The Role of Biomarkers in Temporomandibular Disorders: A Systematic Review
Abstract
1. Introduction
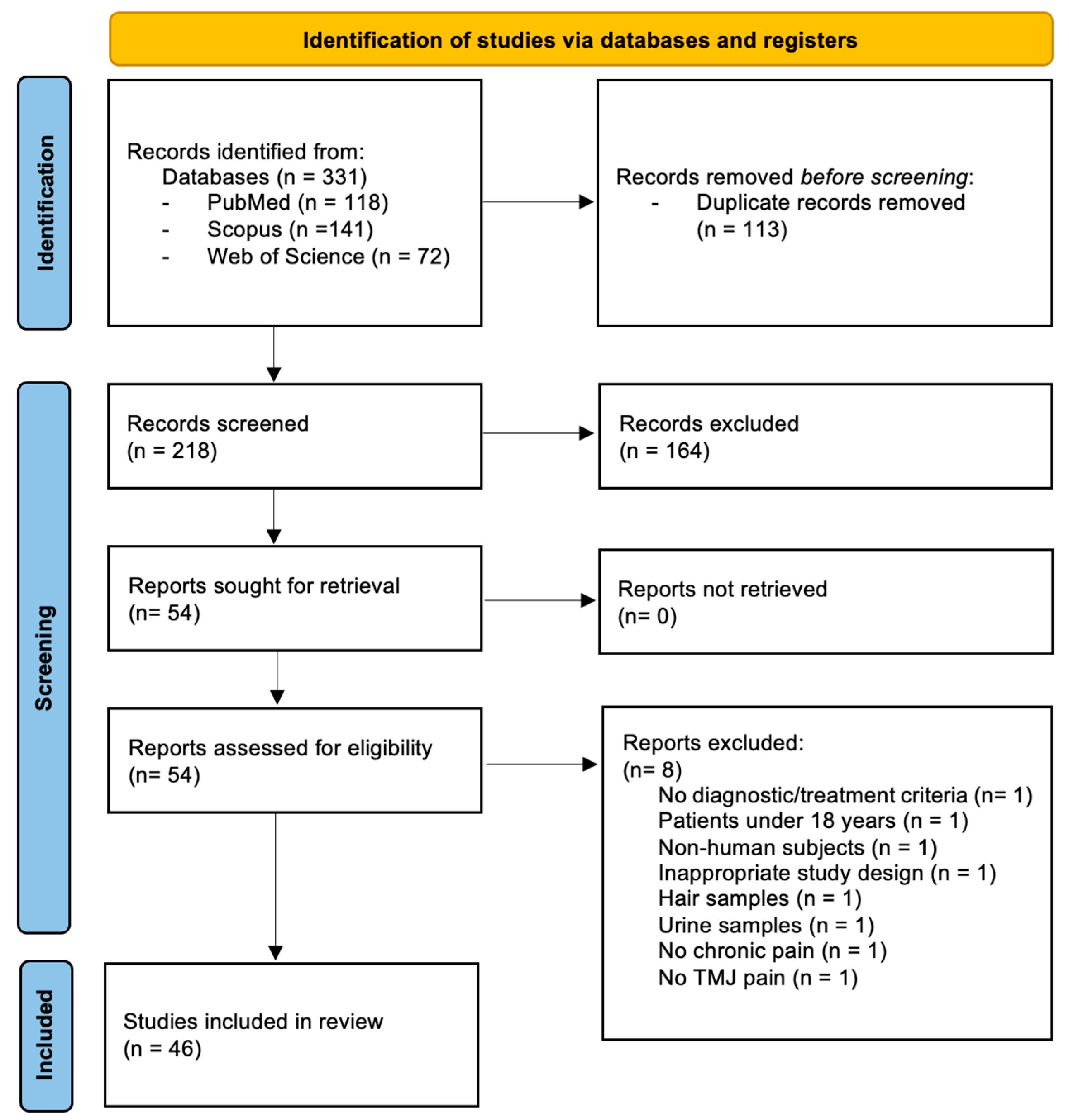
2. Materials and Methods
3. Results
3.1. Characteristics of Included Studies
3.2. Summary of Key Findings
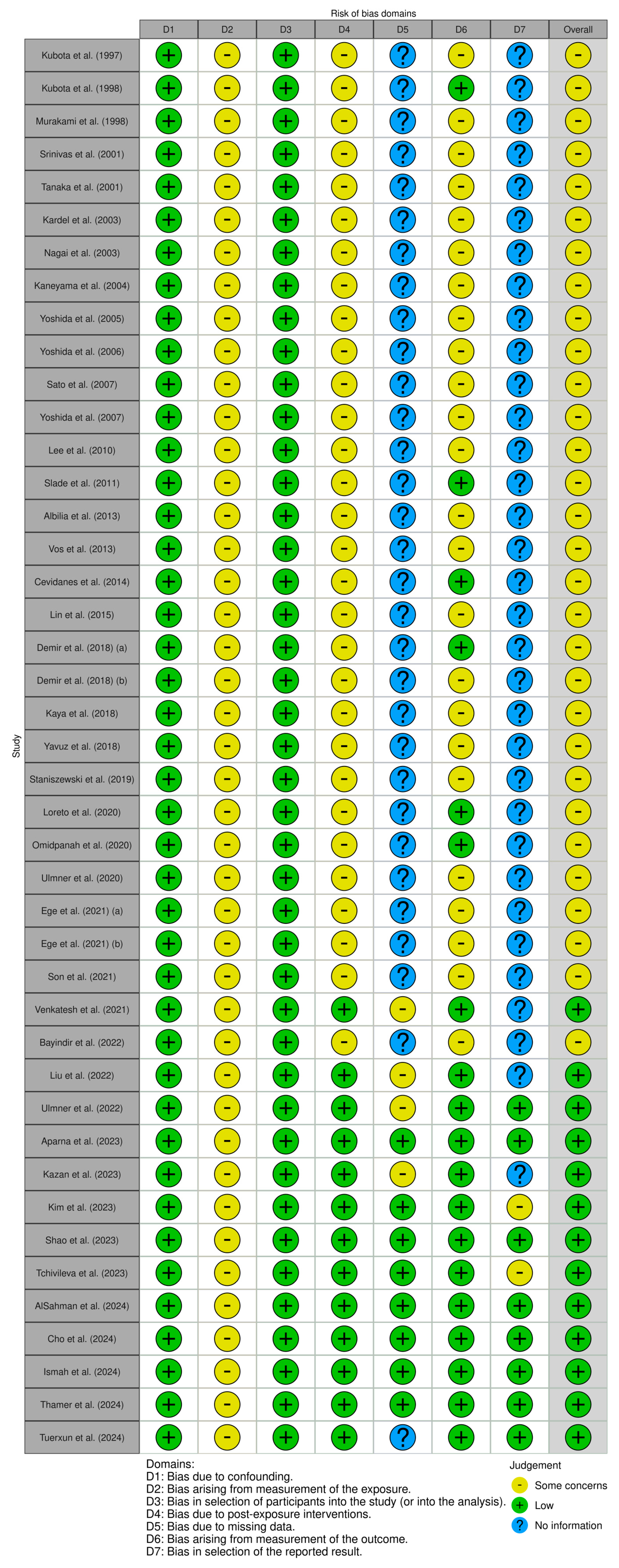
3.3. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMP | Bone morphogenetic protein |
| CAT | Catalase |
| CGRP | Calcitonin gene-related peptide |
| CFHR3 | Complement factor H-related protein 3 |
| CPN2 | Carboxypeptidase N catalytic chain |
| CRP | C-reactive protein |
| DD | Disc displacement |
| DDwR | Disc displacement with reduction |
| DDwoR | Disc displacement without reduction |
| dNLR | Derived NLR |
| ESR | Erythrocyte sedimentation rate |
| GCS | Glucosamine–chondroitin sulphate |
| HA | Hyaluronic acid |
| HMGB1 | High-mobility group box 1 |
| IASP | International Association for the Study of Pain |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LLLT | Low-level laser therapy |
| LMR | Lymphocyte-to-monocyte ratio |
| MDA | Malondialdehyde |
| MMP | Matrix metalloproteinase |
| MMO | Maximum mouth opening |
| NLR | Neutrophil-to-lymphocyte ratio |
| OA | Osteoarthritis |
| OPN | Osteopontin |
| PGE2 | Prostaglandin E2 |
| PLR | Platelet-to-lymphocyte ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRP | Platelet-rich plasma |
| RDX | Radixin |
| RCT | Randomized controlled trial |
| SHA | Sodium hyaluronic acid |
| SII | Systemic immune-inflammation index |
| SOD | Superoxide dismutase |
| SS | Stabilization splint |
| TAC | Total antioxidant capacity |
| TLR4 | Toll-like receptor 4 |
| TMD | Temporomandibular disorders |
| TMJ | Temporomandibular joint |
| TMJ-ID | Temporomandibular joint internal derangement |
| TNF | Tumour necrosis factor |
References
- Chung, M.K.; Wang, S.; Alshanqiti, I.; Hu, J.; Ro, J.Y. The degeneration-pain relationship in the temporomandibular joint: Current understandings and rodent models. Front. Pain Res. 2023, 4, 1038808. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.M.S.; Rady, D.; El Moshy, S.; Ahmed Radwan, I.; Wadan, A.S.; Dorfer, C.E.; El-Sayed, K.M.F. The Temporomandibular Joint and the Human Body: A New Perspective on Cross Talk. Dent J. 2024, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.; Battaglino, R.; Ye, L. A comprehensive review on biomarkers associated with painful temporomandibular disorders. Int. J. Oral. Sci. 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Giano, M.; Franco, C.; Castrezzati, S.; Rezzani, R. Involvement of Oxidative Stress and Nutrition in the Anatomy of Orofacial Pain. Int. J. Mol. Sci. 2023, 24, 13128. [Google Scholar] [CrossRef]
- Pate, J.W.; Noblet, T.; Hush, J.M.; Hancock, M.J.; Sandells, R.; Pounder, M.; Pacey, V. Exploring the concept of pain of Australian children with and without pain: Qualitative study. BMJ Open 2019, 9, e033199. [Google Scholar] [CrossRef]
- Ferreira-Valente, A.; Fontes, F.; Pais-Ribeiro, J.; Jensen, M.P. The Meaning Making Model Applied to Community-Dwelling Adults with Chronic Pain. J. Pain Res. 2021, 14, 2295–2311. [Google Scholar] [CrossRef]
- Borsook, D.; Youssef, A.M.; Simons, L.; Elman, I.; Eccleston, C. When pain gets stuck: The evolution of pain chronification and treatment resistance. Pain 2018, 159, 2421–2436. [Google Scholar] [CrossRef]
- Thamer, S.R.; Diajil, A.R. Therapeutic effect of intra-articular injection of hyaluronic acid and platelet-rich plasma on the expression of salivary matrix metalloproteinases 2 and 9 in tempromandibular internal derrangment patients. J. Emerg. Med. Trauma Acute Care 2024, 2024, 3. [Google Scholar] [CrossRef]
- Cho, I.S.; Jang, J.H.; Park, J.W. Hematological biomarkers of systemic inflammation in predicting long-term treatment response of temporomandibular disorders. BMC Oral Health 2024, 24, 1097. [Google Scholar] [CrossRef]
- Tuerxun, P.; Nijiati, T.; Zhang, K.; Zhang, P. Integration of metabolomics and transcriptomics provides insights into the molecular mechanism of temporomandibular joint osteoarthritis. PLoS ONE 2024, 19, e0301341. [Google Scholar] [CrossRef]
- Shekhar, A.; Maddheshiya, N.; Nair, V.; Rastogi, V.; Srivastava, A.; Singh, A.K. Salivary biomarkers and temporomandibular disorders: A systematic review. Natl. J. Maxillofac. Surg. 2023, 14, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Zaman, M.U.; Alqhtani, N.R.; Alqahtani, A.S.; Alqahtani, F.; Cicciu, M.; Minervini, G. Salivary Biomarkers and Temporomandibular Disorders: A Systematic Review conducted according to PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J. Oral Rehabil. 2024, 51, 416–426. [Google Scholar] [CrossRef] [PubMed]
