Hesperidin Is a Promising Nutraceutical Compound in Counteracting the Progression of NAFLD In Vitro
Abstract
1. Introduction
2. Results
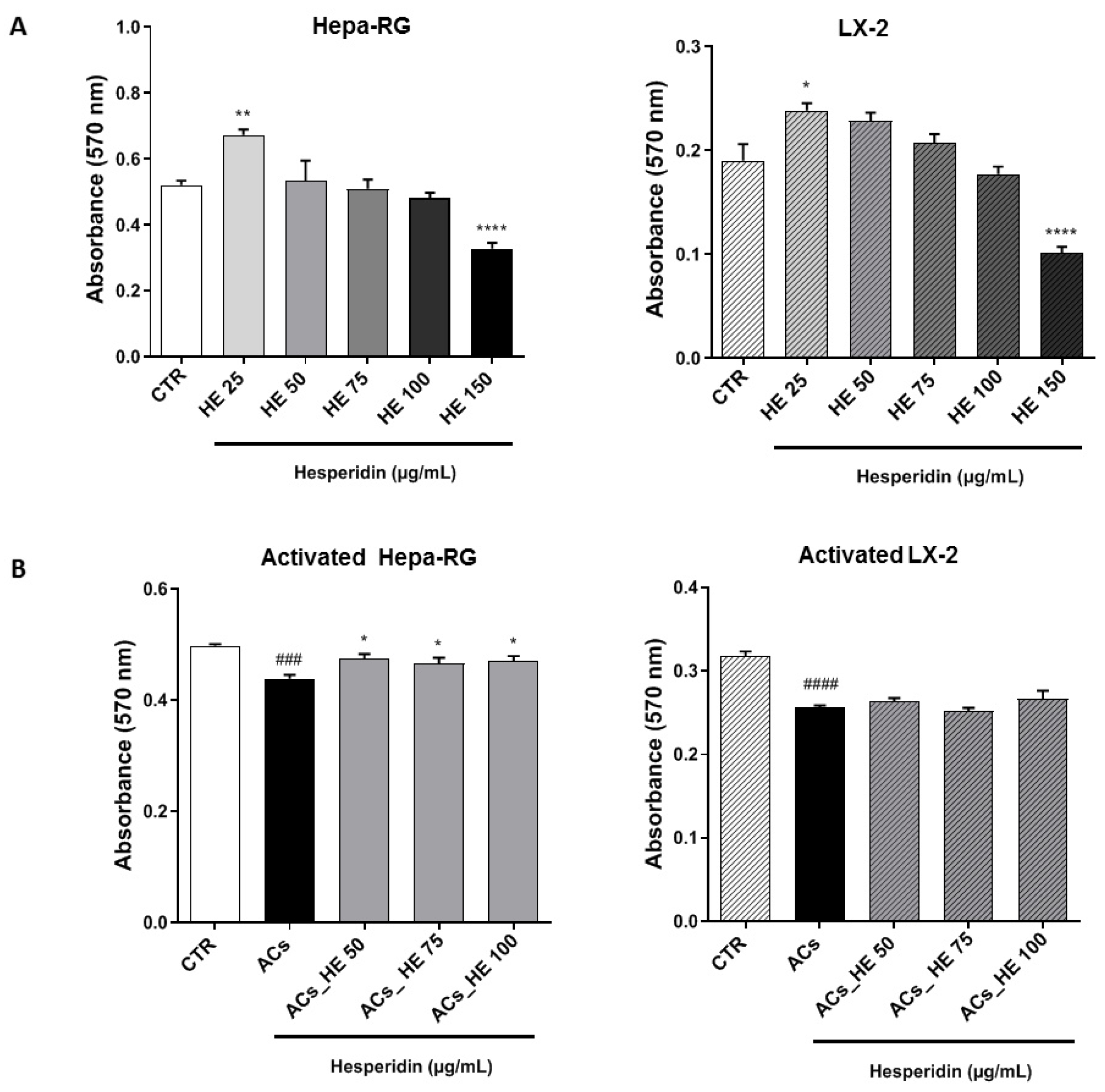
2.1. Effects of Increasing HE Concentrations on the Viability of Hepa-RG and LX-2 Hepatic Cells
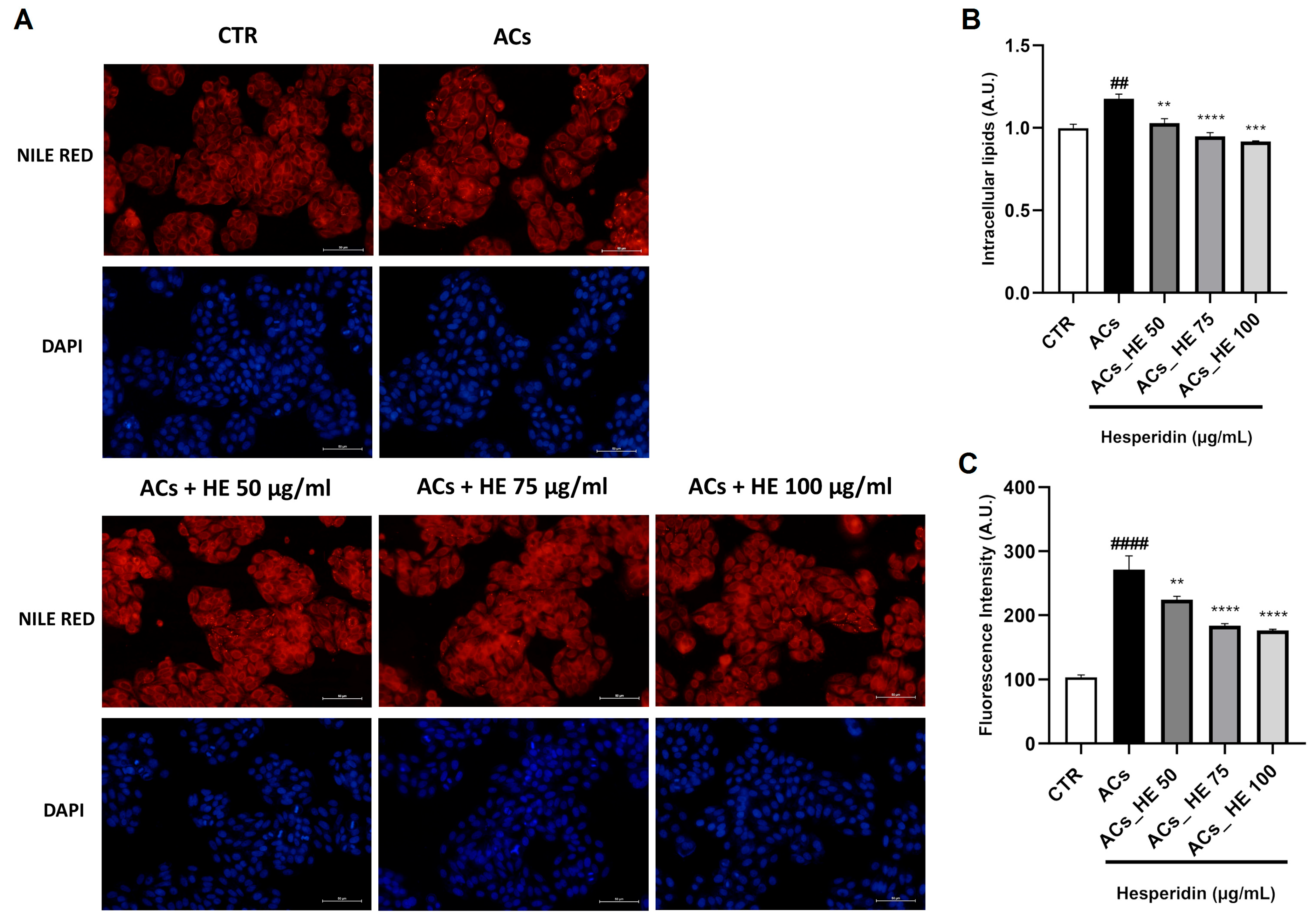
2.2. Intracellular Lipid Accumulation in Hepa-RG Cells Following NPAs Exposure: HE Attenuates Steatosis
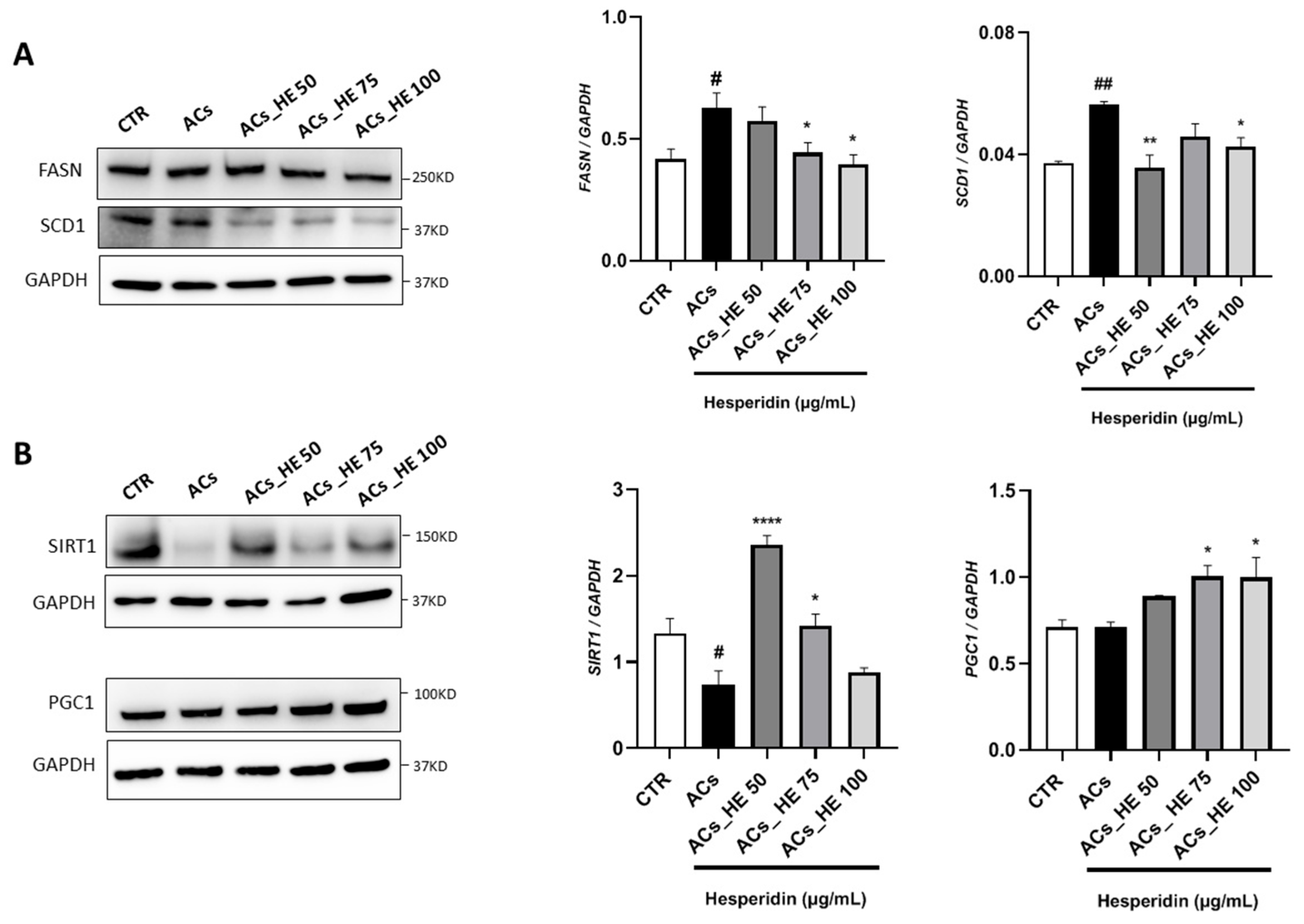
2.3. Effect of HE on the Key Lipid Metabolism Mediators in Hepa-RG Cells
2.4. HE Reduced the SCD1 Activity in Hepa-RG Cell Membranes
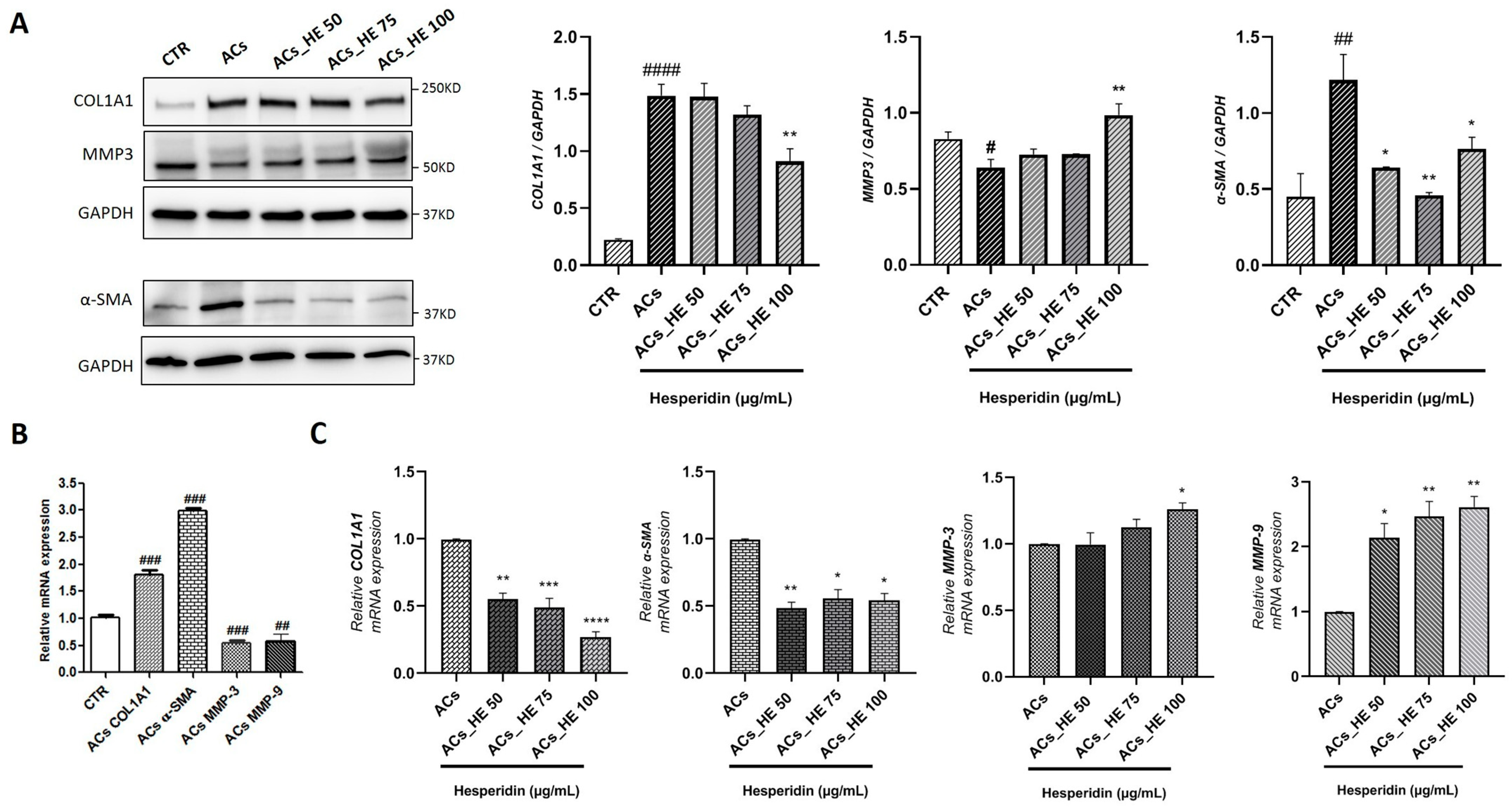
2.5. Effect of HE on the Gene and Protein Expression of Key Mediators Involved in Fibrogenic Processes in Activated LX-2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Line and Culture Conditions
4.2. Cell Viability Assay
4.3. Qualitative Analysis of Intracellular FFA—Nile Red Staining
4.4. Western Blot Analysis
4.5. Lipidomic Analysis
4.6. Nucleic Acid Extraction and RT-qPCR/qPCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| MAFLD | Metabolic Dysfunction-Associated Fatty Liver Disease |
| HE | Hesperidin |
| HCC | Hepatocellular Carcinoma |
| DNL | De Novo Lipogenesis |
| FFA | Free Fatty Acid |
| TG | Triglycerides |
| ACC | Acetyl-CoA Carboxylase |
| FASN | Fatty Acid Synthase |
| SCD1 | Stearoyl-CoA Desaturase-1 |
| PGC1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 alpha |
| SIRT1 | Silent Information Regulator 2 homolog 1 |
| α-SMA | Alpha-Smooth Muscle Actin |
| COL1A1 | Collagen Type 1 Alpha 1 Chain |
| COL1A2 | Collagen Type 1 Alpha 2 Chain |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| ECM | Extracellular Matrix |
| SMAD 2/3 | Small Mother Against decapentaplegic 2 and 3 |
| HSC | Hepatic Stellate Cells |
| HepG2 | Hepatocellular carcinoma cell line |
| LX-2 | Human Hepatic Stellate Cell Line |
