Integrative Wound-Healing Effects of Clinacanthus nutans Extract and Schaftoside Through Anti-Inflammatory, Endothelial-Protective, and Antiviral Mechanisms
Abstract
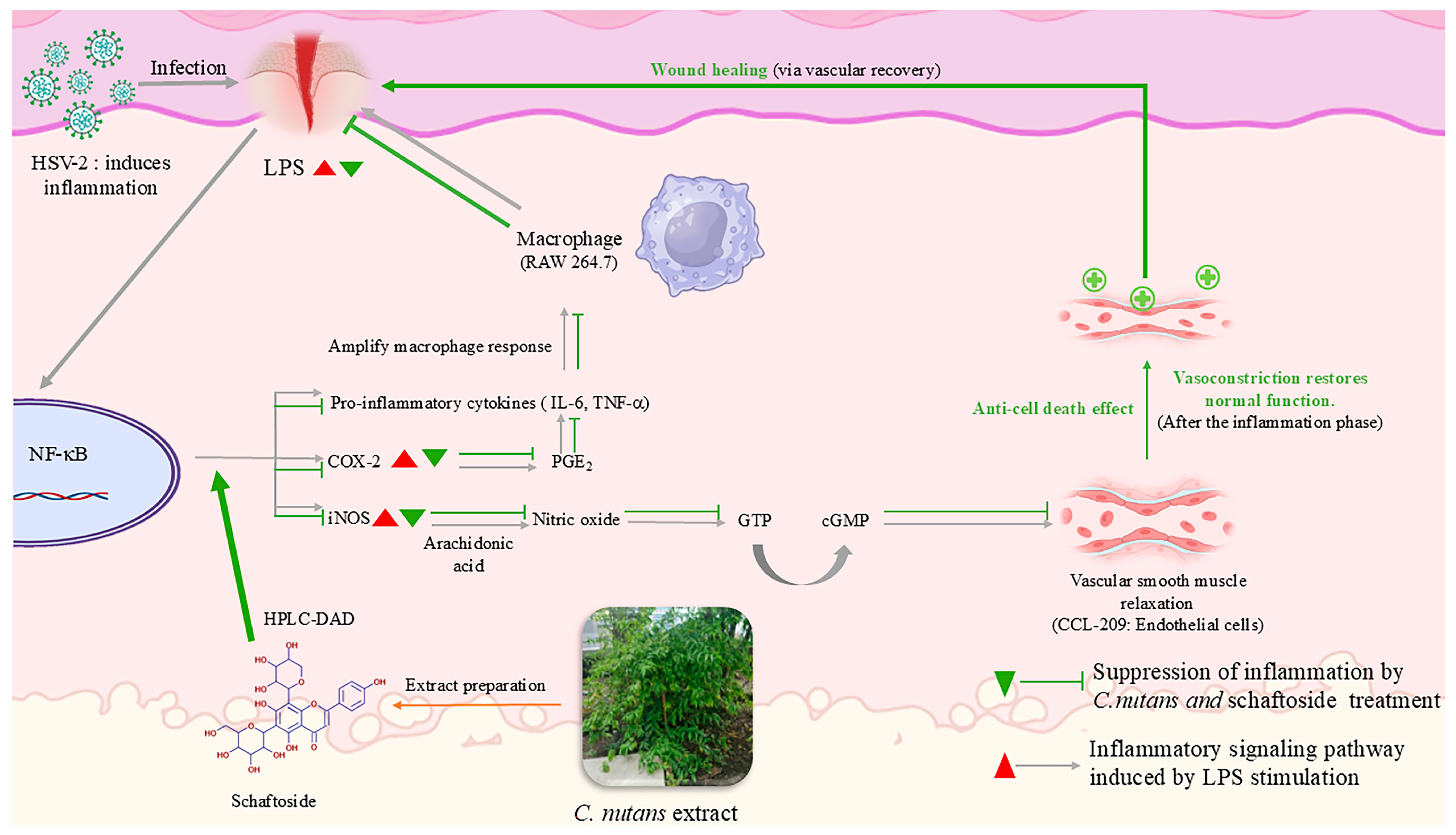
1. Introduction
2. Results
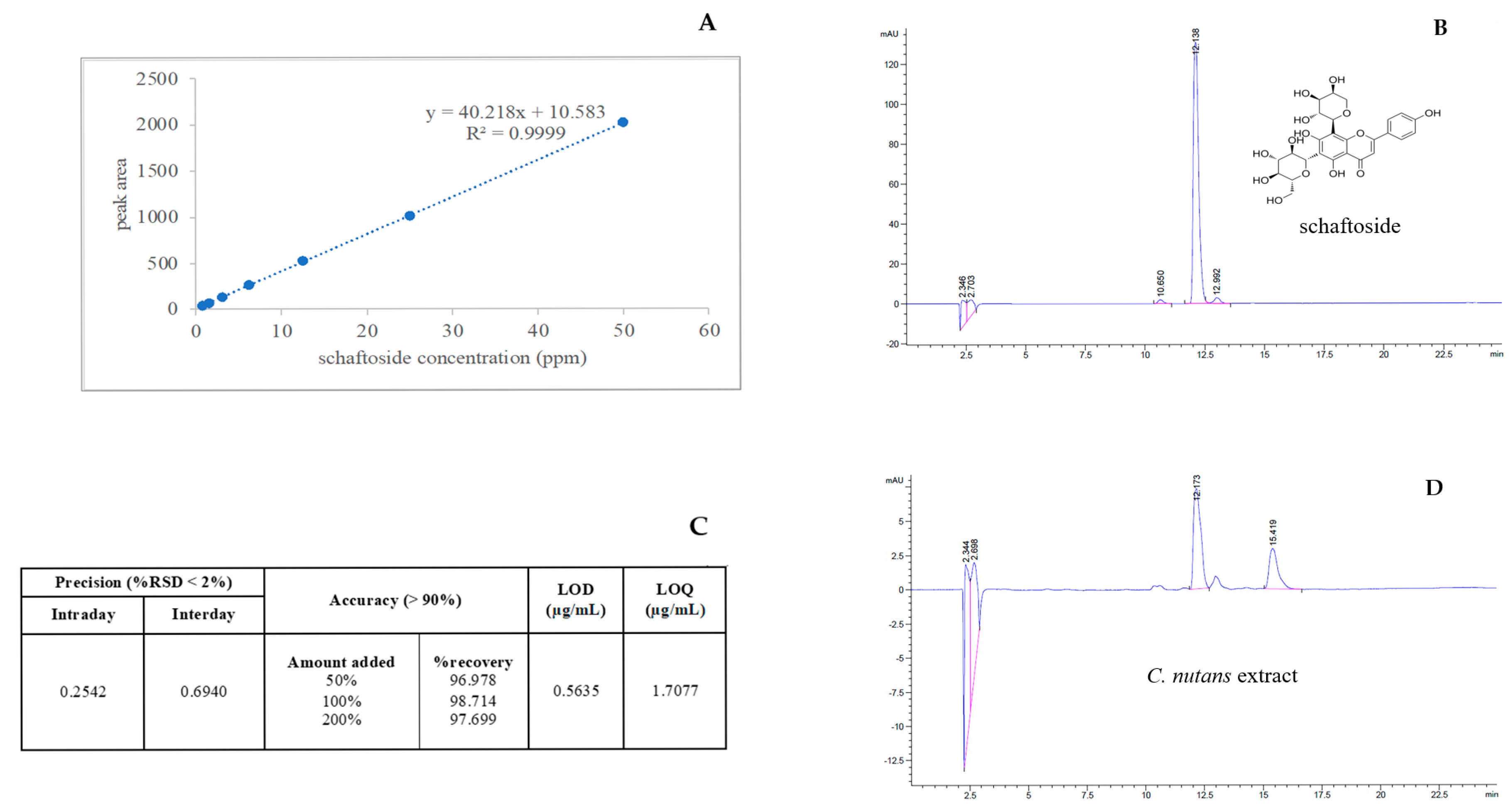
2.1. Optimization and Validation of the High-Performance Liquid Chromatography Method with Diode-Array Detection Method (HPLC-DAD) for Schaftoside Quantification
2.2. Anti-Inflammatory Effects of C. nutans Extract and Schaftoside
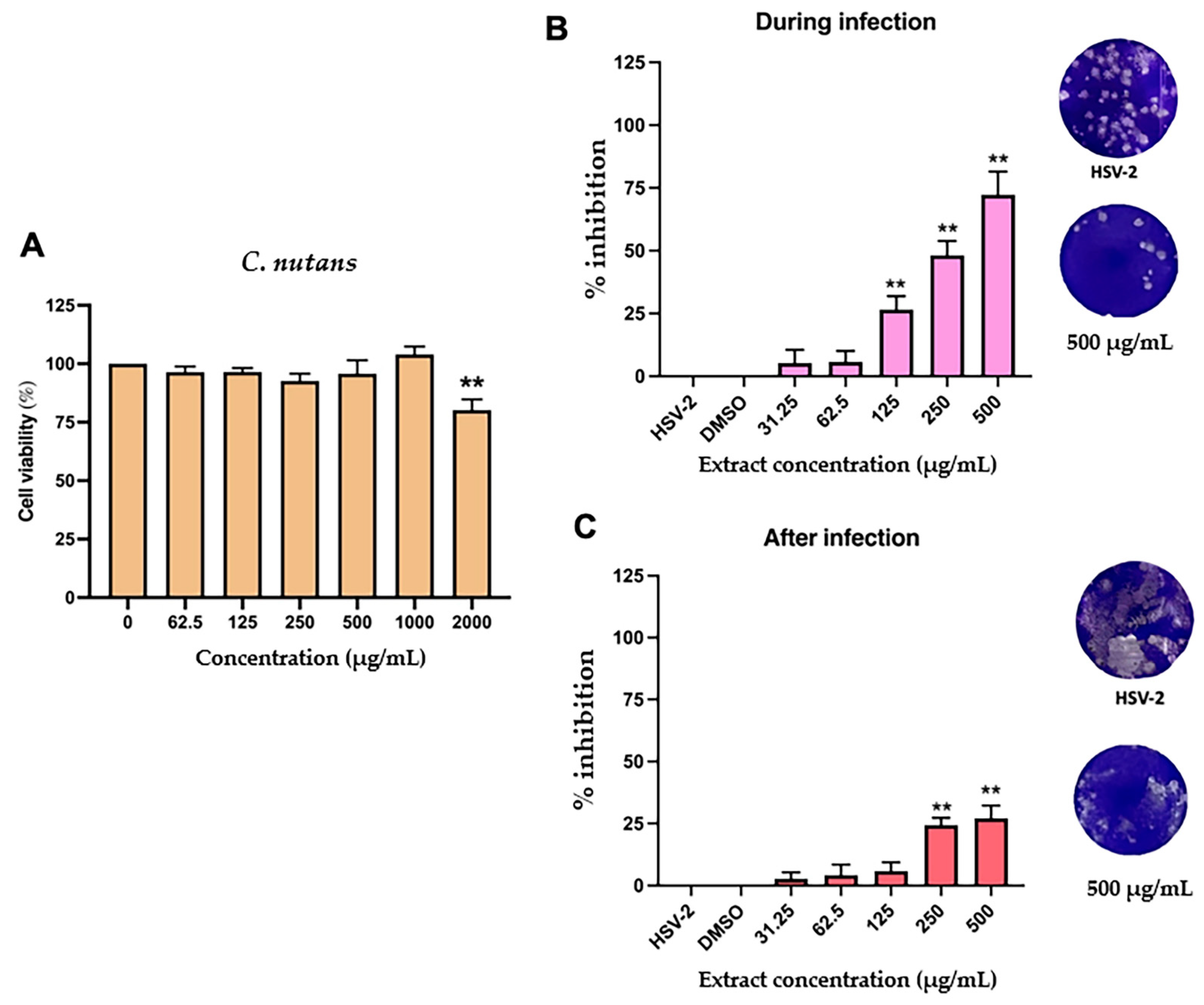
2.2.1. Cytotoxicity and Dose Selection (Figure 2A,B)

2.2.2. Suppression of Inflammatory Proteins (Figure 2C,D)
2.2.3. Inhibitory Effects of C. nutans Extract and Schaftoside on Human Cox-2 Enzyme Activity
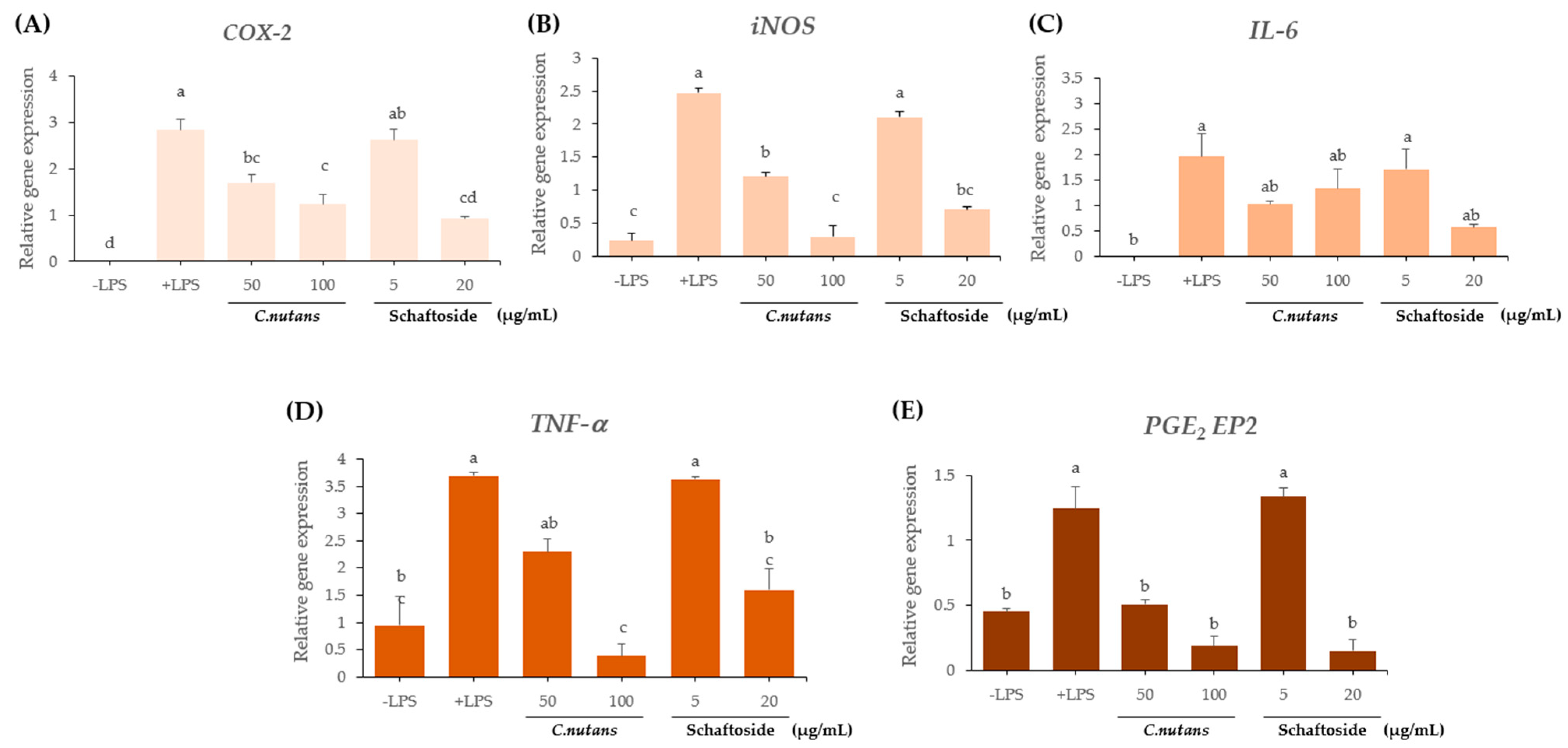
2.2.4. Downregulation of Inflammatory Gene Expression (Figure 4A–E)
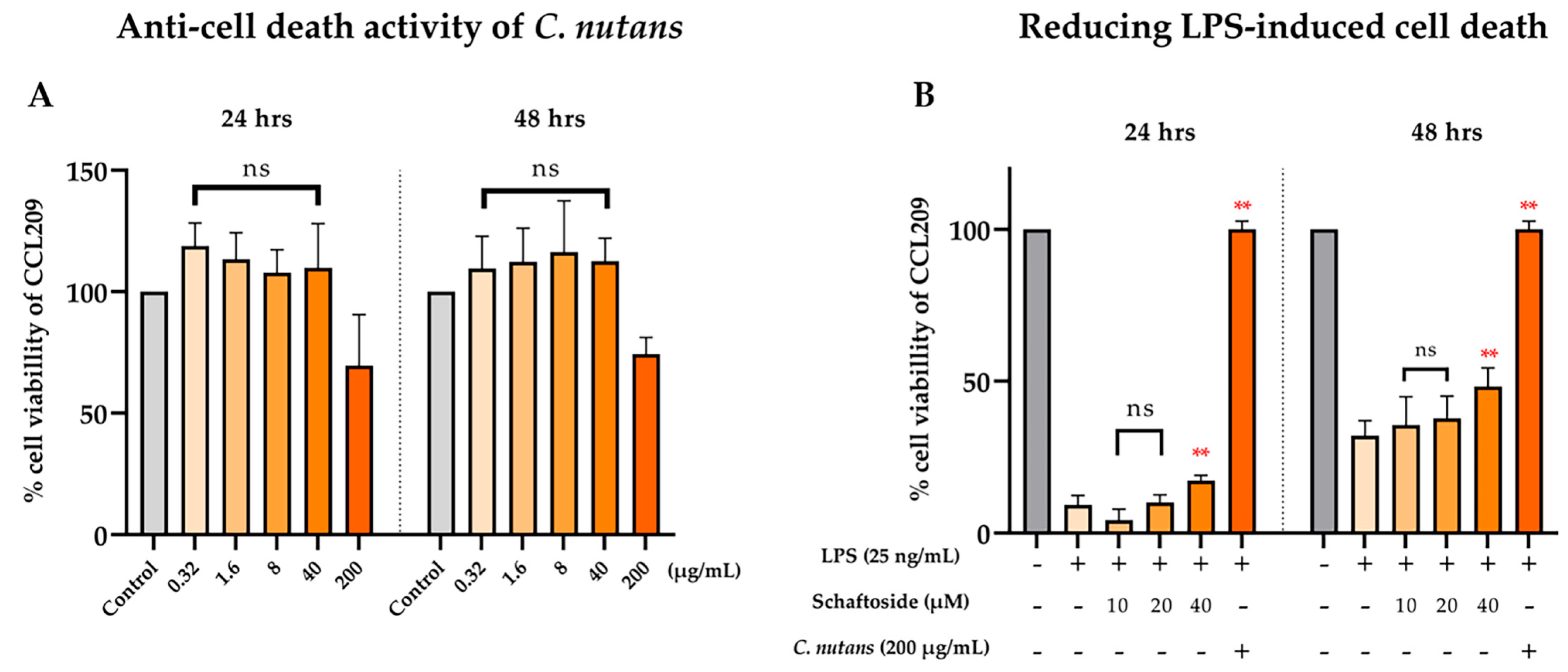
2.3. Anti-Cell Death Activity of C. nutans Extract and Schaftoside Against LPS-Induced Endothelial Cell Death
2.4. Antiviral Activity C. nutans Extract and Schaftoside Against Herpes Simplex Virus (HSV)
3. Discussion
4. Materials and Methods
4.1. Plant Extraction
4.2. Analysis of Bioactive Compound Using HPLC-DAD
4.3. The Effect of C. nutans and Schaftoside on Anti-Inflammation
4.3.1. Reagents and Kits
4.3.2. Cell Cultures
4.3.3. Cell Proliferation Assay
4.3.4. Western Blot Analysis
4.3.5. The Effect of C. nutans and Schaftoside as Human COX-2 Inhibitors
4.3.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.4. Anti-Cell Death Assay of C. nutans Extract and Schaftoside in CCL209 Cell Line
4.5. Anti-HSV Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| % | Percentage |