- AlSahman, L.; AlBagieh, H.; AlSahman, R. Is There a Relationship between Salivary Cortisol and Temporomandibular Disorder: A Systematic Review. Diagnostics 2024, 14, 1435. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Khanzadeh, H.; Jamasbi, S.S.M.; Rezaei, F.; Azadi, A. Vitamin D serum levels and temporomandibular disorders: A systematic review and meta-analysis. Arch. Oral Biol. 2025, 169, 106108. [Google Scholar] [CrossRef]
- Eslami, H.; Katebi, K.; Ghaffaripour Saleh, S.; Mirizadeh, L.; Hashemi, M. The relationship between oxidative stress markers and temporomandibular disorders: A systematic review and meta-analysis. J. Res. Med. Sci. 2024, 29, 33. [Google Scholar] [CrossRef]
- Liu, F.; Steinkeler, A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent. Clin. N. Am. 2013, 57, 465–479. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- AlSahman, L.; AlBagieh, H.; AlSahman, R.; Mokeem, N.R.; Costa, L.P. Does salivary cortisol serve as a potential biomarker for temporomandibular disorders in adults? BMC Oral Health 2024, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Ismah, N.; Bachtiar, E.; Purwanegara, M.; Tanti, I.; Mardiati, E.; Ismah, N.; Bachtiar, E.W.; Purwanegara, M.K.; Tanti, I.; Mardiati, E. Evaluation of IL-1β and CRP mRNA expression levels by RT-PCR in postorthodontic treatment patients with temporomandibular joint disorders: A cross-sectional Study. J. Int. Soc. Prev. Community Dent. 2024, 14, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tchivileva, I.E.; Johnson, K.W.; Chai, X.; Vandam, L.R.; Lim, P.F.; Slade, G.D. Evaluation of Plasma Calcitonin Gene-Related Peptide as a Biomarker for Painful Temporomandibular Disorder and Migraine. J. Pain Res. 2023, 16, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
- Naseem, A.; Raza, S.; Ahmad, R.; Saleem, R. A case-control investigation of the psychological and physiological stress markers with salivary cortisol levels in patients with temporomandibular joint disorders: A short clinical study. J. Indian Prosthodont. Soc. 2023, 23, 163–169. [Google Scholar] [CrossRef]
- Kaya, D.; Bilgili, B.A.; Aydin, A.; Erdem, A. The evaluation of oxidative stress and inflammation markers in serum and saliva of the patients with temporomandibular disorders. Turk. J. Med. Sci. 2023, 53, 1690–1696. [Google Scholar] [CrossRef]
- Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Alstergren, P.; Lund, B.; Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Alstergren, P.; Lund, B. Cytokines in temporomandibular joint synovial fluid and tissue in relation to inflammation. J. Oral Rehabil. 2022, 49, 599–607. [Google Scholar] [CrossRef]
- Bayindir, S.; Asan, C.; Demirbas, A.; Keti, D.; Kütük, N.; Bayindir, S.; Asan, C.Y.; Demirbas, A.E.; Keti, D.B.; Kutuk, N. Evaluation of aggrecan and adipokine levels in temporomandibular joint synovial fluid. J. Cranio-Maxillofac. Surg. 2022, 50, 432–438. [Google Scholar] [CrossRef]
- Venkatesh, S.B.; Shetty, S.S.; Kamath, V. Prevalence of temporomandibular disorders and its correlation with stress and salivary cortisol levels among students. Pesqui. Bras. Odontopediatria Clin. Integr. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Ege, B.; Yumrutas, O.; Bozgeyik, I. Investigation of osteopontin and CD44 levels in patients with temporomandibular joint disorders. Gene Rep. 2021, 24, 101213. [Google Scholar] [CrossRef]
- Ege, B.; Erdogmus, Z.; Bozgeyik, E.; Koparal, M.; Kurt, M.Y.; Gulsun, B. Asporin levels in patients with temporomandibular joint disorders. J. Oral Rehabil. 2021, 48, 1109–1117. [Google Scholar] [CrossRef]
- Song, C.; Park, Y.K.; Park, J.W. Long-term evaluation of temporomandibular disorders in association with cytokine and autoantibody status in young women. Cytokine 2021, 144, 155551. [Google Scholar] [CrossRef]
- Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Suslu, S.; Reseland, J.; Kruger-Weiner, C.; Lund, B.; Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; et al. Synovial tissue cytokine profile in disc displacement of the temporomandibular joint. J. Oral Rehabil. 2020, 47, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Omidpanah, N.; Ebrahimi, S.; Raygani, A.V.; Mozafari, H.; Rezaei, M. Total antioxidant capacity, catalase activity and salivary oxidative parameters in patients with temporomandibular disorders. Front. Dent. 2020, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Filetti, V.; Almeida, L.E.; Rapisarda, G.R.M.; La Rocca, R.; Graziano, C.; La Greca, A. MMP-7 and MMP-9 are overexpressed in the synovial tissue from severe temporomandibular joint dysfunction. Eur. J. Histochem. EJH 2020, 64, 3113. [Google Scholar] [CrossRef]
