Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling
Abstract
1. Introduction
2. Inflammasome Overview: Classification and Mechanisms of Activation
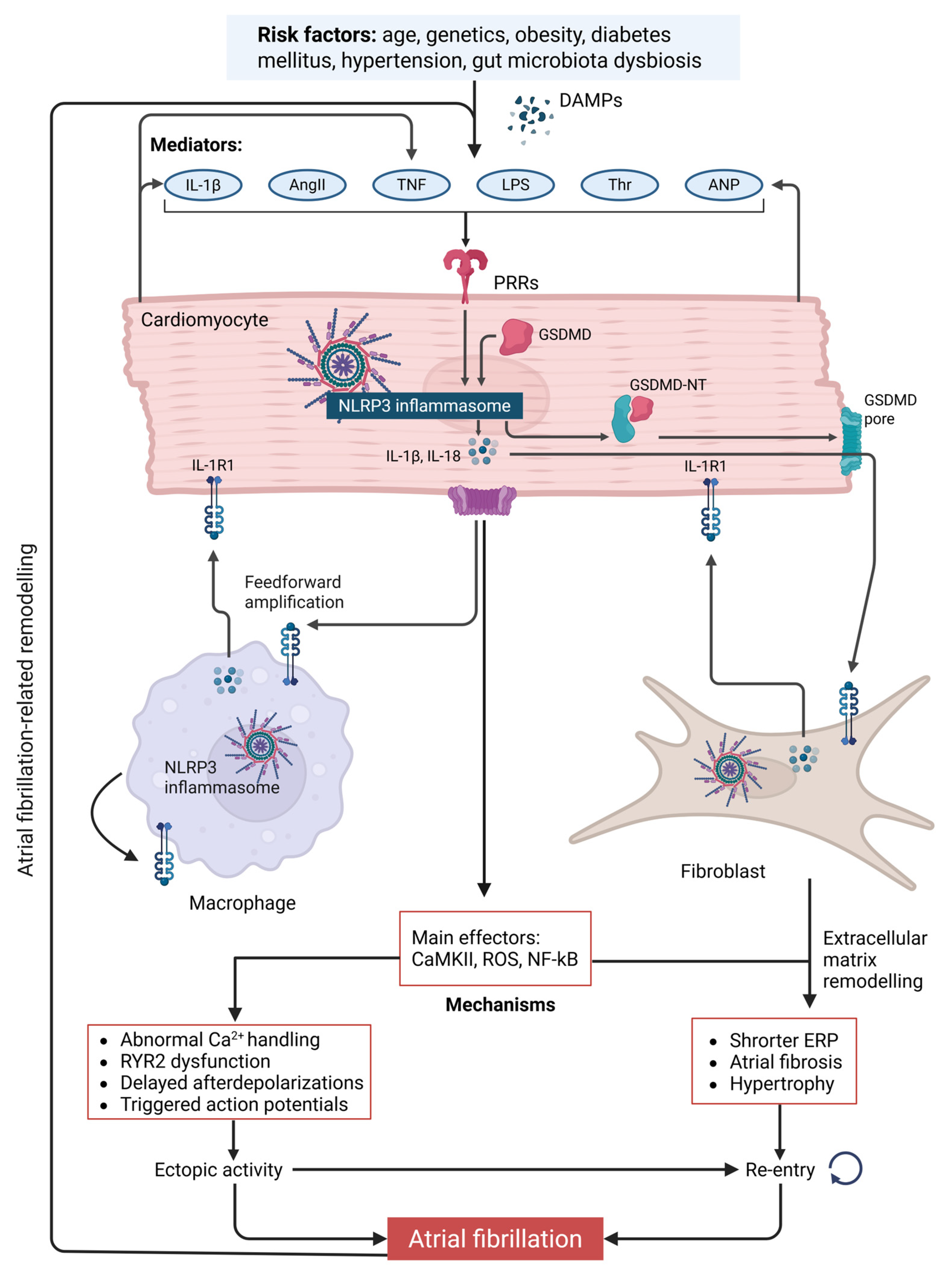
3. Inflammatory Mechanisms Underlying Atrial Fibrillation Pathogenesis
3.1. NLRP3 Inflammasome Activation as a Driver of Electrophysiological Remodeling in Atrial Fibrillation
3.2. Proinflammatory Cytokine Signaling Downstream of Inflammasome Activation in Atrial Fibrillation
3.3. NLRP3 Inflammasome Activation in Atrial Fibroblasts: Implications for Atrial Fibrosis
3.4. Gut Microbiota Dysbiosis and Inflammasome Activation in Atrial Fibrillation
3.5. Obesity-Induced Inflammation and Inflammasome Activation in Atrial Fibrillation
3.6. Diabetes and Inflammasome Activation in Atrial Fibrillation
3.7. Hypertension and Inflammasome Activation in Atrial Fibrillation
3.8. Inflammasome-Independent Innate Immune Pathways in Atrial Fibrillation
4. Inflammasome in Ventricular Arrhythmias
4.1. Mechanistic Pathways Linking NLRP3 Activation to Ventricular Electrophysiology
- Calcium Dysregulation: IL-1β has been shown to enhance SR Ca2+ leak via RyR2 phosphorylation, contributing to delayed afterdepolarizations (DADs) and triggered activity. This is particularly relevant in the failing myocardium, where calcium mishandling already predisposes to arrhythmogenesis [145].
- Modulation of Ion Channels: Inflammasome-derived cytokines suppress repolarizing K+ currents (e.g., I_Kr, I_to) and enhance the late sodium current (I_NaL), prolonging action potential duration (APD) and promoting early afterdepolarizations (EADs) [146], a key substrate for torsade de pointes and ventricular tachyarrhythmias [147].
- Gap Junction Remodeling: NLRP3-driven inflammation has also been linked to the downregulation of connexin-43 and impaired intercellular coupling [148], which contributes to conduction slowing and reentry formation.
4.2. Experimental Evidence
4.3. Structural and Metabolic Context
5. Therapeutic Targeting of the NLRP3 Inflammasome
5.1. Targeting Transcriptional and Post-Translational Regulation of NLRP3
5.2. Targeting IL-1β as a Downstream Effector of NLRP3 Activation
5.3. Targeting ASC and Caspase-1 in Inflammasome Signaling
5.4. Colchicine as a Modulator of Inflammasome-Driven Inflammation and Fibrosis
5.5. Salvianolate as a Modulator of NLRP3-Driven Atrial Remodeling
5.6. Sodium–Glucose Cotransporter 2 Inhibitors
5.7. Mitochondrial Antioxidants: Targeted Redox Modulation to Disrupt AF-Linked Inflammation
5.8. Pro-Resolving Inflammatory Pathways
6. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, S.; Zhou, J.; Veang, T.; Lin, Q.; Liu, Q. Global, Regional, and National Burden of Atrial Fibrillation and Atrial Flutter from 1990 to 2021: Sex Differences and Global Burden Projections to 2046—A Systematic Analysis of the Global Burden of Disease Study 2021. EP Europace 2025, 27, euaf027. [Google Scholar] [CrossRef]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial Fibrillation: Epidemiology, Screening and Digital Health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Alonso, A.; Bengtson, L.G.S. A Rising Tide: The Global Epidemic of Atrial Fibrillation. Circulation 2014, 129, 829–830. [Google Scholar] [CrossRef]
- Linz, D.; Andrade, J.G.; Arbelo, E.; Boriani, G.; Breithardt, G.; Camm, A.J.; Caso, V.; Nielsen, J.C.; De Melis, M.; De Potter, T.; et al. Longer and Better Lives for Patients with Atrial Fibrillation: The 9th AFNET/EHRA Consensus Conference. Europace 2024, 26, euae070. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major Clinical Outcomes in Symptomatic vs. Asymptomatic Atrial Fibrillation: A Meta-Analysis. Eur. Heart J. 2024, 46, 1189–1202. [Google Scholar] [CrossRef]
- Pamporis, K.; Karakasis, P.; Sagris, M.; Theofilis, P.; Milaras, N.; Pantelidaki, A.; Mourouzis, I.; Fragakis, N.; Vlachos, K.; Kordalis, A.; et al. Prevalence of Asymptomatic Atrial Fibrillation and Risk Factors Associated with Asymptomatic Status: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2025, zwaf138. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Karakasis, P.; Tzeis, S.; Pamporis, K.; Schuermans, A.; Theofilis, P.; Milaras, N.; Tsiachris, D.; Efremidis, M.; Antoniadis, A.P.; Fragakis, N. Impact of Catheter Ablation Timing According to Duration of Atrial Fibrillation History on Arrhythmia Recurrences and Clinical Outcomes: A Meta-Analysis. Europace 2025, euaf110. [Google Scholar] [CrossRef]
- Taghdiri, A. Inflammation and Arrhythmogenesis: A Narrative Review of the Complex Relationship. Int. J. Arrhythmia 2024, 25, 4. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Nattel, S.; Lip, G.Y.H.; Ren, J. Inflammasome Signaling in Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2349–2366. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.-H.; Li, F.; Yang, F.; Qian, L.-L.; Wang, R.-X. The Role of NLRP3 Inflammasome Signaling on Arrhythmias in Diabetes. J. Inflamm. Res. 2022, 15, 6883–6889. [Google Scholar] [CrossRef]
- Borim, P.A.; Gatto, M.; Mota, G.A.F.; Meirelles, A.L.B.; Dos Santos, A.C.C.; Pagan, L.U.; Ojopi, E.P.B.; Rodrigues, E.A.; Souza, L.M.; Damatto, F.C.; et al. Nlrc4 Inflammasome Expression After Acute Myocardial Infarction in Rats. Int. J. Mol. Sci. 2025, 26, 3697. [Google Scholar] [CrossRef]
- Garza-González, S.; Nieblas, B.; Solbes-Gochicoa, M.M.; Altamirano, J.; García, N. Intermittent Fasting as Possible Treatment for Heart Failure. Curr. Vasc. Pharmacol. 2022, 20, 260–271. [Google Scholar] [CrossRef]
- Vlachakis, P.K.; Theofilis, P.; Kachrimanidis, I.; Giannakopoulos, K.; Drakopoulou, M.; Apostolos, A.; Kordalis, A.; Leontsinis, I.; Tsioufis, K.; Tousoulis, D. The Role of Inflammasomes in Heart Failure. Int. J. Mol. Sci. 2024, 25, 5372. [Google Scholar] [CrossRef]
- Mo, D.-G.; Liang, M.-T.; Xu, L.; Li, T.; Han, Q.-F.; Chen, C.; Yao, H.-C. The Effect of NLRP3 Inflammasome on Cardiovascular Prognosis in Patients with Acute Coronary Syndrome. Sci. Rep. 2025, 15, 1187. [Google Scholar] [CrossRef]
- Niskala, A.; Heijman, J.; Dobrev, D.; Jespersen, T.; Saljic, A. Targeting the NLRP3 Inflammasome Signalling for the Management of Atrial Fibrillation. Br. J. Pharmacol. 2024, 181, 4939–4957. [Google Scholar] [CrossRef]
- Li, L.; Coarfa, C.; Yuan, Y.; Abu-Taha, I.; Wang, X.; Song, J.; Zeng, Y.; Chen, X.; Koirala, A.; Grimm, S.L.; et al. Fibroblast-Restricted Inflammasome Activation Promotes Atrial Fibrillation and Heart Failure With Diastolic Dysfunction. JACC Basic Transl. Sci. 2025. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.J.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.D.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Milaras, N.; Vlachakis, P.K.; Patoulias, D.; Karamitsos, T.; Antoniadis, A.P.; Fragakis, N. Epigenetic Drivers of Atrial Fibrillation: Mechanisms, Biomarkers, and Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 5253. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Shin, H.J.; Park, E.-J.; Kim, I.S.; Jo, E.-K. Updated Insights into the Molecular Networks for NLRP3 Inflammasome Activation. Cell. Mol. Immunol. 2025, 22, 563–596. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.D. NLRP3 Inflammasome: Structure, Mechanism, Drug-Induced Organ Toxicity, Therapeutic Strategies, and Future Perspectives. RSC Med. Chem. 2025. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.K.; Zhu, X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-Beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The Role of the NLRP3 Inflammasome and Pyroptosis in Cardiovascular Diseases. Nat. Rev. Cardiol. 2024, 21, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Li, N.; Dobrev, D. Role of Inflammatory Signaling in Atrial Fibrillation. Int. J. Cardiol. 2019, 287, 195–200. [Google Scholar] [CrossRef]
