Brain Pericytes Enhance MFSD2A Expression and Plasma Membrane Localization in Brain Endothelial Cells Through the PDGF-BB/PDGFRβ Signaling Pathway
Abstract
1. Introduction
2. Results
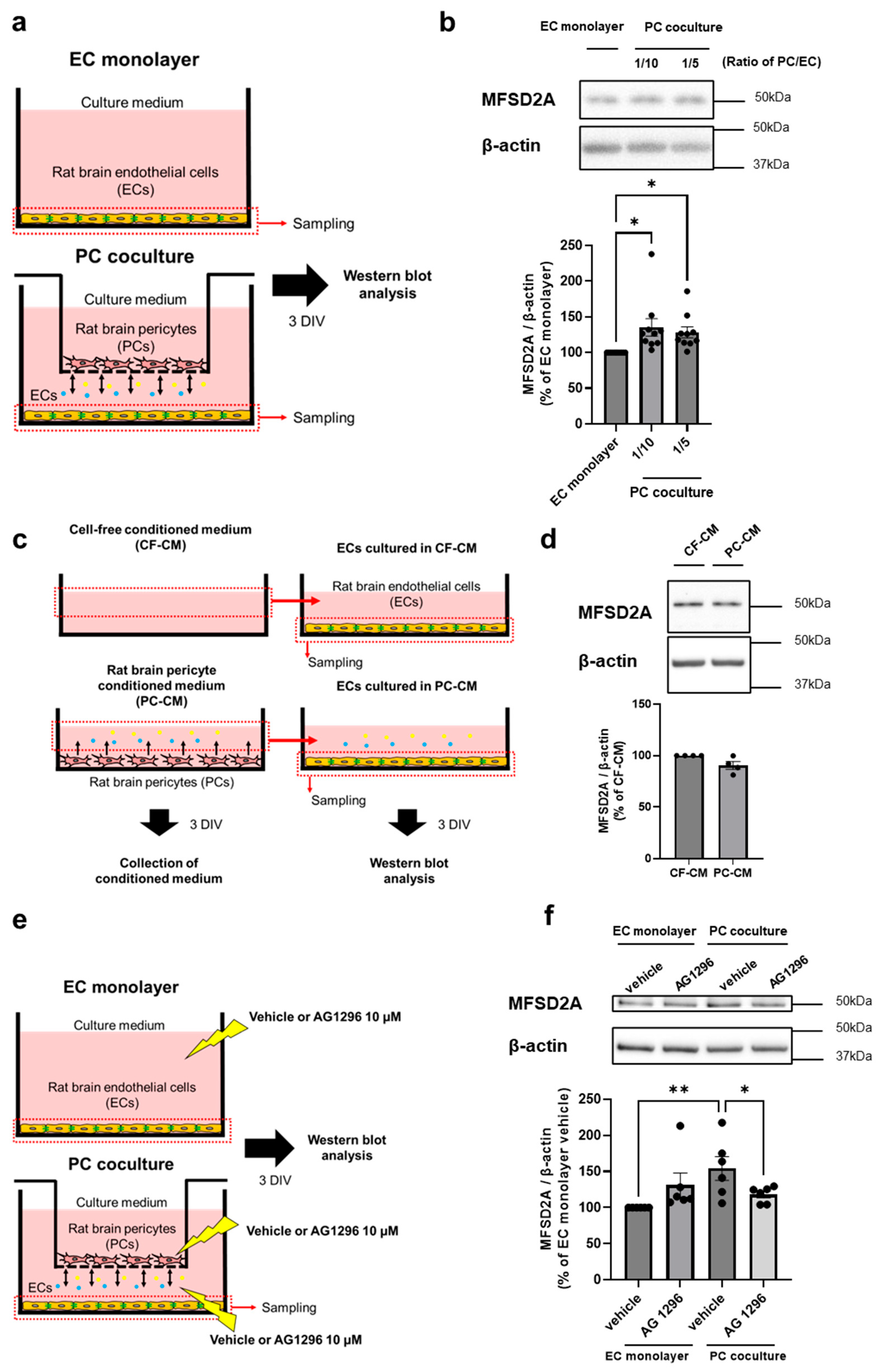
2.1. PCs Regulate MFSD2A Expression on Brain ECs via Cell–Cell Interaction
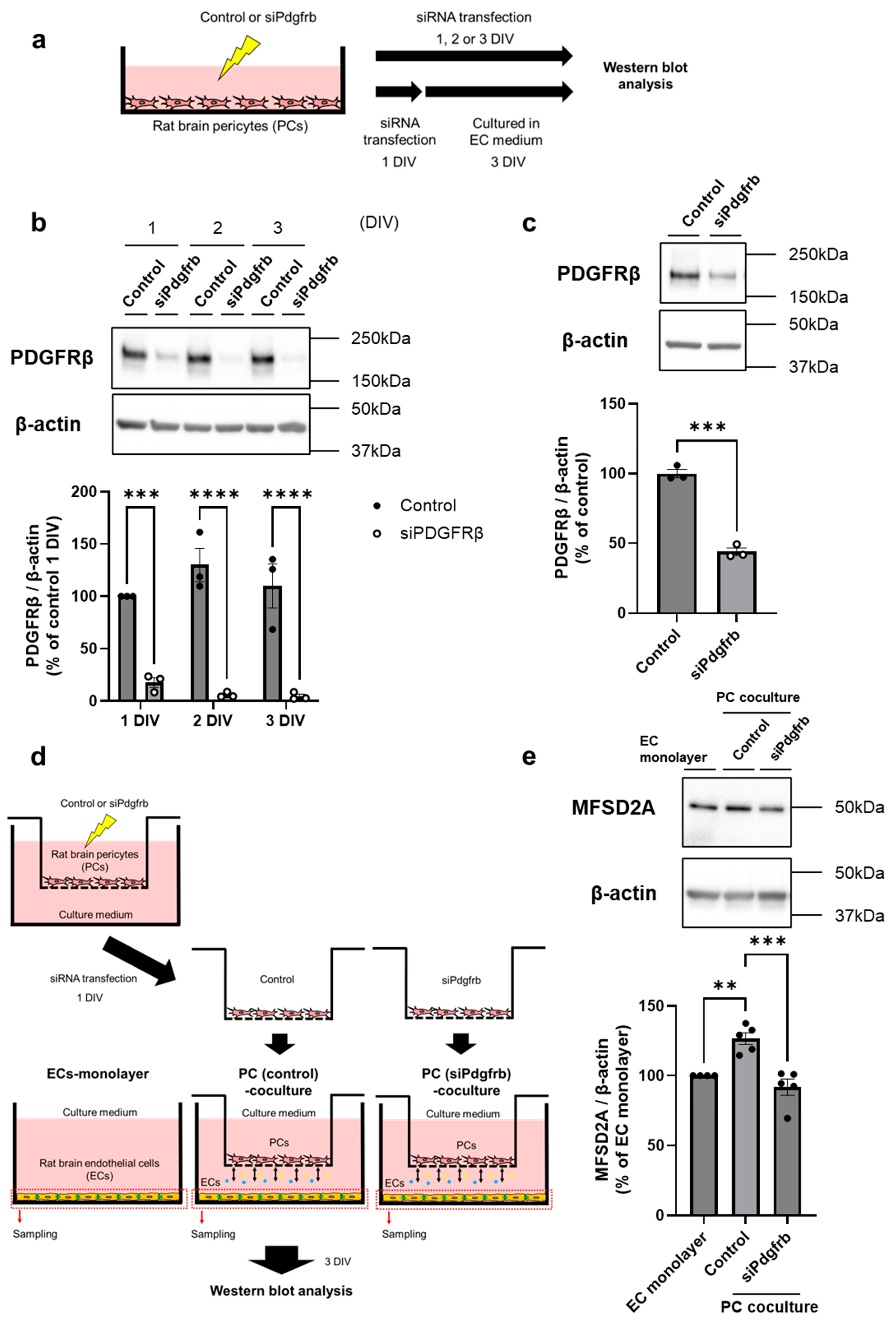
2.2. PDGF-BB/PDGFRβ Signaling Between Brain ECs and PCs Regulates MFSD2A Expression on Brain ECs
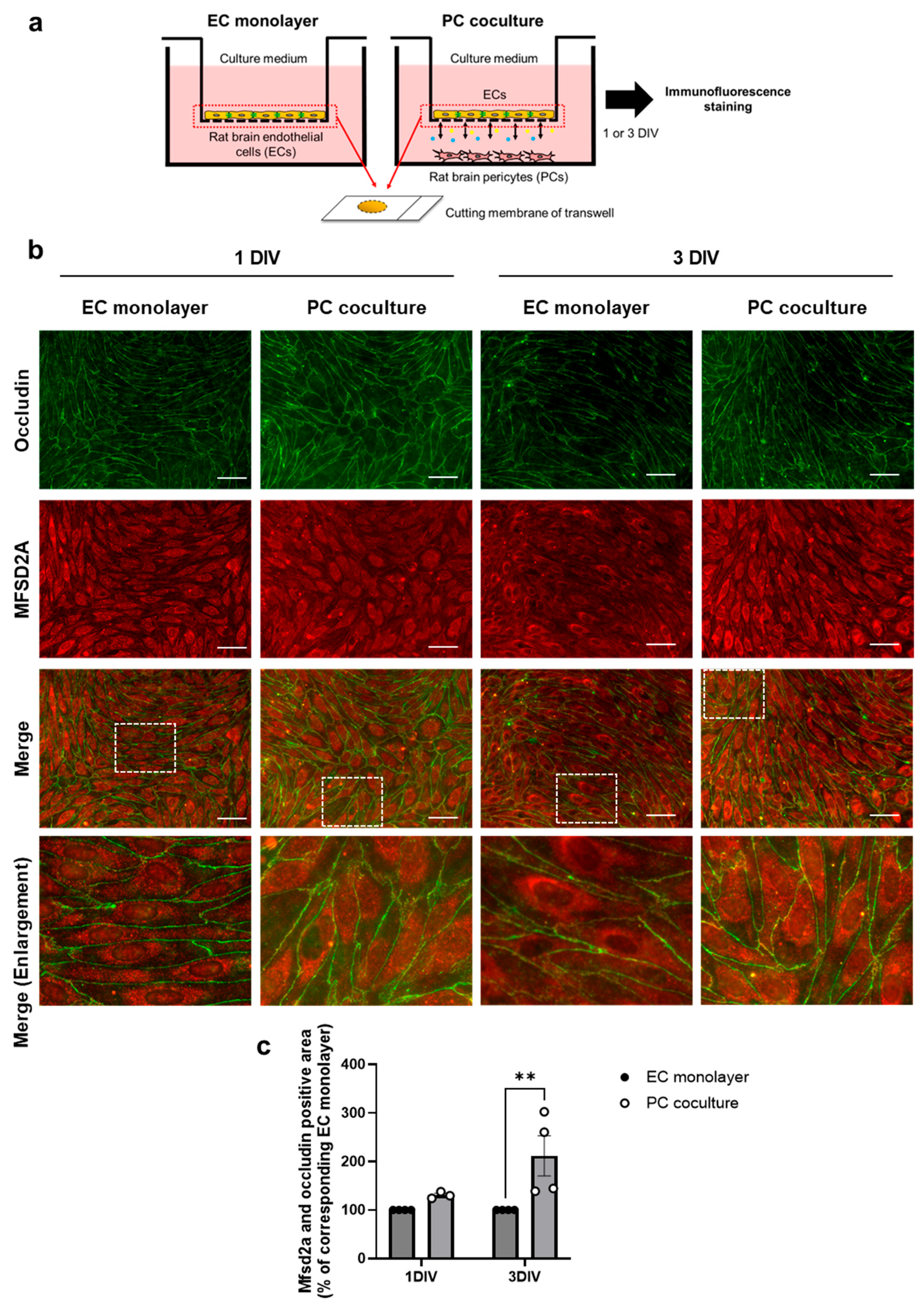
2.3. PCs Increase Plasma Membrane Localization of MFSD2A in Brain ECs
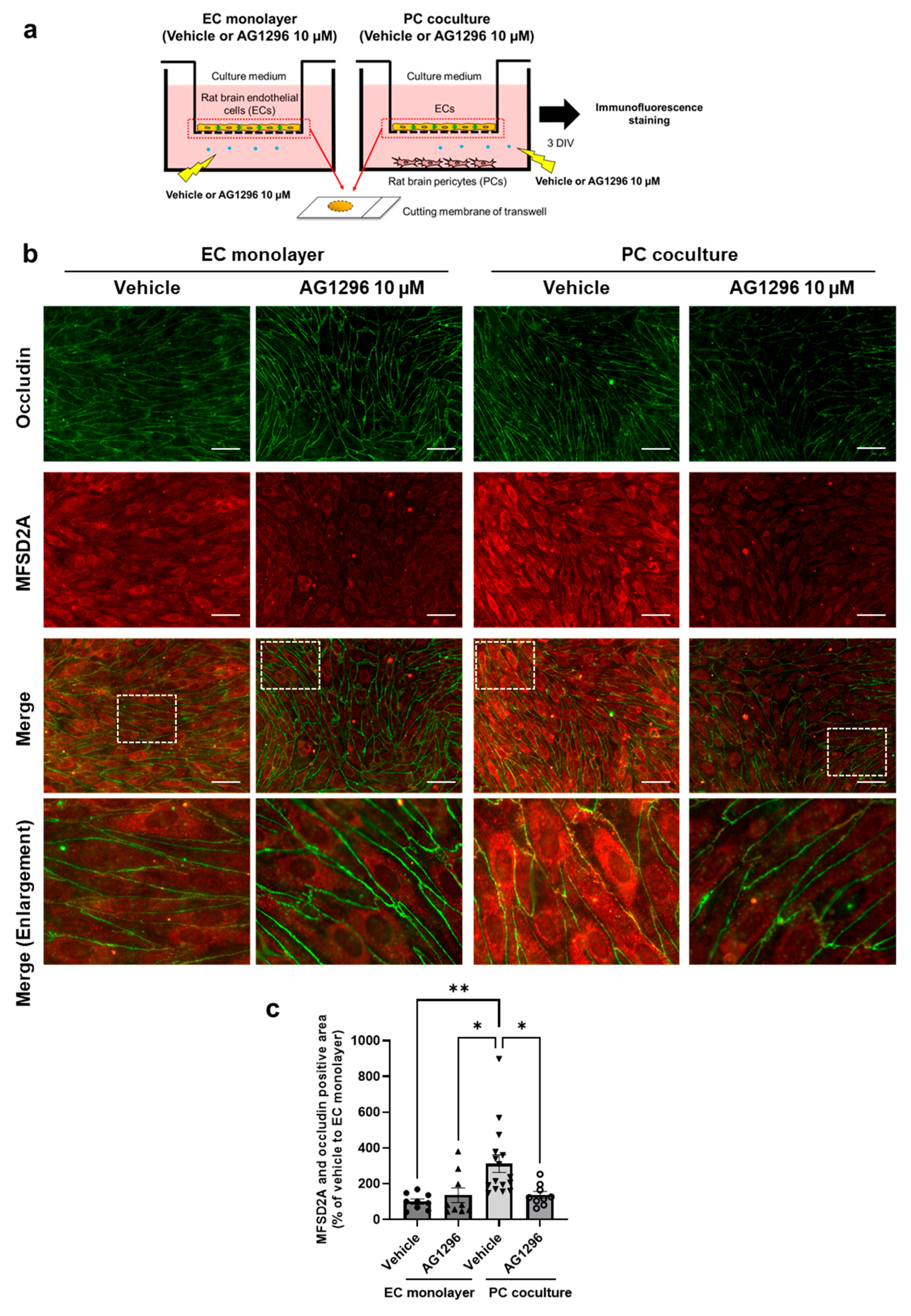
2.4. PDGF-BB/PDGFRβ Signaling Between Brain ECs and PCs Regulates MFSD2A Plasma Membrane Localization in Brain ECs
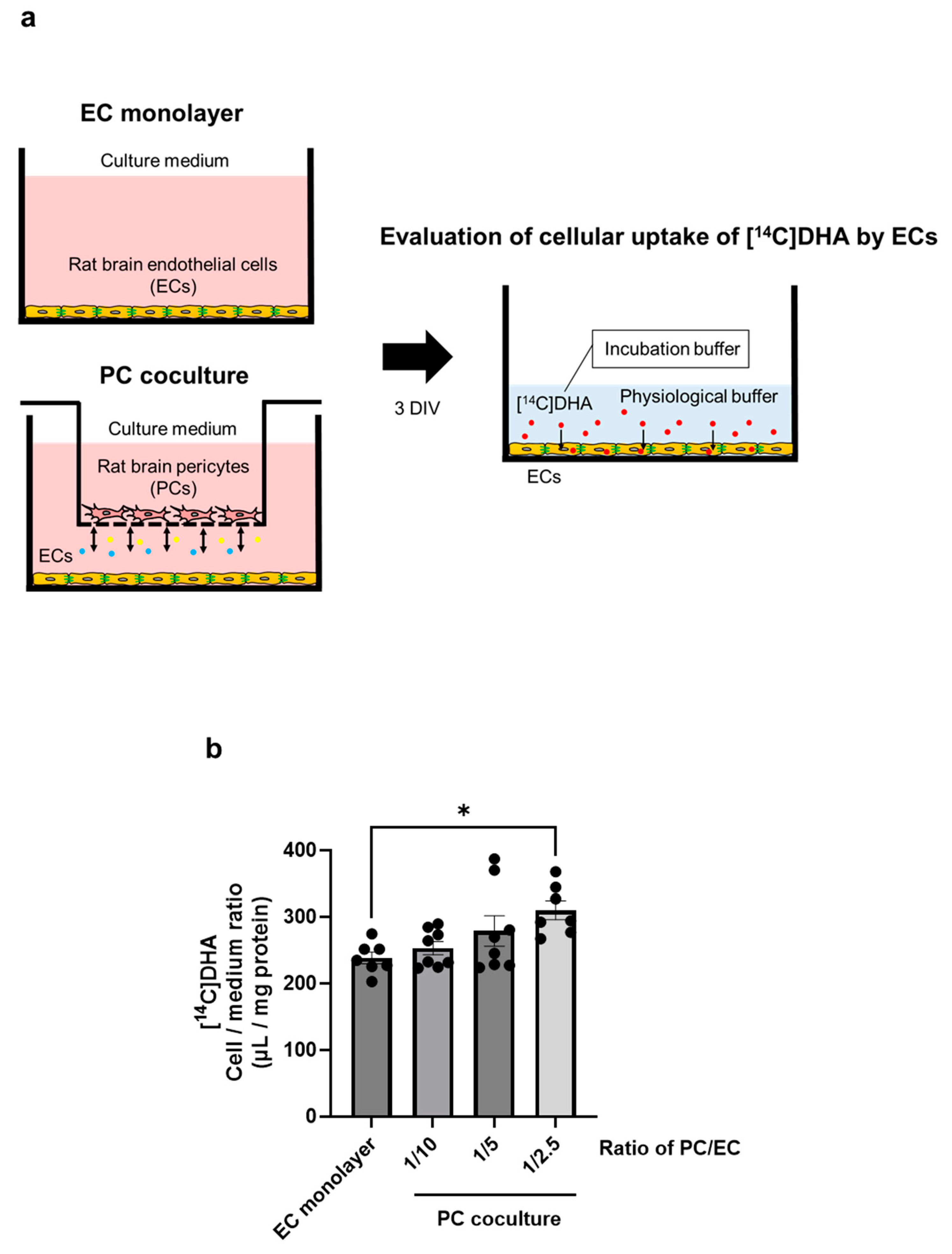
2.5. PCs Upregulate DHA Uptake by Brain ECs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Primary Cultures of Rat Brain ECs and PCs
4.3. Non-Contact Cocultured BBB Models Comprising Rat Brain ECs and PCs
4.4. Treatment of Brain ECs with Conditioned Medium Derived from PCs
4.5. Treatment with AG1296 for Evaluating MFSD2A Protein Levels
4.6. Small Interfering RNA (siRNA) Transfection
4.7. Non-Contact Cocultured BBB Models Comprising Brain ECs and PCs Transfected with siPdgfrb