- Skog, K.; Lundeberg, H.; Berge, T.; Ruggiero, A. Serum Analysis in Patients with Temporomandibular Disorders: A Controlled Cross-Sectional Study in Norway. Pain Res. Manag. 2019, 2019, 1360725. [Google Scholar] [CrossRef]
- Yıldız Güney, G.; Gülsün Kara, Ş.; Koçak, A. Analysis of synovial fluid visfatin level in temporomandibular joint disorders. Cranio 2019, 37, 296–303. [Google Scholar] [CrossRef]
- Demir, C.Y.; Erbaş, M.E. Biochemical changes associated with temporomandibular disorders. J. Int. Med. Res. 2018, 47, 765–771. [Google Scholar] [CrossRef]
- Gülsün Kara, Ş.; Yıldız Güney, G.; Koçak, A. Expression of chemerin in the synovial fluid of patients with temporomandibular joint disorders. J. Oral Rehabil. 2018, 45, 289–294. [Google Scholar] [CrossRef]
- Demir, C.; Kocak, O.; Bozan, N.; Ersoz, M.; Demir, H.; Demir, C.Y.; Kocak, O.F.; Bozan, N.; Ersoz, M.E.; Demir, H. Is There a Role for Oxidative Stress in Temporomandibular Joint Disorders? J. Oral Maxillofac. Surg. 2018, 76, 515–520. [Google Scholar] [CrossRef]
- Lin, S.L.; Wang, S.L.; Tu, C.C.; Kao, S.Y.; Yeh, J.W. Serum cortisol level and disc displacement disorders of the temporomandibular joint. J. Oral Rehabil. 2015, 43, 10–15. [Google Scholar] [CrossRef]
- Chen, L.H.; Wang, D.; Sun, J.; Sun, J.; Gu, W.; Peng, B.; Zhu, H.; Walker, D.; Paniagua, B.; Lim, P.; et al. 3D osteoarthritic changes in TMJ condylar morphology correlates with specific systemic and local biomarkers of disease. Osteoarthr. Cartil. 2014, 22, 1657–1667. [Google Scholar] [CrossRef]
- Voog, L.M.; Kallik, R.; Heffez, J.J.; Schimming, B. Alteration of cartilage degeneration and inflammation markers in temporomandibular joint osteoarthritis occurs proportionally. J. Oral Maxillofac. Surg. 2013, 71, 1659–1664. [Google Scholar]
- Albright, J.B.; Thompson, H.C.; Copen, C.M.; Wysocki, D.R.; Bolognesi, G.I.; Padgett, D.J.; Backstein, D.; Walt, D.R. Serum levels of BMP-2, 4, 7 and AHSG in patients with degenerative joint disease requiring total arthroplasty of the hip and temporomandibular joints. J. Orthop. Res. 2013, 31, 44–52. [Google Scholar] [CrossRef]
- Slade, G.D.; Conrad, M.S.; Diatchenko, L.; Rashid, N.U.; Zhong, S.; Smith, S.; Rhodes, J.; Makarov, S.; Nackley, A.G.; Maixner, W.; et al. Cytokine biomarkers and chronic pain: Association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain 2011, 152, 2802–2812. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Chae, Y.S.; Shin, S.I. Relationship of synovial tumor necrosis factor alpha and interleukin 6 to temporomandibular disorder. J. Oral Maxillofac. Surg. 2010, 68, 1064–1068. [Google Scholar] [CrossRef]
- Saito, J.; Nishimura, N.; Nakagawa, M.; Yamada, Y.; Koseki, K.; Yamashita, Y. Expression of interleukin 8 in synovial tissues in patients with internal derangement of the temporomandibular joint and its relationship with clinical variables. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 467–474. [Google Scholar] [CrossRef]
- Yamamoto, H.; Funakoshi, S.; Nakagawa, M.; Takahashi, T. Angiogenesis in the human temporomandibular joint studied by immunohistochemistry for CD34 antigen. J. Oral Pathol. Med. 2007, 28, 289–292. [Google Scholar] [CrossRef]
- Yoshida, K.; Takahashi, S.; Enomoto, H.; Hirata, H.; Takeuchi, A.; Uematsu, K.; Yoshida, K.; Tanaka, A.; Nakamura, H.; Okada, Y. Expression of matrix metalloproteinases and aggrecanase in the synovial fluids of patients with symptomatic temporomandibular disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 22–27. [Google Scholar] [CrossRef]
- Yoshida, K.; Takahashi, S.; Takeuchi, A.; Enomoto, H.; Hirata, H.; Nakamura, K.; Onodera, Y. Aggrecanase analysis of synovial fluid of temporomandibular joint disorders. Oral Dis. 2005, 11, 299–302. [Google Scholar] [CrossRef]
- Kaneyama, K.; Segami, N.; Suzuki, J.; Nishimura, M.; Hosaka, Y. Interleukin-6 family of cytokines as biochemical markers of osseous changes in the temporomandibular joint disorders. Br. J. Oral Maxillofac. Surg. 2004, 42, 246–250. [Google Scholar] [CrossRef]
- Kopp, R.; Ulmner, A.K.; Reseland, F.P.; Hogstrom, A. Inflammatory cell and cytokine patterns in patients with painful clicking and osteoarthritis in the temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2003, 32, 390–396. [Google Scholar] [CrossRef]
- Nagai, H.; Kaneko, H.; Fujii, M.; Tanimoto, T. Inducible nitric oxide synthase and apoptosis-related factors in the synovial tissues of temporomandibular joints with internal derangement and osteoarthritis. J. Oral Maxillofac. Surg. 2003, 61, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Sugimoto, T.; Takahashi, L.; Nakatani, E.; Ruggiero, A.; Hirata, P.; Tjäderhane, L.; Teronen, O. Matrix metalloproteinases in mild and severe temporomandibular joint internal derangement synovial fluid. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 517–525. [Google Scholar] [CrossRef]