| °C | Degree Celsius |
| A.U. | Arbitrary unit |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| bFGF | Basic fibroblast growth factor |
| C. nutans | Clinacanthus nutans |
| cAMP-PKA-CREB | Cyclic adenosine monophosphate–protein kinase A–cAMP response element-binding protein |
| CCL-209 | Canine endothelial cell line 209 |
| cm | Centimeter |
| CMC | Carboxymethylcellulose |
| CO2 | Carbon dioxide |
| COX-2 | cyclooxygenase-2 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ECL | Enhanced chemiluminescence |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| EP1–EP4 | PGE2 four receptor subtypes |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| EVs | Endothelial-derived extracellular vesicles |
| FBS | Fetal bovine serum |
| g | Gram |
| h | Hour |
| HO-1 | Heme oxygenase-1 |
| HPLC-DAD | High-performance liquid chromatography method with diode-array detection |
| HRP | Horseradish peroxidase |
| HSV-2 | Herpes simplex virus type 2 |
| IL-10 | Interleukin-10 |
| IL-6 | interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| IRF3 | Interferon regulatory factor 3 |
| JNK1/2 | c-Jun N-terminal kinase 1/2 |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen-activated protein kinases |
| MEM | Minimal Essential Medium |
| mg | Milligram |
| min | Minute |
| mL | Milliliter |
| mm | Millimeter |
| μM | Micromolar |
| MMP | Matrix metalloproteinase |
| Na3VO4 | Sodium orthovanadate |
| NaF | Sodium fluoride |
| NF-κB | Transcription factor nuclear factor-kappa B |
| NF-κB p65 | Nuclear factor kappa B subunit p65 |
| ng/mL | Nanogram per milliliter |
| nm | Nanometer |
| NP-40 | Nonidet P-40 |
| Nrf2 | Nuclear factor erythroid 2 |