| Hepa-RG | Human Hepatoma Cell Line |
| CTR | Control |
| AML12 | Alpha Mouse liver 12 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide |
| NPAs | NAFLD Promoting Agents |
| ACs | Activated Cells |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| SFAs | Saturated Fatty Acids |
| MUFAs | Monounsaturated Fatty Acids |
| SCD1 | Stearoyl-CoA Desaturase1 |
| MMP3 | Matrix Metalloproteinase 3 |
| MMP9 | Matrix Metalloproteinase 9 |
| ROS | Reactive Oxygen Species |
| OA | Oleic Acid |
| PA | Palmitic Acid |
| FBS | Fetal Bovine Serum |
| LPS | Lipopolysaccharides |
| TGF-β | Transforming Growth Factor-Beta |
| BSA | Bovine Serum Albumin |
| DMSO | Dimethyl Sulfoxide |
| HCl | Hydrochloric Acid |
| PBS | Phosphate-Buffered Saline |
| HBSS | Hanks’ Balanced Salt Solution |
| RIPA | Radioimmunoprecipitation Assay Buffer |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| TBS | Tris-Buffered Saline |
| TBS-T | Tris-Buffered Saline with Tween 20 |
| ECL | Enhanced Chemiluminescence |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| HBM | Hepatocyte Bullet Kit medium |
| FA | Fatty Acids |
| FAME | Fatty Acid Methyl Esters |
| FID | Flame Ionization Detector |
| SEM | Standard Error of the Mean |
References
- Loomba, R.; Sanyal, A.J. The Global NAFLD Epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Milani, I.; Codini, M.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. Int. J. Mol. Sci. 2024, 25, 10795. [Google Scholar] [CrossRef]
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Wobser, H.; Dorn, C.; Weiss, T.S.; Amann, T.; Bollheimer, C.; Büttner, R.; Schölmerich, J.; Hellerbrand, C. Lipid Accumulation in Hepatocytes Induces Fibrogenic Activation of Hepatic Stellate Cells. Cell Res. 2009, 19, 996–1005. [Google Scholar] [CrossRef]