- Takahashi, A.; Kaneko, S.; Kaneko, S.; Sugimoto, T.; Nakamura, K.; Yamashita, E.; Maeda, N. Expression of matrix metalloproteinase-2 and -9 in synovial fluid of the temporomandibular joint accompanied by anterior disc displacement. J. Oral Pathol. Med. 2001, 30, 59–64. [Google Scholar] [CrossRef]
- Murakami, K.I.; Shibata, T.; Kubota, E.; Maeda, H. Intra-articular levels of prostaglandin E2, hyaluronic acid, and chondroitin-4 and -6 sulfates in the temporomandibular joint synovial fluid of patients with internal derangement. J. Oral Maxillofac. Surg. 1998, 56, 199–203. [Google Scholar] [CrossRef]
- Kubota, E.; Kamata, T.; Murakami, J.; Shibata, T.; Murakami, K.I. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J. Oral. Maxillofac. Surg. 1998, 56, 192–198. [Google Scholar] [CrossRef]
- Kubota, E.; Horiuchi, I.; Kamata, T.; Shibata, T.; Murakami, K. Interleukin 1 beta and stromelysin (MMP3) activity of synovial fluid as possible markers of osteoarthritis in the temporomandibular joint. J. Oral Maxillofac. Surg. 1997, 55, 20–27. [Google Scholar] [CrossRef]
- Shao, B.; Xu, Y.; Jia, M.; Li, C.; Gong, Z.; Shao, B.; Xu, Y.; Jia, M.; Li, C.-x.; Gong, Z.-c. Association of HMGB1 levels in synovial fluid with the severity of temporomandibular joint osteoarthritis. BMC Musculoskelet. Disord. 2023, 24. [Google Scholar] [CrossRef]
- Kim, Y.; Son, C.; Park, Y.K.; Jo, J.H.; Park, J.W. Sleep duration and inflammatory mediator levels associated with long-term prognosis in temporomandibular disorders. J. Oral Rehabil. 2023, 50, 830–839. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Chen, L.; Tian, S.; Abdelrehem, A.; Feng, J.; Fu, G.; Chen, W.; Ding, C.; Luo, Y.; et al. Proteome Analysis of Temporomandibular Joint with Disc Displacement. J. Dent. Res. 2022, 101, 1580–1589. [Google Scholar] [CrossRef]
- Zwiri, A.M.; Al-Hatamleh, M.A.I.; Al-Wakeel, W.M.A.; Asif, J.A.; Khoo, S.P.; Husein, A.; Zwiri, A.M. A Randomized Controlled Trial Evaluating the Levels of the Biomarkers hs-CRP, IL-6, and IL-8 in Patients with Temporomandibular Disorder Treated with LLLT, Traditional Conservative Treatment, and a Combination of Both. Int. J. Environ. Res. Public Health 2022, 19, 8987. [Google Scholar] [CrossRef] [PubMed]
- Alajbeg, I.Z.; Viskic, E.; Laskarin, I.; Alajbeg, I.; Vukojevic, L. Effect of occlusal splint on oxidative stress markers and psychological aspects of chronic temporomandibular pain: A randomized controlled trial. Sci. Rep. 2020, 10, 10981. [Google Scholar] [CrossRef] [PubMed]
- Ganti, S.; Sukhija, P.; Ahmad, A.S.; Kumar, J.M.; Ali, A.; Dhir, A. Evaluation of Effect of Glucosamine-Chondroitin Sulfate, Tramadol, and Sodium Hyaluronic Acid on Expression of Cytokine Levels in Internal Derangement of Temporomandibular Joint. J. Contemp. Dent. Pract. 2018, 19, 1501–1505. [Google Scholar] [PubMed]
- Kollarova, M.; Puzserova, A.; Balis, P.; Radosinska, D.; Tothova, L.; Bartekova, M.; Barancik, M.; Radosinska, J. Age- and Phenotype-Dependent Changes in Circulating MMP-2 and MMP-9 Activities in Normotensive and Hypertensive Rats. Int. J. Mol. Sci. 2020, 21, 7286. [Google Scholar] [CrossRef]
- Park, Y.M.; Ahn, Y.-W.; Jeong, S.-H.; Ju, H.-M.; Jeon, H.-M.; Kim, K.-H.; Ok, S.-m. Interleukin-8 and Matrix Metalloprotease 9 as Salivary Biomarkers of Pain in Patients with Temporomandibular Disorder Myalgia: A Pilot Study. J. Oral Med. Pain 2019, 44, 160–168. [Google Scholar] [CrossRef]
- Zardeneta, G.; Milam, S.B.; Schmitz, J.P. Iron-dependent generation of free radicals: Plausible mechanisms in the progressive deterioration of the temporomandibular joint. J. Oral Maxillofac. Surg. 2000, 58, 302–308. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; 905p. [Google Scholar]
- d’Apuzzo, F.; Rotolo, R.P.; Fordellone, M.; Cuomo, G.; Jamilian, A.; Nucci, L.; Grassia, V. Temporomandibular Disorders and Serological Tests in Patients with Rheumatoid Arthritis. Appl. Sci. 2023, 13, 11488. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- Ruivo, J.; Tavares, I.; Pozza, D.H. Molecular targets in bone cancer pain: A systematic review of inflammatory cytokines. J. Mol. Med. 2024, 102, 1063–1088. [Google Scholar] [CrossRef]
