Sofalcone Suppresses Dengue Virus Replication by Activating Heme Oxygenase-1-Mediated Antiviral Interferon Responses
Abstract
1. Introduction
2. Results
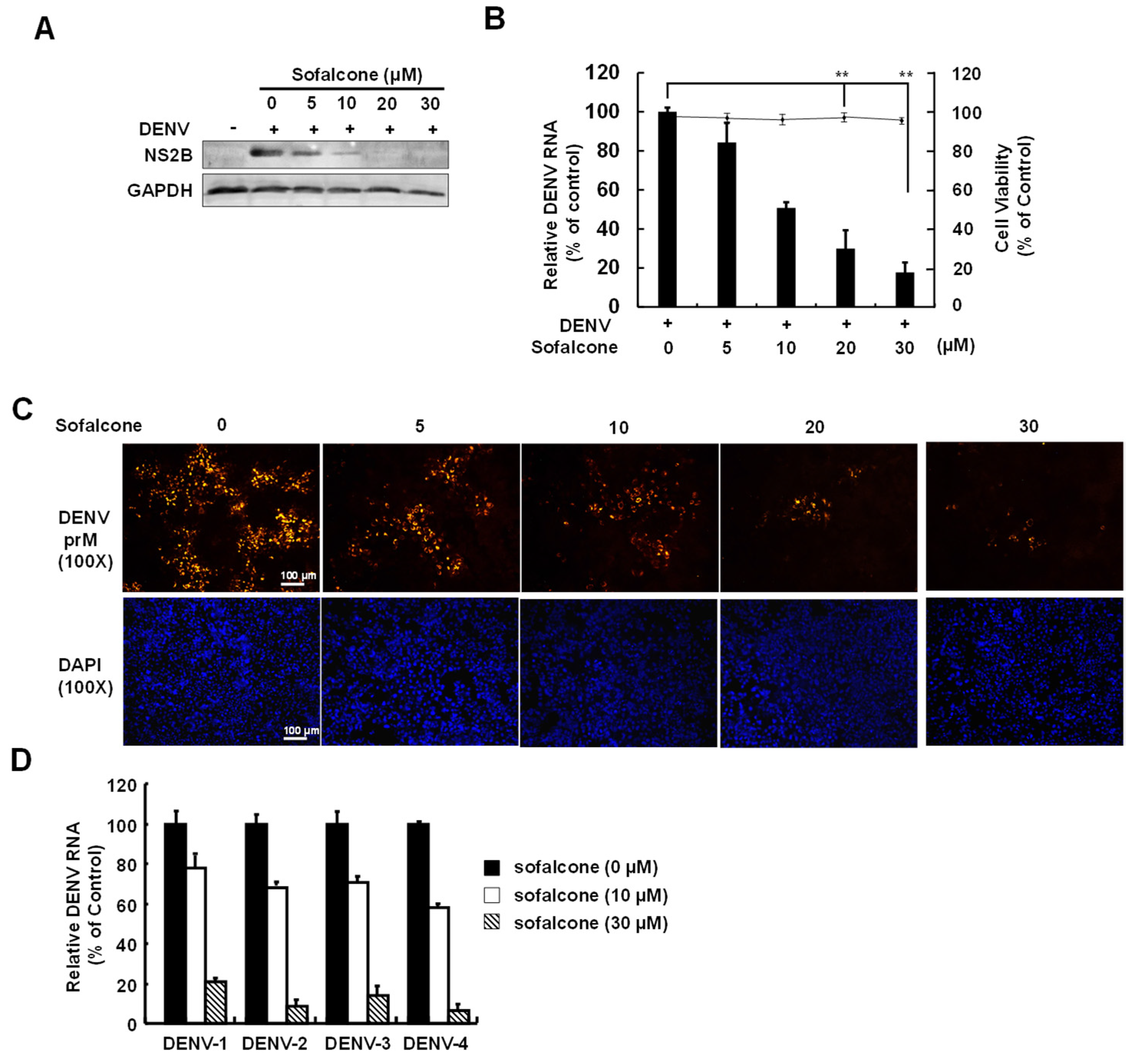
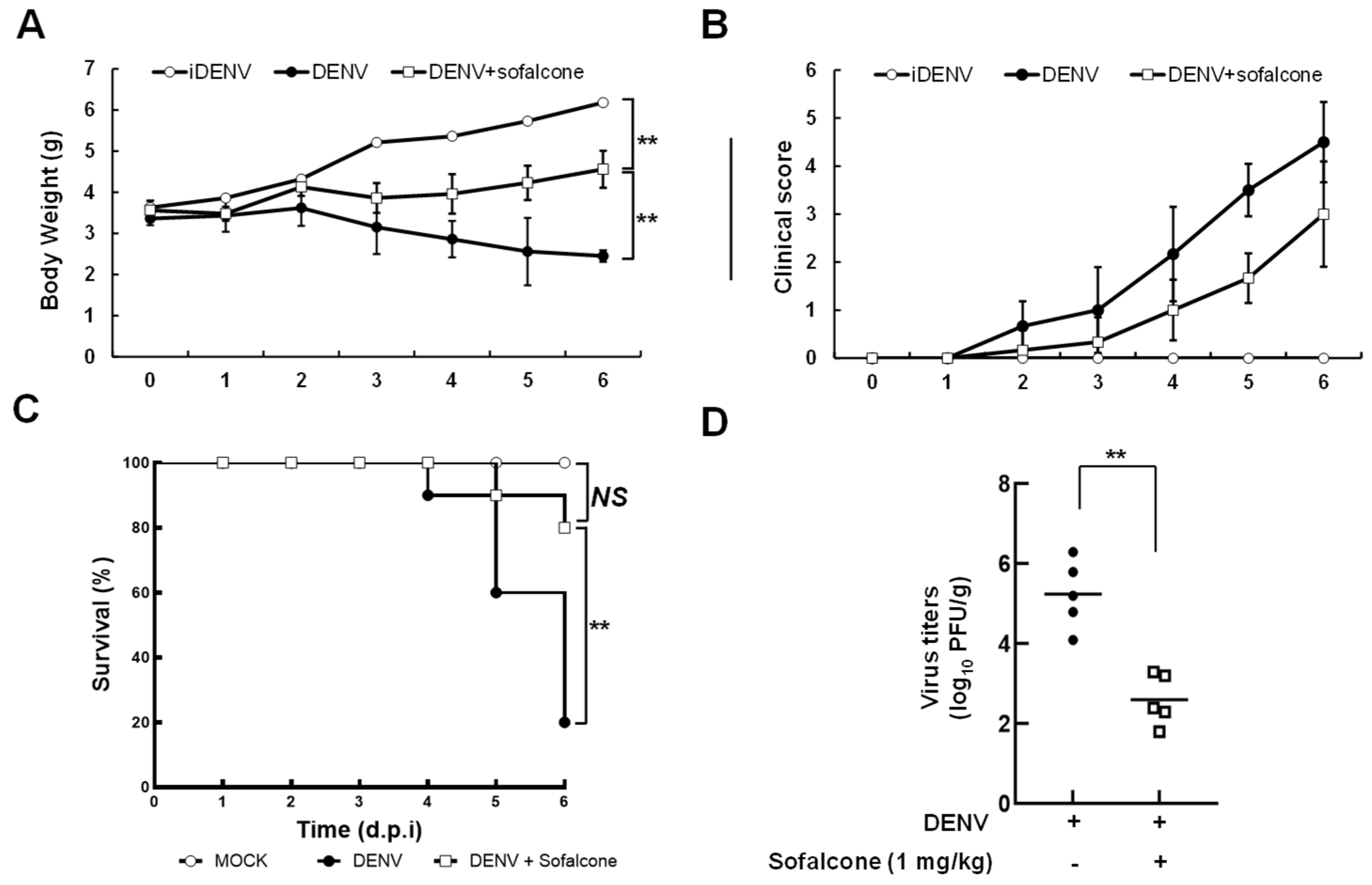
2.1. Sofalcone Suppresses DENV Replication in a Cell-Based and ICR Suckling Mouse Model
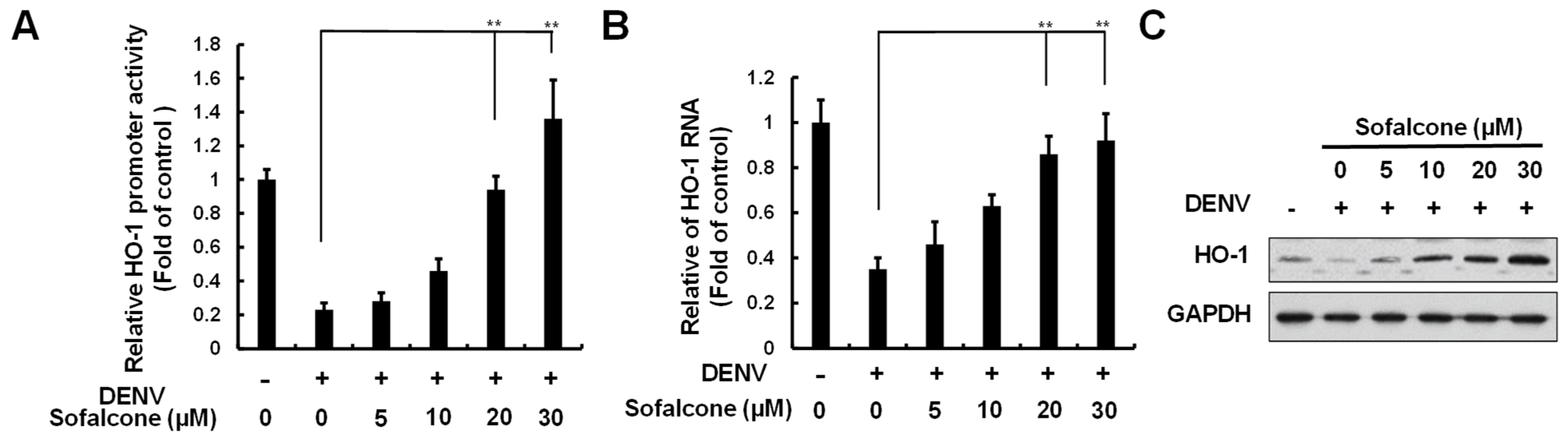
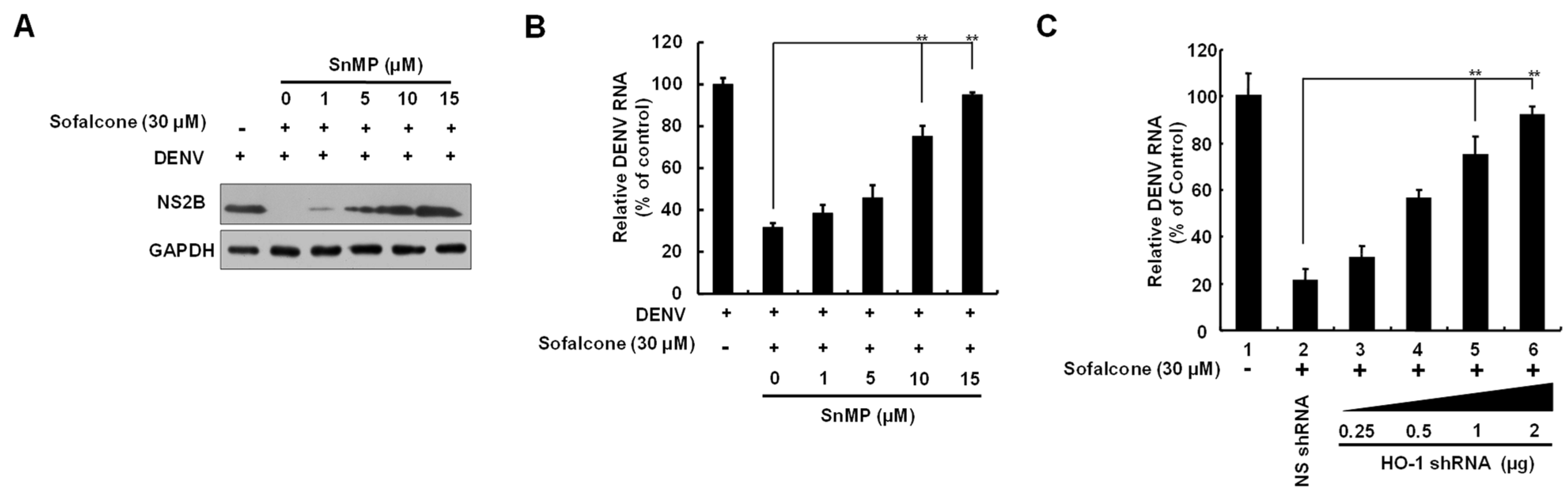
2.2. Sofalcone Suppresses DNV Replication Through the Elevation of HO-1 Expression
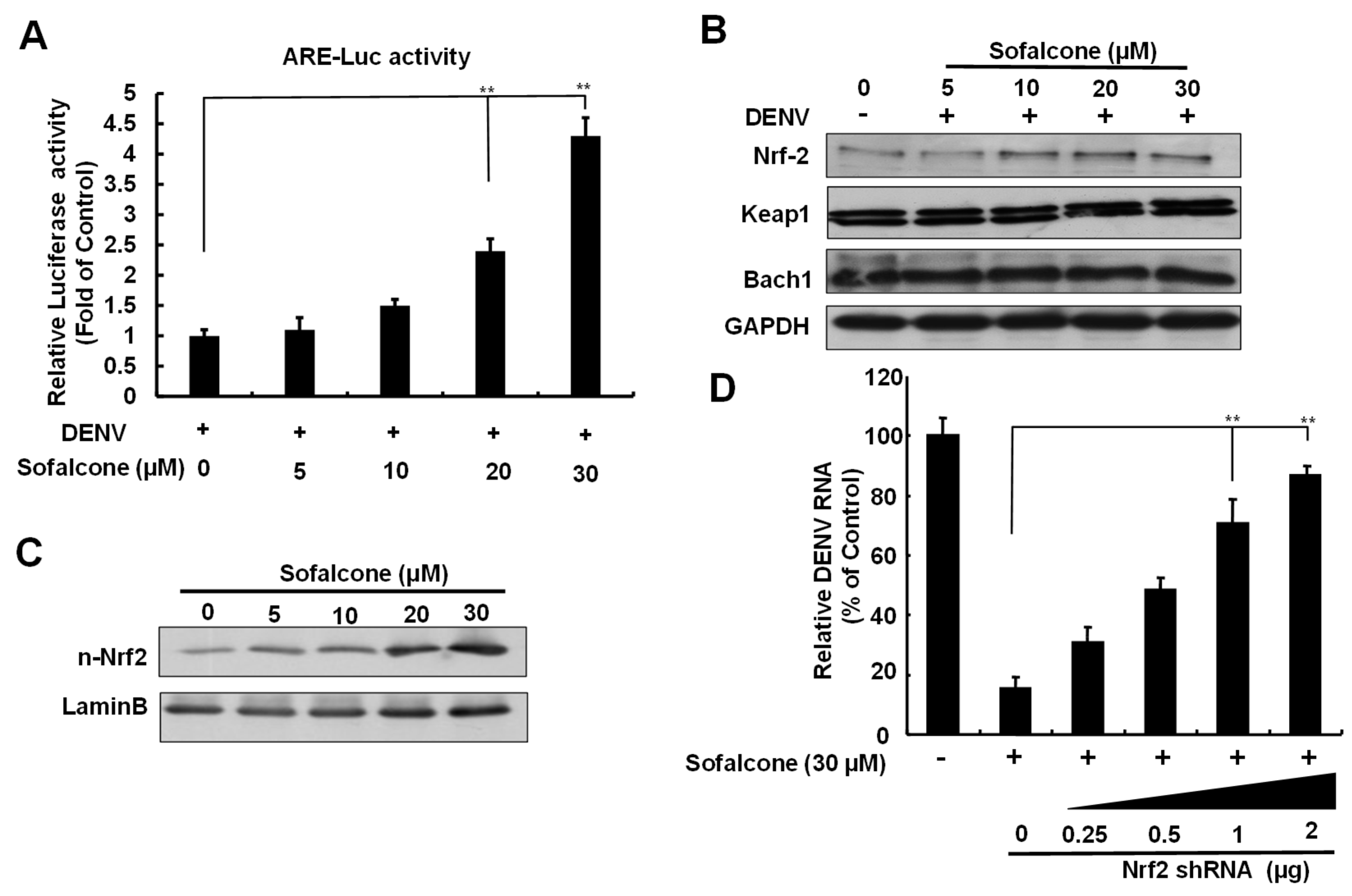
2.3. Sofalcone Exerts an Antiviral Effect Through Nrf2-Mediated HO-1 Expression
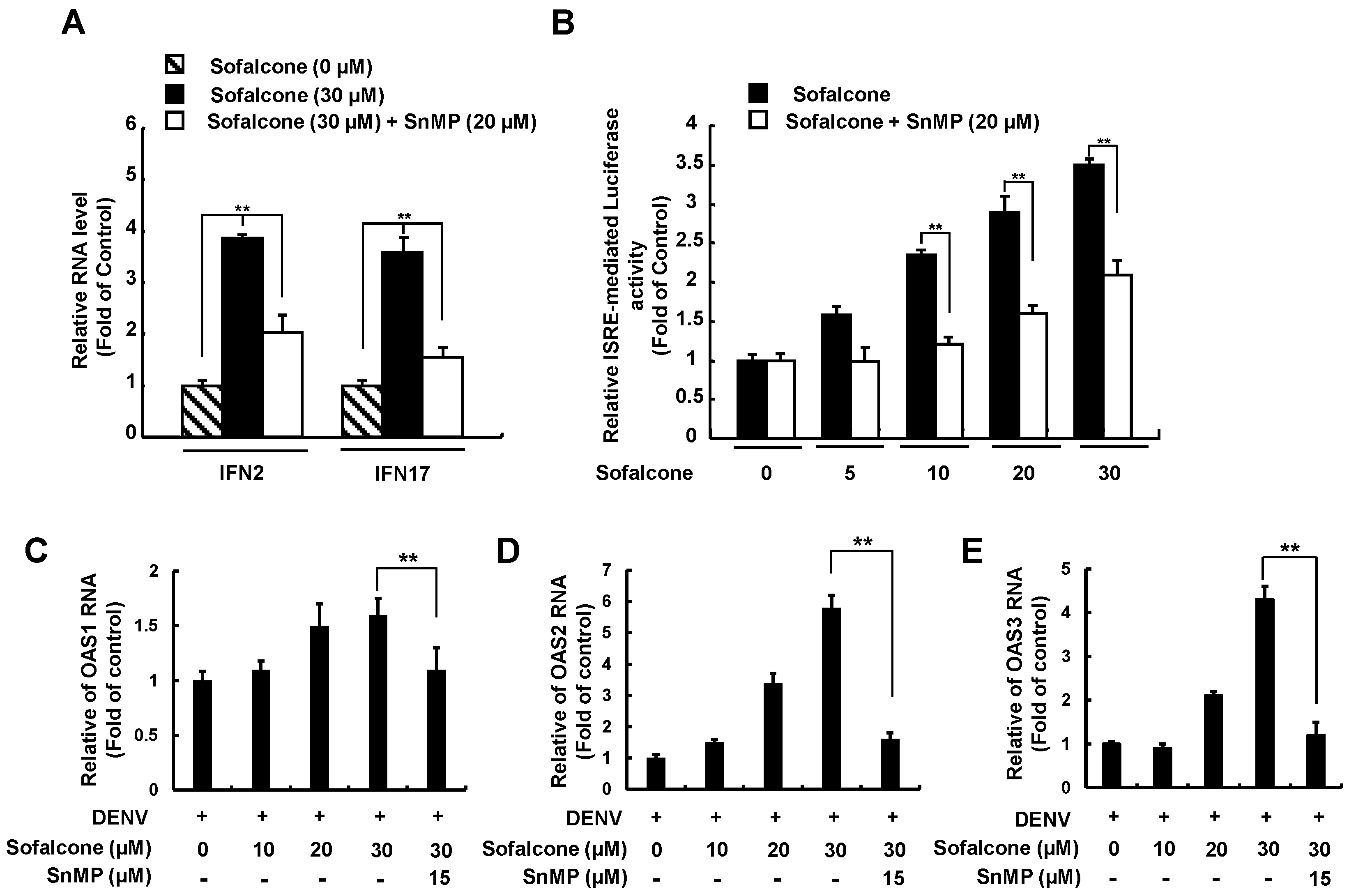
2.4. Sofalcone Inhibits DENV NS3 Protease Activity and Elevated Antiviral IFN Response
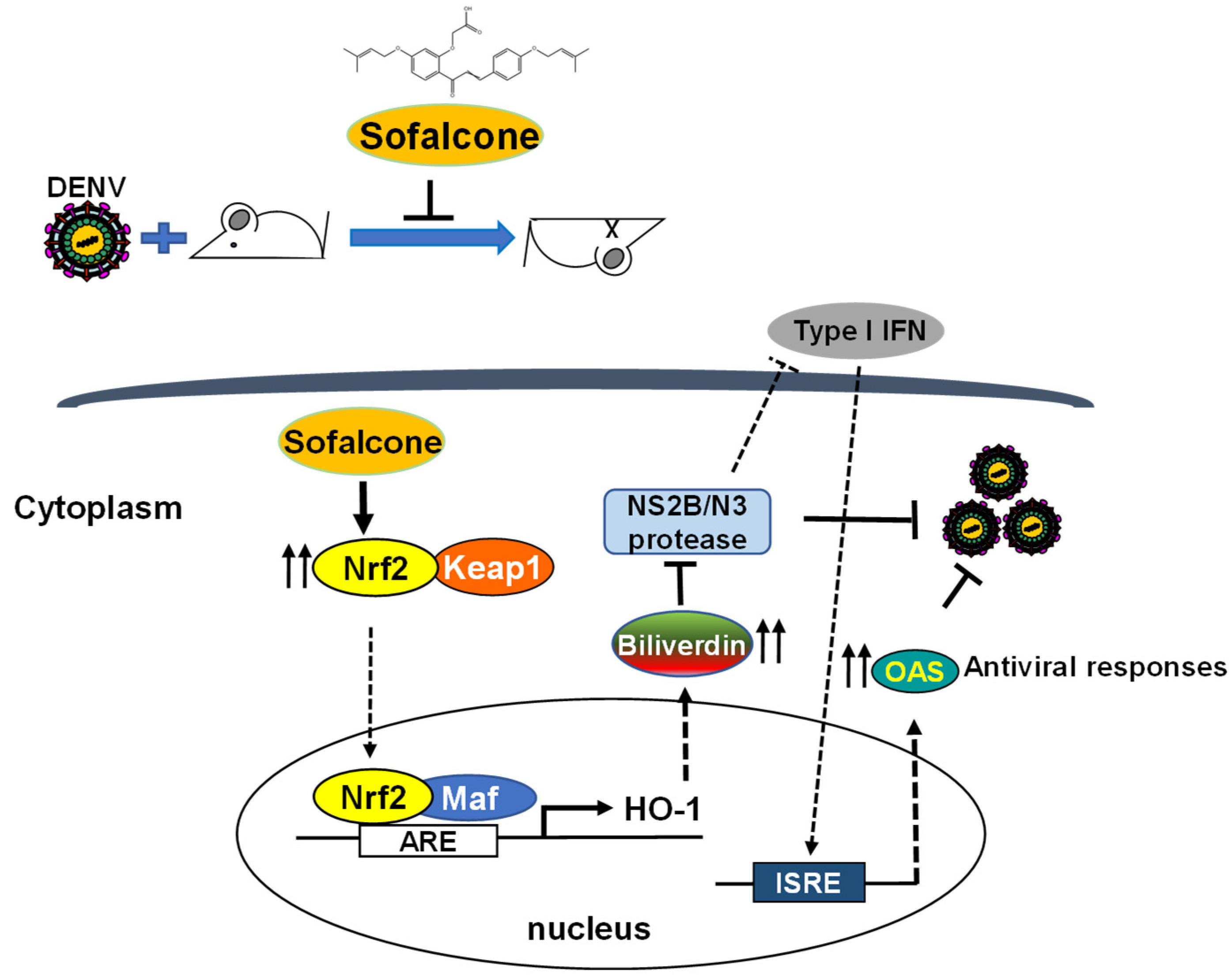
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. DENV-Infected Cell Model
4.3. Animal Ethics and Virus Inoculation Procedures for ICR Suckling Mice
4.4. Plaque Assay
4.5. Preparation of Nuclear Fraction and Western Blot Analysis
4.6. Cell Viability Assessment
4.7. DENV NS2B/NS3 Protease Assay
4.8. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.9. Transient Transfection and Luciferase Reporter Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DENV | dengue virus |
| DENV | dengue virus |
| MOI | multiplicity of infection |
| DHF | hemorrhagic fever |
| DSS | dengue shock syndrome |
| ADE | antibody-dependent enhancement |
| HO-1 | heme oxygenase-1 |
