LncRNA-Encoded Micropeptides: Expression Validation, Translational Mechanisms, and Roles in Cellular Metabolism
Abstract
1. Introduction
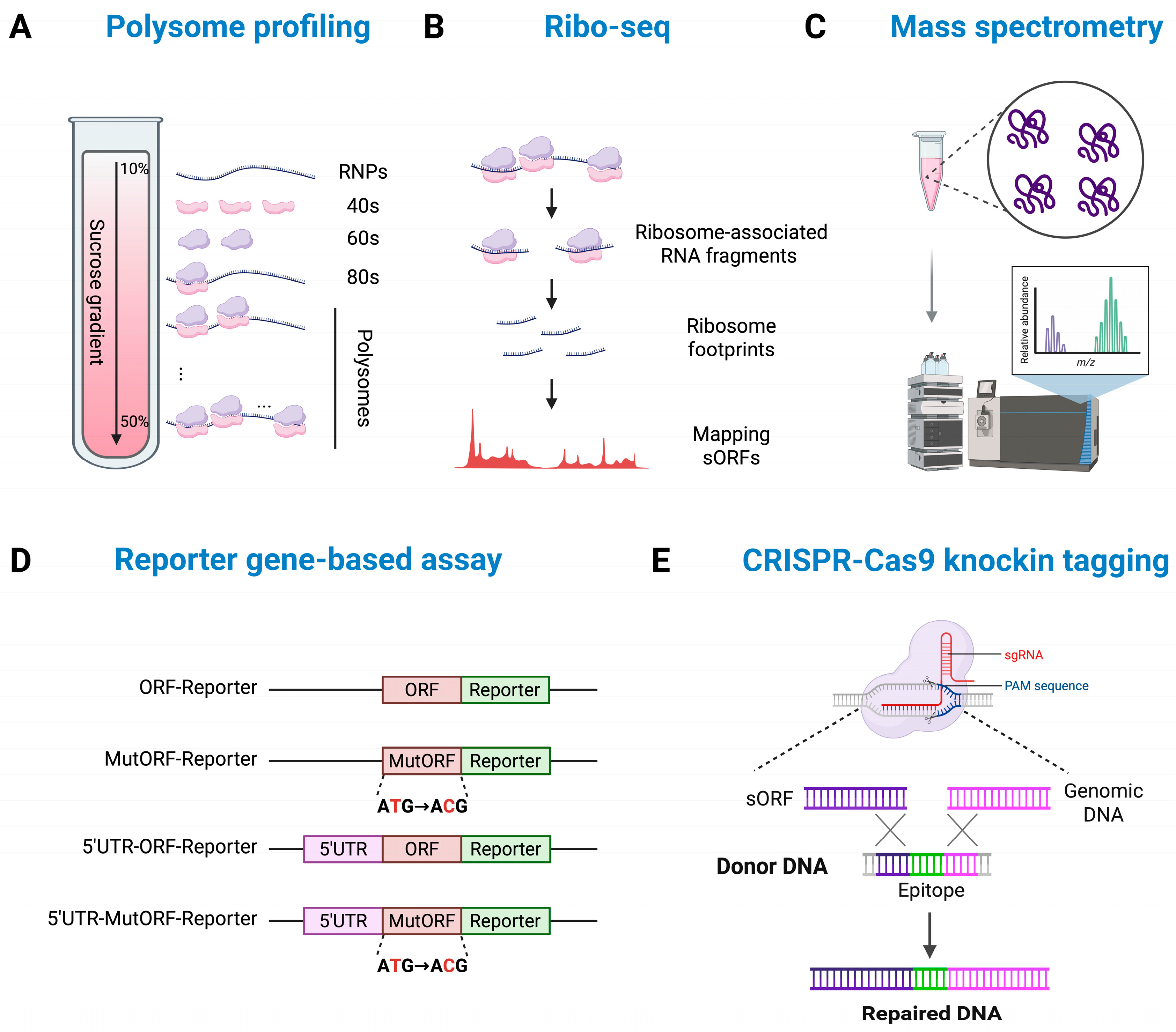
2. Methods for Expression and Functional Validation of lncRNA-Derived Micropeptides
2.1. Computational Approaches for Identifying Micropeptide-Encoding lncRNAs
| Tool | Function | Website | Ref. |
|---|---|---|---|
| CPC2 | Predicts the coding potential of input RNA sequences using a machine learning model. | https://cpc2.gao-lab.org/ (accessed on 17 June 2025) | [38] |
| CPAT | Evaluates coding potential based on ORF length, Fickett score, and codon usage bias. | https://code.google.com/archive/p/cpat/ (accessed on 17 June 2025) | [39] |
| ORF-RATER | Analyzes ribosome occupancy patterns from Ribo-seq data to assess the translation potential of ORFs. | https://github.com/alexfields/ORF-RATER (accessed on 17 June 2025) | [40] |
| RiboTISH | A Ribo-seq-based tool for identifying translation initiation sites (TISs), allowing precise localization of start codons. | https://github.com/zhpn1024/ribotish (accessed on 17 June 2025) | [41] |
| PhyloCSF | Predicts whether an sORF encodes a conserved protein by analyzing codon substitution patterns across multiple species. | https://github.com/mlin/PhyloCSF (accessed on 17 June 2025) | [42,44] |
| RNAcode | Identifies evolutionarily conserved coding regions by analyzing conserved codon patterns from multiple sequence alignments. | https://github.com/ViennaRNA/RNAcode (accessed on 17 June 2025) | [43] |
| PeptideAtlas | A mass spectrometry-based protein/peptide detection database that includes experimentally verified micropeptides. | https://peptideatlas.org/ (accessed on 17 June 2025) | [46] |
| OpenProt | A comprehensive database providing information on non-canonical ORFs (e.g., alternative ORFs, sORFs) and their potential protein products, extending beyond standard annotations. | https://www.openprot.org/ (accessed on 17 June 2025) | [47] |
2.2. In Vitro Validation of Micropeptide Expression
2.3. Molecular Approaches for Understanding Micropeptide Functions
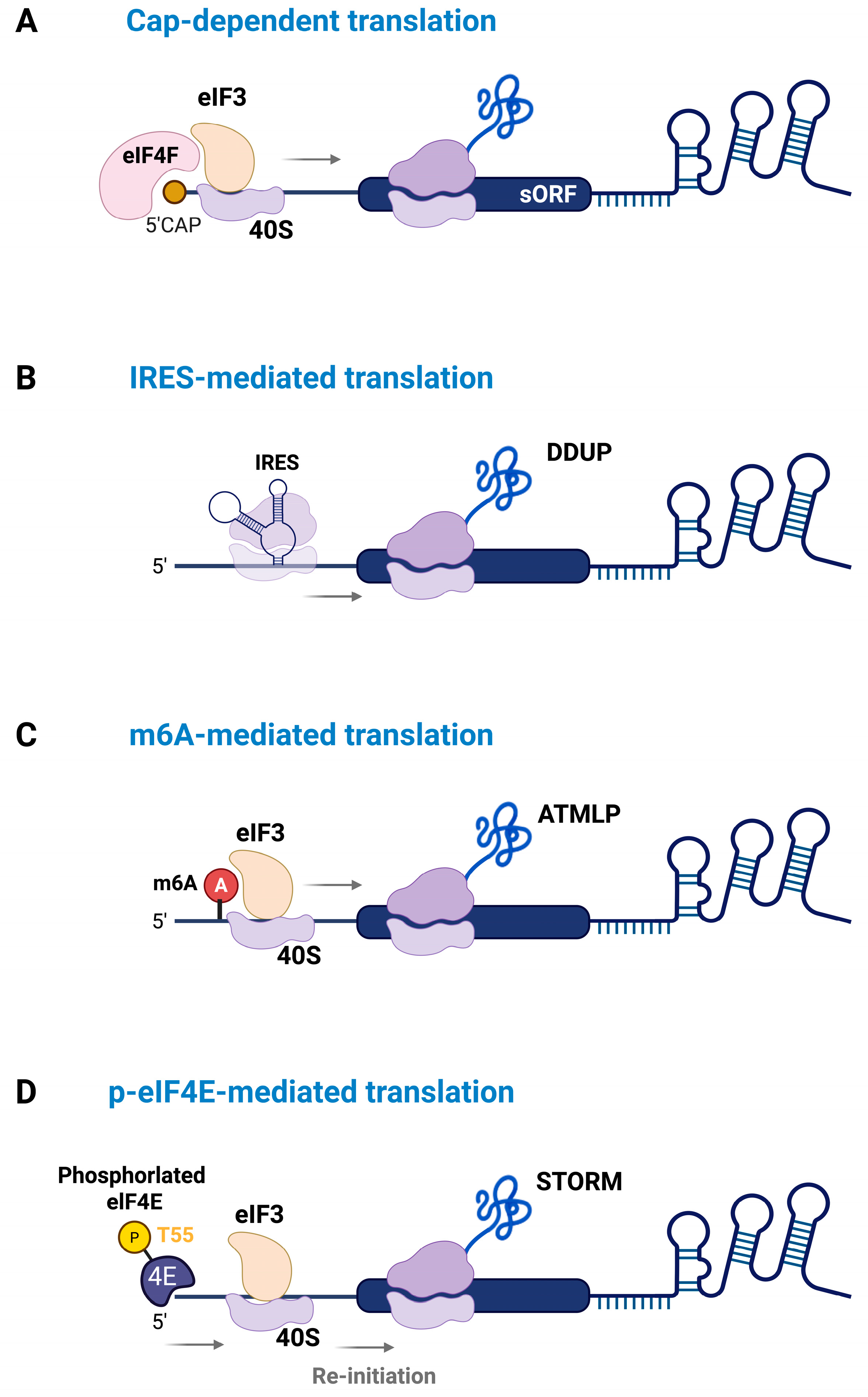
3. Mechanisms Regulating the Translation of lncRNA-Derived Micropeptides
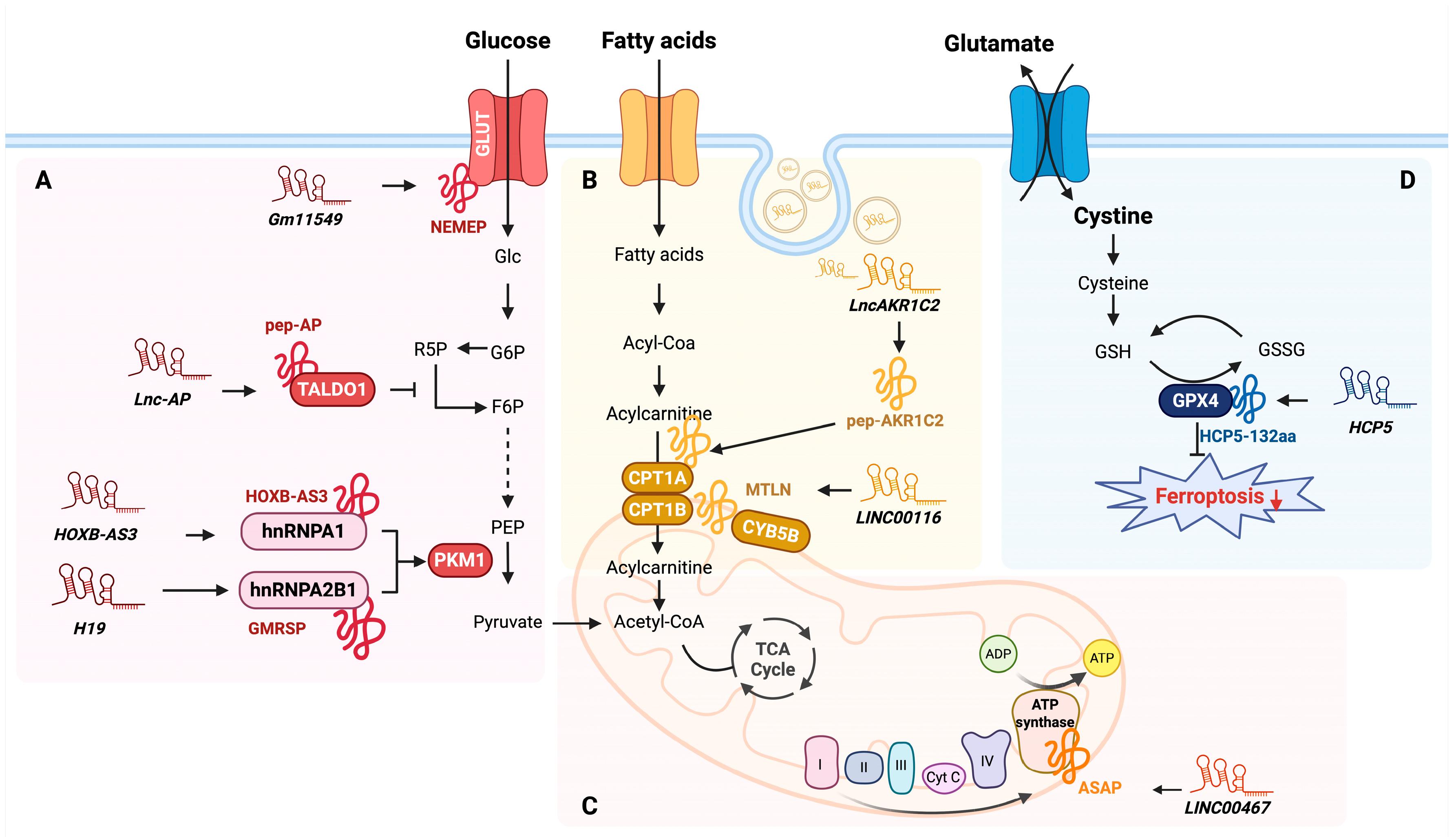
4. Regulatory Roles of lncRNA-Encoded Micropeptides in Cellular Metabolism
| No. | LncRNA | Micropeptide | Size (aa) | Metabolism | Interacting Protein | Cancer/Cell Type | Function and Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Gm11549 | NEMEP | 63 aa | Glucose metabolism | GLUT1/3 | Embryonic stem cells | Interacts with GLUT1 and GLUT3, enhancing glucose uptake. | [79] |
| 2 | HOXB-AS3 | HOXB-AS3 | 53 aa | Glucose metabolism | hnRNP A1 | Colorectal cancer | Suppresses PKM2 isoform by interacting with hnRNP A1, leading to a metabolic shift from glycolysis to oxidative phosphorylation. | [60] |