- Bouloux, G.F. The Use of Synovial Fluid Analysis for Diagnosis of Temporomandibular Joint Disorders. Oral. Maxillofac. Surg. Clin. N. Am. 2018, 30, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Zwiri, A.; Al-Hatamleh, M.A.I.; WMA, W.A.; Ahmed Asif, J.; Khoo, S.P.; Husein, A.; Ab-Ghani, Z.; Kassim, N.K. Biomarkers for Temporomandibular Disorders: Current Status and Future Directions. Diagnostics 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Cordero, M.J.; Sanchez Lopez, A.M.; Mur Villar, N.; Garcia Garcia, I.; Rodriguez Lopez, M.A.; Ortegon Pinero, A.; Cortes Castell, E. Salivary cortisol as an indicator of physological stress in children and adults; a systematic review. Nutr. Hosp. 2014, 29, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Sikora, R.; Duspara, K.; Matić, A.; Petrović, A.; Kralik, K.; Smolić, R.; Sikora, M.; Šarac, M.Č.; Bojanić, K.; Smolić, M. Stabilization Splint Therapy for Patients with Temporomandibular Disorders Improves Opening Movements and Jaw Limitation and Attenuates Pain by Influencing the Levels of IL-7, IL-8, and IL-13 in the Gingival Crevicular Fluid. Medicina 2025, 61, 375. [Google Scholar] [CrossRef]
- Kean, W.F.; Bouchard, S.; Roderich Gossen, E. Women with pain due to osteoarthritis: The efficacy and safety of a once-daily formulation of tramadol. Pain Med. 2009, 10, 1001–1011. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E. HMGB1, IL-1alpha, IL-33 and S100 proteins: Dual-function alarmins. Cell Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert. Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain. Phys. 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Sa, M.; Faria, C.; Pozza, D.H. Conservative versus Invasive Approaches in Temporomandibular Disc Displacement: A Systematic Review of Randomized Controlled Clinical Trials. Dent. J. 2024, 12, 244. [Google Scholar] [CrossRef]
- Ferreira do Couto, M.L.; Fonseca, S.; Pozza, D.H. Pharmacogenetic Approaches in Personalized Medicine for Postoperative Pain Management. Biomedicines 2024, 12, 729. [Google Scholar] [CrossRef]
- Perillo, L.; d’Apuzzo, F.; Illario, M.; Laino, L.; Spigna, G.D.; Lepore, M.; Camerlingo, C. Monitoring Biochemical and Structural Changes in Human Periodontal Ligaments during Orthodontic Treatment by Means of Micro-Raman Spectroscopy. Sensors 2020, 20, 497. [Google Scholar] [CrossRef]

| Reference | Study Type/Country | Type of Sample | Biomarkers | Population Characteristics/Diagnosis | Results |
|---|---|---|---|---|---|
| Tuerxun, P. et al. (2024) [10] | Case–control Observational China | Synovial fluid | 46 metabolites (fatty/organic/amino acids, sugars, amines, and others) | 11 females (41.91± 16.6 years) and 1 male (20 years) TMD: OA or DDwR, pain intensity not reported | OA showed distinct metabolic profiles from DDwR, with L-carnitine, taurine, and adenosine identified as potential biomarkers. TCA cycle and ferroptosis: OA pathogenesis and therapeutics. |
| AlSahman, L. et al. (2024) [21] | Case–control Observational Saudi Arabia | Saliva | Cortisol | 132 patients divided in two groups (TMD: DDwoR vs. control), 18–40 years, pain intensity ≥ 4/10 | ↑ cortisol: Biomarker for specific TMD subtypes, especially in males with DDwoR. |
| Ismah, N. et al. (2024) [22] | Cross-sectional Observational Indonesia | Saliva | IL-1β and C-reactive protein (CRP) | 77 females and 28 males, 26.4 years TMD with arthralgia, pain intensity not reported | Pain-related or joint TMDs with ↑ CRP > IL-1β. Both types were combined ↑ CRP < IL-1β. |
| Tchivileva, I.E. et al. (2023) [23] | Cross-sectional Observational USA | Blood | Calcitonin gene-related peptide (CGRP) | 80 participants from 18 to 64 years Painful TMDs Average pain intensity of 53.2/100 | CGRP associated with age and Body Mass Index, but not chronic painful TMD. |
| Aparna, N. et al. (2023) [24] | Case–control Observational India | Saliva | Cortisol | 50 patients divided in two groups, 18–45 years Painful or symptomatic TMD, pain intensity not reported | No statistically significant difference in salivary cortisol level between cases and controls. |
| Kazan, D. et al. (2023) [25] | Cross-sectional Observational Turkey | Saliva, blood | IL-6, MDA, 8-OHdG | 44 patients, 14–40 years 27 with DDwR/DDwoR vs. 17 controls, pain intensity not reported | Strong positive correlation between pain, 8-OHdG, and IL-6. |
| Ulmner, M. et al. (2022) [26] | Cross-sectional Observational Sweden | Synovial fluid/tissue | ILs, TNF-α | 101 patients, average age of 40.6 years TMD: DDwR, DDwoR, DJD, arthralgia Average pain intensity of 4/10 | IL-1β and TNF-α were significantly associated with TMJ palpation pain. TNF-α also correlated with subjective TMJ pain. IL-1β was linked to synovitis, which contributes to pain. |
| Bayındır, S., et al. (2022) [27] | Cross-sectional Observational Turkey | Synovial fluid | Aggrecan, adiponectin, resistin, apelin, VEGF, and PGE2 | 41 patients, 12–72 years TMD: DDwR, DDwoR and OA Average pain intensity of 6/10 | Aggrecan and PGE2 are linked to localized TMJ pain and are elevated in joints with degenerative changes. |
