The Effect of Freeze-Dried Cherry Pomace and Red Potato Pulp on the Content of Bioactive Substances in Pasta
Abstract
1. Introduction
2. Results and Discussion
2.1. CP and RPP Characteristics
2.2. Effect of CP and RPP on the Content of Polyphenols and Antioxidant Potential of Wheat Pasta
2.3. Effect of CP and RPP on the Content of Phytosterols in Wheat Pasta
3. Materials and Methods
3.1. Materials
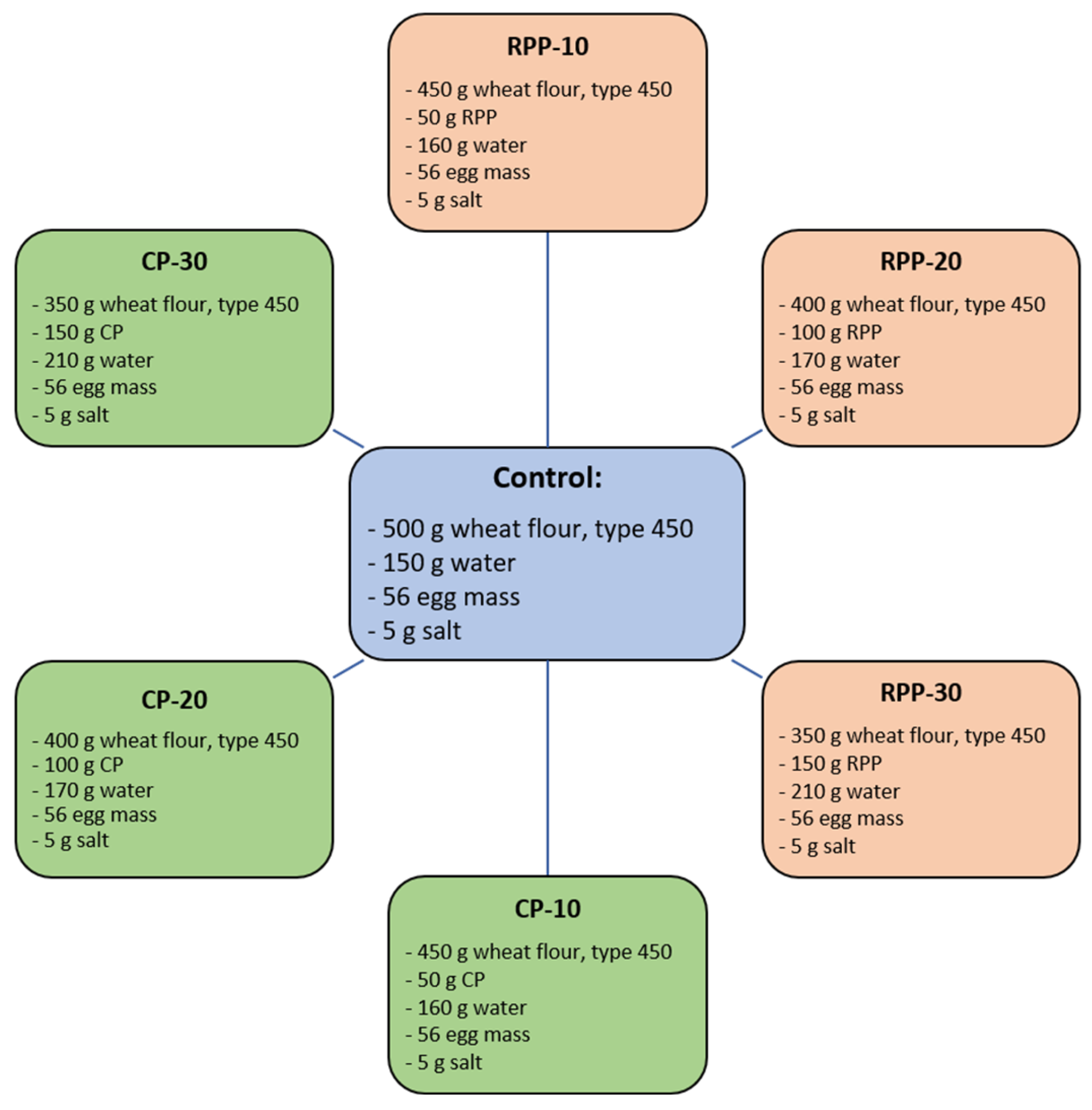
3.2. Pasta Formulation
3.3. Pasta Preparationn
3.4. Analytical Methods
3.5. Phytosterols in Food Determination Using Gas Chromatography
3.6. Phenolic Compound Analysis by UHPLC–DAD–ESI–MS/MS
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Cherry Pomace |
| RPP | Red Potato Pulp |
| Control | pasta with no additions |
| CP-10 | pasta with a 10% share of Cherry Pomace |
| CP-20 | pasta with a 20% share of Cherry Pomace |
| CP-30 | pasta with a 30% share of Cherry Pomace |
| RPP-10 | pasta with a 10% share of Red Potato Pulp |
| RPP-20 | pasta with a 20% share of Red Potato Pulp |
| RPP-30 | pasta with a 30% share of Red Potato Pulp |
References
- Yazdi, A.P.G.; Mohammadi, Z.B.; Khoshtinat, K.; Rousta, L.K.; Jafari, S.M. Application of Spray Dried Encapsulated Bioactives in Food Products. In Spray Drying Encapsulation of Bioactive Materials; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-429-35546-2. [Google Scholar]
- Kamali Rousta, L.; Ghandehari Yazdi, A.P.; Amini, M. Optimization of Athletic Pasta Formulation by D-Optimal Mixture Design. Food Sci. Nutr. 2020, 8, 4546–4554. [Google Scholar] [CrossRef] [PubMed]
- Kamali Rousta, L.; Pouya Ghandehari Yazdi, A.; Khorasani, S.; Tavakoli, M.; Ahmadi, Z.; Amini, M. Optimization of Novel Multigrain Pasta and Evaluation of Physicochemical Properties: Using D-Optimal Mixture Design. Food Sci. Nutr. 2021, 9, 5546–5556. [Google Scholar] [CrossRef] [PubMed]
- Nilusha, R.A.T.; Jayasinghe, J.M.J.K.; Perera, O.D.A.N.; Perera, P.I.P. Development of Pasta Products with Nonconventional Ingredients and Their Effect on Selected Quality Characteristics: A Brief Overview. Int. J. Food Sci. 2019, 2019, 6750726. [Google Scholar] [CrossRef] [PubMed]
- Padalino, L.; Conte, A.; Lecce, L.; Likyovia, D.; Sicari, V.; Pellicanò, T.; Poiana, M.; Del Nobile, M. Durum Wheat Whole-Meal Spaghetti with Tomato Peels: How By-Product Particles Size Can Affect Final Quality of Pasta. J. Food Process. Technol. 2015, 6, 500. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef]
- Oliviero, T.; Fogliano, V. Food Design Strategies to Increase Vegetable Intake: The Case of Vegetable Enriched Pasta. Trends Food Sci. Technol. 2016, 51, 58–64. [Google Scholar] [CrossRef]