- Liu, W.-B.; Wang, S.-S.; Zhang, X.; Ke, Z.-Z.; Wen, X.-Y.; Zhao, J.; Zhuang, X.-D.; Liao, L.-Z. Enhanced Cardiomyocyte NLRP3 Inflammasome-Mediated Pyroptosis Promotes d-Galactose-Induced Cardiac Aging. J. Am. Heart Assoc. 2024, 13, e032904. [Google Scholar] [CrossRef]
- Mao, S.; Chen, P.; Pan, W.; Gao, L.; Zhang, M. Exacerbated Post-Infarct Pathological Myocardial Remodelling in Diabetes Is Associated with Impaired Autophagy and Aggravated NLRP3 Inflammasome Activation. ESC Heart Fail. 2022, 9, 303–317. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in Health and Disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Pharmacological Inhibition of the NLRP3 Inflammasome: Structure, Molecular Activation, and Inhibitor-NLRP3 Interaction. Pharmacol. Rev. 2023, 75, 487–520. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.-D. The Inflammasome: Firing up Innate Immunity. Immunol. Rev. 2015, 265, 1. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. Cells 2021, 10, 951. [Google Scholar] [CrossRef]
- Olsen, M.B.; Gregersen, I.; Sandanger, Ø.; Yang, K.; Sokolova, M.; Halvorsen, B.E.; Gullestad, L.; Broch, K.; Aukrust, P.; Louwe, M.C. Targeting the Inflammasome in Cardiovascular Disease. JACC Basic Transl. Sci. 2022, 7, 84–98. [Google Scholar] [CrossRef]
- Bootman, M.D. Calcium Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011171. [Google Scholar] [CrossRef]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical Role for Calcium Mobilization in Activation of the NLRP3 Inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef]
- Lee, G.-S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The Calcium-Sensing Receptor Regulates the NLRP3 Inflammasome through Ca2+ and CAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef]
- Weber, K.; Schilling, J.D. Lysosomes Integrate Metabolic-Inflammatory Cross-Talk in Primary Macrophage Inflammasome Activation. J. Biol. Chem. 2014, 289, 9158–9171. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The Role of Mitochondria in NLRP3 Inflammasome Activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Horng, T. Calcium Signaling and Mitochondrial Destabilization in the Triggering of the NLRP3 Inflammasome. Trends Immunol. 2014, 35, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.B.; Bloom, H.L.; Shukrullah, I.; Darrow, L.A.; Kleinbaum, D.; Jones, D.P.; Dudley, S.C.J. Oxidative Stress Markers Are Associated with Persistent Atrial Fibrillation. Clin. Chem. 2007, 53, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Bracey, N.A.; Beck, P.L.; Muruve, D.A.; Hirota, S.A.; Guo, J.; Jabagi, H.; Wright, J.R.J.; Macdonald, J.A.; Lees-Miller, J.P.; Roach, D.; et al. The Nlrp3 Inflammasome Promotes Myocardial Dysfunction in Structural Cardiomyopathy through Interleukin-1β. Exp. Physiol. 2013, 98, 462–472. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The Resolution of Inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Kuo, C.-T.; Chang, G.-J.; Qi, X.-Y.; Nattel, S.; Chen, W.-J. Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4 Mediates the Differential Responsiveness of Atrial versus Ventricular Fibroblasts to Transforming Growth Factor-β. Circ. Arrhythm. Electrophysiol. 2013, 6, 790–798. [Google Scholar] [CrossRef]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited-Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Hear. Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Karakasis, P.; Vlachakis, P.K.; Theofilis, P.; Ktenopoulos, N.; Patoulias, D.; Fyntanidou, B.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: A Multimodal Diagnostic Framework. Diagnostics 2025, 15, 1207. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Ktenopoulos, N.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. J. Clin. Med. 2025, 14, 3250. [Google Scholar] [CrossRef]
- Yamashita, T.; Sekiguchi, A.; Iwasaki, Y.; Date, T.; Sagara, K.; Tanabe, H.; Suma, H.; Sawada, H.; Aizawa, T. Recruitment of Immune Cells across Atrial Endocardium in Human Atrial Fibrillation. Circ. J. 2010, 74, 262–270. [Google Scholar] [CrossRef]
- Chen, M.-C.; Chang, J.-P.; Liu, W.-H.; Yang, C.-H.; Chen, Y.-L.; Tsai, T.-H.; Wang, Y.-H.; Pan, K.-L. Increased Inflammatory Cell Infiltration in the Atrial Myocardium of Patients with Atrial Fibrillation. Am. J. Cardiol. 2008, 102, 861–865. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Bellocci, F.; Morgante, E.; Russo, M.A.; Maseri, A. Histological Substrate of Atrial Biopsies in Patients with Lone Atrial Fibrillation. Circulation 1997, 96, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Smorodinova, N.; Bláha, M.; Melenovský, V.; Rozsívalová, K.; Přidal, J.; Ďurišová, M.; Pirk, J.; Kautzner, J.; Kučera, T. Analysis of Immune Cell Populations in Atrial Myocardium of Patients with Atrial Fibrillation or Sinus Rhythm. PLoS ONE 2017, 12, e0172691. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Schloss, M.J.; Lee, I.-H.; Bapat, A.; Iwamoto, Y.; Vinegoni, C.; Paccalet, A.; Yamazoe, M.; Grune, J.; Pabel, S.; et al. Recruited Macrophages Elicit Atrial Fibrillation. Science 2023, 381, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K. Novel Macrophage Targets for the Treatment of Atrial Fibrillation. Nat. Rev. Cardiol. 2023, 20, 648. [Google Scholar] [CrossRef]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory Signalling in Atrial Cardiomyocytes: A Novel Unifying Principle in Atrial Fibrillation Pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef]
- Nattel, S.; Sager, P.T.; Hüser, J.; Heijman, J.; Dobrev, D. Why Translation from Basic Discoveries to Clinical Applications Is so Difficult for Atrial Fibrillation and Possible Approaches to Improving It. Cardiovasc. Res. 2021, 117, 1616–1631. [Google Scholar] [CrossRef]
- Chen, G.; Chelu, M.G.; Dobrev, D.; Li, N. Cardiomyocyte Inflammasome Signaling in Cardiomyopathies and Atrial Fibrillation: Mechanisms and Potential Therapeutic Implications. Front. Physiol. 2018, 9, 1115. [Google Scholar] [CrossRef]
- Luan, Y.; Guo, Y.; Li, S.; Yu, B.; Zhu, S.; Li, S.; Li, N.; Tian, Z.; Peng, C.; Cheng, J.; et al. Interleukin-18 among Atrial Fibrillation Patients in the Absence of Structural Heart Disease. Europace 2010, 12, 1713–1718. [Google Scholar] [CrossRef]
- Gungor, B.; Ekmekci, A.; Arman, A.; Ozcan, K.S.; Ucer, E.; Alper, A.T.; Calik, N.; Yilmaz, H.; Tezel, T.; Coker, A.; et al. Assessment of Interleukin-1 Gene Cluster Polymorphisms in Lone Atrial Fibrillation: New Insight into the Role of Inflammation in Atrial Fibrillation. Pacing Clin. Electrophysiol. 2013, 36, 1220–1227. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Inflammatory Response in Myocardial Injury, Repair, and Remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between Fibroblasts and Inflammatory Cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Packer, M. Characterization, Pathogenesis, and Clinical Implications of Inflammation-Related Atrial Myopathy as an Important Cause of Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e015343. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial Fibrillation Is Associated with the Fibrotic Remodelling of Adipose Tissue in the Subepicardium of Human and Sheep Atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef]
- Greulich, S.; Maxhera, B.; Vandenplas, G.; de Wiza, D.H.; Smiris, K.; Mueller, H.; Heinrichs, J.; Blumensatt, M.; Cuvelier, C.; Akhyari, P.; et al. Secretory Products from Epicardial Adipose Tissue of Patients with Type 2 Diabetes Mellitus Induce Cardiomyocyte Dysfunction. Circulation 2012, 126, 2324–2334. [Google Scholar] [CrossRef]