| Venkates h, S.B. et al. B. et al. (2021) [28] | Cross-sectional Observational India | Saliva | Cortisol | 187 females and 161 males, 18–23 years (20 with TMD vs. 20 controls) TMD: DDwR, DDwoR, arthralgia, and DJD, pain intensity not reported | Cortisol: strong association with stress and TMD severity. |
| Ege, B. et al. (2021) [29] | Case–control Observational Turkey | Blood | OPN, CD44 | 71 patients, 18–57 years (54 with TMD vs. 17 controls) TMD: ID and subluxations, qualitative pain evaluated | ↓ OPN in TMD patients CD44 no statistical difference. |
| Ege, B. et al. (2021) [30] | Case–control Observational Turkey | Blood | Asporin | 43 controls (31.30 ± 7.53) vs. 43 TMD (31.42 ± 13.24) TMD: DDwoR and OA, pain intensity not reported | Asporin significantly upregulated in TMD. |
| Son, C. et al. (2021) [31] | Cross-sectional Observational Republic of Korea | Blood | ILs, IFN-γ, TNF-α, growth factors, PGE2, and THPO | 66 female participants (24.83 ±3.03 years) TMD with arthralgia Average pain intensity of 5.44/10 | TMD—higher pain intensity/duration and ↑ IL-8 and IgG levels link chronic pain and systemic inflammation. |
| Ulmner, M. et al. (2020) [32] | Cross-sectional Observational Sweden | Synovial tissue | BMP, Epidermal Grow Factor (EGF), ILs and OPG, IFN-γ, IP, eotaxin | 51 females and 12 males (41.3 ± 15.1 years) TMD: DDwR (19 patients), DDwoR (44 patients) Pain intensity ≥ 4/10 (DDwoR>DDwR) | DDwoR: IP ↓, OPG ↓ EGF + IL-1 ra ↑ (female > male) sudden onset >delayed onset: BMP 4 ↑, Eotaxin ↑, IL-8 ↑. |
| Omidpan ah, N. et al. (2020) [33] | Case–control Observational Iran | Saliva | MDA, TAC, and Catalase | 30 patients with TMD (30.7 ± 13.2 years) vs. 30 controls (29.16 ± 11.2 years) Painful TMD, pain intensity not reported | TMD: higher MDA levels, no changes in TAC and Catalase. |
| Loreto, C. et al. (2020) [34] | Case–control Observational Italy | Synovial tissue | MMPs | 20 TMD vs. 10 controls DDwoR, pain intensity not reported | MMP-7 and MMP-9 overexpressed in DDwoR. |
| Staniszewski, K. et al. (2019) [35] | Cross-sectional Observational Norway | Blood | Hemoglobin, cobalamin, albumin, PTH, vit D, creatinine, and potassium | 60 patients with TMD vs. 60 controls 20–69 years, mean age 45 years TMD with arthralgia, pain intensity not reported | Serum markers, including vitamin D, were not reliable for TMD diagnosis. |
| Yapıcı, Y.G. et al. (2019) [36] | Cross-sectional Observational Turkey | Synovial fluid | Visfatin | 60 individuals (26.55 ± 8.3 years) with DDwoR and OA Pain intensity > 6/10 | ↑ Vistafin (OA) Positive correlation between pain and visfatin levels. |
| Demir, C.Y. et al. (2018) [37] | Case–control Observational Turkey | Blood | 25(OH) vitamin D, PTH, calcitonin, calcium, phosphorus, magnesium | 50 TMD vs. 50 controls, mean age of 50 years, pain intensity not reported | 25(OH) vitamin D, calcitonin, calcium, magnesium, or phosphorus (no differences) ↑ PTH. |
| Kaya, G.S. et al. (2018) [38] | Cross-sectional Observational Turkey | Synovial fluid | Chemerin | 60 patients (26,55± 8,3 years), 16–52 years TMD: ID and OA Average pain intensity of 70/100 | Positive correlation between pain and chemerin levels. |
| Demir, C.Y. et al. (2018) [39] | Case–control Observational Turkey | Blood | MDA, Catalase superoxide dismutase (SOD), GSH | 32 patients TMD vs. and 32 controls, aged 16–50 years, pain intensity not reported | TMD: higher MDA and lower Catalase, SOD, GSH), no influence from age or gender. |
| Lin, S.L., et al. (2015) [40] | Cross-sectional Observational Taiwan | Blood | Cortisol | 60 DDwoR patients, 37.7 ± 17.22 years vs. 80 patients DD 36.4± 13.08 years, pain intensity not reported | ↑ Cortisol in DDwoR: clinical indicator for distinguishing disc displacement disorders. |
| Cevidanes, L.H. et al. H. et al. (2014) [41] | Case–control Observational USA | Synovial fluid and blood | MMPs, TIMPs, and several others | 24 females (39.9 ± 16 years) 12 OA (47.4 ± 16.1 years) vs. 12 controls (41.8 ± 12.2 years), pain intensity not reported | OA showed bone resorption: ANG and MMPs linked to bone apposition, while IL-6 and TNFα linked to bone resorption. |
| Vos, L.M. et al. (2013) [42] | Cross-sectional case–control Observational Netherlands | Synovial fluid | Collagen type I/II, IL-1β, TNF-α, PGE2 | 30 OA patients (9 males, 21 females; 40.1 ± 15.3 years) vs. 10 controls (5 males, 5 females; 30.3 ± 10.8 years), pain intensity not reported | High collagen-II levels suggest it may be a useful marker for cartilage degradation. |
