Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense
Abstract
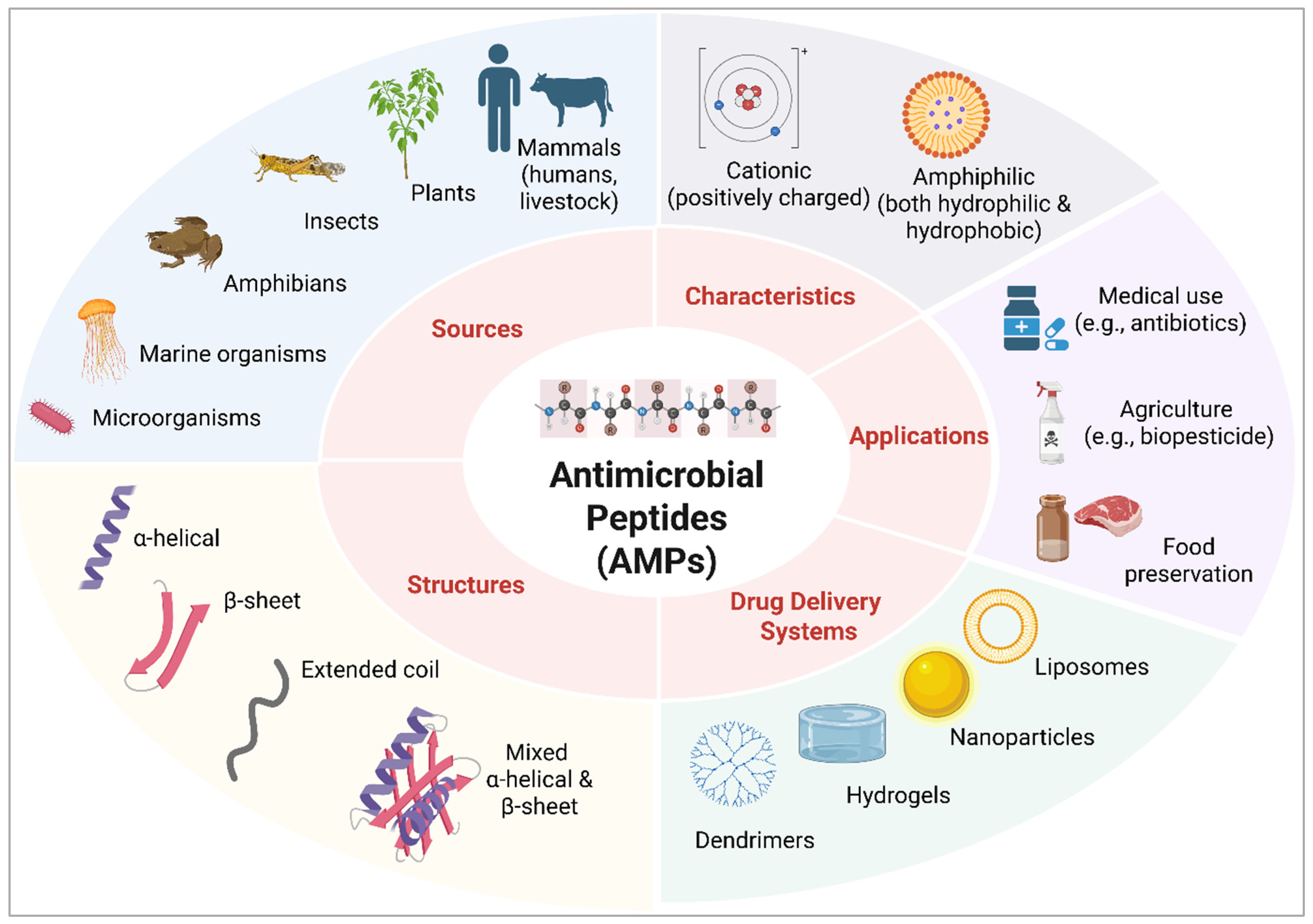
1. Introduction
2. Data Extraction Management
3. Complex Interplay of Immune Responses and Wound Healing
3.1. Overview of Immune Response in Acute Wound Healing
3.2. Overview of Immune Response in Chronic Wound Healing
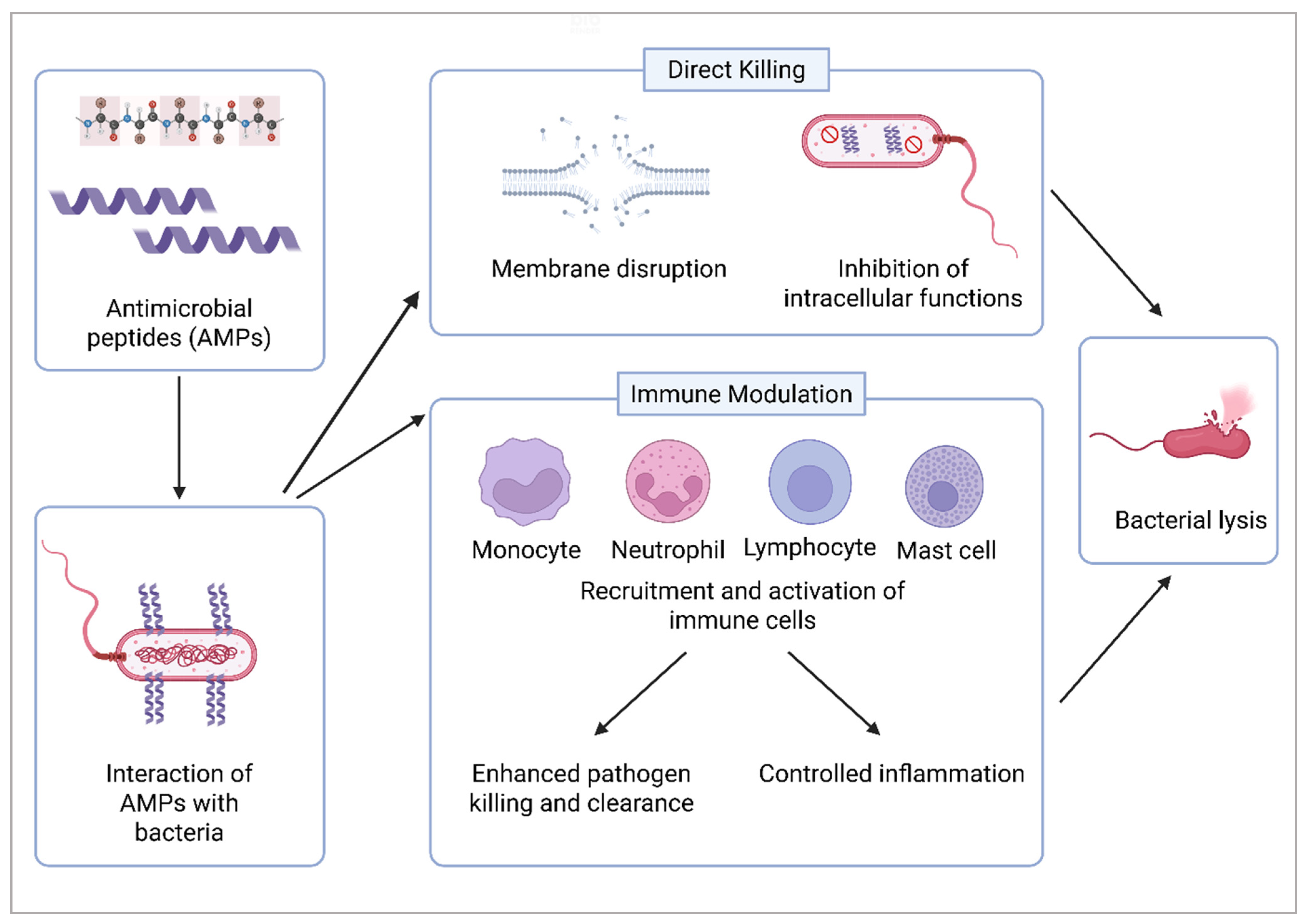
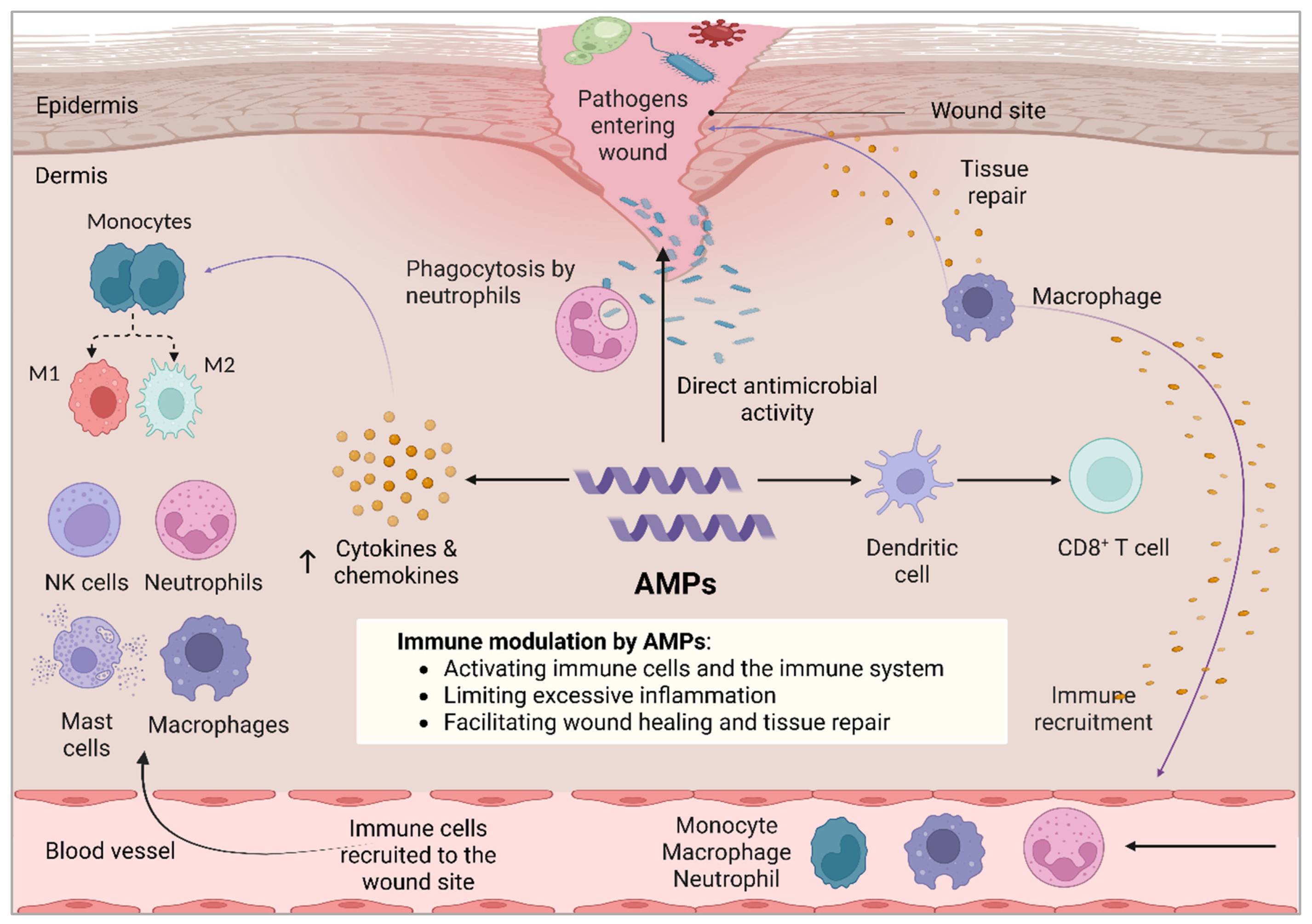
4. Dual Role of Antimicrobial Peptides in Wound Healing and Skin Regeneration
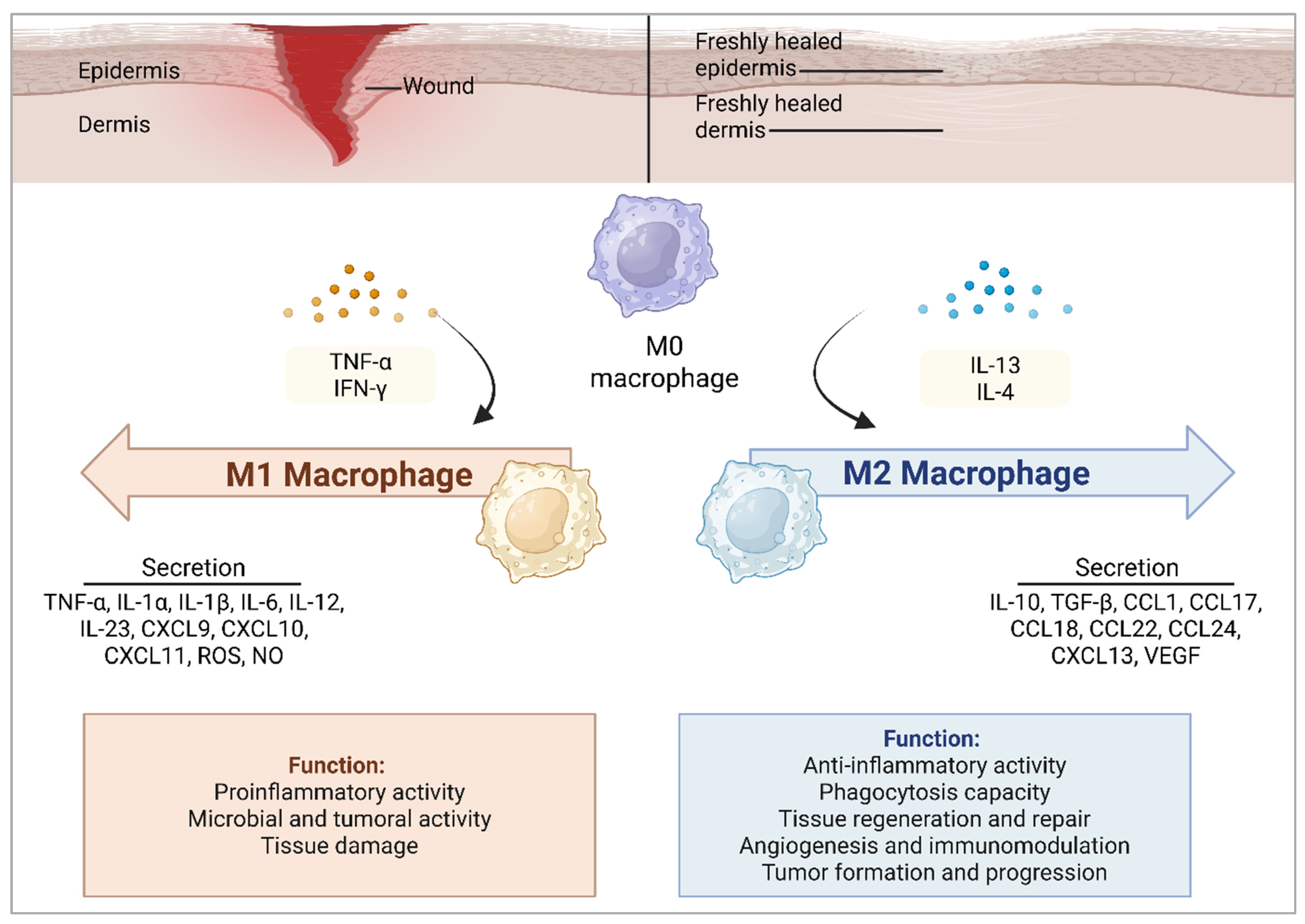
4.1. AMPs Modulate Macrophage Polarization in Wound Healing
4.2. AMPs Modulate Cytokine and Chemokine Regulation in Wound Healing
4.3. AMPs Modulate Responses by NK Cells in Wound Healing
4.4. Other Immunomodulatory Mechanisms of AMPs in Wound Healing
4.5. Bridging Innate Immunity and Adaptive Immunity Using AMPs
5. AMPs in Clinical Development
6. Future Prospects and Challenges in Antimicrobial Peptide Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low. Extrem. Wounds 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D.; Fife, C.E. Chronic wound prevalence and the associated cost of treatment in Medicare beneficiaries: Changes between 2014 and 2019. J. Med. Econ. 2023, 26, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Zawani, M.; Zulkiflee, I.; Salleh, A.; Fadilah, N.I.M.; Maarof, M.; Wen, A.P.Y.; Duman, F.; Tabata, Y.; Aziz, I.A.; et al. Cellular Interaction of Human Skin Cells towards Natural Bioink via 3D-Bioprinting Technologies for Chronic Wound: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 476. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Maarof, M.; Motta, A.; Tabata, Y.; Fauzi, M.B. The Discovery and Development of Natural-Based Biomaterials with Demonstrated Wound Healing Properties: A Reliable Approach in Clinical Trials. Biomedicines 2022, 10, 2226. [Google Scholar] [CrossRef]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burns Trauma. 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Abu-Harirah, H.A.; Al Qudah, A.J.; Daabes, E.; Amawi, K.F.; Qaralleh, H. Multidrug-resistant Bacterial Profile and Patterns for Wound Infections in Nongovernmental Hospitals of Jordan. J. Pure Appl. Microbiol. 2021, 15, 1348–1361. [Google Scholar] [CrossRef]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, B.; Wang, Z. The revitalization of antimicrobial peptides in the resistance era. Pharmacol. Res. 2021, 163, 105276. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- de Souza, G.S.; de Jesus Sonego, L.; Santos Mundim, A.C.; de Miranda Moraes, J.; Sales-Campos, H.; Lorenzon, E.N. Antimicrobial-wound healing peptides: Dual-function molecules for the treatment of skin injuries. Peptides 2022, 148, 170707. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, C. Antimicrobial peptides: Structure, mechanism, and modification. Eur. J. Med. Chem. 2023, 255, 115377. [Google Scholar] [CrossRef] [PubMed]
- Md Fadilah, N.I.; Shahabudin, N.A.; Mohd Razif, R.A.; Sanyal, A.; Ghosh, A.; Baharin, K.I.; Ahmad, H.; Maarof, M.; Motta, A.; Fauzi, M.B. Discovery of bioactive peptides as therapeutic agents for skin wound repair. J. Tissue Eng. 2024, 15, 20417314241280359. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Geitani, R.; Moubareck, C.A.; Xu, Z.; Karam Sarkis, D.; Touqui, L. Expression and Roles of Antimicrobial Peptides in Innate Defense of Airway Mucosa: Potential Implication in Cystic Fibrosis. Front. Immunol. 2020, 11, 1198. [Google Scholar] [CrossRef]
- Dzurová, L.; Holásková, E.; Pospíšilová, H.; Schneider Rauber, G.; Frébortová, J. Cathelicidins: Opportunities and Challenges in Skin Therapeutics and Clinical Translation. Antibiotics 2025, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Meng, G.; Zhu, L.; Ma, L.; Chen, K. Insect Antimicrobial Peptides as Guardians of Immunity and Beyond: A Review. Int. J. Mol. Sci. 2024, 25, 3835. [Google Scholar] [CrossRef]