- Chamoun, N.; Chahine, Y.; Kassar, A.; Al Yasiri, H.; Hensley, T.; Haykal, R.; Boyle, P.M.; Akoum, N. Reduction in Left Atrial Epicardial Adipose Tissue Following Catheter Ablation for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2025, 18, e013590. [Google Scholar] [CrossRef]
- Skoda, I.; Henningsson, M.; Karlsson, L.O.; Carlhäll, C.-J. The Spatial Overlap between Left Atrial Epicardial Adipose Tissue and Fibrosis Is Not Associated to Clinical Stage of Atrial Fibrillation. Sci. Rep. 2024, 14, 24885. [Google Scholar] [CrossRef]
- Takahashi, N.; Abe, I.; Kira, S.; Ishii, Y. Role of Epicardial Adipose Tissue in Human Atrial Fibrillation. J. Arrhythmia 2023, 39, 93–110. [Google Scholar] [CrossRef]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.-J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and Diabetes Are Major Risk Factors for Epicardial Adipose Tissue Inflammation. JCI Insight 2021, 6, e145495. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human Epicardial Adipose Tissue Induces Fibrosis of the Atrial Myocardium through the Secretion of Adipo-Fibrokines. Eur. Heart J. 2015, 36, 795–805. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Chen, Y.-C.; Chen, J.-H.; Chen, S.-A.; Chen, Y.-J. Adipocytes Modulate the Electrophysiology of Atrial Myocytes: Implications in Obesity-Induced Atrial Fibrillation. Basic Res. Cardiol. 2012, 107, 293. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, S.; Li, S.; Wu, B.; Zhao, Q.; Huang, L.; Zheng, Z.; Xie, X.; Liu, J.; Huang, W.; et al. The Adipose-Neural Axis Is Involved in Epicardial Adipose Tissue-Related Cardiac Arrhythmias. Cell Rep. Med. 2024, 5, 101559. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; Verkerk, A.O.; Bayer, J.D.; Aras, K.; Montañés-Agudo, P.; Mohan, R.A.; Veldkamp, M.; Rivaud, M.R.; de Winter, R.; Kawasaki, M.; et al. Secretome of Atrial Epicardial Adipose Tissue Facilitates Reentrant Arrhythmias by Myocardial Remodeling. Heart Rhythm 2022, 19, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial Adipose Tissue in Contemporary Cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Fender, A.C.; Kleeschulte, S.; Stolte, S.; Leineweber, K.; Kamler, M.; Bode, J.; Li, N.; Dobrev, D. Thrombin Receptor PAR4 Drives Canonical NLRP3 Inflammasome Signaling in the Heart. Basic Res. Cardiol. 2020, 115, 10. [Google Scholar] [CrossRef]
- Brydges, S.D.; Mueller, J.L.; McGeough, M.D.; Pena, C.A.; Misaghi, A.; Gandhi, C.; Putnam, C.D.; Boyle, D.L.; Firestein, G.S.; Horner, A.A.; et al. Inflammasome-Mediated Disease Animal Models Reveal Roles for Innate but Not Adaptive Immunity. Immunity 2009, 30, 875–887. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.-B.; Nattel, S. Postoperative Atrial Fibrillation: Mechanisms, Manifestations and Management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Kaireviciute, D.; Aidietis, A.; Lip, G.Y.H. Atrial Fibrillation Following Cardiac Surgery: Clinical Features and Preventative Strategies. Eur. Heart J. 2009, 30, 410–425. [Google Scholar] [CrossRef]
- Heijman, J.; Muna, A.P.; Veleva, T.; Molina, C.E.; Sutanto, H.; Tekook, M.; Wang, Q.; Abu-Taha, I.H.; Gorka, M.; Künzel, S.; et al. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circ. Res. 2020, 127, 1036–1055. [Google Scholar] [CrossRef]
- Song, J.; Wu, J.; Robichaux, D.J.; Li, T.; Wang, S.; Arredondo Sancristobal, M.J.; Dong, B.; Dobrev, D.; Karch, J.; Thomas, S.S.; et al. A High-Protein Diet Promotes Atrial Arrhythmogenesis via Absent-in-Melanoma 2 Inflammasome. Cells 2024, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Feng, F.; Kang, G.-J.; Liu, M.; Guo, Y.; Dudley, S.C.J. Macrophage IL-1β Mediates Atrial Fibrillation Risk in Diabetic Mice. JCI Insight 2024, 9, e171102. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, A.E.; Vatasescu, R.G.; Stanciu, M.M.; Serdarevic, N.; Dorobantu, M. The Role of Pro-Fibrotic Biomarkers in Paroxysmal and Persistent Atrial Fibrillation. Cytokine 2018, 103, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Wang, X.-F.; Hou, Y.-T.; Zhang, L. Correlation between Atrial Fibrillation, Serum Amyloid Protein A and Other Inflammatory Cytokines. Mol. Med. Rep. 2012, 6, 581–584. [Google Scholar] [CrossRef]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of Fibrotic Remodeling and Cytokines/Chemokines Content in Epicardial Adipose Tissue with Atrial Myocardial Fibrosis in Patients with Atrial Fibrillation. Heart Rhythm 2018, 15, 1717–1727. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome Signaling and Regulation of Interleukin-1 Family Cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef]
- Yao, C.; Scott, L.; Wehrens, X.; Dobrev, D.; Li, N. Abstract 14531: Activation of NLRP3 Inflammasome Promotes Atrial Fibrillation. Circulation 2016, 134 (Suppl. S1), A14531. [Google Scholar]
- Dong, Q.; Li, S.; Wang, W.; Han, L.; Xia, Z.; Wu, Y.; Tang, Y.; Li, J.; Cheng, X. FGF23 Regulates Atrial Fibrosis in Atrial Fibrillation by Mediating the STAT3 and SMAD3 Pathways. J. Cell. Physiol. 2019, 234, 19502–19510. [Google Scholar] [CrossRef]
- Liu, C.-C.; Huang, Z.-X.; Li, X.; Shen, K.-F.; Liu, M.; Ouyang, H.-D.; Zhang, S.-B.; Ruan, Y.-T.; Zhang, X.-L.; Wu, S.-L.; et al. Upregulation of NLRP3 via STAT3-Dependent Histone Acetylation Contributes to Painful Neuropathy Induced by Bortezomib. Exp. Neurol. 2018, 302, 104–111. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.-J.; Qian, C.; Dong, Q.; Ding, D.; Wu, Q.-F.; Li, J.; Wang, H.-F.; Li, W.-H.; Xie, Q.; et al. Signal Transducer and Activator of Transcription 3/MicroRNA-21 Feedback Loop Contributes to Atrial Fibrillation by Promoting Atrial Fibrosis in a Rat Sterile Pericarditis Model. Circ. Arrhythm. Electrophysiol. 2016, 9, e003396. [Google Scholar] [CrossRef]
- Xia, H.-J.; Dai, D.-Z.; Dai, Y. Up-Regulated Inflammatory Factors Endothelin, NFkappaB, TNFalpha and INOS Involved in Exaggerated Cardiac Arrhythmias in l-Thyroxine-Induced Cardiomyopathy Are Suppressed by Darusentan in Rats. Life Sci. 2006, 79, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- McGeough, M.D.; Wree, A.; Inzaugarat, M.E.; Haimovich, A.; Johnson, C.D.; Peña, C.A.; Goldbach-Mansky, R.; Broderick, L.; Feldstein, A.E.; Hoffman, H.M. TNF Regulates Transcription of NLRP3 Inflammasome Components and Inflammatory Molecules in Cryopyrinopathies. J. Clin. Investig. 2017, 127, 4488–4497. [Google Scholar] [CrossRef] [PubMed]
- Aschar-Sobbi, R.; Izaddoustdar, F.; Korogyi, A.S.; Wang, Q.; Farman, G.P.; Yang, F.; Yang, W.; Dorian, D.; Simpson, J.A.; Tuomi, J.M.; et al. Increased Atrial Arrhythmia Susceptibility Induced by Intense Endurance Exercise in Mice Requires TNFα. Nat. Commun. 2015, 6, 6018. [Google Scholar] [CrossRef]
- Kalifa, J.; Jalife, J.; Zaitsev, A.V.; Bagwe, S.; Warren, M.; Moreno, J.; Berenfeld, O.; Nattel, S. Intra-Atrial Pressure Increases Rate and Organization of Waves Emanating from the Superior Pulmonary Veins during Atrial Fibrillation. Circulation 2003, 108, 668–671. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chen, Y.-C.; Chen, Y.-J.; Chang, S.-L.; Tai, C.-T.; Wongcharoen, W.; Yeh, H.-I.; Lin, C.-I.; Chen, S.-A. Tumor Necrosis Factor-Alpha Alters Calcium Handling and Increases Arrhythmogenesis of Pulmonary Vein Cardiomyocytes. Life Sci. 2007, 80, 1806–1815. [Google Scholar] [CrossRef]