- Rafiei, H.; Yeung, M.; Kowalski, S.; Krystal, G.; Elisia, I. Development of a Novel Human Triculture Model of Non-Alcoholic Fatty Liver Disease and Identification of Berberine as Ameliorating Steatosis, Oxidative Stress and Fibrosis. Front. Pharmacol. 2023, 14, 1234300. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and Liver Cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Gambino, R. Non-Alcoholic Steatohepatitis: Emerging Molecular Targets and Therapeutic Strategies. Nat. Rev. Drug Discov. 2016, 15, 249–274. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic Pipeline in Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Palasca, O.; Yarani, R.; Litman, T.; Anthon, C.; Groenen, M.A.M.; Stadler, P.F.; Pociot, F.; Jensen, L.J.; Gorodkin, J. Human Pathways in Animal Models: Possibilities and Limitations. Nucleic Acids Res. 2021, 49, 1859–1871. [Google Scholar] [CrossRef]
- Gnoni, A.; Di Chiara Stanca, B.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Damiano, F. Quercetin Reduces Lipid Accumulation in a Cell Model of NAFLD by Inhibiting De Novo Fatty Acid Synthesis through the Acetyl-CoA Carboxylase 1/AMPK/PP2A Axis. Int. J. Mol. Sci. 2022, 23, 1044. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, B.; Yang, C.; Yang, L. Hepatic Lipid Homeostasis in NAFLD. In Non-Alcoholic Fatty Liver Disease—New Insight and Glance into Disease Pathogenesis; IntechOpen: London, UK, 2023. [Google Scholar]
- Grattagliano, I.; Montezinho, L.P.; Oliveira, P.J.; Frühbeck, G.; Gómez-Ambrosi, J.; Montecucco, F.; Carbone, F.; Wieckowski, M.R.; Wang, D.Q.-H.; Portincasa, P. Targeting Mitochondria to Oppose the Progression of Nonalcoholic Fatty Liver Disease. Biochem. Pharmacol. 2019, 160, 34–45. [Google Scholar] [CrossRef]
- Francque, S.; Vonghia, L. Pharmacological Treatment for Non-Alcoholic Fatty Liver Disease. Adv. Ther. 2019, 36, 1052–1074. [Google Scholar] [CrossRef]