| ns | Not significant |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PDGF | Platelet-derived growth factor |
| PFU | Plaque-forming unit |
| PGE2 | prostaglandin E2 |
| PGF2α | Prostaglandin F2-alpha |
| PGH2 | Prostaglandin H2 |
| PMSF | Phenylmethylsulfonyl fluoride |
| R2 | Correlation coefficient |
| RAW 264.7 cell line | RAW 264.7 murine macrophage cell line |
| RIPA | Radioimmunoprecipitation assay |
| RNA | Ribonucleic acid |
| RT-qPCR | Reverse transcription polymerase chain reaction |
| SD | Standard deviation |
| SDS | Sodium dodecyl sulfate |
| SE | Standard error |
| TBS-T | Tris-buffered saline with Tween 20 |
| TGF-β | Transforming growth factor-beta |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor-alpha |
| Tris | Tris(hydroxymethyl)aminomethane |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
| VZV | Varicella-zoster virus |
| μg | Microgram |
References
- Alimyar, O.; Sediqi, S.; Azizi, A.; Hejran, A.B.; Niazi, P.; Monib, A.W. Medicinal Plants and Bioactive Compounds: Traditional and Modern Approaches to Wound Healing. Univers. Publ. Index E-Libr. 2024, 11, 29–59. [Google Scholar] [CrossRef]
- Kuo, X.; Herr, D.; Ong, W.Y. Anti-Inflammatory and Cytoprotective Effect of Clinacanthus nutans Leaf but Not Stem Extracts on 7-Ketocholesterol Induced Brain Endothelial Cell Injury. Neuromolecular Med. 2020, 23, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Daduang, S.; Uawonggul, N.; Watson, R.; Preedy, V. Herbal Therapies of Snake and Insect Bites in Thailand. Bot. Med. Clin. Pract. 2008, 814, 814–822. [Google Scholar]
- Aslam, M.S.; Ahmad, M.; Mamat, A. A Review on Phytochemical Constituents and Pharmacological Activities of Clinacanthus nutans. Int. J. Pharm. Pharm. Sci. 2014, 7, 30–33. [Google Scholar]