- Sawaya, S.E.; Rajawat, Y.S.; Rami, T.G.; Szalai, G.; Price, R.L.; Sivasubramanian, N.; Mann, D.L.; Khoury, D.S. Downregulation of Connexin40 and Increased Prevalence of Atrial Arrhythmias in Transgenic Mice with Cardiac-Restricted Overexpression of Tumor Necrosis Factor. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1561–H1567. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Wu, Y.; Gao, S.; Livingston, J.; Sheppard, B.B.; Chen, M.-H.H. Do the Combined Blood Pressure Effects of Exercise and Antihypertensive Medications Add up to the Sum of Their Parts? A Systematic Meta-Review. BMJ Open Sport Exerc. Med. 2021, 7, e000895. [Google Scholar] [CrossRef]
- Cunha, P.S.; Laranjo, S.; Heijman, J.; Oliveira, M.M. The Atrium in Atrial Fibrillation—A Clinical Review on How to Manage Atrial Fibrotic Substrates. Front. Cardiovasc. Med. 2022, 9, 879984. [Google Scholar] [CrossRef]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC. Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef]
- Sandanger, Ø.; Ranheim, T.; Vinge, L.E.; Bliksøen, M.; Alfsnes, K.; Finsen, A.V.; Dahl, C.P.; Askevold, E.T.; Florholmen, G.; Christensen, G.; et al. The NLRP3 Inflammasome Is Up-Regulated in Cardiac Fibroblasts and Mediates Myocardial Ischaemia-Reperfusion Injury. Cardiovasc. Res. 2013, 99, 164–174. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial Fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Coarfa, C.; Yuan, Y.; Abu-Taha, I.; Wang, X.; Song, J.; Koirala, A.; Grimm, S.L.; Kamler, M.; Mullany, L.K.; et al. Fibroblast-Specific Inflammasome Activation Predisposes to Atrial Fibrillation. bioRxiv Prepr. Serv. Biol. 2023. [Google Scholar] [CrossRef]
- Scott, L.J.; Fender, A.C.; Saljic, A.; Li, L.; Chen, X.; Wang, X.; Linz, D.; Lang, J.; Hohl, M.; Twomey, D.; et al. NLRP3 Inflammasome Is a Key Driver of Obesity-Induced Atrial Arrhythmias. Cardiovasc. Res. 2021, 117, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Gawałko, M.; Sanders, P.; Penders, J.; Li, N.; Nattel, S.; Dobrev, D. Does Gut Microbiota Affect Atrial Rhythm? Causalities and Speculations. Eur. Heart J. 2021, 42, 3521–3525. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Zuo, K.; Li, J.; Li, K.; Hu, C.; Gao, Y.; Chen, M.; Hu, R.; Liu, Y.; Chi, H.; Wang, H.; et al. Disordered Gut Microbiota and Alterations in Metabolic Patterns Are Associated with Atrial Fibrillation. Gigascience 2019, 8, giz058. [Google Scholar] [CrossRef]
- Zuo, K.; Yin, X.; Li, K.; Zhang, J.; Wang, P.; Jiao, J.; Liu, Z.; Liu, X.; Liu, J.; Li, J.; et al. Different Types of Atrial Fibrillation Share Patterns of Gut Microbiota Dysbiosis. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Li, B.; Luo, Y.; Gong, Y.; Jin, X.; Zhang, J.; Zhou, Y.; Zhuo, X.; Wang, Z.; et al. Gut Microbiota Dysbiosis Promotes Age-Related Atrial Fibrillation by Lipopolysaccharide and Glucose-Induced Activation of NLRP3-Inflammasome. Cardiovasc. Res. 2022, 118, 785–797. [Google Scholar] [CrossRef]
- Gawałko, M.; Agbaedeng, T.A.; Saljic, A.; Müller, D.N.; Wilck, N.; Schnabel, R.; Penders, J.; Rienstra, M.; van Gelder, I.; Jespersen, T.; et al. Gut Microbiota, Dysbiosis and Atrial Fibrillation. Arrhythmogenic Mechanisms and Potential Clinical Implications. Cardiovasc. Res. 2022, 118, 2415–2427. [Google Scholar] [CrossRef]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Sanders, P.; Kottkamp, H.; Kalman, J.M. The Role of Obesity in Atrial Fibrillation. Eur. Heart J. 2016, 37, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Davies, G.; Woods, R.; Budge, H.; Sacks, H.S.; Symonds, M.E. “Browning” the Cardiac and Peri-Vascular Adipose Tissues to Modulate Cardiovascular Risk. Int. J. Cardiol. 2017, 228, 265–274. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Brooks, A.G.; Shipp, N.J.; Manavis, J.; Wood, J.P.M.; Finnie, J.W.; Samuel, C.S.; Royce, S.G.; Twomey, D.J.; et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J. Am. Coll. Cardiol. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Scridon, A.; Dobreanu, D.; Chevalier, P.; Şerban, R.C. Inflammation, a Link between Obesity and Atrial Fibrillation. Inflamm. Res. 2015, 64, 383–393. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative Stress and Inflammation as Central Mediators of Atrial Fibrillation in Obesity and Diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Goudis, C.A.; Korantzopoulos, P.; Ntalas, I.V.; Kallergis, E.M.; Ketikoglou, D.G. Obesity and Atrial Fibrillation: A Comprehensive Review of the Pathophysiological Mechanisms and Links. J. Cardiol. 2015, 66, 361–369. [Google Scholar] [CrossRef]
- Batal, O.; Schoenhagen, P.; Shao, M.; Ayyad, A.E.; Van Wagoner, D.R.; Halliburton, S.S.; Tchou, P.J.; Chung, M.K. Left Atrial Epicardial Adiposity and Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 230–236. [Google Scholar] [CrossRef]
- Sha, R.; Baines, O.; Hayes, A.; Tompkins, K.; Kalla, M.; Holmes, A.P.; O’Shea, C.; Pavlovic, D. Impact of Obesity on Atrial Fibrillation Pathogenesis and Treatment Options. J. Am. Heart Assoc. 2024, 13, e032277. [Google Scholar] [CrossRef]
- Huxley, R.R.; Filion, K.B.; Konety, S.; Alonso, A. Meta-Analysis of Cohort and Case-Control Studies of Type 2 Diabetes Mellitus and Risk of Atrial Fibrillation. Am. J. Cardiol. 2011, 108, 56–62. [Google Scholar] [CrossRef]
- Goudis, C.A.; Korantzopoulos, P.; Ntalas, I.V.; Kallergis, E.M.; Liu, T.; Ketikoglou, D.G. Diabetes Mellitus and Atrial Fibrillation: Pathophysiological Mechanisms and Potential Upstream Therapies. Int. J. Cardiol. 2015, 184, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Tu, D.; Liu, X.; Niu, S.; Suo, Y.; Liu, T.; Li, G.; Liu, C. Role of NLRP3-Inflammasome/Caspase-1/Galectin-3 Pathway on Atrial Remodeling in Diabetic Rabbits. J. Cardiovasc. Transl. Res. 2020, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Xie, A.; Kim, T.-Y.; Terentyeva, R.; Liu, M.; Shi, G.; Feng, F.; Choi, B.-R.; Terentyev, D.; et al. Interleukin-1β, Oxidative Stress, and Abnormal Calcium Handling Mediate Diabetic Arrhythmic Risk. JACC Basic Transl. Sci. 2021, 6, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hiram, R.; Xiong, F.; Naud, P.; Xiao, J.; Sosnowski, D.K.; Le Quilliec, E.; Saljic, A.; Abu-Taha, I.H.; Kamler, M.; LeBlanc, C.-A.; et al. An Inflammation Resolution-Promoting Intervention Prevents Atrial Fibrillation Caused by Left Ventricular Dysfunction. Cardiovasc. Res. 2024, 120, 345–359. [Google Scholar] [CrossRef]
- E Castor, R.G.M.; Bruno, A.S.; Pereira, C.A.; Bello, F.L.M.; Rodrigues, Y.B.; Silva, M.G.; Bernardes, S.S.; e Castor, M.G.M.; Ferreira, A.J.; de Cassia Tostes, R.; et al. Glibenclamide Reverses Cardiac Damage and NLRP3 Inflammasome Activation Associated with a High Refined Sugar Diet. Eur. J. Pharmacol. 2024, 984, 177035. [Google Scholar] [CrossRef]
- Aune, D.; Mahamat-Saleh, Y.; Kobeissi, E.; Feng, T.; Heath, A.K.; Janszky, I. Blood Pressure, Hypertension and the Risk of Atrial Fibrillation: A Systematic Review and Meta-Analysis of Cohort Studies. Eur. J. Epidemiol. 2023, 38, 145–178. [Google Scholar] [CrossRef]
- Antoun, I.; Layton, G.R.; Nizam, A.; Barker, J.; Abdelrazik, A.; Eldesouky, M.; Koya, A.; Lau, E.Y.M.; Zakkar, M.; Somani, R.; et al. Hypertension and Atrial Fibrillation: Bridging the Gap Between Mechanisms, Risk, and Therapy. Medicina 2025, 61, 362. [Google Scholar] [CrossRef]
- Gawałko, M.; Linz, D. Atrial Fibrillation Detection and Management in Hypertension. Hypertension 2023, 80, 523–533. [Google Scholar] [CrossRef]
- Kamioka, M.; Narita, K.; Watanabe, T.; Watanabe, H.; Makimoto, H.; Okuyama, T.; Yokota, A.; Komori, T.; Kabutoya, T.; Imai, Y.; et al. Hypertension and Atrial Fibrillation: The Clinical Impact of Hypertension on Perioperative Outcomes of Atrial Fibrillation Ablation and Its Optimal Control for the Prevention of Recurrence. Hypertens. Res. 2024, 47, 2800–2810. [Google Scholar] [CrossRef]