- Abbasi Parizad, P.; Marengo, M.; Bonomi, F.; Scarafoni, A.; Cecchini, C.; Pagani, M.A.; Marti, A.; Iametti, S. Bio-Functional and Structural Properties of Pasta Enriched with a Debranning Fraction from Purple Wheat. Foods 2020, 9, 163. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; Zamudio-Flores, P.B.; Tirado-Gallegos, J.M.; Ramírez-Mancinas, S.; Olivas-Orozco, G.I.; Espino-Díaz, M.; Hernández-González, M.; García-Cano, V.G.; Sánchez-Ortíz, O.; Buenrostro-Figueroa, J.J.; et al. Evaluation of Cooking Quality, Nutritional and Texture Characteristics of Pasta Added with Oat Bran and Apple Flour. Foods 2019, 8, 299. [Google Scholar] [CrossRef]
- Attanzio, A.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Cascioferro, S.M.; Allegra, M.; Cirrincione, G.; Tesoriere, L.; et al. Quality, Functional and Sensory Evaluation of Pasta Fortified with Extracts from Opuntia Ficus-Indica Cladodes. J. Sci. Food Agric. 2019, 99, 4242–4247. [Google Scholar] [CrossRef]
- Lalegani, S.; Ahmadi Gavlighi, H.; Azizi, M.H.; Amini Sarteshnizi, R. Inhibitory Activity of Phenolic-Rich Pistachio Green Hull Extract-Enriched Pasta on Key Type 2 Diabetes Relevant Enzymes and Glycemic Index. Food Res. Int. 2018, 105, 94–101. [Google Scholar] [CrossRef]
- Marinelli, V.; Padalino, L.; Nardiello, D.; Del Nobile, M.A.; Conte, A. New Approach to Enrich Pasta with Polyphenols from Grape Marc. J. Chem. 2015, 2015, 734578. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals Report; United Nations: Rome, Italy, 2021. [Google Scholar]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.-J.; Ganjyal, G.M. Utilization of Food Processing By-Products in Extrusion Processing: A Review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Teslić, N.; Kojić, J.; Đermanović, B.; Šarić, L.; Maravić, N.; Pestorić, M.; Šarić, B. Sour Cherry Pomace Valorization as a Bakery Fruit Filling: Chemical Composition, Bioactivity, Quality and Sensory Properties. Antioxidants 2023, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bartek, L.; Sundin, N.; Strid, I.; Andersson, M.; Hansson, P.-A.; Eriksson, M. Environmental Benefits of Circular Food Systems: The Case of Upcycled Protein Recovered Using Genome Edited Potato. J. Clean. Prod. 2022, 380, 134887. [Google Scholar] [CrossRef]
- Kita, A.; Bąkowska-Barczak, A.; Lisińska, G.; Hamouz, K.; Kułakowska, K. Antioxidant Activity and Quality of Red and Purple Flesh Potato Chips. LWT-Food Sci. Technol. 2015, 62, 525–531. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Šulc, M.; Orsák, M.; Pivec, V.; Hejtmánková, A.; Dvořák, P.; Čepl, J. Cultivar Differences of Total Anthocyanins and Anthocyanidins in Red and Purple-Fleshed Potatoes and Their Relation to Antioxidant Activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]