| 3 | H19 | GMRSP | 131 aa | Glucose metabolism | hnRNP A2B1 | Vascular smooth muscle cell | Interacts with hnRNP A2B1 to suppress PKM2 isoform, modulating metabolic flux toward oxidative phosphorylation. | [83] |
| 4 | Lnc-AP | pep-AP | 37 aa | Pentose phosphate pathway | TALDO1 | Colorectal cancer | Interacts with TALDO1, reducing NADPH and glutathione levels, inducing apoptosis, and enhancing chemotherapy sensitivity. | [85] |
| 5 | LINC00116 | MTLN | 56 aa | Fatty acid metabolism | CPT1B, CYB5B | Human Embryo Kidney cells | Interacts with CPT1B and CYB5B at the mitochondrial outer membrane, regulating fatty acid oxidation and lipid metabolic flux. | [89] |
| 6 | LncAKR1C2 | pep-AKR1C2 | 163 aa | Fatty acid metabolism | CPT1A | Gastric cancer | Activates YAP signaling, which increases CPT1A expression and promotes fatty acid oxidation, leading to higher ATP production and facilitating lymphatic metastasis. | [90] |
| 7 | LINC00467 | ASAP | 94 aa | Mitochondrial activity | ATP5A and ATP5C | Colorectal cancer | Enhances interaction between ATP5A and ATP5C, promoting ATP synthase activity and mitochondrial oxygen consumption. | [86] |
| 8 | HCP5 | HCP5-132aa | 132 aa | Glutathione metabolism (ferroptosis) | GPX4 | Triple-negative breast cancer | Regulates ferroptosis by modulating GPX4 expression, which increases ROS levels and lipid peroxidation. | [92] |
5. Conclusions
6. Perspectives
Funding
Conflicts of Interest
References
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Kapranov, P.; Drenkow, J.; Cheng, J.; Long, J.; Helt, G.; Dike, S.; Gingeras, T.R. Examples of the complex architecture of the human transcriptome revealed by RACE and high-density tiling arrays. Genome Res. 2005, 15, 987–997. [Google Scholar] [CrossRef]
- Kapranov, P.; Cheng, J.; Dike, S.; Nix, D.A.; Duttagupta, R.; Willingham, A.T.; Stadler, P.F.; Hertel, J.; Hackermüller, J.; Hofacker, I.L.; et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007, 316, 1484–1488. [Google Scholar] [CrossRef]
- Pinkney, H.R.; Ross, C.R.; Hodgson, T.O.; Pattison, S.T.; Diermeier, S.D. Discovery of prognostic lncRNAs in colorectal cancer using spatial transcriptomics. NPJ Precis. Oncol. 2024, 8, 230. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef]
- International Human Genome Sequencing, C. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Birney, E.; Stamatoyannopoulos, J.A.; Dutta, A.; Guigo, R.; Gingeras, T.R.; Margulies, E.H.; Weng, Z.; Snyder, M.; Dermitzakis, E.T.; ENCODE Project Consortium; et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007, 447, 799–816. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, W.; Zhao, Q.; Xiao, S.; Linghu, H.; Zhang, L.; Du, J.; Cui, H.; Yang, X.; Ling, S.; et al. Deep annotation of long noncoding RNAs by assembling RNA-seq and small RNA-seq data. J. Biol. Chem. 2023, 299, 105130. [Google Scholar] [CrossRef]