- Lau, Y.-F.; Yiu, K.-H.; Siu, C.-W.; Tse, H.-F. Hypertension and Atrial Fibrillation: Epidemiology, Pathophysiology and Therapeutic Implications. J. Hum. Hypertens. 2012, 26, 563–569. [Google Scholar] [CrossRef]
- Yagi, S.; Akaike, M.; Aihara, K.; Ishikawa, K.; Iwase, T.; Ikeda, Y.; Soeki, T.; Yoshida, S.; Sumitomo-Ueda, Y.; Matsumoto, T.; et al. Endothelial Nitric Oxide Synthase-Independent Protective Action of Statin against Angiotensin II-Induced Atrial Remodeling via Reduced Oxidant Injury. Hypertension 2010, 55, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Hypertension, Inflammation and Atrial Fibrillation. J. Hypertens. 2014, 32, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Suetomi, T.; Willeford, A.; Brand, C.S.; Cho, Y.; Ross, R.S.; Miyamoto, S.; Brown, J.H. Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca2+/Calmodulin-Dependent Protein Kinase II δ Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation 2018, 138, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Ishida, N.; Ibi, M.; Saito, M.; Takahashi, M.; Taniguchi, S.; Iwakura, Y.; Morino, Y.; Taira, E.; Sawa, Y.; et al. IL-1β Plays an Important Role in Pressure Overload-Induced Atrial Fibrillation in Mice. Biol. Pharm. Bull. 2019, 42, 543–546. [Google Scholar] [CrossRef]
- Ge, C.; Zhao, Y.; Liang, Y.; He, Y. Silencing of TLR4 Inhibits Atrial Fibrosis and Susceptibility to Atrial Fibrillation via Downregulation of NLRP3-TGF-β in Spontaneously Hypertensive Rats. Dis. Markers 2022, 2022, 2466150. [Google Scholar] [CrossRef]
- Ye, T.; Zhou, Y.; Yang, J.; Yu, F.; Song, Z.; Shi, J.; Wang, L.; Huang, Z.; Yang, B.; Wang, X. P2X7 Receptor Inhibition Prevents Atrial Fibrillation in Rodent Models of Depression. Europace 2024, 26, euae022. [Google Scholar] [CrossRef]
- Xing, Y.; Yan, L.; Li, X.; Xu, Z.; Wu, X.; Gao, H.; Chen, Y.; Ma, X.; Liu, J.; Zhang, J. The Relationship between Atrial Fibrillation and NLRP3 Inflammasome: A Gut Microbiota Perspective. Front. Immunol. 2023, 14, 1273524. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhang, Y.; Gao, P.; Fleishman, J.S.; Wang, H. CGAS-STING Targeting Offers a Novel Therapeutic Paradigm in Cardiovascular Diseases. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2025, 211, 107137. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, L.; Ruan, H.; Huang, Z. CGAS-STING Signaling in Cardiovascular Diseases. Front. Immunol. 2024, 15, 1402817. [Google Scholar] [CrossRef]
- Hu, D.; Cui, Y.-X.; Wu, M.-Y.; Li, L.; Su, L.-N.; Lian, Z.; Chen, H. Cytosolic DNA Sensor CGAS Plays an Essential Pathogenetic Role in Pressure Overload-Induced Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1525–H1537. [Google Scholar] [CrossRef]
- Mezzaroma, E.; Abbate, A.; Toldo, S. The Inflammasome in Heart Failure. Curr. Opin. Physiol. 2021, 19, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ren, M.; Adhikari, B.K.; Wang, H.; He, Y. The NLRP3 Inflammasome as a Novel Therapeutic Target for Cardiac Fibrosis. J. Inflamm. Res. 2022, 15, 3847–3858. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The Role of Inflammasomes in Human Diseases and Their Potential as Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 Inflammasome: Contributions to Inflammation-Related Diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Hegyi, B.; Pölönen, R.-P.; Hellgren, K.T.; Ko, C.Y.; Ginsburg, K.S.; Bossuyt, J.; Mercola, M.; Bers, D.M. Cardiomyocyte Na+ and Ca2+ Mishandling Drives Vicious Cycle Involving CaMKII, ROS, and Ryanodine Receptors. Basic Res. Cardiol. 2021, 116, 58. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.; Ou, D.; Huang, L.; Li, H.; Lang, M. MCC950 Ameliorates Ventricular Arrhythmia Vulnerability Induced by Heart Failure. Bioengineered 2022, 13, 8593–8604. [Google Scholar] [CrossRef]
- Lei, M.; Salvage, S.C.; Jackson, A.P.; Huang, C.L.-H. Cardiac Arrhythmogenesis: Roles of Ion Channels and Their Functional Modification. Front. Physiol. 2024, 15, 1342761. [Google Scholar] [CrossRef]
- Roger, E.; Chadjichristos, C.E.; Kavvadas, P.; Price, G.W.; Cliff, C.L.; Hadjadj, S.; Renciot, J.; Squires, P.E.; Hills, C.E. Connexin-43 Hemichannels Orchestrate NOD-like Receptor Protein-3 (NLRP3) Inflammasome Activation and Sterile Inflammation in Tubular Injury. Cell Commun. Signal. 2023, 21, 263. [Google Scholar] [CrossRef]
- Higashikuni, Y.; Liu, W.; Numata, G.; Tanaka, K.; Fukuda, D.; Tanaka, Y.; Hirata, Y.; Imamura, T.; Takimoto, E.; Komuro, I.; et al. NLRP3 Inflammasome Activation Through Heart-Brain Interaction Initiates Cardiac Inflammation and Hypertrophy During Pressure Overload. Circulation 2023, 147, 338–355. [Google Scholar] [CrossRef]
- Toldo, S.; Marchetti, C.; Mauro, A.G.; Chojnacki, J.; Mezzaroma, E.; Carbone, S.; Zhang, S.; Van Tassell, B.; Salloum, F.N.; Abbate, A. Inhibition of the NLRP3 Inflammasome Limits the Inflammatory Injury Following Myocardial Ischemia-Reperfusion in the Mouse. Int. J. Cardiol. 2016, 209, 215–220. [Google Scholar] [CrossRef]
- Duan, F.; Li, H.; Lu, B.; Wang, X.; Xu, X. Loss of Trim31 Worsens Cardiac Remodeling in a Mouse Model of Heart Failure by Enhancing the Activation of the NLRP3 Inflammasome. Inflammation 2024. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shi, H.; Chang, S.; Gao, Y.; Li, X.; Lv, C.; Yang, H.; Xiang, H.; Yang, J.; Xu, L.; et al. The Selective NLRP3-Inflammasome Inhibitor MCC950 Reduces Myocardial Fibrosis and Improves Cardiac Remodeling in a Mouse Model of Myocardial Infarction. Int. Immunopharmacol. 2019, 74, 105575. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, M.; Yu, J.; Xu, Y.; Zhang, J.; Liu, J.; Zheng, Z.; Ye, J.; Wang, Z.; Ye, D.; et al. MCC950, a Selective NLRP3 Inhibitor, Attenuates Adverse Cardiac Remodeling Following Heart Failure Through Improving the Cardiometabolic Dysfunction in Obese Mice. Front. Cardiovasc. Med. 2022, 9, 727474. [Google Scholar] [CrossRef]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M.; et al. Macrophage-Dependent IL-1β Production Induces Cardiac Arrhythmias in Diabetic Mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef]
- Balan, A.I.; Halațiu, V.B.; Scridon, A. Oxidative Stress, Inflammation, and Mitochondrial Dysfunction: A Link between Obesity and Atrial Fibrillation. Antioxidants 2024, 13, 117. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Q.; Lin, D.; Ma, S. The Role of Infarct Border Zone Remodelling in Ventricular Arrhythmias: Bridging Basic Research and Clinical Applications. J. Cell. Mol. Med. 2025, 29, e70526. [Google Scholar] [CrossRef]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef]
- Pinar, A.A.; Scott, T.E.; Huuskes, B.M.; Tapia Cáceres, F.E.; Kemp-Harper, B.K.; Samuel, C.S. Targeting the NLRP3 Inflammasome to Treat Cardiovascular Fibrosis. Pharmacol. Ther. 2020, 209, 107511. [Google Scholar] [CrossRef]
- Mo, B.; Ding, Y.; Ji, Q. NLRP3 Inflammasome in Cardiovascular Diseases: An Update. Front. Immunol. 2025, 16, 1550226. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation-Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 Inflammasome in Inflammatory Diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.S.; Kaur, M.; Silakari, P.; Silakari, O. Interleukin-1 Receptor Associated Kinase Inhibitors: Potential Therapeutic Agents for Inflammatory- and Immune-Related Disorders. Cell. Signal. 2015, 27, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Kasper, S.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Scharl, S.; Raselli, T.; Frey-Wagner, I.; Gutte, P.M.; et al. NLRP3 Tyrosine Phosphorylation Is Controlled by Protein Tyrosine Phosphatase PTPN22. J. Clin. Investig. 2016, 126, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Liu, Z.-S.; Xue, W.; Bai, Z.-F.; Wang, Q.-Y.; Dai, J.; Liu, X.; Huang, Y.-J.; Cai, H.; Zhan, X.-Y.; et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell 2017, 68, 185–197.e6. [Google Scholar] [CrossRef]