- Kita, A.; Bąkowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The Effect of Frying on Anthocyanin Stability and Antioxidant Activity of Crisps from Red- and Purple-Fleshed Potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Nemś, A.; Pęksa, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kita, A.; Drożdż, W.; Hamouz, K. Anthocyanin and Antioxidant Activity of Snacks with Coloured Potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef]
- Fernando Reyes, L.; Emilio Villarreal, J.; Cisneros-Zevallos, L. The Increase in Antioxidant Capacity after Wounding Depends on the Type of Fruit or Vegetable Tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Dvořák, P. The Influence of Flesh Colour and Growing Locality on Polyphenolic Content and Antioxidant Activity in Potatoes. Sci. Hortic. 2008, 117, 109–114. [Google Scholar] [CrossRef]
- Zieliński, H.; Kozłowska, H. Antioxidant Activity and Total Phenolics in Selected Cereal Grains and Their Different Morphological Fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Okur, İ.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste Biomass Valor. 2019, 10, 3545–3555. [Google Scholar] [CrossRef]
- Brown, C.R.; Wrolstad, R.; Durst, R.; Yang, C.-P.; Clevidence, B. Breeding Studies in Potatoes Containing High Concentrations of Anthocyanins. Am. J. Potato Res. 2003, 80, 241–249. [Google Scholar] [CrossRef]
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial Phytochemicals in Potato—A Review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Brown, C.R. Antioxidants in Potato. Am. J. Potato Res. 2005, 82, 163–172. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Fenolokwasy Jako Bioaktywne Składniki Żywności. Żywność Nauka Technol. Jakość 2004, 4, 29–40. [Google Scholar]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Mystkowska, I.; Baranowska, A.; Niewęgłowski, M.; Dołęga, H. The Effect of Herbicides and Biostimulants on Polyphenol Content of Potato (Solanum tuberosum L.) Tubers and Leaves. J. Saudi Soc. Agric. Sci. 2019, 18, 102–106. [Google Scholar] [CrossRef]
- Rytel, E.; Tajner-Czopek, A.; Kita, A.; Aniołowska, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Hamouz, K. Content of Polyphenols in Coloured and Yellow Fleshed Potatoes during Dices Processing. Food Chem. 2014, 161, 224–229. [Google Scholar] [CrossRef]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and Glycoalkaloid Contents in Potato Cultivars Grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef]
- Mäder, J.; Rawel, H.; Kroh, L.W. Composition of Phenolic Compounds and Glycoalkaloids α-Solanine and α-Chaconine during Commercial Potato Processing. J. Agric. Food Chem. 2009, 57, 6292–6297. [Google Scholar] [CrossRef]
- Panza, O.; Conte, A.; Del Nobile, M.A. Recycling of Fig Peels to Enhance the Quality of Handmade Pasta. LWT 2022, 168, 113872. [Google Scholar] [CrossRef]