- Michira, B.B.; Wang, Y.; Mwangi, J.; Wang, K.; Asmamaw, D.; Tadese, D.A.; Gao, J.; Khalid, M.; Lu, Q.M.; Lai, R.; et al. A Tachyplesin Antimicrobial Peptide from Theraphosidae Spiders with Potent Antifungal Activity Against Cryptococcus neoformans. Microorganisms 2024, 12, 2648. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Balandin, S.V.; Belogurova-Ovchinnikova, O.Y.; Ovchinnikova, T.V. Marine Invertebrate Antimicrobial Peptides and Their Potential as Novel Peptide Antibiotics. Mar. Drugs 2023, 21, 503. [Google Scholar] [CrossRef]
- Ghazvini, K.; Neshani, A.; Farsiani, H.; Youssefi, M.; Keikha, M. Preparation and evaluation of antibacterial properties of Pexiganan, a Magainin analogue with Broadly-Spectrum Antimicrobial Activity. Pak. J. Med. Health Sci. 2021, 15, 1778–1784. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X.A. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial peptide biological activity, delivery systems and clinical translation status and challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef]
- Deshayes, C.; Arafath, M.N.; Apaire-Marchais, V.; Roger, E. Drug Delivery Systems for the Oral Administration of Antimicrobial Peptides: Promising Tools to Treat Infectious Diseases. Front. Med. Technol. 2021, 3, 778645. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, C.K.; Dibua, M.E.U. Anti-biofilm, drug delivery and cytotoxicity properties of dendrimers. ADMET DMPK 2024, 12, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Kawmudhi, P.A.S.; Chathurika, S.; Weerasinghe, L. Applications of antimicrobial peptides in plant pest and disease control. Discov. Plants 2025, 2, 55. [Google Scholar] [CrossRef]
- de la Lastra, J.M.P.; González-Acosta, S.; Otazo-Pérez, A.; Asensio-Calavia, P.; Rodríguez-Borges, V.M. Antimicrobial Peptides for Food Protection: Leveraging Edible Mushrooms and Nano-Innovation. Dietetics 2025, 4, 9. [Google Scholar] [CrossRef]
- Li, H.; Niu, J.; Wang, X.; Niu, M.; Liao, C. The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances. Pharmaceutics 2023, 15, 2278. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, K.A.; Mishra, N.; Rajinikanth, P.; Saraf, S.A. Antimicrobial peptides as antimicrobials for wound care management: A comprehensive review. J. Drug Deliv. Sci. Technol. 2024, 95, 105570. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef]
- Maouia, A.; Rebetz, J.; Kapur, R.; Semple, J.W. The Immune Nature of Platelets Revisited. Transfus. Med. Rev. 2020, 34, 209–220. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of Platelets, the Coagulation and Fibrinolytic Systems to Cutaneous Wound Healing. Thromb. Res. 2020, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chan, K.W.; Huang, H.; Wang, Z.; Fang, X.; Guo, C.; Li, F.; Zhang, L.; Yao, Y.; Chen, Z.; et al. Platelet-derived alpha-granules are associated with inflammation in patients with NK/T-cell lymphoma-associated hemophagocytic syndrome. Cytokine 2020, 126, 154878. [Google Scholar] [CrossRef]
- Muire, P.J.; Mangum, L.H.; Wenke, J.C. Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds. Front. Immunol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Cioce, A.; Cavani, A.; Cattani, C.; Scopelliti, F. Role of the Skin Immune System in Wound Healing. Cells 2024, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory Microenvironment of Skin Wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, L.; Colciago, A.; Castiglioni, S.; Maier, J.A. Platelets in Wound Healing: What Happens in Space? Front. Bioeng. Biotechnol. 2021, 9, 716184. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landen, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Land, W.G. Use of DAMPs and SAMPs as Therapeutic Targets or Therapeutics: A Note of Caution. Mol. Diagn. Ther. 2020, 24, 251–262. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage Polarization in Cardiac Tissue Repair Following Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.I.M.; Ahmad, H.; Abdul Rahman, M.B.; Chia, S.L.; Ng, S.-F.; Leong, S.W. Synthesis and in vitro biological evaluations of novel tetrapeptide as therapeutic agent for wound treatment. J. Saudi Chem. Soc. 2020, 24, 606–619. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Rahman, M.B.A.; Yusof, L.M.; Mustapha, N.M.; Ahmad, H. The Therapeutic Effect and In Vivo Assessment of Palmitoyl-GDPH on the Wound Healing Process. Pharmaceutics 2021, 13, 193. [Google Scholar] [CrossRef]