- Chiquitto, A.G.; Silva, L.O.L.; Oliveira, L.S.; Domingues, D.S.; Paschoal, A.R. Impact of sequencing technologies on long non-coding RNA computational identification. In Proceedings of the 2022 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Las Vegas, NV, USA, 6–8 December 2022; pp. 3299–3306. [Google Scholar]
- Wang, K.C.; Yang, Y.W.; Liu, B.; Sanyal, A.; Corces-Zimmerman, R.; Chen, Y.; Lajoie, B.R.; Protacio, A.; Flynn, R.A.; Gupta, R.A.; et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011, 472, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Zhang, L.; Min, K.-W.; Cho, J.-H.; Yeh, C.-C.; Moon, H.; Hormaechea-Agulla, D.; Mun, H.; Ko, S.; Lee, J.W.; et al. NEAT1 is essential for metabolic changes that promote breast cancer growth and metastasis. Cell Metab. 2021, 33, 2380–2397.e9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lin, L.; Luo, D.; Shi, L.; Chen, W.; Fan, H.; Li, Z.; Ma, X.; Ni, P.; Yang, L.; et al. Long noncoding RNA TRPM2-AS acts as a microRNA sponge of miR-612 to promote gastric cancer progression and radioresistance. Oncogenesis 2020, 9, 29. [Google Scholar] [CrossRef]
- Bazzini, A.A.; Johnstone, T.G.; Christiano, R.; MacKowiak, S.D.; Obermayer, B.; Fleming, E.S.; E Vejnar, C.; Lee, M.T.; Rajewsky, N.; Walther, T.C.; et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014, 33, 981–993. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Fickett, J.W. ORFs and Genes: How Strong a Connection? J. Comput. Biol. 1995, 2, 117–123. [Google Scholar] [CrossRef]
- Olexiouk, V.; Van Criekinge, W.; Menschaert, G. An update on sORFs.org: A repository of small ORFs identified by ribosome profiling. Nucleic Acids Res. 2017, 46, D497–D502. [Google Scholar] [CrossRef]
- Tharakan, R.; Kreimer, S.; Ubaida-Mohien, C.; Lavoie, J.; Olexiouk, V.; Menschaert, G.; Ingolia, N.T.; Cole, R.N.; Ishizuka, K.; Sawa, A.; et al. A methodology for discovering novel brain-relevant peptides: Combination of ribosome profiling and peptidomics. Neurosci. Res. 2020, 151, 31–37. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Hussmann, J.A.; Weissman, J.S. Ribosome Profiling: Global Views of Translation. Cold Spring Harb. Perspect. Biol. 2019, 11, a032698. [Google Scholar] [CrossRef]
- Stringer, A.; Smith, C.; Mangano, K.; Wade, J.T. Identification of novel translated small ORFs in Escherichia coli using complementary ribosome profiling approaches. J. Bacteriol. 2021, 204, Jb0035221. [Google Scholar] [CrossRef] [PubMed]
- Tomuro, K.; Mito, M.; Toh, H.; Kawamoto, N.; Miyake, T.; Chow, S.Y.A.; Doi, M.; Ikeuchi, Y.; Shichino, Y.; Iwasaki, S. Calibrated ribosome profiling assesses the dynamics of ribosomal flux on transcripts. Nat. Commun. 2024, 15, 7061. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef]
- Thompson, A.; Schäfer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Hamon, C. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef]
- Wu, Q.; Wright, M.; Gogol, M.M.; Bradford, W.D.; Zhang, N.; A Bazzini, A. Translation of small downstream ORFs enhances translation of canonical main open reading frames. EMBO J. 2020, 39, e104763. [Google Scholar] [CrossRef]