| IFN | interferon |
| OAS1 | 2′-5′-oligoadenylate synthetase 1 |
| ROS | reactive oxygen species |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| Nrf2 | nuclear factor (erythroid-derived 2) |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Bach1 | BTB and CNC homolog 1 |
| ARE | antioxidant response element |
| IRF3 | interferon regulatory factor 3 |
| SnMP | tin mesoporphyrin |
| DMSO | dimethyl sulfoxide |
| RT-qPCR | quantitative reverse transcription polymerase chain reaction |
| SD | standard deviation |
| DAA | direct-acting antivirals |
References
- Paixao, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2018, 3 (Suppl. 1), e000530. [Google Scholar] [CrossRef]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology, biology, and disease aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef]
- Reiner, R.C., Jr.; Achee, N.; Barrera, R.; Burkot, T.R.; Chadee, D.D.; Devine, G.J.; Endy, T.; Gubler, D.; Hombach, J.; Kleinschmidt, I.; et al. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Negl. Trop. Dis. 2016, 10, e0004588. [Google Scholar] [CrossRef]
- Osorio, J.E.; Partidos, C.D.; Wallace, D.; Stinchcomb, D.T. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine 2015, 33, 7112–7120. [Google Scholar] [CrossRef]
- Aguirre, S.; Maestre, A.M.; Pagni, S.; Patel, J.R.; Savage, T.; Gutman, D.; Maringer, K.; Bernal-Rubio, D.; Shabman, R.S.; Simon, V.; et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012, 8, e1002934. [Google Scholar] [CrossRef]