4.8. Treatment with AG1296 for Evaluating Plasma Membrane Localization of MFSD2A
4.9. Western Blotting
4.10. Immunofluorescence Staining
4.11. Cellular Uptake of [14C]DHA by Brain ECs Cocultured with PCs
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| BMECs | Brain microvessel endothelial cells |
| PCs | Pericytes |
| MFSD2A | Major facilitator superfamily domain–containing protein 2a |
| DHA | Docosahexaenoic acid |
| BBB | Blood–brain barrier |
| DIV | Day in vitro |
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. Febs J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B. 2021, 11, 2306–2325. [Google Scholar] [CrossRef]
- Angers, M.; Uldry, M.; Kong, D.; Gimble, J.M.; Jetten, A.M. Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem. J. 2008, 416, 347–355. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Iwao, T.; Takata, F.; Matsumoto, J.; Aridome, H.; Yasunaga, M.; Yokoya, M.; Kataoka, Y.; Dohgu, S.; Aridome, H.; Yasunaga, M.; et al. Aging decreases docosahexaenoic acid transport across the blood-brain barrier in C57BL/6J mice. PLoS ONE 2023, 18, e0281946. [Google Scholar] [CrossRef]
- Eser Ocak, P.; Ocak, U.; Sherchan, P.; Zhang, J.H.; Tang, J. Insights into major facilitator superfamily domain-containing protein-2a (Mfsd2a) in physiology and pathophysiology. What do we know so far? J. Neurosci. Res. 2020, 98, 29–41. [Google Scholar] [CrossRef]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 2017, 94, 581–594.e585. [Google Scholar] [CrossRef]
- Yang, Y.R.; Xiong, X.Y.; Liu, J.; Wu, L.R.; Zhong, Q.; Zhou, K.; Meng, Z.Y.; Liu, L.; Wang, F.X.; Gong, Q.W.; et al. Mfsd2a (Major Facilitator Superfamily Domain Containing 2a) attenuates intracerebral hemorrhage-induced blood-brain barrier disruption by inhibiting vesicular transcytosis. J. Am. Heart Assoc. 2017, 6, e005811. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, X. The role of Mfsd2a in nervous system Diseases. Front. Neurosci. 2021, 15, 730534. [Google Scholar] [CrossRef]
- Zheng, Z.; Chopp, M.; Chen, J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J. Cereb. Blood Flow Metab. 2020, 40, 1381–1401. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow. Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Johansson, B.R.; Levéen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef]
- Huang, H. Pericyte-Endothelial Interactions in the retinal microvasculature. Int. J. Mol. Sci. 2020, 21, 7413. [Google Scholar] [CrossRef]