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of Grape Pomace Addition on the Technological, Sensory, and Nutritional Properties of Durum Wheat Pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- la Gatta, B.; Rutigliano, M.; Liberatore, M.T.; Dilucia, F.; Spadaccino, G.; Quinto, M.; Di Luccia, A. Preservation of Bioactive Compounds Occurring in Fresh Pasta Fortified with Artichoke Bracts and Tomato Powders Obtained with a Novel Pre-Treatment. LWT 2023, 187, 115298. [Google Scholar] [CrossRef]
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango Peel Powder: A Potential Source of Antioxidant and Dietary Fiber in Macaroni Preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of Different Drying Techniques on Physical Properties, Total Polyphenols and Antioxidant Capacity of Blackcurrant Pomace Powders. LWT 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The Effect of Cooking on the Phytochemical Content of Vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyhydroquinone-Free Coffee: A Double-Blind, Randomized Controlled Dose–Response Study of Blood Pressure. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Muñoa, L.; Sosa, P.; Cayhualla, E.; Sanchez, C.; Díaz, C.; Bonierbale, M. Total Phenolic, Total Anthocyanin and Phenolic Acid Concentrations and Antioxidant Activity of Purple-Fleshed Potatoes as Affected by Boiling. J. Food Compos. Anal. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and Anthocyanin-Rich Extracts: Role in Diabetes and Eye Function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Reddivari, L.; Vanamala, J.; Safe, S.H.; Miller, J.C., Jr. The Bioactive Compounds α-Chaconine and Gallic Acid in Potato Extracts Decrease Survival and Induce Apoptosis in LNCaP and PC3 Prostate Cancer Cells. Nutr. Cancer 2010, 62, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Hale, A.L.; Miller, J.C. Determination of Phenolic Content, Composition and Their Contribution to Antioxidant Activity in Specialty Potato Selections. Am. J. Potato Res. 2007, 84, 275–282. [Google Scholar] [CrossRef]
- Stushnoff, C.; Holm, D.; Thompson, M.D.; Jiang, W.; Thompson, H.J.; Joyce, N.I.; Wilson, P. Antioxidant Properties of Cultivars and Selections from the Colorado Potato Breeding Program. Am. J. Potato Res. 2008, 85, 267–276. [Google Scholar] [CrossRef]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A Review on Dietary Phytosterols: Their Occurrence, Metabolism and Health Benefits. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Rajhi, I.; Baccouri; Mhadhbi, H. Phytosterols in Wheat: Composition, Contents and Role in Human Health. Op. Acc. J. Bio Sci. Res. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Lee, W.; Yeo, Y.; Oh, S.; Cho, K.-S.; Park, Y.-E.; Park, S.K.; Lee, S.M.; Cho, H.S.; Park, S.-Y. Compositional Analyses of Diverse Phytochemicals and Polar Metabolites from Different-Colored Potato (Solanum tubersum L.) Tubers. Food Sci. Biotechnol. 2017, 26, 1379–1389. [Google Scholar] [CrossRef]