- Stutz, A.; Kolbe, C.-C.; Stahl, R.; Horvath, G.L.; Franklin, B.S.; van Ray, O.; Brinkschulte, R.; Geyer, M.; Meissner, F.; Latz, E. NLRP3 Inflammasome Assembly Is Regulated by Phosphorylation of the Pyrin Domain. J. Exp. Med. 2017, 214, 1725–1736. [Google Scholar] [CrossRef]
- Huang, X.; Dixit, V.M. Drugging the Undruggables: Exploring the Ubiquitin System for Drug Development. Cell Res. 2016, 26, 484–498. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, A.; Lv, J.; Zhang, Q.; Ran, Y.; Wei, C.; Wu, J. Development of Small Molecule Inhibitors Targeting NLRP3 Inflammasome Pathway for Inflammatory Diseases. Eur. J. Med. Chem. 2020, 185, 111822. [Google Scholar] [CrossRef]
- Jesus, A.A.; Goldbach-Mansky, R. IL-1 Blockade in Autoinflammatory Syndromes. Annu. Rev. Med. 2014, 65, 223–244. [Google Scholar] [CrossRef]
- Saljic, A.; Heijman, J.; Dobrev, D. Emerging Antiarrhythmic Drugs for Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 4096. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W.M. Treating Inflammation by Blocking Interleukin-1 in a Broad Spectrum of Diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Calabrese, L.H. Anakinra Treatment of Patients with Rheumatoid Arthritis. Ann. Pharmacother. 2002, 36, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Granowitz, E.V.; Porat, R.; Mier, J.W.; Pribble, J.P.; Stiles, D.M.; Bloedow, D.C.; Catalano, M.A.; Wolff, S.M.; Dinarello, C.A. Pharmacokinetics, Safety and Immunomodulatory Effects of Human Recombinant Interleukin-1 Receptor Antagonist in Healthy Humans. Cytokine 1992, 4, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Tannenbaum, S.; Rordorf, C.; Lowe, P.J.; Floch, D.; Gram, H.; Roy, S. Pharmacokinetic and Pharmacodynamic Properties of Canakinumab, a Human Anti-Interleukin-1β Monoclonal Antibody. Clin. Pharmacokinet. 2012, 51, e1–e18. [Google Scholar] [CrossRef]
- Rossi-Semerano, L.; Fautrel, B.; Wendling, D.; Hachulla, E.; Galeotti, C.; Semerano, L.; Touitou, I.; Koné-Paut, I. Tolerance and Efficacy of Off-Label Anti-Interleukin-1 Treatments in France: A Nationwide Survey. Orphanet J. Rare Dis. 2015, 10, 19. [Google Scholar] [CrossRef]
- Moran, A.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; Raskin, P.; et al. Interleukin-1 Antagonism in Type 1 Diabetes of Recent Onset: Two Multicentre, Randomised, Double-Blind, Placebo-Controlled Trials. Lancet 2013, 381, 1905–1915. [Google Scholar] [CrossRef]
- Wannamaker, W.; Davies, R.; Namchuk, M.; Pollard, J.; Ford, P.; Ku, G.; Decker, C.; Charifson, P.; Weber, P.; Germann, U.A.; et al. (S)-1-((S)-2-{[1-(4-Amino-3-Chloro-Phenyl)-Methanoyl]-Amino}-3,3-Dimethyl-Butanoyl)-Pyrrolidine-2-Carboxylic Acid ((2R,3S)-2-Ethoxy-5-Oxo-Tetrahydro-Furan-3-Yl)-Amide (VX-765), an Orally Available Selective Interleukin (IL)-Converting Enzyme/Caspase-1 Inhibitor, Exhibits Potent Anti-Inflammatory Activities by Inhibiting the Release of IL-1β and IL-18. J. Pharmacol. Exp. Ther. 2007, 321, 509–516. [Google Scholar] [CrossRef]
- Boxer, M.B.; Quinn, A.M.; Shen, M.; Jadhav, A.; Leister, W.; Simeonov, A.; Auld, D.S.; Thomas, C.J. A Highly Potent and Selective Caspase 1 Inhibitor That Utilizes a Key 3-Cyanopropanoic Acid Moiety. ChemMedChem 2010, 5, 730–738. [Google Scholar] [CrossRef]
- MacKenzie, S.H.; Schipper, J.L.; Clark, A.C. The Potential for Caspases in Drug Discovery. Curr. Opin. Drug Discov. Devel. 2010, 13, 568–576. [Google Scholar]
- Conen, D.; Ke Wang, M.; Popova, E.; Chan, M.T.V.; Landoni, G.; Cata, J.P.; Reimer, C.; McLean, S.R.; Srinathan, S.K.; Reyes, J.C.T.; et al. Effect of Colchicine on Perioperative Atrial Fibrillation and Myocardial Injury after Non-Cardiac Surgery in Patients Undergoing Major Thoracic Surgery (COP-AF): An International Randomised Trial. Lancet 2023, 402, 1627–1635. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Rovere, M.E.; Gandino, A.; Cemin, R.; Ferrua, S.; Belli, R.; Maestroni, S.; Simon, C.; et al. Colchicine Reduces Postoperative Atrial Fibrillation: Results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) Atrial Fibrillation Substudy. Circulation 2011, 124, 2290–2295. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, H.; Liao, J.; Zhao, N.; Tse, G.; Han, B.; Chen, L.; Huang, Z.; Du, Y. Colchicine Prevents Atrial Fibrillation Promotion by Inhibiting IL-1β-Induced IL-6 Release and Atrial Fibrosis in the Rat Sterile Pericarditis Model. Biomed. Pharmacother. 2020, 129, 110384. [Google Scholar] [CrossRef]
- Dong, P.; Hu, H.; Guan, X.; Ung, C.O.L.; Shi, L.; Han, S.; Yu, S. Cost-Consequence Analysis of Salvianolate Injection for the Treatment of Coronary Heart Disease. Chin. Med. 2018, 13, 28. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, W.; Lan, T.; Pan, W.; Chen, X.; Wu, H.; Xu, D. Salvianolate Reduces Atrial Fibrillation through Suppressing Atrial Interstitial Fibrosis by Inhibiting TGF-Β1/Smad2/3 and TXNIP/NLRP3 Inflammasome Signaling Pathways in Post-MI Rats. Phytomedicine 2018, 51, 255–265. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Pamporis, K.; Sagris, M.; Ktenopoulos, N.; Kassimis, G.; Antoniadis, A.P.; Fragakis, N. Sodium–Glucose Cotransporter 2 Inhibitors in Aortic Stenosis: Toward a Comprehensive Cardiometabolic Approach. Int. J. Mol. Sci. 2025, 26, 4494. [Google Scholar] [CrossRef]
- Mylonas, N.; Nikolaou, P.E.; Karakasis, P.; Stachteas, P.; Fragakis, N.; Andreadou, I. Endothelial Protection by Sodium-Glucose Cotransporter 2 Inhibitors: A Literature Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 7274. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Stachteas, P.; Patoulias, D.; Bougioukas, K.I.; Fragakis, N. Efficacy and Safety of Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: An Overview of 36 Systematic Reviews. Heart Fail. Rev. 2023, 28, 1033–1051. [Google Scholar] [CrossRef]
- Stachteas, P.; Nasoufidou, A.; Patoulias, D.; Karakasis, P.; Karagiannidis, E.; Mourtzos, M.-A.; Samaras, A.; Apostolidou, X.; Fragakis, N. The Role of Sodium-Glucose Co-Transporter-2 Inhibitors on Diuretic Resistance in Heart Failure. Int. J. Mol. Sci. 2024, 25, 3122. [Google Scholar] [CrossRef]
- Stachteas, P.; Nasoufidou, A.; Karagiannidis, E.; Patoulias, D.; Karakasis, P.; Alexiou, S.; Samaras, A.; Zormpas, G.; Stavropoulos, G.; Tsalikakis, D.; et al. The Role of Sodium Glucose Co-Transporter 2 Inhibitors in Atrial Fibrillation: A Comprehensive Review. J. Clin. Med. 2024, 13, 5408. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Schuermans, A.; Vlachakis, P.K.; Klisic, A.; Rizzo, M.; Fragakis, N. Sodium-Glucose Cotransporter 2 Inhibitors and Outcomes in Transthyretin Amyloid Cardiomyopathy: Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2025, e14392. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Vlachakis, P.K.; Karakasis, P.; Dimitriadis, K.; Sagris, M.; Pamporis, K.; Drakopoulou, M.; Siasos, G.; Tsioufis, K.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Changes in Epicardial Adipose Tissue: A Systematic Literature Review And Meta-Analysis. Curr. Vasc. Pharmacol. 2025, 23, 204–212. [Google Scholar] [CrossRef]
- Gao, J.; Xue, G.; Zhan, G.; Wang, X.; Li, J.; Yang, X.; Xia, Y. Benefits of SGLT2 Inhibitors in Arrhythmias. Front. Cardiovasc. Med. 2022, 9, 1011429. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Wang, Q. Effects and Mechanisms of SGLT2 Inhibitors on the NLRP3 Inflammasome, with a Focus on Atherosclerosis. Front. Endocrinol. 2022, 13, 992937. [Google Scholar] [CrossRef]