- Nizam, A.A.K.; Md Fadilah, N.I.; Ahmad, H.; Maarof, M.; Fauzi, M.B. Injectable Gelatin–Palmitoyl–GDPH Hydrogels as Bioinks for Future Cutaneous Regeneration: Physicochemical Characterization and Cytotoxicity Assessment. Polymers 2025, 17, 41. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Jimi, S.; Saparov, A.; Takagi, S. Editorial: Cellular and Molecular Mechanisms at the Proliferation Stage in Wound Healing: From Scarring to Tissue Regeneration. Front. Cell Dev. Biol. 2021, 9, 659089. [Google Scholar] [CrossRef]
- Wu, X.; He, W.; Mu, X.; Liu, Y.; Deng, J.; Liu, Y.; Nie, X. Macrophage polarization in diabetic wound healing. Burns Trauma 2022, 10, tkac051. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Wolny, D.; Stepanek, L.; Horakova, D.; Thomas, J.; Zapletalova, J.; Patel, M.S. Risk Factors for Non-Healing Wounds-A Single-Centre Study. J. Clin. Med. 2024, 13, 1003. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef] [PubMed]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef]
- Shen, S.; Miskolci, V.; Dewey, C.N.; Sauer, J.D.; Huttenlocher, A. Infection induced inflammation impairs wound healing through IL-1beta signaling. iScience 2024, 27, 109532. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; Ma, X.; Jiang, Z.; Huang, W.; Gao, C.; Pu, Y.; Luo, L.; Xu, Y.; Xu, Y. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Front. Pharmacol. 2021, 12, 653940. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Q.; Huang, X.; Feng, J.; Wang, Y.; Shao, T.; Deng, X.; Cao, Y.; Chen, X.; Zhou, M.; et al. Wounds under diabetic milieu: The role of immune cellar components and signaling pathways. Biomed. Pharmacother. 2023, 157, 114052. [Google Scholar] [CrossRef]
- Tan, J.L.; Lash, B.; Karami, R.; Nayer, B.; Lu, Y.Z.; Piotto, C.; Julier, Z.; Martino, M.M. Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun. Biol. 2021, 4, 422. [Google Scholar] [CrossRef]
- Versey, Z.; da Cruz Nizer, W.S.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Ren, Y.; Xu, L.; Wang, H.; Ling, X.; Jin, L.; Hu, Y.; Zhang, H.; Miao, C.; et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 2021, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Reno, F.; Pagano, C.A.; Bignotto, M.; Sabbatini, M. Neutrophil Heterogeneity in Wound Healing. Biomedicines 2025, 13, 694. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, X.; Jin, L.; Shan, S.; Yang, J.; Zhou, J. Recent advances in strategies to target the behavior of macrophages in wound healing. Biomed. Pharmacother. 2023, 165, 115199. [Google Scholar] [CrossRef]
- Nazari, M.; Taremi, S.; Elahi, R.; Mostanadi, P.; Esmeilzadeh, A. Therapeutic Properties of M2 Macrophages in Chronic Wounds: An Innovative Area of Biomaterial-Assisted M2 Macrophage Targeted Therapy. Stem Cell Rev. Rep. 2025, 21, 390–422. [Google Scholar] [CrossRef] [PubMed]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Pavlov, S.; Babenko, N.; Kumetchko, M.; Litvinova, O.; Valilshchykov, M. Features of cellular and molecular mechanisms of re-gulation of reparative processes in chronic wounds using photobiomodulation therapy. Folia Med. 2022, 64, 260–266. [Google Scholar] [CrossRef]
- Roy, R.; Zayas, J.; Mohamed, M.F.; Aboonabi, A.; Delgado, K.; Wallace, J.; Bayat, M.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. IL-10 Dysregulation Underlies Chemokine Insufficiency, Delayed Macrophage Response, and Impaired Healing in Diabetic Wounds. J. Invest. Dermatol. 2022, 142 Pt A, 692–704.e614. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabe-Garcia, A.; Nicolas, F.J. Role of TGF-beta in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating Inflammatory Markers in Wound Healing: Understanding Implications and Identifying Artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef]
- Wong, R.S.Y.; Tan, T.; Pang, A.S.-R.; Srinivasan, D.K. The role of cytokines in wound healing: From mechanistic insights to therapeutic applications. Explor. Immunol. 2025, 5, 1003183. [Google Scholar] [CrossRef]