- Ruiz-Orera, J.; Messeguer, X.; Subirana, J.A.; Alba, M.M. Long non-coding RNAs as a source of new peptides. eLife 2014, 3, e03523. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Zhou, Y.; Wang, Q.; Zheng, Z.; Xu, B.; Wu, C.; Zhou, Q.; Hu, W.; Wu, C.; et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol. Cancer 2019, 18, 47. [Google Scholar] [CrossRef]
- Rodriguez, C.M.; Chun, S.Y.; Mills, R.E.; Todd, P.K. Translation of upstream open reading frames in a model of neuronal differentiation. BMC Genom. 2019, 20, 391. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.-B.; Jaffrey, S.R. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- King, H.A.; Cobbold, L.C.; Willis, A.E. The role of IRES trans-acting factors in regulating translation initiation. Biochem. Soc. Trans. 2010, 38, 1581–1586. [Google Scholar] [CrossRef]
- Min, K.W.; Davila, S.; Zealy, R.W.; Lloyd, L.T.; Lee, I.Y.; Lee, R.; Roh, K.H.; Jung, A.; Jemielity, J.; Choi, E.-J.; et al. eIF4E phosphorylation by MST1 reduces translation of a subset of mRNAs, but increases lncRNA translation. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 761–772. [Google Scholar] [CrossRef]
- Patraquim, P.; Magny, E.G.; Pueyo, J.I.; Platero, A.I.; Couso, J.P. Translation and natural selection of micropeptides from long non-canonical RNAs. Nat. Commun. 2022, 13, 6515. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.-P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef]
- Fields, A.P.; Rodriguez, E.H.; Jovanovic, M.; Stern-Ginossar, N.; Haas, B.J.; Mertins, P.; Raychowdhury, R.; Hacohen, N.; Carr, S.A.; Ingolia, N.T.; et al. A Regression-Based Analysis of Ribosome-Profiling Data Reveals a Conserved Complexity to Mammalian Translation. Mol. Cell 2015, 60, 816–827. [Google Scholar] [CrossRef]
- Zhang, P.; He, D.; Xu, Y.; Hou, J.; Pan, B.-F.; Wang, Y.; Liu, T.; Davis, C.M.; Ehli, E.A.; Tan, L.; et al. Genome-wide identification and differential analysis of translational initiation. Nat. Commun. 2017, 8, 1749. [Google Scholar] [CrossRef]
- Lin, M.F.; Jungreis, I.; Kellis, M. PhyloCSF: A comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics 2011, 27, i275–i282. [Google Scholar] [CrossRef] [PubMed]
- Washietl, S.; Findeiß, S.; Müller, S.A.; Kalkhof, S.; von Bergen, M.; Hofacker, I.L.; Stadler, P.F.; Goldman, N. RNAcode: Robust discrimination of coding and noncoding regions in comparative sequence data. Rna 2011, 17, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Pockrandt, C.; Steinegger, M.; Salzberg, S.L. PhyloCSF++: A fast and user-friendly implementation of PhyloCSF with annotation tools. Bioinformatics 2022, 38, 1440–1442. [Google Scholar] [CrossRef]
- Brito-Estrada, O.; Hassel, K.R.; Makarewich, C.A. An Integrated Approach for Microprotein Identification and Sequence Analysis. J. Vis. Exp. 2022, 185. [Google Scholar] [CrossRef]