- Muto, T.; Tien, T.; Kim, D.; Sarthy, V.P.; Roy, S. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Müller cells: Promotes Müller cell and pericyte apoptosis. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4327–4337. [Google Scholar] [CrossRef]
- Monickaraj, F.; McGuire, P.; Das, A. Cathepsin D plays a role in endothelial-pericyte interactions during alteration of the blood-retinal barrier in diabetic retinopathy. Faseb J. 2018, 32, 2539–2548. [Google Scholar] [CrossRef]
- McGuire, P.G.; Rangasamy, S.; Maestas, J.; Das, A. Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e107–e115. [Google Scholar] [CrossRef]
- Mendelson, K.; Swendeman, S.; Saftig, P.; Blobel, C.P. Stimulation of platelet-derived growth factor receptor beta (PDGFRbeta) activates ADAM17 and promotes metalloproteinase-dependent cross-talk between the PDGFRbeta and epidermal growth factor receptor (EGFR) signaling pathways. J. Biol. Chem. 2010, 285, 25024–25032. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance, and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cedervall, J.; Hamidi, A.; Herre, M.; Viitaniemi, K.; D’Amico, G.; Miao, Z.; Unnithan, R.V.M.; Vaccaro, A.; van Hooren, L.; et al. Platelet-specific PDGFB ablation impairs tumor vessel integrity and promotes metastasis. Cancer Res. 2020, 80, 3345–3358. [Google Scholar] [CrossRef] [PubMed]