- Alam, A.; Ferdosh, S.; Ghafoor, K.; Hakim, A.; Juraimi, A.S.; Khatib, A.; Sarker, Z.I. Clinacanthus nutans: A Review of the Medicinal Uses, Pharmacology and Phytochemistry. Asian Pac. J. Trop. Med. 2016, 9, 402–409. [Google Scholar] [CrossRef]
- Yeo, C.S.S.; Tan, W.P.; Choo, K.J.L.; Lee, B.W.; Koh, H.L.; Goh, B. A Review of the Antiviral and Anti-Inflammatory Properties of Clinacanthus nutans. Trop. J. Pharm. Res. 2018, 17, 377–384. [Google Scholar] [CrossRef]
- Mai, C.W.; Yap, K.S.I.; Kho, M.T.; Ismail, N.H.; Yusoff, K.; Shaari, K.; Chin, S.Y.; Lim, E.S.H. Mechanisms Underlying the Anti-Inflammatory Effects of Clinacanthus nutans Lindau Extracts: Inhibition of Cytokine Production and Toll-like Receptor-4 Activation. Front. Pharmacol. 2016, 7, 7. [Google Scholar] [CrossRef]
- Liew, C.X.Q.; Le, C.F.; Ling, S.K.; Chow, S.C.; Fang, C.M. Anti-Inflammatory and Immunomodulatory Properties of Clinacanthus nutans. Curr. Pharmacol. Rep. 2024, 10, 360–370. [Google Scholar] [CrossRef]
- Ong, W.Y.; Herr, D.R.; Sun, G.; Lin, T.N. Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans. Molecules 2022, 27, 3607. [Google Scholar] [CrossRef]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Federici, M. Microarray Analysis of Human Endothelial Cells Exposed to High Glucose Reveals Alteration of TGF-β Signaling. Cardiovasc. Diabetol. 2005, 4, 6. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Li, J.; Wu, Y. Targeting the Endothelial Dysfunction Induced by LPS In Vitro Using Natural Products: A Potential Strategy for Sepsis-Associated Vascular Injury. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef]