- Stryjecka, M.; Michalak, M.; Cymerman, J.; Kiełtyka-Dadasiewicz, A. Comparative Assessment of Phytochemical Compounds and Antioxidant Properties of Kernel Oil from Eight Sour Cherry (Prunus cerasus L.) Cultivars. Molecules 2022, 27, 696. [Google Scholar] [CrossRef]
- Gumul, D.; Ivanišová, E.; Oracz, J.; Sabat, R.; Wywrocka-Gurgul, A.; Ziobro, R. Red Potato Pulp and Cherry Pomace for Pasta Enrichment: Health-Promoting Compounds, Physical Properties and Quality. Appl. Sci. 2024, 14, 4873. [Google Scholar] [CrossRef]
- Wischmann, B.; Ahmt, T.; Bandsholm, O.; Blennow, A.; Young, N.; Jeppesen, L.; Thomsen, L. Testing Properties of Potato Starch from Different Scales of Isolations—A Ringtest. J. Food Eng. 2007, 79, 970–978. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, Phenolics, and Color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and Antioxidative Activities in Common Beans (Phaseolus vulgaris L). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of Flavonoids in the Resistance of Two Poplar Cultivars to Mistletoe (Viscum album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Hussain, N.; Jabeen, Z.; Li, Y.; Chen, M.; Li, Z.; Guo, W.; Shamsi, I.H.; Chen, X.; Jiang, L. Detection of Tocopherol in Oilseed Rape (Brassica napus L.) Using Gas Chromatography with Flame Ionization Detector. J. Integr. Agric. 2013, 12, 803–814. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Effect of Roasting Conditions on the Fat, Tocopherol, and Phytosterol Content and Antioxidant Capacity of the Lipid Fraction from Cocoa Beans of Different Theobroma cacao L. Cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, W.; Wei, X.; Zhang, F.; Shen, C.; Wu, B.; Zhao, Z.; Liu, H.; Deng, X. Simultaneous Determination of Tocopherols and Tocotrienols in Vegetable Oils by GC-MS. Anal. Methods 2016, 8, 7341–7346. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and Quantification of Free and Bound Phenolic Compounds Contained in the High-Molecular Weight Melanoidin Fractions Derived from Two Different Types of Cocoa Beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef]
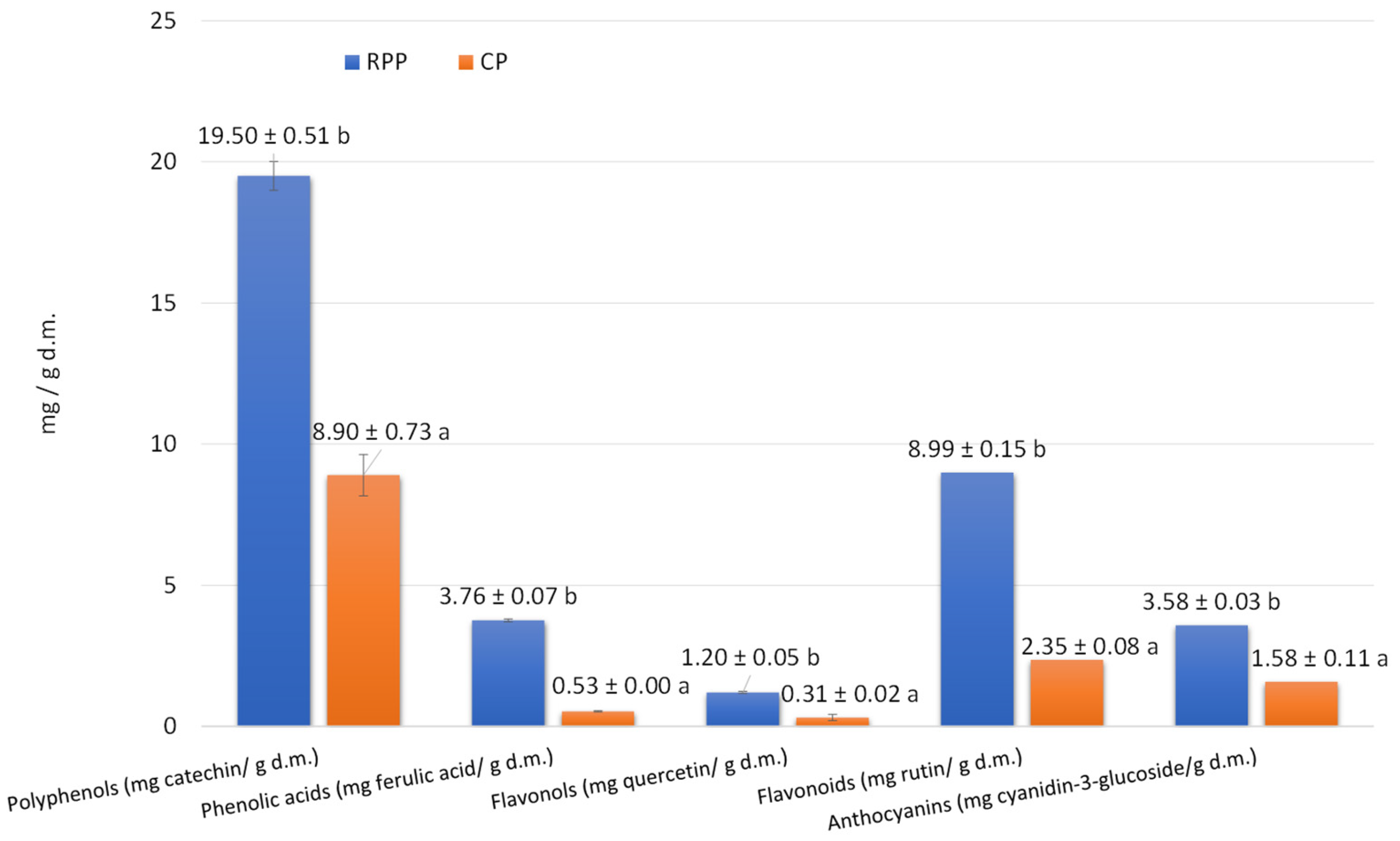
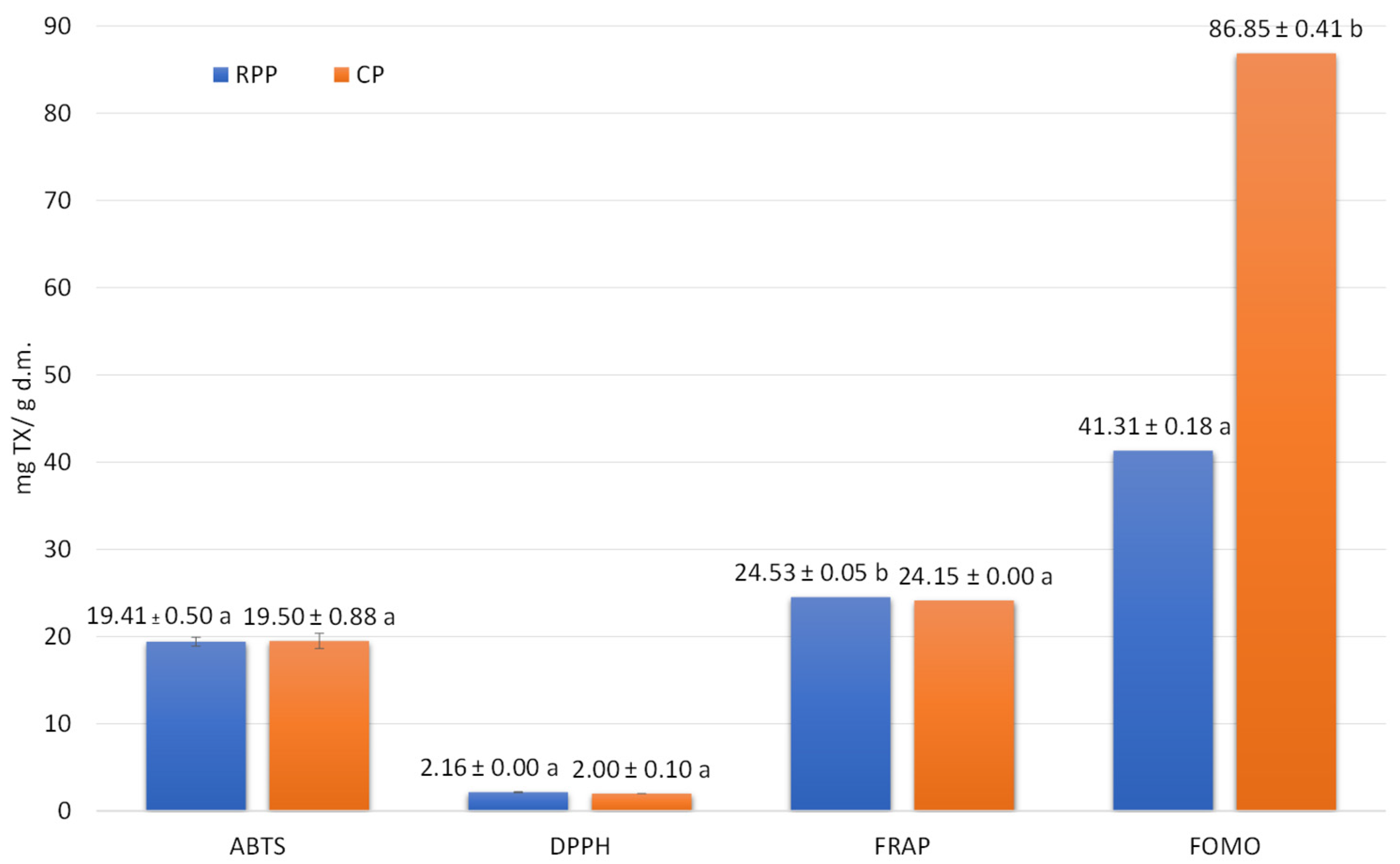
| Compound * | Content (mg/100 g d.m.) | |
|---|---|---|
| CP | RPP | |
| Polyphenols | ||
| Gallic acid | 1.75 ± 0.03 b* | 1.32 ± 0.01 a |
| Procyanidin B1 | 9.44 ± 0.18 b | 4.68 ± 0.04 a |
| 3-O-Caffeoylquinic acid (neochlorogenic acid) | 349.65 ± 6.71 b | 35.41 ± 0.32 a |
| (−)-Epigallocatechin | 26.44 ± 0.51 a | 0.00 ± 0.00 |