- Las Heras, K.; Garcia-Orue, I.; Rancan, F.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Modulating the immune system towards a functional chronic wound healing: A biomaterials and Nanomedicine perspective. Adv. Drug Deliv. Rev. 2024, 210, 115342. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Nizam, N.A.A.K.; Fauzi, M.B. Antibacterial compounds-incorporated functional biomaterials for chronic wound healing application via 3D bioprinting: The mechanism of action. Int. J. Bioprinting 2024, 10, 3372. [Google Scholar] [CrossRef]
- Takahashi, M.; Umehara, Y.; Yue, H.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Ikutama, R.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The Antimicrobial Peptide Human beta-Defensin-3 Accelerates Wound Healing by Promoting Angiogenesis, Cell Migration, and Proliferation Through the FGFR/JAK2/STAT3 Signaling Pathway. Front. Immunol. 2021, 12, 712781. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Q.; Feng, J.; Wang, J.; Wang, Y.; Huang, X.; Shao, T.; Deng, X.; Cao, Y.; Zhou, M.; et al. Recent insights into the role of defensins in diabetic wound healing. Biomed. Pharmacother. 2022, 155, 113694. [Google Scholar] [CrossRef]
- Tan, Z.X.; Tao, R.; Li, S.C.; Shen, B.Z.; Meng, L.X.; Zhu, Z.Y. Role of defensins in diabetic wound healing. World J. Diabetes 2022, 13, 962–971. [Google Scholar] [CrossRef]
- Balaji, S.K.; Balasundarasekar, B.; Khuwaja, W.M.; Dolan, K.M.; Dong, X. Antimicrobial Peptide Signaling in Skin Diseases. JID Innov. 2025, 5, 100354. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xie, Y.; Zhong, J.; Fu, Z.; Wu, P.; Chen, X.; Xiao, Z.; Yuan, J.; Shi, X.; Liang, D. Antimicrobial peptides loaded collagen nanosheets with enhanced antibacterial activity, corneal wound healing and M1 macrophage polarization in bacterial keratitis. Compos. Part B Eng. 2024, 275, 111283. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, J.; Xing, J.; He, L.; Xu, C.; Wu, A.; Li, J. Unlocking the Potential of Antimicrobial Peptides: Cutting-Edge Advances and Therapeutic Potential in Combating Bacterial Keratitis. Bioconjug Chem. 2025, 36, 311–331. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.L.; Han, J.; Si, R.J.; Liu, P.P.; Zhang, Z.R.; Wang, A.M.; Zhang, J. Chitosan hydrogel encapsulated with LL-37 peptide promotes deep tissue injury healing in a mouse model. Mil. Med. Res. 2020, 7, 20. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Alessandrini, V.; Hardisty, G.; Melrose, L.; Jackson-Jones, L.; MacDonald, A.S.; Davidson, D.J.; Gwyer Findlay, E. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef]
- Silva, O.N.; Torres, M.D.T.; Cao, J.; Alves, E.S.F.; Rodrigues, L.V.; Resende, J.M.; Liao, L.M.; Porto, W.F.; Fensterseifer, I.C.M.; Lu, T.K.; et al. Repurposing a peptide toxin from wasp venom into antiinfectives with dual antimicrobial and immunomodulatory properties. Proc. Natl. Acad. Sci. USA 2020, 117, 26936–26945. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Y.; Fu, Z.; Ji, Y.; Yan, J.; Kong, Y.; Peng, Y.; Ru, Z.; Huang, Y.; Li, Y.; et al. The direct binding of bioactive peptide Andersonin-W1 to TLR4 expedites the healing of diabetic skin wounds. Cell Mol. Biol. Lett. 2024, 29, 24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.T.; Peng, G.; Trujillo-Paez, J.V.; Yue, H.; Ikutama, R.; Takahashi, M.; Umehara, Y.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The Antimicrobial Peptide AMP-IBP5 Suppresses Dermatitis-like Lesions in a Mouse Model of Atopic Dermatitis through the Low-Density Lipoprotein Receptor-Related Protein-1 Receptor. Int. J. Mol. Sci. 2023, 24, 5200. [Google Scholar] [CrossRef]
- Yue, H.; Song, P.; Sutthammikorn, N.; Umehara, Y.; Trujillo-Paez, J.V.; Nguyen, H.L.T.; Takahashi, M.; Peng, G.; Ikutama, R.; Okumura, K.; et al. Antimicrobial peptide derived from insulin-like growth factor-binding protein 5 improves diabetic wound healing. Wound Repair. Regen. 2022, 30, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Riool, M.; de Breij, A.; Kwakman, P.H.S.; Schonkeren-Ravensbergen, E.; de Boer, L.; Cordfunke, R.A.; Malanovic, N.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Thrombocidin-1-derived antimicrobial peptide TC19 combats superficial multi-drug resistant bacterial wound infections. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183282. [Google Scholar] [CrossRef]