- Madavaraju, K.K.; Koganti, R.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2021, 11, 614770. [Google Scholar] [CrossRef]
- Azemi, A.K.; Mokhtar, S.S.; Rasool, A.H.G. Clinacanthus nutans Leaves Extract Reverts Endothelial Dysfunction in Type 2 Diabetes Rats by Improving Protein Expression of eNOS. Plants 2020, 9, 1299. [Google Scholar] [CrossRef]
- Roeslan, B.O.; Ayudhya, Y.G. Antibiofilm, Nitric Oxide Inhibition and Wound Healing Activities of Purpurin-18 Phytyl Ester Isolated from Clinacanthus nutans. Biomed. Pharmacother. 2019, 117, 109169. [Google Scholar] [CrossRef]
- Chiangchin, S.; Thongyim, S.; Pandith, H.; Kaewkod, T.; Tragoolpua, Y.; Inta, A.; Watthana, S.; Pongamornkul, W.; Jangsutthivorawat, S.; Panya, A. Clinacanthus nutans genetic diversity and its association with anti-apoptotic, antioxidant, and anti-bacterial activities. Sci. Rep. 2023, 13, 19566. [Google Scholar] [CrossRef]
- Chelyn, J.L.; Omar, M.H.; Mohd Yousof, N.S.A.; Ranggasamy, R.; Wasiman, M.I.; Ismail, Z. Analysis of Flavone C-Glycosides in the Leaves of Clinacanthus nutans (Burm.f.) Lindau by HPTLC and HPLC-UV/DAD. Sci. World J. 2014, 2014, 724267. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.; Xue, H.; Yu, R.; Bao, Y.; Kuang, Y.; Chai, Y.; Ma, W.; Wang, J.; Shi, X.; et al. Schaftoside Inhibits 3CLpro and PLpro of SARS-CoV-2 Virus and Regulates Immune Response and Inflammation of Host Cells for the Treatment of COVID-19. Acta Pharm. Sin. B 2022, 12, 4154–4164. [Google Scholar] [CrossRef]