| Albilia, J.B. et al. (2013) [43] | Case–control Observational Canada | Blood | BMPs, Alpha-2-heremans-schmid glycoprotein (AHSG) | 30 patients with DJD (hip patients— 64.6 ± 12.1, TMJ patients—41.6 ± 9.8) vs. 120 controls (mean age 38.8 years) Average pain intensity of 6.6/10 | ↑ BMP-2, BMP-4, ↓ AHSG levels. These markers may help guide treatment decisions. |
| Slade, G.D. et al. D. et al. (2011) [44] | Case–control Observational USA | Blood | MCPs, MIPs, ILs, and several others | 344 females, 18–60 years TMD, pain intensity not reported | Localized TMD linked to IL-1ra and widespread TMD linked to IL-8. Positive correlation between pain intensity and MCP-1 levels. |
| Lee, J.K. et al. (2010) [45] | Cross-sectional Observational USA | Synovial fluid | TNF-α and IL-6 | 24 TMD vs. 5 controls TMD symptomatic, pain intensity not reported | ↑ TNF-α and ↑ IL-6 in TMD without significant correlation. |
| Sato, J. et al. (2007) [46] | Cross-sectional Observational Japan | Synovial tissue | IL-8 | 44 patients, 6 males and 38 females (mean age of 43 years, 17–84 years), with DDwoR vs. 7 controls Average pain intensity of 6/10 | ↑ IL-8 in TMD, no significant link to pain or inflammation severity. |
| Yoshida, H. et al. (2007) [47] | Cross-sectional Observational Japan | TMJ specimens | CD34 | 20 DD and OA patients vs. 10 controls, 20–72 years, pain intensity not reported | ↑ CD34 in TMJ internal derangement linked to angiogenesis. |
| Yoshida, K. et al. (2006) [48] | Cross-sectional Observational Japan | Synovial fluid | MMPs and aggrecanase | 35 patients (17–74 years, mean 36.6 years) with DDwR, DDwoR, and OA vs. 10 controls (16–44 years, mean 23.1 years) Average pain intensity of 60/100 (DDwR), 63.5/100 (DDwoR), and 65/100 in (OA) | ↑ MMP-9 in severe TMJ OA and disc displacement. ↑ MMP-2 and aggrecanase were elevated in early OA. Aggrecanase—marker for cartilage degradation. |
| Yoshida, K. et al. (2005) [49] | Cross-sectional Observational Japan | Synovial fluid | Aggrecanase | 35 patients (17–74 years, mean 36.6 years) with TMD vs. 10 controls (16–44 years, mean 23.1 years) TMD: DDwR, DDwoR, and OA Average pain intensity of 61.7/100 | ↑ Aggrecanase in TMD, especially in severe OA and disc displacement. Aggrecanase—marker for cartilage degradation. |
| Kaneyama, K. et al. (2004) [50] | Cross-sectional Observational Japan | Synovial fluid | ILs | 61 patients (52 females and 9 males) with DDwoR and OA vs. 7 controls, pain intensity not reported | ↑ IL-6 and ↑ IL-11 in joints with condylar bone changes: osseous degeneration. |
| Kardel, R. et al. (2003) [51] | Cross-sectional Observational Sweden | Synovial tissue | ILs, TNF-α, IFN-γ, TGF-β1,2,3, CD68, CD45RO, proliferating cell nuclear antigen | 39 patients (19 with arthralgia: 18–66 years and 20 with OA: 26–62 years) Average pain intensity of 5.6/10 in painful clicking and 6.7 in OA | OA joints: ↑ IL-1α, ↑ IL-1β, ↑ IFN-γ, ↑ IL-1ra, ↑ CD68+ macrophages, ↑ inflammation, and ↑ immune activity. |
| Nagai, H. et al. (2003) [52] | Cross-sectional Observational Japan | Synovial tissue | iNOS, Fas, CD68, and ssDNA | 33 patients with TMD (ID and OA), 17–75 years vs. 33 controls, 17 to 54 years old, pain intensity not reported | ↑ iNOS, ↑ CD68, ↑ Fas, ↑ ssDNA were linked to synovial changes in TMD disease progression. |
| Srinivas, R. et al. (2001) [53] | Cross-sectional Observational Canada | Synovial fluid | MMPs | 44 TMD with ID patients (33 females and 11 males) with a mean age of 36 years (16–76 years), pain intensity not reported | ↑ MMP-1, ↑ MMP-2, ↑ MMP-8, ↑ MMP-9 ↑, MMP-13 in mild TMJ-ID—active collagen degradation. |
| Tanaka, A. et al. (2001) [54] | Cross-sectional Observational Japan | Synovial fluid | MMPs | 38 DDwR, DDwoR, and OA patients, 15–69 years (34.8 ± 14.7 years), vs. 20 controls, 22 to 47 years (26.8 ± 3.7 years), pain intensity not reported | ↑ MMP-2 and ↑ MMP-9 in DDwoR>DDwR—diagnostic markers. |
| Murakami, K.I. et al. (1998) [55] | Cross-sectional Observational Japan | Synovial fluid | PGE2, HA, C4S, and C6S | 15 females with painful TMD, mean age of 36.7 years Average pain intensity from 5.1/10 to 6.6/10 | ↑ PGE2 linked to pain scores ↑ C4S and ↑ C6S linked to TMJ degeneration—markers of proteoglycan breakdown pain-related joint changes. |
| Kubota, E. et al. (1998) [56] | Cross-sectional Observational Japan | Synovial fluid | MMPs and ILs | 22 DDwoR and OA patients vs. 11 controls, pain intensity not reported | IL-1β ↑ (DDwoR), IL-1β ↑ (OA), IL-6 ↑ (OA) —catabolic markers linked to cartilage degradation and pain in TMD. |