- Ji, S.Y.; Lee, H.; Hwangbo, H.; Hong, S.H.; Cha, H.J.; Park, C.; Kim, D.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; et al. A Novel Peptide Oligomer of Bacitracin Induces M1 Macrophage Polarization by Facilitating Ca2+ Influx. Nutrients 2020, 12, 1603. [Google Scholar] [CrossRef]
- Buijs, N.P.; Vlaming, H.C.; Kotsogianni, I.; Arts, M.; Willemse, J.; Duan, Y.; Alexander, F.M.; Cochrane, S.A.; Schneider, T.; Martin, N.I. A classic antibiotic reimagined: Rationally designed bacitracin variants exhibit potent activity against vancomycin-resistant pathogens. Proc. Natl. Acad. Sci. USA 2024, 121, e2315310121. [Google Scholar] [CrossRef]
- Letafati, A.; Ardekani, O.S.; Naderisemiromi, M.; Norouzi, M.; Shafiei, M.; Nik, S.; Mozhgani, S.H. Unraveling the dynamic mechanisms of natural killer cells in viral infections: Insights and implications. Virol. J. 2024, 21, 18. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, L.; Jiang, J.; Sun, J.; Xue, L.; Ma, X.; Kuai, L.; Li, B.; Li, Y. M2 macrophage-polarized anti-inflammatory microneedle patch for accelerating biofilm-infected diabetic wound healing via modulating the insulin pathway. J. Nanobiotechnol. 2024, 22, 489. [Google Scholar] [CrossRef]
- Sobecki, M.; Krzywinska, E.; Nagarajan, S.; Audige, A.; Huynh, K.; Zacharjasz, J.; Debbache, J.; Kerdiles, Y.; Gotthardt, D.; Takeda, N.; et al. NK cells in hypoxic skin mediate a trade-off between wound healing and antibacterial defence. Nat. Commun. 2021, 12, 4700. [Google Scholar] [CrossRef] [PubMed]
- Adib, Y.; Bensussan, A.; Michel, L. Cutaneous Wound Healing: A Review about Innate Immune Response and Current Therapeutic Applications. Mediat. Inflamm. 2022, 2022, 5344085. [Google Scholar] [CrossRef]
- Sadeghi, M.; Moghaddam, A.; Amiri, A.M.; Charoghdoozi, K.; Mohammadi, M.; Dehnavi, S.; Orazizadeh, M. Improving the Wound Healing Process: Pivotal role of Mesenchymal stromal/stem Cells and Immune Cells. Stem Cell Rev. Rep. 2025, 21, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Adib, Y.; Boy, M.; Serror, K.; Dulphy, N.; des Courtils, C.; Duciel, L.; Boccara, D.; Mimoun, M.; Samardzic, M.; Bagot, M.; et al. Modulation of NK cell activation by exogenous calcium from alginate dressings in vitro. Front. Immunol. 2023, 14, 1141047. [Google Scholar] [CrossRef]
- Irina, A.; Rakityanskaya, T.S.R.A.A.K. Effects of Alloferon versus Valaciclovir for Treating Chronic Epstein-Barr Virus Infection. Explor. Res. Hypothesis Med. 2023, 8, 202–214. [Google Scholar] [CrossRef]
- Bacci, S. Fine Regulation During Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef]
- Ma, J.Y.; Shao, S.; Wang, G. Antimicrobial peptides: Bridging innate and adaptive immunity in the pathogenesis of psoriasis. Chin. Med J. 2020, 133, 2966–2975. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R.; et al. Significance of LL-37 on Immunomodulation and Disease Outcome. Biomed. Res. Int. 2020, 2020, 8349712. [Google Scholar] [CrossRef]
- Moreno-Angarita, A.; Aragon, C.C.; Tobon, G.J. Cathelicidin LL-37: A new important molecule in the pathophysiology of systemic lupus erythematosus. J. Transl. Autoimmun. 2020, 3, 100029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cao, L.; Melino, S.; Candi, E.; Wang, Y.; Shao, C.; Melino, G.; Shi, Y.; Chen, X. Orchestration of Mesenchymal Stem/Stromal Cells and Inflammation During Wound Healing. Stem Cells Transl. Med. 2023, 12, 576–587. [Google Scholar] [CrossRef]
- Gao, M.; Guo, H.; Dong, X.; Wang, Z.; Yang, Z.; Shang, Q.; Wang, Q. Regulation of inflammation during wound healing: The function of mesenchymal stem cells and strategies for therapeutic enhancement. Front. Pharmacol. 2024, 15, 1345779. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, X. IL-17 signaling in skin repair: Safeguarding metabolic adaptation of wound epithelial cells. Signal Transduct. Target. Ther. 2022, 7, 359. [Google Scholar] [CrossRef]