- Lu, D.; Liu, W.; Yang, H.; Zong, Y.; Sun, J.; Sun, X.; Song, S.; Liu, M.; Kan, J.; Che, C. Schaftoside Reduces Inflammation in Aspergillus fumigatus Keratitis through the Inhibition of the TLR4/MyD88 Pathway. Cytokine 2023, 175, 156483. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, M.; Chen, Z. Schaftoside Improves Cerebral Ischemia-Reperfusion Injury by Enhancing Autophagy and Reducing Apoptosis and Inflammation through the AMPK/mTOR Pathway. Adv. Clin. Exp. Med. 2022, 31, 1343–1354. [Google Scholar] [CrossRef]
- Thongyim, S.; Chiangchin, S.; Pandith, H.; Tragoolpua, Y.; Jangsutthivorawat, S.; Panya, A. Anti-Inflammatory Activity of Glyceryl 1,3-Distearate Identified from Clinacanthus nutans Extract against Bovine Mastitis Pathogens. Antibiotics 2023, 12, 549. [Google Scholar] [CrossRef]
- Thongyim, S.; Wright, T.A.; Sattayawat, P.; Kaewkod, T.; Baillie, G.S.; Tragoolpua, Y.; Jangsutthivorawat, S.; Panya, A. Clinacanthus nutans Extract Lowers Periodontal Inflammation under High-Glucose Conditions via Inhibiting NF-κB Signaling Pathway. Front. Pharmacol. 2024, 15, 1410419. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.F.; Cheng, Y.Y.; Tang, T.W.H.; Shih, C.; Chen, J.H.; Hsieh, P.C.H. Prostaglandin E2 Receptor 2 Modulates Macrophage Activity for Cardiac Repair. J. Am. Heart Assoc. 2018, 7, e002261. [Google Scholar] [CrossRef] [PubMed]
- Thongchai, S.; Ekalaksananan, T.; Pientong, C.; Aromdee, C.; Seubsasana, S.; Sukpol, C.; Kongyingyoes, B. Anti-Herpes Simplex Virus Type 1 Activity of Crude Ethyl Acetate Extract Derived from Leaves of Clinacanthus nutans Lindau. Nat. Prod. Res. 2010, 27, 318–326. [Google Scholar]
- Jantakee, K.; Panwong, S.; Sattayawat, P.; Sumankan, R.; Saengmuang, S.; Choowongkomon, K.; Panya, A. Clinacanthus nutans (Burm.f.) Lindau Extract Inhibits Dengue Virus Infection and Inflammation in the Huh7 Hepatoma Cell Line. Antibiotics 2024, 13, 705. [Google Scholar] [CrossRef]