- Chen, H.; Nie, T.; Zhang, P.; Ma, J.; Shan, A. Hesperidin Attenuates Hepatic Lipid Accumulation in Mice Fed High-Fat Diet and Oleic Acid Induced HepG2 via AMPK Activation. Life Sci. 2022, 296, 120428. [Google Scholar] [CrossRef]
- Paglialunga, S.; Dehn, C.A. Clinical Assessment of Hepatic de Novo Lipogenesis in Non-Alcoholic Fatty Liver Disease. Lipids Health Dis. 2016, 15, 159. [Google Scholar] [CrossRef]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of Acetyl-CoA Carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef]
- Esler, W.P.; Cohen, D.E. Pharmacologic Inhibition of Lipogenesis for the Treatment of NAFLD. J. Hepatol. 2024, 80, 362–377. [Google Scholar] [CrossRef]
- O’Farrell, M.; Duke, G.; Crowley, R.; Buckley, D.; Martins, E.B.; Bhattacharya, D.; Friedman, S.L.; Kemble, G. FASN Inhibition Targets Multiple Drivers of NASH by Reducing Steatosis, Inflammation and Fibrosis in Preclinical Models. Sci. Rep. 2022, 12, 15661. [Google Scholar] [CrossRef]
- Harriman, G.; Greenwood, J.; Bhat, S.; Huang, X.; Wang, R.; Paul, D.; Tong, L.; Saha, A.K.; Westlin, W.F.; Kapeller, R.; et al. Acetyl-CoA Carboxylase Inhibition by ND-630 Reduces Hepatic Steatosis, Improves Insulin Sensitivity, and Modulates Dyslipidemia in Rats. Proc. Natl. Acad. Sci. USA 2016, 113, E1796–E1805. [Google Scholar] [CrossRef]
- Nie, T.; Wang, X.; Li, A.; Shan, A.; Ma, J. The Promotion of Fatty Acid β-Oxidation by Hesperidin via Activating SIRT1/PGC1α to Improve NAFLD Induced by a High-Fat Diet. Food Funct. 2024, 15, 372–386. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, D.; Gong, Q.; Xu, Q.; Pan, D.; Lu, F.; Tang, Q. Elucidation of SIRT-1/PGC-1α-Associated Mitochondrial Dysfunction and Autophagy in Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, M.; Bellner, L.; Singh, S.P.; Favero, G.; Rezzani, R.; Rodella, L.F.; Falck, J.R.; Abraham, N.G.; Vanella, L. Epoxyeicosatrienoic Intervention Improves NAFLD in Leptin