- Mu, X.; Gu, R.; Tang, M.; Wu, X.; He, W.; Nie, X. IL-17 in wound repair: Bridging acute and chronic responses. Cell Commun. Signal 2024, 22, 288. [Google Scholar] [CrossRef]
- Asif, F.; Zaman, S.U.; Arnab, K.H.; Hasan, M.; Islam, M. Antimicrobial peptides as therapeutics: Confronting delivery challenges to optimize efficacy. Microbe 2024, 2, 100051. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.; van den Munckhof, E.H.A.; de Kam, M.L.; Feiss, G.L.; Prens, E.P.; et al. Pharmacodynamic Effects of Topical Omiganan in Patients With Mild to Moderate Atopic Dermatitis in a Randomized, Placebo-Controlled, Phase II Trial. Clin. Transl. Sci. 2020, 13, 994–1003. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.; Yu, J.; Wen, L.; Chen, L.; An, H.; Xiao, W.; Zhang, B.; Feng, H.; Zhou, M.; et al. Potent synergy and sustained bactericidal activity of polymyxins combined with Gram-positive only class of antibiotics versus four Gram-negative bacteria. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 60. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Kang, C.; Syed, Y.Y. Bulevirtide: First Approval. Drugs 2020, 80, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, K.; Farasat, A.; Rostamian, M.; Johari, B.; Madanchi, H. Enfuvirtide, an HIV-1 fusion inhibitor peptide, can act as a potent SARS-CoV-2 fusion inhibitor: An in silico drug repurposing study. J. Biomol. Struct. Dyn. 2022, 40, 5566–5576. [Google Scholar] [CrossRef] [PubMed]
- Botelho Sampaio de Oliveira, K.; Lopes Leite, M.; Albuquerque Cunha, V.; Brito da Cunha, N.; Luiz Franco, O. Challenges and advances in antimicrobial peptide development. Drug Discov. Today 2023, 28, 103629. [Google Scholar] [CrossRef]
- Luo, X.; Chen, H.; Song, Y.; Qin, Z.; Xu, L.; He, N.; Tan, Y.; Dessie, W. Advancements, challenges and future perspectives on peptide-based drugs: Focus on antimicrobial peptides. Eur. J. Pharm. Sci. 2023, 181, 106363. [Google Scholar] [CrossRef] [PubMed]
- Mulukutla, A.; Shreshtha, R.; Kumar Deb, V.; Chatterjee, P.; Jain, U.; Chauhan, N. Recent advances in antimicrobial peptide-based therapy. Bioorg Chem. 2024, 145, 107151. [Google Scholar] [CrossRef]
- Gu, J.; Isozumi, N.; Yuan, S.; Jin, L.; Gao, B.; Ohki, S.; Zhu, S. Evolution-Based Protein Engineering for Antifungal Peptide Improvement. Mol. Biol. Evol. 2021, 38, 5175–5189. [Google Scholar] [CrossRef]
- Lu, J.; Xu, H.; Xia, J.; Ma, J.; Xu, J.; Li, Y.; Feng, J. D- and Unnatural Amino Acid Substituted Antimicrobial Peptides With Improved Proteolytic Resistance and Their Proteolytic Degradation Characteristics. Front. Microbiol. 2020, 11, 563030. [Google Scholar] [CrossRef]
- Mitra, S.; Chen, M.T.; Stedman, F.; Hernandez, J.; Kumble, G.; Kang, X.; Zhang, C.; Tang, G.; Reed, I.; Daugherty, I.Q.; et al. Cyclization of Two Antimicrobial Peptides Improves Their Activity. ACS Omega 2025, 10, 9728–9740. [Google Scholar] [CrossRef]
- Zhang, Q. Antimicrobial peptides: From discovery to developmental applications. Appl. Environ. Microbiol. 2025, 91, e0211524. [Google Scholar] [CrossRef]
- Fang, P.; Yu, S.; Ma, X.; Hou, L.; Li, T.; Gao, K.; Wang, Y.; Sun, Q.; Shang, L.; Liu, Q.; et al. Applications of tandem mass spectrometry (MS/MS) in antimicrobial peptides field: Current state and new applications. Heliyon 2024, 10, e28484. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Feng, Z.; Ma, Y. The Role and Mechanisms of Antimicrobial Peptides in Overcoming Multidrug-Resistant Bacteria Molecules. Molecules 2025, 30, 128. [Google Scholar] [CrossRef]