- Desiere, F.; Deutsch, E.W.; King, N.L.; Nesvizhskii, A.I.; Mallick, P.; Eng, J.; Chen, S.; Eddes, J.; Loevenich, S.N.; Aebersold, R. The PeptideAtlas project. Nucleic Acids Res. 2006, 34, D655–D658. [Google Scholar] [CrossRef]
- Leblanc, S.; Yala, F.; Provencher, N.; Lucier, J.-F.; Levesque, M.; Lapointe, X.; Jacques, J.-F.; Fournier, I.; Salzet, M.; Ouangraoua, A.; et al. OpenProt 2.0 builds a path to the functional characterization of alternative proteins. Nucleic Acids Res. 2023, 52, D522–D528. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Brar, G.A.; Rouskin, S.; McGeachy, A.M.; Weissman, J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 2012, 7, 1534–1550. [Google Scholar] [CrossRef]
- Ingolia, N.T. Ribosome profiling: New views of translation, from single codons to genome scale. Nat. Rev. Genet. 2014, 15, 205–213. [Google Scholar] [CrossRef]
- Chassé, H.; Boulben, S.; Costache, V.; Cormier, P.; Morales, J. Analysis of translation using polysome profiling. Nucleic Acids Res. 2017, 45, e15. [Google Scholar] [CrossRef]
- Slavoff, S.A.; Mitchell, A.J.; Schwaid, A.G.; Cabili, M.N.; Ma, J.; Levin, J.Z.; Karger, A.D.; A Budnik, B.; Rinn, J.L.; Saghatelian, A. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat. Chem. Biol. 2013, 9, 59–64. [Google Scholar] [CrossRef]
- Peng, M.; Zhou, Y.; Wan, C. Identification of phosphorylated small ORF-encoded peptides in Hep3B cells by LC/MS/MS. J. Proteom. 2024, 303, 105214. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, E.; Tang, Y.; Cai, T.; Zhang, L.; Wang, J.; Hao, Y.; Zhang, B.; Zhou, Y.; Guo, X.; et al. Deeply Mining a Universe of Peptides Encoded by Long Noncoding RNAs. Mol. Cell Proteom. 2021, 20, 100109. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Anderson, K.M.; Chang, C.-L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, J.; Han, L.; Qi, H.; Wang, Y.; Wang, H.; Chen, S.; Du, L.; Li, S.; Zhang, Y.; et al. The micropeptide LEMP plays an evolutionarily conserved role in myogenesis. Cell Death Dis. 2020, 11, 357. [Google Scholar] [CrossRef]
- Guo, B.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Wang, Y.; Zhang, S.; Wu, R.; Lu, J.; et al. Micropeptide CIP2A-BP encoded by LINC00665 inhibits triple-negative breast cancer progression. EMBO J. 2020, 39, e102190. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Xiao, M.-H.; Chen, H.-X.; Meng, Y.; Zhao, N.; Yang, L.; Tang, H.; Wang, J.-L.; Liu, X.; Zhu, Y.; et al. A novel mitochondrial micropeptide MPM enhances mitochondrial respiratory activity and promotes myogenic differentiation. Cell Death Dis. 2019, 10, 528. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, Z.; Wang, W.; Liu, Y.; Zhu, H.; Liang, H.; Zhang, B.; Chen, X. A micropeptide JunBP regulated by TGF-β promotes hepatocellular carcinoma metastasis. Oncogene 2023, 42, 113–123. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Huang, J.Z.; Chen, M.; Chen, D.; Gao, X.-C.; Zhu, S.; Huang, H.; Hu, M.; Zhu, H.; Yan, G.-R. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol. Cell 2017, 68, 171–184.e176. [Google Scholar] [CrossRef]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K.I.; Clohessy, J.G.; Pandolfi, P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017, 541, 228–232. [Google Scholar] [CrossRef]