- Kunsorn, P.; Ruangrungsi, N.; Lipipun, V.; Khanboon, A.; Rungsihirunrat, K. The Identities and Anti-Herpes Simplex Virus Activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac. J. Trop. Biomed. 2013, 3, 284–290. [Google Scholar] [CrossRef]
- Lokare, M.S.; Keshamma, E.; Hugar, B.S. Applications of Plant-Based Nanomedicines for Wound Healing. Recent Pat. Biotechnol. 2022, 16, 38–48. [Google Scholar] [CrossRef]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Farooqi, A.A.; Rahman, M.M.; Koppula, S.; Nabavi, S.M. Natural Product-Based Nanomedicines for Wound Healing Purposes: Therapeutic Targets and Future Perspectives. J. Cell. Physiol. 2019, 234, 8578–8596. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, M.; Kong, Y.; Zhang, F.; Wang, Y.; Huang, Y.; Song, W.; Li, Z.; Hou, L.; Liang, L.; et al. Fibroblasts and Endothelial Cells Interplay Drives Hypertrophic Scar Formation: Insights from In Vitro and In Vivo Models. Bioeng. Transl. Med. 2023, 9, e10630. [Google Scholar] [CrossRef]

| Genes | Primer Sequences | |
|---|---|---|
| Sense Strand (5′–3′) | Anti-Sense Strand (5′–3′) | |
| COX-2 | CCCCCACAGTCAAAGACACT | GAGTCCATGTTCCAGGAGGA |
| iNOS | GTCTTGCAAGCTGATGGTC | CATGATGGTCACATTCTGC |
| IL-6 | CCGGAGAGGAGACTTCACAG | GGAAATTGGGGTAGGAAGGA |
| TNF-α | CGTCAGCCGATTTGCTATCT | CGGACTCCGCAAAGTCTAAG |
| PGE2 EP2 | GTGGCCCTGGCTCCCGAAAGTC | GGCAAGGAGCATATGGCGAAGGTG |