- Wang, C.; Hong, T.; Cui, P.; Wang, J.; Xia, J. Antimicrobial peptides towards clinical application: Delivery and formulation. Adv. Drug Deliv. Rev. 2021, 175, 113818. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.M.; Monges, B.E.D.; Oshiro, K.G.N.; Candido, E.S.; Pimentel, J.P.F.; Franco, O.L.; Cardoso, M.H. Advantages and Challenges of Using Antimicrobial Peptides in Synergism with Antibiotics for Treating Multidrug-Resistant Bacteria. ACS Infect. Dis. 2025, 11, 323–334. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial peptides: Opportunities and challenges in overcoming resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Singh, J.; Trivedi, R.; Ranade, P. Shaping the Future of Antimicrobial Therapy: Harnessing the Power of Antimicrobial Peptides in Biomedical Applications. J. Funct. Biomater. 2023, 14, 539. [Google Scholar] [CrossRef]
- Wan, F.; Wong, F.; Collins, J.J.; de la Fuente-Nunez, C. Machine learning for antimicrobial peptide identification and design. Nat. Rev. Bioeng. 2024, 2, 392–407. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Xue, Y.; Huang, Y.; Li, X.; Chen, X.; Xu, Y.; Zhang, D.; Zhang, P.; Zhao, J.; et al. Identification of potent antimicrobial peptides via a machine-learning pipeline that mines the entire space of peptide sequences. Nat. Biomed. Eng. 2023, 7, 797–810. [Google Scholar] [CrossRef]
- Maasch, J.; Torres, M.D.T.; Melo, M.C.R.; de la Fuente-Nunez, C. Molecular de-extinction of ancient antimicrobial peptides enabled by machine learning. Cell Host Microbe 2023, 31, 1260–1274.e6. [Google Scholar] [CrossRef]
- Yan, J.; Cai, J.; Zhang, B.; Wang, Y.; Wong, D.F.; Siu, S.W.I. Recent Progress in the Discovery and Design of Antimicrobial Peptides Using Traditional Machine Learning and Deep Learning. Antibiotics 2022, 11, 1451. [Google Scholar] [CrossRef]
- Capecchi, A.; Cai, X.; Personne, H.; Kohler, T.; van Delden, C.; Reymond, J.L. Machine learning designs non-hemolytic antimicrobial peptides. Chem. Sci. 2021, 12, 9221–9232. [Google Scholar] [CrossRef]
- Khabbaz, H.; Karimi-Jafari, M.H.; Saboury, A.A.; BabaAli, B. Prediction of antimicrobial peptides toxicity based on their physico-chemical properties using machine learning techniques. BMC Bioinform. 2021, 22, 549. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, H.; Gan, T.; Ma, X.; Geng, Q.; Yin, S.; Zhang, H.; Wu, Y. Smart Hydrogel Dressing Enhances the Healing of Chronic Infectious Diabetic Wounds Through Dual-Barrier Drug Delivery Action. Biomacromolecules 2024, 25, 6814–6829. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D. Rational Design of Peptide-Based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9, 770102. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New Smart Antimicrobial Hydrogels, Nanomaterials, and Coatings: Earlier Action, More Specific, Better Dosing? Adv. Healthc. Mater. 2021, 10, e2001199. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M.; Liu, F.; Liang, Z.; Hong, R.; Dong, Y.; Luan, H.; Fu, X.; Yuan, W.; Fang, W.; et al. Artificial intelligence using a latent diffusion model enables the generation of diverse and potent antimicrobial peptides. Sci. Adv. 2025, 11, eadp7171. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.A.; Liu, G.; Stokes, J.M.; de la Fuente-Nunez, C. AI Methods for Antimicrobial Peptides: Progress and Challenges. Microb. Biotechnol. 2025, 18, e70072. [Google Scholar] [CrossRef]
- Bakare, O.O.; Gokul, A.; Niekerk, L.A.; Aina, O.; Abiona, A.; Barker, A.M.; Basson, G.; Nkomo, M.; Otomo, L.; Keyster, M.; et al. Recent Progress in the Characterization, Synthesis, Delivery Procedures, Treatment Strategies, and Precision of Antimicrobial Peptides. Int. J. Mol. Sci. 2023, 24, 11864. [Google Scholar] [CrossRef]

| Antimicrobial Peptide | Antimicrobial Mechanisms | Immunomodulatory Function | References |
|---|---|---|---|
| Human β-defensins (hBDs) | Exhibit broad-spectrum antimicrobial activity against various pathogens (i.e., bacteria, fungi, and viruses) | Release pro-inflammatory cytokines and chemokines that attract immune cells such as neutrophils and macrophages to the wound site, modulate macrophage polarization, and suppress neutrophil apoptosis | [83,84,85,86] |
| Tet213-CN | Improves bacterial phagocytosis | Promotes M1 macrophage polarization, increases intracellular ROS generation and proinflammatory cytokine secretion, and modulates macrophage polarization | [87,88] |