- Lin, J.S.; Lai, E.M. Protein-Protein Interactions: Co-Immunoprecipitation. Methods Mol. Biol. 2017, 1615, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, S.; Lu, S.; Gong, J.; Wang, L.; Ding, L.; Chen, Q.; Liu, W. The development of proximity labeling technology and its applications in mammals, plants, and microorganisms. Cell Commun. Signal. 2023, 21, 269. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Martell, J.D.; Kamer, K.J.; Deerinck, T.J.; Ellisman, M.H.; Mootha, V.K.; Ting, A.Y. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 2015, 12, 51–54. [Google Scholar] [CrossRef]
- Branon, T.C.; Bosch, J.A.; Sanchez, A.D.; Udeshi, N.D.; Svinkina, T.; Carr, S.A.; Feldman, J.L.; Perrimon, N.; Ting, A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018, 36, 880–887. [Google Scholar] [CrossRef]
- Chu, Q.; Rathore, A.; Diedrich, J.K.; Donaldson, C.J.; Yates, J.R.; Saghatelian, A. Identification of Microprotein-Protein Interactions via APEX Tagging. Biochemistry 2017, 56, 3299–3306. [Google Scholar] [CrossRef]
- Stoneley, M.; Willis, A.E. Cellular internal ribosome entry segments: Structures, trans-acting factors and regulation of gene expression. Oncogene 2004, 23, 3200–3207. [Google Scholar] [CrossRef]
- Spriggs, K.A.; Bushell, M.; Mitchell, S.A.; Willis, A.E. Internal ribosome entry segment-mediated translation during apoptosis: The role of IRES-trans-acting factors. Cell Death Differ. 2005, 12, 585–591. [Google Scholar] [CrossRef]
- Godet, A.C.; David, F.; Hantelys, F.; Tatin, F.; Lacazette, E.; Garmy-Susini, B.; Prats, A.-C. IRES Trans-Acting Factors, Key Actors of the Stress Response. Int. J. Mol. Sci. 2019, 20, 924. [Google Scholar] [CrossRef]
- Yu, R.; Hu, Y.; Zhang, S.; Li, X.; Tang, M.; Yang, M.; Wu, X.; Li, Z.; Liao, X.; Xu, Y.; et al. LncRNA CTBP1-DT-encoded microprotein DDUP sustains DNA damage response signalling to trigger dual DNA repair mechanisms. Nucleic Acids Res. 2022, 50, 8060–8079. [Google Scholar] [CrossRef]
- Pei, H.; Dai, Y.; Yu, Y.; Tang, J.; Cao, Z.; Zhang, Y.; Li, B.; Nie, J.; Hei, T.K.; Zhou, G. The Tumorigenic Effect of lncRNA AFAP1-AS1 is Mediated by Translated Peptide ATMLP Under the Control of m(6) A Methylation. Adv. Sci. 2023, 10, e2300314. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, L.; Deng, J.; Guo, B.; Li, F.; Wang, Y.; Wu, R.; Zhang, S.; Lu, J.; Zhou, Y. A Novel Micropeptide Encoded by Y-Linked LINC00278 Links Cigarette Smoking and AR Signaling in Male Esophageal Squamous Cell Carcinoma. Cancer Res. 2020, 80, 2790–2803. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17, 651–662. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, K.; Yu, H.; Zhang, L.; Xu, Y.; Chen, L.; Sun, Z.; Zhu, Y.; Zhang, C.; Qian, Y.; et al. Metabolic remodelling during early mouse embryo development. Nat. Metab. 2021, 3, 1372–1384. [Google Scholar] [CrossRef]