| 3-O-p-Coumaroylquinic acid | 0.00 ± 0.00 | 59.88 ± 0.54 a |
| 5-O-Caffeoylquinic acid (chlorogenic acid) | 1010.78 ± 19.4 b | 33.69 ± 0.3 a |
| (+)-Catechin | 9.40 ± 0.18 a | 0.00 ± 0.00 |
| Cyanidin 3-O-sophoroside | 11.13 ± 0.21 a | 0.00 ± 0.00 |
| 4-O-Caffeoylquinic acid | 116.81 ± 2.24 b | 2.72 ± 0.02 a |
| Cyanidin-3-O-glucoside | 11.38 ± 0.22 a | 0.00 ± 0.00 |
| (−)-Epicatechin | 8.31 ± 0.16 a | 0.00 ± 0.00 |
| 4-O-coumaroylquinic acid | 4.36 ± 0.08 a | 0.00 ± 0.00 |
| Procyanidin B2 | 0.00 ± 0.00 | 12.77 ± 0.11 a |
| p-Coumaric acid | 0.00 ± 0.00 | 1.96 ± 0.02 a |
| Pelargonidin 3-(4‴-p-coumaroylrutinoside)-5-glucoside | 0.00 ± 0.00 | 428.21 ± 3.84 a |
| Procyanidin C1 | 0.00 ± 0.00 | 7.68 ± 0.07 a |
| Quercetin 3-O-rutinoside-7-O-glucoside | 53.00 ± 1.02 a | 0.00 ± 0.00 |
| Quercetin 3-O-(2′-glucosyl)-rutinoside | 0.00 | 15.60 ± 0.14 a |
| Quercetin 3-O-galactoside | 5.56 ± 0.11 a | 39.84 ± 0.36 b |
| Quercetin 3-O-rutinoside | 8.17 ± 0.16 b | 6.39 ± 0.06 a |
| Cyanidin 3-O-rutinoside | 473.25 ± 9.08 a | 0.00 |
| Quercetin 3-O-glucoside | 2.78 ± 0.05 a | 9.66 ± 0.09 b |
| Quercetin 3-O-sulfate | 0.00 ± 0.00 | 23.0 ± 0.21 b |
| Kaempferol-3-O-rutinoside | 45.82 ± 0.88 a | 0.00 ± 0.00 |
| 3,5-Di-O-caffeoylquinic acid | 6.75 ± 0.13 a | 0.00 ± 0.00 |
| Quercetin | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Phytosterols | ||
| Cholesterol | 2.71 ± 0.00 a | 0.00 ± 0.00 |
| Stignasterol | 1.07 ± 0.12 a | 1.01 ± 0.07 a |
| Sitosterol | 17.04 ± 0.27 b | 1.53 ± 0.10 a |
| Campesterol | 2.53 ± 0.03 a | 0.00 ± 0.00 |
| Δ 5avenasterol | 1.52 ± 0.04 a | 0.00 ± 0.00 |
| Δ 7avenasterol | 0.84 ± 0.11 a | 0.00 ± 0.00 |
| Total Polyphenol Content (mg Catechin/g d.m.) | Phenolic Acids (mg Ferulic Acid/g d.m.) | Flavonols (mg Quercetin/g d.m.) | Anthocyanins (mg Glucoside Cyjanidyn/g d.m.) | Flavonoids (mg Rutin/g d.m.) | |
|---|---|---|---|---|---|
| Control | 13.04 ± 0.40 a* | 0.07 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 13.2 ± 0.60 a |
| CP-10 | 123.67 ± 1.19 c | 5.42 ± 0.13 b | 4.10 ± 0.21 c | 11.19 ± 0.00 c | 29.16 ± 1.22 b |
| CP-20 | 203.80 ± 1.47 e | 11.22 ± 0.09 d | 5.80 ± 0.53 d | 24.9 ± 1.14 d | 42.70 ± 1.80 d |
| CP-30 | 216.08 ± 0.34 f | 14.08 ± 0.00 e | 8.08 ± 0.16 e | 33.73 ± 1.10 f | 69.00 ± 1.52 f |
| RPP-10 | 109.76 ± 0.00 b | 10.25 ± 0.12 c | 2.39 ± 0.12 b | 8.82 ± 0.50 b | 33.90 ± 0.34 c |
| RPP-20 | 182.40 ± 1.40 d | 13.03 ± 0.64 f | 9.79 ± 0.87 f | 26.60 ± 1.01 e | 57.18 ± 0.61 e |
| RPP-30 | 323.93 ± 0.89 g | 23.78 ± 0.23 g | 19.49 ± 0.34 g | 36.16 ± 0.81 g | 110.26 ± 1.52 g |
| Compound * | Control | CP-10 | CP-20 | CP-30 | RPP-10 | RPP-20 | RPP-30 |
|---|---|---|---|---|---|---|---|
| Gallic acid | 0.00 ± 0.0 a* | 1.65 ± 0.03 d | 2.74 ± 0.05 e | 4.20 ± 0.08 f | 0.18 ± 0 b | 0.22 ± 0 b | 1.07 ± 0.01 c |
