. Biomed. Technol. 2025, 9, 100071. [Google Scholar] [CrossRef]
- Bose, I.; Roy, S.; Pandey, V.K.; Singh, R. A comprehensive review on significance and advancements of antimicrobial agents in biodegradable food packaging. Antibiotics 2023, 12, 968. [Google Scholar] [CrossRef]
- Pachiyappan, J.; Gnanansundaram, N.; Sivamani, S.; Sankari, N.P.B.P.; Senthilnathan, N.; Kerga, G.A. Preparation and characterization of magnesium oxide nanoparticles and its application for photocatalytic removal of rhodamine B and methylene blue dyes. J. Nanomater. 2022, 2022, 6484573. [Google Scholar] [CrossRef]
- Gurule, A.C.; Gaikwad, S.S.; Kajale, D.D.; Shinde, V.S.; Jadhav, G.R.; Gaikwad, V.B. Synthesis of magnesium oxide nanoparticles via hydrothermal and sol-gel methods: Charaterization and their application for H2S and NO2 gas sensing. J. Indian Chem. Soc. 2025, 102, 101496. [Google Scholar] [CrossRef]
- Thamizharasan, S.; Gurunathan, K.; Varaprasad, K.; Chandrasekaran, K. Green engineering of MgO nanoparticles: Assessment of their antioxidant and antibacterial activity against dental pathogens. Inorg. Chem. Commun. 2024, 169, 113025. [Google Scholar] [CrossRef]
- Muhaymin, A.; Mohamed, H.E.A.; Hkiri, K.; Safdar, A.; Azizi, S.; Maaza, M. Green synthesis of magnesium oxide nanoparticles using Hyphaene thebaica extract and their photocatalytic activities. Sci. Rep. 2024, 14, 20135. [Google Scholar] [CrossRef]
- Perera, H.; Gurunanthanan, V.; Singh, A.; Mantilaka, M.; Das, G.; Arya, S. Magnesium oxide (MgO) nanoadsorbents in wastewater treatment: A comprehensive review. J. Magnes. Alloys 2024, 12, 1709–1773. [Google Scholar] [CrossRef]
- Nejati, M.; Rostami, M.; Mirzaei, H.; Rahimi-Nasrabadi, M.; Vosoughifar, M.; Nasab, A.S.; Ganjali, M.R. Green methods for the preparation of MgO nanomaterials and their drug delivery, anti-cancer and anti-bacterial potentials: A review. Inorg. Chem. Commun. 2022, 136, 109107. [Google Scholar] [CrossRef]
- Diana, P.; Saravanakumar, S.; Prasad, K.H.; Sivaganesh, D.; Chidhambaram, N.; Isaac, R.R.; Alshahrani, T.; Shkir, M.; AIFaify, S.; Ali, K.S. Enhanced photocatalytic decomposition efficacy of novel MgO NPs: Impact of annealing temperatures. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3027–3036. [Google Scholar] [CrossRef]
- Hegde, V.N.; Manju, V.; Lumbini, J.; Shanika, S.; Somashekar, R. Effect of calcination temperature on structural, morphological elastic and electrical properties of MgO nanoparticles synthesized by combustion method. J. Phys. Chem. Solids 2024, 192, 112071. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Suresh, J.; Gandhi, R.R. A comparative study on antibacterial properties of MgO nanoparticles prepared under different calcination temperature. Dig. J. Nanomater. Biostruct. 2012, 7, 983–989. [Google Scholar]
- Huang, L.; Yang, Z.; Wang, S. Influence of calcination temperature on the structure and hydration of MgO. Constr. Build. Mater. 2020, 262, 120776. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Lu, K. Nanoparticulate Materials: Synthesis, Characterization, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zahir, M.H.; Rahman, M.M.; Irshad, K.; Rahman, M.M. Shape-stabilized phase change materials for solar energy storage: MgO and Mg(OH)2 mixed with polyethylene glycol. Nanomaterials 2019, 9, 1773. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Feng, X.; Han, X.; Bai, Z.; Zhang, Z. Calcination temperature-dependent surface structure and physicochemical properties of magnesium oxide. RSC Adv. 2015, 5, 86102–86112. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Wang, D.; Zhang, X.; Qian, S.; Liu, X. A facile and universal strategy to endow implant materials with antibacterial ability via alkalinity disturbing bacterial respiration. Biomater. Sci. 2020, 8, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Enhanced antimicrobial activity of silver nanoparticles with controlled particle size by pH variation. Powder Technol. 2015, 269, 110–117. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, É.; De Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Kasi, G.; Thanakkasaranee, S.; Stalin, N.; Arumugam, A.; Jantanasakulwong, K.; Panyathip, R.; Sukunta, J.; Tanadchangsaeng, N.; Worajittiphon, P.; Rachtanapun, P. Enhancement of antimicrobial properties and cytocompatibility through silver and magnesium doping strategies on copper oxide nanocomposites. J. Alloys Compd. 2024, 1007, 176481. [Google Scholar] [CrossRef]