- Agostini, M.; Romeo, F.; Inoue, S.; Niklison-Chirou, M.V.; Elia, A.J.; Dinsdale, D.; Morone, N.; Knight, R.A.; Mak, T.W.; Melino, G. Metabolic reprogramming during neuronal differentiation. Cell Death Differ. 2016, 23, 1502–1514. [Google Scholar] [CrossRef]
- Pantaleon, M.; Kaye, P.L. Glucose transporters in preimplantation development. Rev. Reprod. 1998, 3, 77–81. [Google Scholar] [CrossRef]
- Fu, H.; Wang, T.; Kong, X.; Yan, K.; Yang, Y.; Cao, J.; Yuan, Y.; Wang, N.; Kee, K.; Lu, Z.J.; et al. A Nodal enhanced micropeptide NEMEP regulates glucose uptake during mesendoderm differentiation of embryonic stem cells. Nat. Commun. 2022, 13, 3984. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Tamada, M.; Suematsu, M.; Saya, H. Pyruvate kinase M2: Multiple faces for conferring benefits on cancer cells. Clin. Cancer Res. 2012, 18, 5554–5561. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Yang, F.; Sun, Y.; Chen, J.; Liu, J.; Sun, T.; Fan, R.; Pei, F.; Luo, S.; et al. GMRSP encoded by lncRNA H19 regulates metabolic reprogramming and alleviates aortic dissection. Nat. Commun. 2025, 16, 1719. [Google Scholar] [CrossRef] [PubMed]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Yin, S.; Yang, Y.; Yang, H.; Yang, J.; Zhou, Z.; Li, S.; Ying, G.; Ba, Y. lncRNA-encoded pep-AP attenuates the pentose phosphate pathway and sensitizes colorectal cancer cells to Oxaliplatin. EMBO Rep. 2022, 23, e53140. [Google Scholar] [CrossRef]
- Ge, Q.; Jia, D.; Cen, D.; Qi, Y.; Shi, C.; Li, J.; Sang, L.; Yang, L.-J.; He, J.; Lin, A.; et al. Micropeptide ASAP encoded by LINC00467 promotes colorectal cancer progression by directly modulating ATP synthase activity. J. Clin. Invest. 2021, 131, e152911. [Google Scholar] [CrossRef]
- Makarewich, C.A.; Baskin, K.K.; Munir, A.Z.; Bezprozvannaya, S.; Sharma, G.; Khemtong, C.; Shah, A.M.; McAnally, J.R.; Malloy, C.R.; Szweda, L.I.; et al. MOXI Is a Mitochondrial Micropeptide That Enhances Fatty Acid β-Oxidation. Cell Rep. 2018, 23, 3701–3709. [Google Scholar] [CrossRef]
- Stein, C.S.; Jadiya, P.; Zhang, X.; McLendon, J.M.; Abouassaly, G.M.; Witmer, N.H.; Anderson, E.J.; Elrod, J.W.; Boudreau, R.L. Mitoregulin: A lncRNA-Encoded Microprotein that Supports Mitochondrial Supercomplexes and Respiratory Efficiency. Cell Rep. 2018, 23, 3710–3720.e3718. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Fidelito, G.; Robinson, D.R.; Liang, C.; Lim, R.; Bichler, Z.; Guo, R.; Wu, G.; Xu, H.; et al. LINC00116-encoded microprotein mitoregulin regulates fatty acid metabolism at the mitochondrial outer membrane. iScience 2023, 26, 107558. [Google Scholar] [CrossRef]