- Wu, B.; Wu, Y.; Tang, W. Heme Catabolic Pathway in Inflammation and Immune Disorders. Front. Pharmacol. 2019, 10, 825. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Lee, C. Therapeutic Modulation of Virus-Induced Oxidative Stress via the Nrf2-Dependent Antioxidative Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 6208067. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, W.C.; Tseng, C.K.; Chen, Y.H.; Lin, C.K.; Lee, J.C. Heme oxygenase-1 inhibits DENV-induced endothelial hyperpermeability and serves as a potential target against dengue hemorrhagic fever. FASEB J. 2022, 36, e22110. [Google Scholar] [CrossRef]
- Medina, M.V.; Sapochnik, D.; Garcia Sola, M.; Coso, O. Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and Beyond. Antioxid. Redox Signal. 2020, 32, 1033–1044. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Gonzalez, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Tzima, S.; Victoratos, P.; Kranidioti, K.; Alexiou, M.; Kollias, G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J. Exp. Med. 2009, 206, 1167–1179. [Google Scholar] [CrossRef]
- Tseng, C.K.; Lin, C.K.; Wu, Y.H.; Chen, Y.H.; Chen, W.C.; Young, K.C.; Lee, J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016, 6, 32176. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, S.; Onda, K.; Tazaki, T.; Hirano, T. Sofalcone, an anti-ulcer chalcone derivative, suppresses inflammatory crosstalk between macrophages and adipocytes and adipocyte differentiation: Implication of heme-oxygenase-1 induction. Biochem. Biophys. Res. Commun. 2009, 381, 566–571. [Google Scholar] [CrossRef]
- Shibuya, A.; Onda, K.; Kawahara, H.; Uchiyama, Y.; Nakayama, H.; Omi, T.; Nagaoka, M.; Matsui, H.; Hirano, T. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2-heme-oxygenase-1 dependent pathway in gastric epithelial cells. Biochem. Biophys. Res. Commun. 2010, 398, 581–584. [Google Scholar] [CrossRef]
- Shen, T.J.; Chen, C.L.; Jhan, M.K.; Tseng, P.C.; Lin, C.F. CNS Immune Profiling in a Dengue Virus-Infected Immunocompetent Outbred ICR Mice Strain. Front. Cell. Infect. Microbiol. 2020, 10, 557610. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Kim, S.; Joo, S.; Jeong, S.; Yoo, J.W.; Jung, Y. Sofalcone, a gastroprotective drug, covalently binds to KEAP1 to activate Nrf2 resulting in anti-colitic activity. Eur. J. Pharmacol. 2019, 865, 172722. [Google Scholar] [CrossRef]
- Akagi, R.; Kubo, T.; Hatori, Y.; Miyamoto, T.; Inouye, S. Heme oxygenase-1 induction by heat shock in rat hepatoma cell line is regulated by the coordinated function of HSF1, NRF2 and BACH1. J. Biochem. 2021, 170, 501–510. [Google Scholar] [CrossRef]
- Sander, W.J.; Fourie, C.; Sabiu, S.; O’Neill, F.H.; Pohl, C.H.; O’Neill, H.G. Reactive oxygen species as potential antiviral targets. Rev. Med. Virol. 2022, 32, e2240. [Google Scholar] [CrossRef]