| Kubota, E. et al. (1997) [57] | Case–control Observational USA | Synovial fluid | IL-1β, MMP, TNF-α | 22 TMD with OA patients, 15–77 years vs. 15 controls, 18–66 years, pain intensity not reported | ↑ IL-1β in TMD (osteolytic changes TMJs) ↑ MMP-3 linked to cartilage degradation —early markers of TMJ deterioration and pain. |
| Reference | Study Type/ Country | Type of Sample | Biomarkers | Population Characteristics/Treatment | Results |
|---|---|---|---|---|---|
| Thamer, S.R. and Diajil, A.R. (2024) [8] | Non-RCT Iraq | Saliva | Matrix metalloproteinases (MMPs) | 32 females and 20 males, 18–55 years with TMD resistant to conservative therapy Intra-articular HA (30 patients) and PRP (22 patients) treatment | MMP-2 and MMP-9 positively correlated with pain and joint click/negatively with mouth opening. HA and PRP therapies reduce inflammation and improve TMD symptoms. |
| Cho, I.S. et al. (2024) [9] | Cross-sectional Observational South Korea | Blood | Total protein, neutrophils, lymphocytes, monocytes, platelets, and ratios | 154 patients, 30.2 ± 10.6 years TMD with arthralgia Average pain intensity of 4/10 | 69.5% showed significant pain improvement. Hematologic markers, particularly low hemoglobin, may help predict long-term treatment outcomes in TMD. |
| Shao, B. et al. (2023) [58] | Cross-sectional Observational China | Synovial fluid | HMGB1, interleukins (ILs), PGE2, RAGE, TLR4, and iNOS | Two TMD groups: OA: 77% females, 40.36 ± 9.67 years ID: 70% females, 31.5 ± 10.62 years Intra-articular HA injection 1x/week for 2 weeks Average pain intensity of 5.97/10 in OA and 3.6/10 in DD | High levels of biomarkers, OA > DD HMGB1 levels, pain scores, and jaw dysfunction scores improved after HA treatment. |
| Kim, Y. et al. (2023) [59] | Cross-sectional Observational South Korea | Blood | ILs, ESR, high-sensitivity C-reactive protein (hs- CRP), cortisol, ACTH, norepinephrine, and epinephrine. | 63 females, 24.84 ± 3.00 years, TMD with arthralgia Conservative treatment Hematological analysis at 3 and 6 months post-treatment. | Significant pain improvements (≥2/10) of 64.29%, 41.67%, and 66.67% in normal-, short-, and long-sleep group, respectively. ↑ IL-1β, ↑ IL-4, ↑ IL-8, and ↑ IL-17 showed sufficient strength in predicting significant pain improvement with long-term TMD treatment. |
| Liu, X. et al. (2022) [60] | Cross-sectional Observational China | Synovial fluid | 1714 proteins in the cytosol (43%), plasma membrane (31%), and extracellular space (25%) | 95 females and 14 males, 21.31 ± 7.95 years with TMD DDwoR Conservative treatment or disc reposition | ↑ ACACB during pain, ↑ HADHA in bruxism, ↑ TGFB1-impaired bone formation. Higher pain levels related to ↑ radixin, ↑ LCP1, ↑ CPN2, ↓ CFHR3, ↓ Factor 11, ↓ INADL, ↓ MBL2. |
| Zwiri, A.M. et al. (2022) [61] | RCT Malaysia | Blood | hs-CRP, ILs | 12 males and 20 females, 20.9 years with painful TMD Conservative treatment (CT), low-level taser therapy (LLLT), and a combination of both | IL-8 may serve as a potential biomarker for TMJ pain: hs-CRP ↑ (LLT, C) ≈ (CT) IL-6 ↑ (LLLT, CT) ↓ (C) IL-8 ↓ (LLLT) ↑ (CT, C); no significant correlation between pain intensity and biomarker levels except for IL6 at baseline and after treatment. |
| Alajbeg, I.Z. et al. (2020) [62] | RCT Croatia | Saliva | Oxidative stress | 20 females, 36.1 ± 11.95 years, TMD with arthralgia Oral splint or placebo for 6 months Average pain in the last 10 days > 30/100 | Splint improved pain and depressive symptoms in TMD with associated reductions in oxidative stress. |
| Ganti, S. et al. (2018) [63] | RCT India | Synovial fluid | ILs, TNF-α, PGE2 | 30 males and 30 females, 20.9 years with DDwR Treated with glucosamine–chondroitin sulphate, tramadol, or sodium hyaluronate | Treatments improved mouth opening and pain in all groups, with associated reductions in inflammatory markers IL-1β, TNF-α, PGE2, and IL-6. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, J.M.; Carneiro, B.D.; Pozza, D.H. The Role of Biomarkers in Temporomandibular Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5971. https://doi.org/10.3390/ijms26135971
Soares JM, Carneiro BD, Pozza DH. The Role of Biomarkers in Temporomandibular Disorders: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(13):5971. https://doi.org/10.3390/ijms26135971
Chicago/Turabian StyleSoares, Joana Maria, Bruno Daniel Carneiro, and Daniel Humberto Pozza. 2025. "The Role of Biomarkers in Temporomandibular Disorders: A Systematic Review" International Journal of Molecular Sciences 26, no. 13: 5971. https://doi.org/10.3390/ijms26135971
APA StyleSoares, J. M., Carneiro, B. D., & Pozza, D. H. (2025). The Role of Biomarkers in Temporomandibular Disorders: A Systematic Review. International Journal of Molecular Sciences, 26(13), 5971. https://doi.org/10.3390/ijms26135971