- Bjarnegård, M.; Enge, M.; Norlin, J.; Gustafsdottir, S.; Fredriksson, S.; Abramsson, A.; Takemoto, M.; Gustafsson, E.; Fässler, R.; Betsholtz, C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 2004, 131, 1847–1857. [Google Scholar] [CrossRef]
- Heldin, C.H.; Ostman, A.; Rönnstrand, L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1998, 1378, F79–F113. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.H.; Huang, S.; Fu, Z.; Tomita, Y.; Britton, W.R.; Cho, S.S.; Chen, C.T.; Sun, Y.; Ma, J.X.; et al. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci. Adv. 2020, 6, eaba7457. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Song, X.; Ning, H.; Zhang, Y.; Teng, Y.; Wang, J.; Yang, X. Brain endothelial PTEN/AKT/NEDD4-2/MFSD2A axis regulates blood-brain barrier permeability. Cell Rep. 2021, 36, 109327. [Google Scholar] [CrossRef]
- Erdő, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef]
- Stewart, P.A.; Magliocco, M.; Hayakawa, K.; Farrell, C.L.; Del Maestro, R.F.; Girvin, J.; Kaufmann, J.C.; Vinters, H.V.; Gilbert, J. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc. Res. 1987, 33, 270–282. [Google Scholar] [CrossRef]