- Tao, L.; Lemoff, A.; Wang, G.; Zarek, C.; Lowe, A.; Yan, N.; Reese, T.A. Reactive oxygen species oxidize STING and suppress interferon production. eLife 2020, 9, e57837. [Google Scholar] [CrossRef]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafa, Y.M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef]
- Castro, R.; Pinzon, H.S.; Alvis-Guzman, N. A systematic review of observational studies on oxidative/nitrosative stress involvement in dengue pathogenesis. Colomb. Med. 2015, 46, 135–143. [Google Scholar] [CrossRef]
- Toro, A.; Ruiz, M.S.; Lage-Vickers, S.; Sanchis, P.; Sabater, A.; Pascual, G.; Seniuk, R.; Cascardo, F.; Ledesma-Bazan, S.; Vilicich, F.; et al. A Journey into the Clinical Relevance of Heme Oxygenase 1 for Human Inflammatory Disease and Viral Clearance: Why Does It Matter on the COVID-19 Scene? Antioxidants 2022, 11, 276. [Google Scholar] [CrossRef]
- Schumacher, A.; Zenclussen, A.C. Effects of heme oxygenase-1 on innate and adaptive immune responses promoting pregnancy success and allograft tolerance. Front. Pharmacol. 2014, 5, 288. [Google Scholar] [CrossRef]
- Lebeau, G.; El Safadi, D.; Hoarau, M.; Meilhac, O.; Krejbich-Trotot, P.; Viranaicken, W. Zika virus restriction of host antioxidant response is mediated by intracellular NS1 and reveals its ability to upregulate Bach1 expression. Biochem. Biophys. Res. Commun. 2024, 690, 149312. [Google Scholar] [CrossRef]