- Zhu, K.G.; Yang, J.; Zhu, Y.; Zhu, Q.; Pan, W.; Deng, S.; He, Y.; Zuo, D.; Wang, P.; Han, Y.; et al. The microprotein encoded by exosomal lncAKR1C2 promotes gastric cancer lymph node metastasis by regulating fatty acid metabolism. Cell Death Dis. 2023, 14, 708. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Tong, X.; Yu, Z.; Xing, J.; Liu, H.; Zhou, S.; Huang, Y.; Lin, J.; Jiang, W.; Wang, L. LncRNA HCP5-Encoded Protein Regulates Ferroptosis to Promote the Progression of Triple-Negative Breast Cancer. Cancers 2023, 15, 1880. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Wegener, M.; Müller-McNicoll, M. Nuclear retention of mRNAs—Quality control, gene regulation and human disease. Semin. Cell Dev. Biol. 2018, 79, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F. Nonsense-Mediated mRNA Decay, a Finely Regulated Mechanism. Biomedicines 2022, 10, 141. [Google Scholar] [CrossRef]
- Miyagi, M.; Yamaji, M.; Kurokawa, N.; Yohda, M.; Kawano, R. Redesign of Translocon EXP2 Nanopore for Detecting Peptide Fragments. Small Methods 2025, 9, e2401562. [Google Scholar] [CrossRef]
- Huang, G.; Voet, A.; Maglia, G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat. Commun. 2019, 10, 835. [Google Scholar] [CrossRef]
- Iesu, L.; Sai, M.; Torbeev, V.; Kieffer, B.; Pelta, J.; Cressiot, B. Single-molecule nanopore sensing of proline cis/trans amide isomers. Chem. Sci. 2025, 16, 9730–9738. [Google Scholar] [CrossRef]
- Stickels, R.R.; Murray, E.; Kumar, P.; Li, J.; Marshall, J.L.; Di Bella, D.J.; Arlotta, P.; Macosko, E.Z.; Chen, F. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat. Biotechnol. 2021, 39, 313–319. [Google Scholar] [CrossRef]
- Mund, A.; Coscia, F.; Kriston, A.; Hollandi, R.; Kovács, F.; Brunner, A.-D.; Migh, E.; Schweizer, L.; Santos, A.; Bzorek, M.; et al. Deep Visual Proteomics defines single-cell identity and heterogeneity. Nat. Biotechnol. 2022, 40, 1231–1240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.W.; Lee, J.W.; Shin, C.H.; Min, K.-W. LncRNA-Encoded Micropeptides: Expression Validation, Translational Mechanisms, and Roles in Cellular Metabolism. Int. J. Mol. Sci. 2025, 26, 5913. https://doi.org/10.3390/ijms26125913
Ho CW, Lee JW, Shin CH, Min K-W. LncRNA-Encoded Micropeptides: Expression Validation, Translational Mechanisms, and Roles in Cellular Metabolism. International Journal of Molecular Sciences. 2025; 26(12):5913. https://doi.org/10.3390/ijms26125913
Chicago/Turabian StyleHo, Chul Woong, Ji Won Lee, Chang Hoon Shin, and Kyung-Won Min. 2025. "LncRNA-Encoded Micropeptides: Expression Validation, Translational Mechanisms, and Roles in Cellular Metabolism" International Journal of Molecular Sciences 26, no. 12: 5913. https://doi.org/10.3390/ijms26125913
APA StyleHo, C. W., Lee, J. W., Shin, C. H., & Min, K.-W. (2025). LncRNA-Encoded Micropeptides: Expression Validation, Translational Mechanisms, and Roles in Cellular Metabolism. International Journal of Molecular Sciences, 26(12), 5913. https://doi.org/10.3390/ijms26125913