| GAPDH | CAGGAGCGAGACCCCACTAACAT | GTCAGATCCACGACGGACACATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limpanich, N.; Chayapakdee, P.; Mekawan, K.; Thongyim, S.; Yongsawas, R.; Khamwong, P.; Tragoolpua, Y.; Kaewkod, T.; Jangsutthivorawat, S.; Jungklang, J.; et al. Integrative Wound-Healing Effects of Clinacanthus nutans Extract and Schaftoside Through Anti-Inflammatory, Endothelial-Protective, and Antiviral Mechanisms. Int. J. Mol. Sci. 2025, 26, 6029. https://doi.org/10.3390/ijms26136029
Limpanich N, Chayapakdee P, Mekawan K, Thongyim S, Yongsawas R, Khamwong P, Tragoolpua Y, Kaewkod T, Jangsutthivorawat S, Jungklang J, et al. Integrative Wound-Healing Effects of Clinacanthus nutans Extract and Schaftoside Through Anti-Inflammatory, Endothelial-Protective, and Antiviral Mechanisms. International Journal of Molecular Sciences. 2025; 26(13):6029. https://doi.org/10.3390/ijms26136029
Chicago/Turabian StyleLimpanich, Nipitpawn, Pattarasuda Chayapakdee, Kullanun Mekawan, Saruda Thongyim, Rujipas Yongsawas, Phanuwit Khamwong, Yingmanee Tragoolpua, Thida Kaewkod, Siriphorn Jangsutthivorawat, Jarunee Jungklang, and et al. 2025. "Integrative Wound-Healing Effects of Clinacanthus nutans Extract and Schaftoside Through Anti-Inflammatory, Endothelial-Protective, and Antiviral Mechanisms" International Journal of Molecular Sciences 26, no. 13: 6029. https://doi.org/10.3390/ijms26136029
APA StyleLimpanich, N., Chayapakdee, P., Mekawan, K., Thongyim, S., Yongsawas, R., Khamwong, P., Tragoolpua, Y., Kaewkod, T., Jangsutthivorawat, S., Jungklang, J., Chanasut, U., Inta, A., Arjinajarn, P., Panya, A., & Pandith, H. (2025). Integrative Wound-Healing Effects of Clinacanthus nutans Extract and Schaftoside Through Anti-Inflammatory, Endothelial-Protective, and Antiviral Mechanisms. International Journal of Molecular Sciences, 26(13), 6029. https://doi.org/10.3390/ijms26136029