- Walker, G.M. The roles of magnesium in biotechnology. Crit. Rev. Biotechnol. 1994, 14, 311–354. [Google Scholar] [CrossRef]
- Kumar, A.; Mehan, S.; Tiwari, A.; Khan, Z.; Das Gupta, G.; Narula, A.S.; Samant, R. Magnesium (Mg2+): Essential mineral for neuronal health: From cellular biochemistry to cognitive health and behavior regulation. Curr. Pharm. Des. 2024, 30, 3074–3107. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Stillwell, A.A.; Tochilovsky, B.V.; Tanzman, J.V.; Limage, R.; Kolba, N.; Tako, E.; Marques, C.N.; Mahler, G.J. The mechanistic effects of human digestion on magnesium oxide nanoparticles: Implications for probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium bifidum VPI 1124. Environ. Sci. Nano 2022, 9, 4540–4557. [Google Scholar] [CrossRef]
- Ge, S.; Wang, G.; Shen, Y.; Zhang, Q.; Jia, D.; Wang, H.; Dong, Q.; Yin, T. Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro. IET Nanobiotechnol. 2011, 5, 36–40. [Google Scholar] [CrossRef]
- Mazaheri, N.; Naghsh, N.; Karimi, A.; Salavati, H. In vivo toxicity investigation of magnesium oxide nanoparticles in rat for environmental and biomedical applications. Iran. J. Biotechnol. 2019, 17, e1543. [Google Scholar] [CrossRef]
- Mittag, A.; Schneider, T.; Westermann, M.; Glei, M. Toxicological assessment of magnesium oxide nanoparticles in HT29 intestinal cells. Arch. Toxicol. 2019, 93, 1491–1500. [Google Scholar] [CrossRef]
- Patel, M.K.; Zafaryab, M.; Rizvi, M.; Agrawal, V.V.; Ansari, Z.; Malhotra, B.; Ansari, S. Antibacterial and cytotoxic effect of magnesium oxide nanoparticles on bacterial and human cells. J. Nanoeng. Nanomanuf. 2013, 3, 162–166. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Rachtanapun, P.; Rachtanapun, C.; Kanthiya, T.; Kasi, G.; Sommano, S.R.; Jantanasakulwong, K.; Seo, J. Bio-Composite Films Based on Carboxymethyl Chitosan Incorporated with Calcium Oxide: Synthesis and Antimicrobial Activity. Polymers 2024, 16, 2393. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-T.; Cho, K.-H.; Sharma, A.; Devi, S.; Park, T.-S. Annona muricata leaf extract attenuates hepatic lipogenesis and adipogenesis. Food Funct. 2021, 12, 4621–4629. [Google Scholar] [CrossRef] [PubMed]


| Sample | Element | Weight (%) | Atomic (%) |
|---|---|---|---|
| MgO-400 °C | Mg | 49.37 | 39.09 |
| O | 50.63 | 60.91 | |
| MgO-500 °C | Mg | 51.47 | 41.11 |
| O | 48.53 | 58.89 | |
| MgO-600 °C | Mg | 60.16 | 49.84 |
| O | 39.84 | 50.16 |
| Samples | Escherichia coli | Staphylococcus aureus | ||
|---|---|---|---|---|
| Variable Cell (CFUs/mL) | R (%) | Variable Cell (CFUs/mL) | R (%) | |
| Control | 5.0 × 108 | - | 7.0 × 108 | - |
| MgO-400 °C | ND | 100 | ND | 100 |
| MgO-500 °C | ND | 100 | ND | 100 |
| MgO-600 °C | ND | 100 | 4.0 × 103 | >99.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasi, G.; Stalin, N.; Rachtanapun, P.; Jantanasakulwong, K.; Halder, J.N.; Phongthai, S.; Worajittiphon, P.; Seo, J.; Thanakkasaranee, S. Effect of Calcination Temperatures on Crystallite Size, Particle Size, and Antimicrobial Activity of Synthesized MgO and Its Cytotoxicity. Int. J. Mol. Sci. 2025, 26, 4868. https://doi.org/10.3390/ijms26104868
Kasi G, Stalin N, Rachtanapun P, Jantanasakulwong K, Halder JN, Phongthai S, Worajittiphon P, Seo J, Thanakkasaranee S. Effect of Calcination Temperatures on Crystallite Size, Particle Size, and Antimicrobial Activity of Synthesized MgO and Its Cytotoxicity. International Journal of Molecular Sciences. 2025; 26(10):4868. https://doi.org/10.3390/ijms26104868
Chicago/Turabian StyleKasi, Gopinath, Nattan Stalin, Pornchai Rachtanapun, Kittisak Jantanasakulwong, Joshua Nizel Halder, Suphat Phongthai, Patnarin Worajittiphon, Jongchul Seo, and Sarinthip Thanakkasaranee. 2025. "Effect of Calcination Temperatures on Crystallite Size, Particle Size, and Antimicrobial Activity of Synthesized MgO and Its Cytotoxicity" International Journal of Molecular Sciences 26, no. 10: 4868. https://doi.org/10.3390/ijms26104868
APA StyleKasi, G., Stalin, N., Rachtanapun, P., Jantanasakulwong, K., Halder, J. N., Phongthai, S., Worajittiphon, P., Seo, J., & Thanakkasaranee, S. (2025). Effect of Calcination Temperatures on Crystallite Size, Particle Size, and Antimicrobial Activity of Synthesized MgO and Its Cytotoxicity. International Journal of Molecular Sciences, 26(10), 4868. https://doi.org/10.3390/ijms26104868