Receptor Deficient Mice by an Increase in HO-1-PGC1α Mitochondrial Signaling. Exp. Cell Res. 2019, 380, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Swe, M.T.; Thongnak, L.; Jaikumkao, K.; Pongchaidecha, A.; Chatsudthipong, V.; Lungkaphin, A. Dapagliflozin Not Only Improves Hepatic Injury and Pancreatic Endoplasmic Reticulum Stress, but Also Induces Hepatic Gluconeogenic Enzymes Expression in Obese Rats. Clin. Sci. 2019, 133, 2415–2430. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhu, L.; Shen, Y.; Mou, Q.; Lin, T.; Feng, H. Protection of Hepatocyte Mitochondrial Function by Blueberry Juice and Probiotics via SIRT1 Regulation in Non-Alcoholic Fatty Liver Disease. Food Funct. 2019, 10, 1540–1551. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X.; Jiang, Y.; Wen, J.; Yang, L.; Chen, D.; Cao, X.; Peng, C. A Comprehensive Review of Natural Products against Liver Fibrosis: Flavonoids, Quinones, Lignans, Phenols, and Acids. Evid. Based Complement. Altern. Med. 2020, 2020, 7171498. [Google Scholar] [CrossRef]
- Li, J.-J.; Jiang, H.-C.; Wang, A.; Bu, F.-T.; Jia, P.-C.; Zhu, S.; Zhu, L.; Huang, C.; Li, J. Hesperetin Derivative-16 Attenuates CCl4-Induced Inflammation and Liver Fibrosis by Activating AMPK/SIRT3 Pathway. Eur. J. Pharmacol. 2022, 915, 174530. [Google Scholar] [CrossRef]
- Saponara, I.; Aloisio Caruso, E.; Cofano, M.; De Nunzio, V.; Pinto, G.; Centonze, M.; Notarnicola, M. Anti-Inflammatory and Anti-Fibrotic Effects of a Mixture of Polyphenols Extracted from “Navelina” Orange in Human Hepa-RG and LX-2 Cells Mediated by Cannabinoid Receptor 2. Int. J. Mol. Sci. 2025, 26, 512. [Google Scholar] [CrossRef]