- Wyler, E.; Franke, V.; Menegatti, J.; Kocks, C.; Boltengagen, A.; Praktiknjo, S.; Walch-Ruckheim, B.; Bosse, J.; Rajewsky, N.; Grasser, F.; et al. Single-cell RNA-sequencing of herpes simplex virus 1-infected cells connects NRF2 activation to an antiviral program. Nat. Commun. 2019, 10, 4878. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Herengt, A.; Thyrsted, J.; Holm, C.K. NRF2 in Viral Infection. Antioxidants 2021, 10, 1491. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Effects of oxidative stress on viral infections: An overview. Npj Viruses 2025, 3, 27. [Google Scholar] [CrossRef]
- Batra, N.; De Souza, C.; Batra, J.; Raetz, A.G.; Yu, A.M. The HMOX1 Pathway as a Promising Target for the Treatment and Prevention of SARS-CoV-2 of 2019 (COVID-19). Int. J. Mol. Sci. 2020, 21, 6412. [Google Scholar] [CrossRef]
- Green, A.M.; Beatty, P.R.; Hadjilaou, A.; Harris, E. Innate immunity to dengue virus infection and subversion of antiviral responses. J. Mol. Biol. 2014, 426, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, V.V.; Infante, E.; Li, L.; Campbell, G.A.; Wang, T.; Paessler, S.; Robert Beatty, P.; Harris, E.; Milligan, G.N.; Bourne, N.; et al. Characterization of lethal dengue virus type 4 (DENV-4) TVP-376 infection in mice lacking both IFN-alpha/beta and IFN-gamma receptors (AG129) and comparison with the DENV-2 AG129 mouse model. J. Gen. Virol. 2015, 96, 3035–3048. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Maasoumy, B. Direct-Acting Antiviral Agents for Hepatitis C Virus Infection-From Drug Discovery to Successful Implementation in Clinical Practice. Viruses 2022, 14, 1325. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.I.; Correia, I.; Seagal, J.; DeGoey, D.A.; Schrimpf, M.R.; Hardee, D.J.; Noey, E.L.; Kati, W.M. Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses 2022, 14, 961. [Google Scholar] [CrossRef]
- Caldwell, H.S.; Pata, J.D.; Ciota, A.T. The Role of the Flavivirus Replicase in Viral Diversity and Adaptation. Viruses 2022, 14, 1076. [Google Scholar] [CrossRef]
- Zhang, H.; Quadeer, A.A.; McKay, M.R. Direct-acting antiviral resistance of Hepatitis C virus is promoted by epistasis. Nat. Commun. 2023, 14, 7457. [Google Scholar] [CrossRef]
- Wang, L.; Song, L.; Jiang, X. Quantification of sofalcone in human plasma and urine by high performance liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 1179–1185. [Google Scholar] [CrossRef]

| Oligonucleotide Name | Sequence 5′-3′ |
|---|---|
| 3′NS5B | 5′-GGAAACCA GCTGCCCATCA |
| 3′NS5B | 5′-CCTCCACGGATAGAAGTTTAA |
| 5′GAPDH | 5′-GTCTTCACCACCATGGAGAA |
| 3′GAPDH | 5′-ATGGCATGGACTGTGGTCAT |
| 5′IFN-2 | 5′-GCAAGTCAAGCTGCTCTGTG |
| 3′IFN-2 | 5′-GATGGTTTCAGCCTTTTGGA |
| 5′IFN-17 | 5′-AGGAGTTTGATGGCAACCAG |
| 3′IFN-17 | 5′-CATCAGGGGAGTCTCTTCCA |
| 5′OAS1 | 5′-CAAGCTTAAGAGCCTCATCC |
| 3′OAS1 | 5′-TGGGCTGTGTTGAAATGTGT |