- Ke, Q.; Shi, C.; Lv, Y.; Wang, L.; Luo, J.; Jiang, L.; Yang, J.; Zhou, Y. SGLT2 Inhibitor Counteracts NLRP3 Inflammasome via Tubular Metabolite Itaconate in Fibrosis Kidney. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22078. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Safa, B.I.; Gonzalez, M.S.C.; Kim, S.F.; Echouffo-Tcheugui, J.B. Systemic and Organ-Specific Anti-Inflammatory Effects of Sodium-Glucose Cotransporter-2 Inhibitors. Trends Endocrinol. Metab. 2024, 35, 425–438. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ang, S.P.; Kumar, D.; Deb, N.; Jaiswal, A.; Joshi, A.; Nasir, Y.M.; Bandyopadhyay, D.; Michos, E.D.; Benjamin, E.J.; et al. Sodium-Glucose Cotransporter-2 Inhibitors and Arrhythmias: A Meta-Analysis of 38 Randomized Controlled Trials. JACC. Adv. 2025, 4, 101615. [Google Scholar] [CrossRef]
- Chen, S.; Overberg, K.; Ghouse, Z.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; Zuurbier, C.J. Empagliflozin Mitigates Cardiac Hypertrophy through Cardiac RSK/NHE-1 Inhibition. Biomed. Pharmacother. 2024, 174, 116477. [Google Scholar] [CrossRef]
- Chen, X.; Hocher, C.-F.; Shen, L.; Krämer, B.K.; Hocher, B. Reno- and Cardioprotective Molecular Mechanisms of SGLT2 Inhibitors beyond Glycemic Control: From Bedside to Bench. Am. J. Physiol. Cell Physiol. 2023, 325, C661–C681. [Google Scholar] [CrossRef]
- Chung, Y.J.; Park, K.C.; Tokar, S.; Eykyn, T.R.; Fuller, W.; Pavlovic, D.; Swietach, P.; Shattock, M.J. Off-Target Effects of Sodium-Glucose Co-Transporter 2 Blockers: Empagliflozin Does Not Inhibit Na+/H+ Exchanger-1 or Lower [Na+]i in the Heart. Cardiovasc. Res. 2021, 117, 2794–2806. [Google Scholar] [CrossRef]
- Ma, H.-X.; Wu, K.; Dong, F.-H.; Cai, B.-K.; Wu, D.; Lu, H.-Y. Effects of Empagliflozin and Dapagliflozin in Alleviating Cardiac Fibrosis through SIRT6-Mediated Oxidative Stress Reduction. Sci. Rep. 2024, 14, 30764. [Google Scholar] [CrossRef]
- Rykova, E.Y.; Klimontov, V.V.; Shmakova, E.; Korbut, A.I.; Merkulova, T.I.; Kzhyshkowska, J. Anti-Inflammatory Effects of SGLT2 Inhibitors: Focus on Macrophages. Int. J. Mol. Sci. 2025, 26, 1670. [Google Scholar] [CrossRef]
- Duan, H.-Y.; Barajas-Martinez, H.; Antzelevitch, C.; Hu, D. The Potential Anti-Arrhythmic Effect of SGLT2 Inhibitors. Cardiovasc. Diabetol. 2024, 23, 252. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, M.; Bie, M.; Wang, X.; Guo, J.; Xiao, H. Galectin-3 Induces Atrial Fibrosis by Activating the TGF-Β1/Smad Pathway in Patients with Atrial Fibrillation. Cardiology 2020, 145, 446–455. [Google Scholar] [CrossRef]
- Gohari, S.; Ismail-Beigi, F.; Mahjani, M.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S. The Effect of Sodium-Glucose Co-Transporter-2 (SGLT2) Inhibitors on Blood Interleukin-6 Concentration: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Endocr. Disord. 2023, 23, 257. [Google Scholar] [CrossRef]
- Kang, S.; Verma, S.; Hassanabad, A.F.; Teng, G.; Belke, D.D.; Dundas, J.A.; Guzzardi, D.G.; Svystonyuk, D.A.; Pattar, S.S.; Park, D.S.J.; et al. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can. J. Cardiol. 2020, 36, 543–553. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 Inhibition Modulates NLRP3 Inflammasome Activity via Ketones and Insulin in Diabetes with Cardiovascular Disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Kasperova, B.J.; Mraz, M.; Svoboda, P.; Hlavacek, D.; Kratochvilova, H.; Modos, I.; Vrzackova, N.; Ivak, P.; Janovska, P.; Kobets, T.; et al. Sodium-Glucose Cotransporter 2 Inhibitors Induce Anti-Inflammatory and Anti-Ferroptotic Shift in Epicardial Adipose Tissue of Subjects with Severe Heart Failure. Cardiovasc. Diabetol. 2024, 23, 223. [Google Scholar] [CrossRef]
- Takano, M.; Kondo, H.; Harada, T.; Takahashi, M.; Ishii, Y.; Yamasaki, H.; Shan, T.; Akiyoshi, K.; Shuto, T.; Teshima, Y.; et al. Empagliflozin Suppresses the Differentiation/Maturation of Human Epicardial Preadipocytes and Improves Paracrine Secretome Profile. JACC Basic Transl. Sci. 2023, 8, 1081–1097. [Google Scholar] [CrossRef]
- Ionică, L.N.; Lința, A.V.; Bătrîn, A.D.; Hâncu, I.M.; Lolescu, B.M.; Dănilă, M.D.; Petrescu, L.; Mozoș, I.M.; Sturza, A.; Muntean, D.M. The Off-Target Cardioprotective Mechanisms of Sodium-Glucose Cotransporter 2 Inhibitors: An Overview. Int. J. Mol. Sci. 2024, 25, 7711. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Qi, X.; Xiong, F.; Xiao, J.; Muthukumarasamy, K.M.; Altuntas, Y.; Zhong, Y.; Abu-Taha, I.; Bruns, F.; Tekook, M.; Kamler, M.; et al. Time-Dependent Mitochondrial Remodeling in Experimental Atrial Fibrillation and Potential Therapeutic Relevance. bioRxiv Prepr. Serv. Biol. 2025. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, R.; Chakrabarti, S.; Su, Z. Resident Macrophages as Potential Therapeutic Targets for Cardiac Ageing and Injury. Clin. Transl. Immunol. 2020, 9, e1167. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study Model | Cell Type(s) Involved | Key Findings | Implications for AF |
|---|---|---|---|---|
| Yao et al., 2018 [19] | Human, Dog, and Mouse models (including CM-specific knock-in and CREM-TG mice) | Cardiomyocytes | Enhanced NLRP3 inflammasome activation in atrial cardiomyocytes from AF patients and animal models; CM-specific NLRP3 activation induced ectopic activity, shortened AERP, structural remodeling, and increased AF susceptibility. Inhibition (MCC950, AAV-shRNA, or knockout) reduced AF burden. | NLRP3 inflammasome in cardiomyocytes promotes both arrhythmic triggers and substrate for AF; targeting NLRP3 may represent a novel therapeutic approach. |
| Heijman et al., 2020 [80] | Human atrial tissue from cardiac surgery patients | Cardiomyocytes | Patients developing POAF showed increased atrial NLRP3-inflammasome activation and CaMKII-mediated RyR2 dysfunction; Ca2+-handling abnormalities, including SR Ca2+-leak and spontaneous Ca2+-release events, were present preoperatively. Acute IL-1β exposure exacerbated arrhythmogenic Ca2+ disturbances in atrial myocytes. | NLRP3/CaMKII signaling constitutes a latent arrhythmogenic substrate for POAF, unmasked by postoperative inflammation; targeting these pathways may prevent POAF and long-term AF progression. |
| Fender et al., 2020 [76] | Mouse (HFD model), Human atrial tissue, Human ventricular fibroblasts | Cardiac fibroblasts, cardiomyocytes | PAR4 expression is upregulated in diabetic hearts and mediates thrombin-induced activation of canonical NLRP3 inflammasome via caspase-1, IL-1β, and GSDMD in both mouse and human cardiac tissues. Genetic deletion or pharmacologic inhibition of PAR4 blunted this pathway. | Diabetes-associated upregulation of PAR4 links hypercoagulability to sterile cardiac inflammation through the NLRP3 inflammasome; PAR4 antagonists may mitigate thromboinflammatory contributions to AF substrate development. |
| Song et al., 2024 [81] | Mouse (WT and Aim2−/−), atrial myocytes | Atrial cardiomyocytes | High-protein diet (HPD) enhanced AF inducibility via AIM2 inflammasome activation; AIM2−/− mice were protected from HPD-induced AF. HPD promoted mitochondrial ROS, cytoplasmic dsDNA, and abnormal SR Ca2+ release, which were suppressed in AIM2-deficient mice. | AIM2 inflammasome links dietary triggers (HPD) to arrhythmogenesis via mitochondrial stress and Ca2+ dysregulation; AIM2 may serve as a novel therapeutic target for metabolically driven AF. |
| Yao et al., 2016 [87] | Mouse (CM-specific NLRP3 A350V knock-in, CREM-IbΔC-X Tg) | Cardiomyocytes, Fibroblasts, Macrophages | CM-specific activation of NLRP3 inflammasome increased AF inducibility and premature atrial contractions. Upregulation of active caspase-1, IL-1β, and collagen 1a indicated fibroblast and macrophage activation. NLRP3 deletion in CREM-Tg mice reduced spontaneous AF. | Constitutive NLRP3 activation in cardiomyocytes drives AF initiation and promotes atrial remodeling; therapeutic targeting of NLRP3 could interrupt inflammatory and fibrotic AF substrates. |