- Matilainen, J.; Mustonen, A.-M.; Rilla, K.; Käkelä, R.; Sihvo, S.P.; Nieminen, P. Orotic Acid-Treated Hepatocellular Carcinoma Cells Resist Steatosis by Modification of Fatty Acid Metabolism. Lipids Health Dis. 2020, 19, 70. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Xu, Y.; Du, J.; Ling, C. Roles of Hepatic Stellate Cells in NAFLD: From the Perspective of Inflammation and Fibrosis. Front. Pharmacol. 2022, 13, 958428. [Google Scholar] [CrossRef]
- Rizzo, M.; Colletti, A.; Penson, P.E.; Katsiki, N.; Mikhailidis, D.P.; Toth, P.P.; Gouni-Berthold, I.; Mancini, J.; Marais, D.; Moriarty, P.; et al. Nutraceutical Approaches to Non-Alcoholic Fatty Liver Disease (NAFLD): A Position Paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2023, 189, 106679. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Cisternino, A.M.; Cofano, M.; Donghia, R.; Giannuzzi, V.; Zappimbulso, M.; Milella, R.A.; Giannelli, G.; et al. Daily Orange Consumption Reduces Hepatic Steatosis Prevalence in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: Exploratory Outcomes of a Randomized Clinical Trial. Nutrients 2024, 16, 3191. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xing, X.; Wang, H.; Wan, K.; Fan, R.; Liu, C.; Wang, Y.; Wu, W.; Wang, Y.; Wang, R. SCD1 Is the Critical Signaling Hub to Mediate Metabolic Diseases: Mechanism and the Development of Its Inhibitors. Biomed. Pharmacother. 2024, 170, 115586. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Lupuliasa, D.; Neacșu, S.M.; Olteanu, G.; Busnatu, Ș.S.; Mihai, A.; Popovici, V.; Măru, N.; Boroghină, S.C.; Mihai, S.; et al. Polyunsaturated Fatty Acids and Human Health: A Key to Modern Nutritional Balance in Association with Polyphenolic Compounds from Food Sources. Foods 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Gigante, I.; Scavo, M.P.; Refolo, M.G.; De Nunzio, V.; Milella, R.A.; Caruso, M.G.; Notarnicola, M. Stearoyl-CoA Desaturase-1 Enzyme Inhibition by Grape Skin Extracts Affects Membrane Fluidity in Human Colon Cancer Cell Lines. Nutrients 2020, 12, 693. [Google Scholar] [CrossRef]