| Human cathelicidins LL-37 | Directly kill bacteria, fungi, and viruses by disrupting their cell membranes | Regulate inflammation by balancing between pro-inflammatory and anti-inflammatory actions, modulate macrophage polarization, act as a chemoattractant to guide immune cells such as neutrophils to the wound site, stimulate cytokine production, bridge innate immunity and adaptive immunity, and suppress neutrophil apoptosis | [86,89,90] |
| Mast-MO | Compromises bacterial cell membrane integrity | Stimulates cytokine production, reduces excessive inflammation promotes a balanced immune response by suppressing pro-inflammatory factors (e.g., TNF-α and IL-6) | [91] |
| Andersonin-W1 (AW1) | Directly kills pathogens and prevents biofilm formation | Balances inflammation, enhances immune cell recruitment, promotes tissue repair, and modulates inflammation by macrophages via the TLR4/NF-κB molecular axis by directly binding to TLR4 in the macrophage extracellular region | [92] |
| Antimicrobial peptide derived from insulin-like growth factor-binding protein 5 (AMP-IBP5) | Exhibits broad-spectrum antimicrobial effects against Gram-positive and Gram-negative bacteria, kills antibiotic-resistant strains such as MRSA and Pseudomonas aeruginosa, and inhibits biofilm formation | Regulates inflammation and induces macrophage polarization | [93,94] |
| Human α-defensins | Directly kill or inhibit bacterial growth via membrane disruption and inhibition of bacterial cell wall synthesis | Act as chemokines to recruit cells such as neutrophils, eosinophils, mast cells, monocytes, and lymphocytes to the infection or wound site and suppress neutrophil apoptosis | [86,95] |
| Thrombocidin-1-derived peptide TC19 | Eradicates superficial multidrug-resistant bacterial strains, including Staphylococcus aureus and Acinetobacter baumannii | Induces neutrophil chemotaxis | [96] |
| Peptide-Based Antimicrobial Medication | Brand Name | Application | Target Species | References |
|---|---|---|---|---|
| Bacitracin | Neosporin | To treat skin infections | Mainly Gram-positive bacteria, including Staphylococcus, Streptococcus, Corynebacterium, Clostridium, and Actinomyces | [98] |
| Vancomycin | Firvanq, Vancocin | To treat serious Gram-positive infections, including MRSA infections | Staphylococcus aureus | [116] |
| Dalbavancin | Dalvance, Xydalba | To treat skin infections | Gram-positive pathogens, including Staphylococcus aureus (including MRSA), Streptococcus pyogenes, Streptococcus agalactiae, and Enterococcus faecalis (vancomycin-susceptible strains) | [116,119] |
| Polymyxin E | Colistin | To treat multidrug-resistant (MDR) Gram-negative bacterial infections | Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae | [116,120] |
| Bulevirtide | Hepcludex | To treat chronic Hepatitis D (HDV) infection in adults with compensated liver disease | Hepatitis D virus | [116,121] |
| Enfuvirtide | Fuzeon | An HIV-1 fusion inhibitor used in the treatment of human immunodeficiency virus (HIV) infection, primarily prescribed for patients who have developed resistance to other antiretroviral therapies (ART) | Human immunodeficiency virus (HIV) | [116,122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, S.B.; Maarof, M.; Fauzi, M.B.; Md Fadilah, N.I. Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense. Int. J. Mol. Sci. 2025, 26, 5920. https://doi.org/10.3390/ijms26135920
Adnan SB, Maarof M, Fauzi MB, Md Fadilah NI. Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense. International Journal of Molecular Sciences. 2025; 26(13):5920. https://doi.org/10.3390/ijms26135920
Chicago/Turabian StyleAdnan, Siti Balqis, Manira Maarof, Mh Busra Fauzi, and Nur Izzah Md Fadilah. 2025. "Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense" International Journal of Molecular Sciences 26, no. 13: 5920. https://doi.org/10.3390/ijms26135920
APA StyleAdnan, S. B., Maarof, M., Fauzi, M. B., & Md Fadilah, N. I. (2025). Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense. International Journal of Molecular Sciences, 26(13), 5920. https://doi.org/10.3390/ijms26135920