| 5′OAS2 | 5′-ACAGCTGAAAGCCTTTTGGA |
| 3′OAS2 | 5′-GCATTAAAGGCAGGA AGCAC |
| 5′OAS3 | 5′-CACTGACATCCCAGACGATG |
| 3′OAS3 | 5′-GATCAGGCTCTTCAGCTTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.-L.; Chen, W.-C.; Yen, C.-H.; Liu, W.; Lin, C.-K.; Yu, S.-C.; Lee, M.-Y.; Lee, J.-C. Sofalcone Suppresses Dengue Virus Replication by Activating Heme Oxygenase-1-Mediated Antiviral Interferon Responses. Int. J. Mol. Sci. 2025, 26, 5921. https://doi.org/10.3390/ijms26135921
Ou Y-L, Chen W-C, Yen C-H, Liu W, Lin C-K, Yu S-C, Lee M-Y, Lee J-C. Sofalcone Suppresses Dengue Virus Replication by Activating Heme Oxygenase-1-Mediated Antiviral Interferon Responses. International Journal of Molecular Sciences. 2025; 26(13):5921. https://doi.org/10.3390/ijms26135921
Chicago/Turabian StyleOu, Yu-Lun, Wei-Chun Chen, Chia-Hung Yen, Wangta Liu, Chun-Kuang Lin, Shun-Chieh Yu, Mei-Yueh Lee, and Jin-Ching Lee. 2025. "Sofalcone Suppresses Dengue Virus Replication by Activating Heme Oxygenase-1-Mediated Antiviral Interferon Responses" International Journal of Molecular Sciences 26, no. 13: 5921. https://doi.org/10.3390/ijms26135921
APA StyleOu, Y.-L., Chen, W.-C., Yen, C.-H., Liu, W., Lin, C.-K., Yu, S.-C., Lee, M.-Y., & Lee, J.-C. (2025). Sofalcone Suppresses Dengue Virus Replication by Activating Heme Oxygenase-1-Mediated Antiviral Interferon Responses. International Journal of Molecular Sciences, 26(13), 5921. https://doi.org/10.3390/ijms26135921