- Angelucci, C.; D’Alessio, A.; Iacopino, F.; Proietti, G.; Di Leone, A.; Masetti, R.; Sica, G. Pivotal Role of Human Stearoyl-CoA Desaturases (SCD1 and 5) in Breast Cancer Progression: Oleic Acid-Based Effect of SCD1 on Cell Migration and a Novel pro-Cell Survival Role for SCD5. Oncotarget 2018, 9, 24364–24380. [Google Scholar] [CrossRef]
- Choe, M.; Jackson, C.; Yu, B.P. Lipid Peroxidation Contributes to Age-Related Membrane Rigidity. Free Radic. Biol. Med. 1995, 18, 977–984. [Google Scholar] [CrossRef]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary Polyphenols Protect Against Oleic Acid-Induced Steatosis in an in Vitro Model of NAFLD by Modulating Lipid Metabolism and Improving Mitochondrial Function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef]
- Xu, H.; Wan, S.; An, Y.; Wu, Q.; Xing, Y.; Deng, C.; Zhang, P.; Long, Y.; Xu, B.; Jiang, Z. Targeting Cell Death in NAFLD: Mechanisms and Targeted Therapies. Cell Death Discov. 2024, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding Lipotoxicity in NAFLD Pathogenesis: Is CD36 a Key Driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
| CTR | ACs | ACs HE 50 μg/mL | ACs HE 75 μg/mL | ACs HE 100 μg/mL | |
|---|---|---|---|---|---|
| Palmitic acid | 22.11 ± 0.02 | 22.03 ± 0.10 | 23.64 ± 0.29 *** | 24.72 ± 0.01 *** | 23.65 ± 0.30 *** |
| Palmitoleic acid | 0.77 ± 0.01 | 0.80 ± 0.02 | 0.60 ± 0.002 | 0.31 ± 0.08 *** | 0.43 ± 0.10 *** |
| Stearic acid | 15.32 ± 0.15 | 13.22 ± 0.21 ## | 17.11 ± 0.36 *** | 18.25 ± 0.05 *** | 16.29 ± 0.60 *** |
| Oleic acid | 31.96 ± 0.12 | 35.55 ± 0.07 ### | 34.43 ± 0.37 | 35.76 ± 0.80 | 35.47 ± 0.10 |
| SFAs | 42.44 ± 0.18 | 39.88 ± 0.35 ## | 45.47 ± 0.58 *** | 46.73 ± 0.24 *** | 44.31 ± 0.76 *** |
| MUFAs | 40.62 ± 0.10 | 43.01 ± 0.17 ## | 40.61 ± 0.47 ** | 41.38 ± 0.75 | 42.26 ± 0.08 |
| SCD1-C16 | 0.035 ± 0.002 | 0.037 ± 0.002 | 0.027 ± 0.002 | 0.012 ± 0.003 *** | 0.018 ± 0.005 ** |