| Huang et al., 2016 [90] | Rat sterile pericarditis model, primary cultured cardiac fibroblasts | Cardiac fibroblasts | STAT3 and miR-21 form a positive feedback loop that promotes atrial fibrosis. Inhibition of STAT3 (S3I-201) or miR-21 (antagomir-21) reduced atrial fibrosis, conduction inhomogeneity, and inducible AF. IL-6 stimulated CF activation via increased STAT3 phosphorylation and miR-21 expression; blockade of either reduced fibrotic gene expression and fibroblast proliferation. | While not directly implicating inflammasomes, this study highlights IL-6/STAT3/miR-21 signaling as a crucial inflammatory–fibrotic axis in AF substrate formation; targeting this pathway could indirectly modulate inflammasome-mediated profibrotic signaling. |
| Li et al., 2023 [102] | Human, Canine, and FB-specific NLRP3 KI Mouse Model (Tcf21iCre:Nlrp3A350V) | Cardiac Fibroblasts | NLRP3 and IL1B upregulated in human atrial FBs from AF patients and canine AF model. FB-specific NLRP3 activation in mice caused atrial dilation, fibrosis, hypocontractility, and enhanced AF inducibility. Connexin 43 remodeling and impaired intercellular communication contributed to reduced conduction velocity. | Fibroblast-restricted NLRP3 activation drives atrial cardiomyopathy and arrhythmogenesis via fibrotic and gap junction remodeling; suggests NLRP3 as a unifying target across cardiac cell types in AF therapy. |
| Scott et al., 2021 [103] | Human, Sheep, Mouse (WT and NLRP3−/− with HFD) | Cardiomyocytes | Obesity enhanced atrial NLRP3 inflammasome activation in humans, sheep, and HFD-fed mice; NLRP3−/− mice were protected from AF inducibility, atrial refractoriness shortening, abnormal Ca2+ handling, and atrial fibrosis. ER stress and Kv1.5 upregulation contributed to the proarrhythmic substrate. | NLRP3 inflammasome mediates obesity-induced atrial arrhythmogenesis through inflammatory, electrical, and structural remodeling; targeting NLRP3 may mitigate AF risk in obese individuals. |
| Author, Year | Study Model | Cell Type(s) Involved | Key Findings | Implications for Ventricular Arrhythmias |
|---|---|---|---|---|
| Suetomi et al., 2018 [133] | Mouse (CM-specific CaMKIIδ KO, TAC model), Human | Cardiomyocytes | Pressure overload triggered NLRP3 inflammasome activation in cardiomyocytes via CaMKIIδ-mediated NFκB and ROS signaling. This led to early cytokine production, macrophage recruitment, fibrosis, and ventricular dysfunction. CaMKIIδ deletion or NLRP3 inhibition prevented remodeling. | CM-specific NLRP3 activation initiates inflammatory cascades that promote adverse ventricular remodeling and dysfunction; early inhibition may prevent heart failure and reduce arrhythmogenic substrate development. |
| Jiang et al., 2022 [146] | Mouse (TAC-induced HF model) | Cardiomyocytes | MCC950, a selective NLRP3 inhibitor, reduced QTc and APD90, suppressed VA inducibility, and ameliorated HF-induced cardiac hypertrophy, fibrosis, and ion channel remodeling (Kv4.2, KChIP2, Cav1.2). MCC950 downregulated NLRP3, ASC, caspase-1, IL-1β, and IL-18 expression, indicating suppression of inflammasome signaling. | NLRP3 inflammasome inhibition by MCC950 prevents electrical and structural remodeling and reduces susceptibility to HF-induced ventricular arrhythmias; suggests translational potential for targeted anti-inflammatory therapy in arrhythmia prevention. |
| Higashikuni et al., 2023 [149] | Mouse (WT, Nlrp3−/−, P2rx7−/−, Slc17a9 conditional KO), Human heart tissue, in vitro cardiomyocyte, fibroblast, endothelial models | Cardiomyocytes, Fibroblasts, Endothelial Cells | Pressure overload induces cardiac NLRP3 activation through ATP release from sympathetic efferent nerves via P2X7 receptors. NLRP3 deficiency or ATP/P2X7 blockade reduced IL-1β, hypertrophy, fibrosis, macrophage infiltration, and capillary density. Neural signals (afferent/efferent) and β-blockers modulate inflammasome activity. | Reveals a novel heart–brain axis in inflammasome-driven cardiac remodeling; modulation of neural pathways and NLRP3 inhibition may prevent structural arrhythmogenic substrate formation. |
| Toldo et al., 2016 [150] | Mouse (Iischemia–reperfusion model, ICR male mice) | Cardiomyocytes | NLRP3 expression and caspase-1 activity increased progressively after reperfusion. Pharmacologic NLRP3 inhibition reduced infarct size and caspase-1 activation when administered at or 1 h after reperfusion. No benefit observed if administered 3 h post-reperfusion. Infarct size reduction confirmed even in prolonged ischemia model. | NLRP3 activation exacerbates post-reperfusion myocardial injury and inflammation. Timely NLRP3 inhibition may preserve ventricular integrity and limit arrhythmogenic remodeling following AMI. |
| Gao et al., 2019 [152] | Mouse (MI model via coronary ligation), in vitro cardiac fibroblast model | Cardiomyocytes, Cardiac Fibroblasts | MCC950 significantly reduced myocardial fibrosis, preserved ejection fraction, and suppressed expression of NLRP3, IL-1β, and IL-18. In vitro, MCC950 attenuated hypoxia-induced fibroblast activation and inflammatory cytokine production without affecting proliferation. | Post-MI NLRP3 activation in fibroblasts and cardiomyocytes contributes to inflammation and remodeling. MCC950 reduces fibrosis and may help prevent arrhythmogenic substrate development post-infarction. |
| Monnerat et al., 2016 [154] | Mouse (streptozotocin-induced DM model), Rat and Human cardiomyocytes | Macrophages, Cardiomyocytes | TLR2/NLRP3 activation in diabetic heart macrophages promotes IL-1β production, which prolongs QT/APD, reduces Ito, increases Ca2+ sparks, and enhances spontaneous arrhythmias. IL-1β-induced CaMKII oxidation/phosphorylation underlies arrhythmogenic remodeling. Genetic or pharmacologic targeting (IL-1R KO, NLRP3 KO, Casp1 KO, Anakinra, MCC950) prevented arrhythmias. | Demonstrates inflammasome-mediated macrophage–cardiomyocyte crosstalk in diabetic arrhythmogenesis; highlights therapeutic potential of IL-1 axis and NLRP3 inhibition in preventing ventricular arrhythmias in diabetes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Pamporis, K.; Theofilis, P.; Milaras, N.; Vlachakis, P.K.; Grigoriou, K.; Patoulias, D.; Karamitsos, T.; Antoniadis, A.P.; Fragakis, N. Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling. Int. J. Mol. Sci. 2025, 26, 5954. https://doi.org/10.3390/ijms26135954
Karakasis P, Pamporis K, Theofilis P, Milaras N, Vlachakis PK, Grigoriou K, Patoulias D, Karamitsos T, Antoniadis AP, Fragakis N. Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling. International Journal of Molecular Sciences. 2025; 26(13):5954. https://doi.org/10.3390/ijms26135954
Chicago/Turabian StyleKarakasis, Paschalis, Konstantinos Pamporis, Panagiotis Theofilis, Nikias Milaras, Panayotis K. Vlachakis, Konstantinos Grigoriou, Dimitrios Patoulias, Theodoros Karamitsos, Antonios P. Antoniadis, and Nikolaos Fragakis. 2025. "Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling" International Journal of Molecular Sciences 26, no. 13: 5954. https://doi.org/10.3390/ijms26135954
APA StyleKarakasis, P., Pamporis, K., Theofilis, P., Milaras, N., Vlachakis, P. K., Grigoriou, K., Patoulias, D., Karamitsos, T., Antoniadis, A. P., & Fragakis, N. (2025). Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling. International Journal of Molecular Sciences, 26(13), 5954. https://doi.org/10.3390/ijms26135954