| ProcyanidinB1 | 0.00 ± 0.0 a | 2.29 ± 0.04 b | 3.67 ± 0.07 b | 7.88 ± 0.15 f | 4.32 ± 0.04 c | 4.76 ± 0.04 d | 5.94 ± 0.05 e |
| 3-O-Caffeoylquinic acid (neochlorogenic acid) | 0.00 ± 0.0 a | 0.52 ± 0.01 b | 6.08 ± 0.12 e | 9.31 ± 0.18 f | 1.34 ± 0.01 c | 3.81 ± 0.03 d | 9.92 ± 0.09 g |
| (−)-Epigallocatechin | 0.00 ± 0.0 a | 0 a | 0 a | 0 a | - | - | - |
| 3-O-p-Coumaroylquinic acid | 0.00 ± 0.0 a | - | - | - | 2.41 ± 0.02 b | 6.55 ± 0.06 c | 17.09 ± 0.15 d |
| 5-O-Caffeoylquinic acid (chlorogenic acid) | 0.00 ± 0.0 a | 4.12 ± 0.08 d | 24.6 ± 0.47 f | 37.64 ± 0.72 g | 1.94 ± 0.02 b | 3.79 ± 0.03 c | 9.69 ± 0.09 e |
| (+)-Catechin | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a | - | - | - |
| Cyanidin3-O-sophoroside | - | - | - | - | - | - | - |
| 4-O-Caffeoylquinic acid | 0.00 ± 0.0 a | 0.44 ± 0.01 c | 3.86 ± 0.07 e | 5.91 ± 0.11 f | 1.94 ± 0.02 d | 0.27 ± 0 b | 0.44 ± 0 c |
| Cyanidin-3-O-glucoside | 0.00 ± 0.0 | 0 | 0 | 0 | - | - | - |
| (−)-Epicatechin | 0.00 ± 0.0 | 0 | 0 | 0 | - | - | - |
| 4-O-coumaroylquinic acid | 0.00 ± 0.0 a | 0.03 ± 0 b | 0.05 ± 0 c | 0.08 ± 0 d | - | - | - |
| Procyanidin B2 | 0.00 ± 0.0 a | - | - | - | 1.04 ± 0.01 b | 3.44 ± 0.03 c | 9.34 ± 0.08 d |
| p-Coumaric acid | 0.00 ± 0.0 a | - | - | - | 0.14 ± 0 b | 0.47 ± 0 c | 1.27 ± 0.01 d |
| Pelargonidin3-(4‴-p-coumaroylrutinoside)-5-glucoside | 0.00 ± 0.0 a | - | - | - | 11.80 ± 0.11 b | 67.16 ± 0.6 c | 189.52 ± 1.7 d |
| Procyanidin C1 | 0.00 ± 0.0 a | - | - | - | 0.51 ± 0 a | 1.31 ± 0.01 b | 4.23 ± 0.04 d |
| Quercetin3-O-rutinoside-7-O-glucoside | 0.00 ± 0.0 a | 0.97 ± 0.02 a | 2.94 ± 0.06 b | 4.5 ± 0.09 c | - | - | - |
| Quercetin3-O-(2′-glucosyl)-rutinoside | 0.00 ± 0.0 a | - | - | - | 0.77 ± 0.01 a | 2.11 ± 0.02 b | 4.96 ± 0.04 c |
| Quercetin3-O-galactoside | 0.00 ± 0.0 a | 0 a | 0 a | 0 a | 4.74 ± 0.04 b | 10.43 ± 0.09 c | 23.55 ± 0.21 d |
| Quercetin3-O-rutinoside | 0.00 ± 0.0 a | 1.44 ± 0.03 b | 2.88 ± 0.06 d | 4.32 ± 0.08 e | 2.37 ± 0.02 c | 5.22 ± 0.05 f | 11.77 ± 0.11 g |
| Cyanidin3-O-rutinoside | 0.00 ± 0.0 a | 3.54 ± 0.07 b | 7.06 ± 0.14 c | 17.51 ± 0.34 d | - | - | - |
| Quercetin3-O-glucoside | 0.00 ± 0.0 a | 0 a | 0 a | 0 a | 0.77 ± 0.01 a | 1.29 ± 0.01 b | 3.04 ± 0.03 c |
| Quercetin3-O-sulfate | 0.00 ± 0.0 a | - | - | - | 1.25 ± 0.01 a | 2.95 ± 0.03 b | 6.86 ± 0.06 c |
| Kaempferol-3-O-rutinoside | 0.00 ± 0.0 a | 3.81 ± 0.07 b | 10.35 ± 0.2 c | 16.47 ± 0.32 d | - | - | - |
| 3,5-Di-O-caffeoylquinic acid | 0.00 ± 0.0 a | 0.14 ± 0 a | 0.61 ± 0.01 b | 0.94 ± 0.02 c | - | - | - |
| Quercetin | 0.00 ± 0.0 a | 0.14 ± 0 a | 0.61 ± 0.01 b | 0.92 ± 0.02 c | - | - | - |
| Dipcoumaroylspermidine | 0.35 ± 0.01 d | 0.21 ± 0.03 c | 0.16 ± 0.02 c | 0 ± 0 a | 0.24 ± 0.02 c | 0.13 ± 0 b | 0 ± 0 a |