| SCD1-C18 | 2.09 ± 0.03 | 2.70 ± 0.05 ### | 2.03 ± 0.06 *** | 1.96 ± 0.05 *** | 2.20 ± 0.08 *** |
| SCD1 | 2.13 ± 0.03 | 2.73 ± 0.05 ### | 2.05 ± 0.07 *** | 1.97 ± 0.05 *** | 2.22 ± 0.08 *** |
| Gene | Unique Assay ID | Chromosome Location | Amplicon Length |
|---|---|---|---|
| COL1A1 | qHsaCED0043248 | 17:48277174–48278779 | 113 |
| α-SMA | qHsaCID0013300 | 10:90699374–90701011 | 78 |
| MMP3 | qHsaCID0006170 | 11:102711183–102712920 | 148 |
| MMP9 | qHsaCID0011597 | 20:44641165–44641948 | 82 |
| GAPDH | qHsaCED0038674 | 12:6647267–6647413 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofano, M.; Saponara, I.; De Nunzio, V.; Pinto, G.; Aloisio Caruso, E.; Centonze, M.; Notarnicola, M. Hesperidin Is a Promising Nutraceutical Compound in Counteracting the Progression of NAFLD In Vitro. Int. J. Mol. Sci. 2025, 26, 5982. https://doi.org/10.3390/ijms26135982
Cofano M, Saponara I, De Nunzio V, Pinto G, Aloisio Caruso E, Centonze M, Notarnicola M. Hesperidin Is a Promising Nutraceutical Compound in Counteracting the Progression of NAFLD In Vitro. International Journal of Molecular Sciences. 2025; 26(13):5982. https://doi.org/10.3390/ijms26135982
Chicago/Turabian StyleCofano, Miriam, Ilenia Saponara, Valentina De Nunzio, Giuliano Pinto, Emanuela Aloisio Caruso, Matteo Centonze, and Maria Notarnicola. 2025. "Hesperidin Is a Promising Nutraceutical Compound in Counteracting the Progression of NAFLD In Vitro" International Journal of Molecular Sciences 26, no. 13: 5982. https://doi.org/10.3390/ijms26135982
APA StyleCofano, M., Saponara, I., De Nunzio, V., Pinto, G., Aloisio Caruso, E., Centonze, M., & Notarnicola, M. (2025). Hesperidin Is a Promising Nutraceutical Compound in Counteracting the Progression of NAFLD In Vitro. International Journal of Molecular Sciences, 26(13), 5982. https://doi.org/10.3390/ijms26135982