- Yang, A.C.; Stevens, M.Y.; Chen, M.B.; Lee, D.P.; Stähli, D.; Gate, D.; Contrepois, K.; Chen, W.; Iram, T.; Zhang, L. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 2020, 583, 425–430. [Google Scholar] [CrossRef]
- Zhou, J.; Chi, X.; Cheng, M.; Huang, X.; Liu, X.; Fan, J.; Xu, H.; Lin, T.; Shi, L.; Qin, C.; et al. Zika virus degrades the ω-3 fatty acid transporter Mfsd2a in brain microvascular endothelial cells and impairs lipid homeostasis. Sci. Adv. 2019, 5, eaax7142. [Google Scholar] [CrossRef] [PubMed]
- Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Yamauchi, A.; Kataoka, Y. Monomeric α-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc. Res. 2019, 124, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Dohgu, S.; Yamauchi, A.; Matsumoto, J.; Machida, T.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Sato, K.; Kataoka, Y.; et al. In vitro blood-brain barrier models using brain capillary endothelial cells isolated from neonatal and adult rats retain age-related barrier properties. PLoS ONE 2013, 8, e55166. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwao, T.; Takata, F.; Aridome, H.; Yasunaga, M.; Yokoya, M.; Mizoguchi, J.; Dohgu, S. Brain Pericytes Enhance MFSD2A Expression and Plasma Membrane Localization in Brain Endothelial Cells Through the PDGF-BB/PDGFRβ Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 5949. https://doi.org/10.3390/ijms26135949
Iwao T, Takata F, Aridome H, Yasunaga M, Yokoya M, Mizoguchi J, Dohgu S. Brain Pericytes Enhance MFSD2A Expression and Plasma Membrane Localization in Brain Endothelial Cells Through the PDGF-BB/PDGFRβ Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(13):5949. https://doi.org/10.3390/ijms26135949
Chicago/Turabian StyleIwao, Takuro, Fuyuko Takata, Hisataka Aridome, Miho Yasunaga, Miki Yokoya, Junko Mizoguchi, and Shinya Dohgu. 2025. "Brain Pericytes Enhance MFSD2A Expression and Plasma Membrane Localization in Brain Endothelial Cells Through the PDGF-BB/PDGFRβ Signaling Pathway" International Journal of Molecular Sciences 26, no. 13: 5949. https://doi.org/10.3390/ijms26135949
APA StyleIwao, T., Takata, F., Aridome, H., Yasunaga, M., Yokoya, M., Mizoguchi, J., & Dohgu, S. (2025). Brain Pericytes Enhance MFSD2A Expression and Plasma Membrane Localization in Brain Endothelial Cells Through the PDGF-BB/PDGFRβ Signaling Pathway. International Journal of Molecular Sciences, 26(13), 5949. https://doi.org/10.3390/ijms26135949