| Feruloylquinic acid | 0.10 ± 0.00 d | 0.07 ± 0.01 c | 0 ± 0 a | 0 ± 0 a | 0.05 ± 0 b | 0 ± 0 a | 0 ± 0 a |
| DPPH | FRAP | FOMO | ABTS | |
|---|---|---|---|---|
| Control | 0.61 ± 0.01 a* | 0.06 ± 0.00 a | 2.57 ± 0.15 a | 6.36 ± 0.53 a |
| CP-10 | 0.64 ± 0.07 a | 2.10 ± 0.42 b | 8.88 ± 0.31 d | 9.45 ± 0.21 c |
| CP-20 | 0.85 ± 0.10 a | 4.60 ± 0.40 c | 16.75 ± 0.28 e | 14.15 ± 0.13 f |
| CP-30 | 1.02 ± 0.15 a | 6.89 ± 0.36 d | 21.79 ± 0.80 f | 15.89 ± 0.24 g |
| RPP-10 | 0.62 ± 0.00 a | 0.43 ± 0.00 a | 4.58 ± 0.62 bc | 7.63 ± 0.19 b |
| RPP-20 | 0.68 ± 0.04 a | 0.50 ± 0.00 a | 3.76 ± 0.07 ab | 11.88 ± 0.00 d |
| RPP-30 | 0.78 ± 0.09 a | 1.11 ± 0.11 ab | 5.97 ± 0.01 c | 13.45 ± 0.35 e |
| Cholesterol (mg/100 g) | Campesterol (mg/100 g) | Stigmasterol (mg/100 g) | β-Sitosterol (mg/100 g) | Δ-5-Avenasterol (mg/100 g) | Δ-7-Avenasterol (mg/100 g) | |
|---|---|---|---|---|---|---|
| Control | 53.85 ± 1.60 d* | 3.04 ± 0.09 d | 0.26 ± 0.00 a | 13.15 ± 0.37 d | 0.35 ± 0.00 b | 0.54 ± 0.03 c |
| CP-10 | 34.59 ± 0.02 a | 1.67 ± 0.01 ab | 0.36 ± 0.19 b | 7.8 ± 0.03 b | 0.39 ± 0.01 c | 0.76 ± 0.01 e |
| CP-20 | 51.71 ± 1.22 d | 2.46 ± 0.05 c | 0.56 ± 0.00 c | 11.39 ± 0.26 c | 0.48 ± 0.04 d | 0.62 ± 0.02 d |
| CP-30 | 73.49 ± 0.75 e | 2.48 ± 0.05 c | 0.63 ± 0.01 d | 10.79 ± 0.23 c | 0.69 ± 0.00 e | 0.80 ± 0.02 f |
| RPP-10 | 39.99 ± 0.27 b | 1.79 ± 0.01 b | 0.36 ± 0.01 b | 7.82 ± 0.06 b | 0.18 ± 0.01 a | 0.21 ± 0.04 a |
| RPP-20 | 34.21 ± 0.12 a | 1.60 ± 0.01 a | 0.24 ± 0.04 a | 6.57 ± 0.01 a | 0.17 ± 0.01 a | 0.23 ± 0.01 a |
| RPP-30 | 45.46 ± 0.19 c | 1.69 ± 0.02 ab | 0.32 ± 0.01 b | 7.31 ± 0.00 ab | 0.17 ± 0.01 a | 0.32 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumul, D.; Berski, W.; Ivanišová, E.; Oracz, J.; Kruczek, M. The Effect of Freeze-Dried Cherry Pomace and Red Potato Pulp on the Content of Bioactive Substances in Pasta. Int. J. Mol. Sci. 2025, 26, 6020. https://doi.org/10.3390/ijms26136020
Gumul D, Berski W, Ivanišová E, Oracz J, Kruczek M. The Effect of Freeze-Dried Cherry Pomace and Red Potato Pulp on the Content of Bioactive Substances in Pasta. International Journal of Molecular Sciences. 2025; 26(13):6020. https://doi.org/10.3390/ijms26136020
Chicago/Turabian StyleGumul, Dorota, Wiktor Berski, Eva Ivanišová, Joanna Oracz, and Marek Kruczek. 2025. "The Effect of Freeze-Dried Cherry Pomace and Red Potato Pulp on the Content of Bioactive Substances in Pasta" International Journal of Molecular Sciences 26, no. 13: 6020. https://doi.org/10.3390/ijms26136020
APA StyleGumul, D., Berski, W., Ivanišová, E., Oracz, J., & Kruczek, M. (2025). The Effect of Freeze-Dried Cherry Pomace and Red Potato Pulp on the Content of Bioactive Substances in Pasta. International Journal of Molecular Sciences, 26(13), 6020. https://doi.org/10.3390/ijms26136020









