A Bridge Too Far? Towards Medical Therapy for Clinically Nonfunctioning Pituitary Tumors
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. CNFPTs Represent a Heterogenous Group of Pituitary Tumors
3.1.1. Gonadotroph Tumors
3.1.2. Silent Corticotroph Tumors
3.1.3. Silent Somatotroph Tumors
3.1.4. Silent Thyrotroph Tumors
3.1.5. Silent Lactotroph Tumors
3.1.6. Null Cell Tumors
3.2. Current Management of CNFPTs
3.3. Current Role of Medical Therapy in CNFPTs
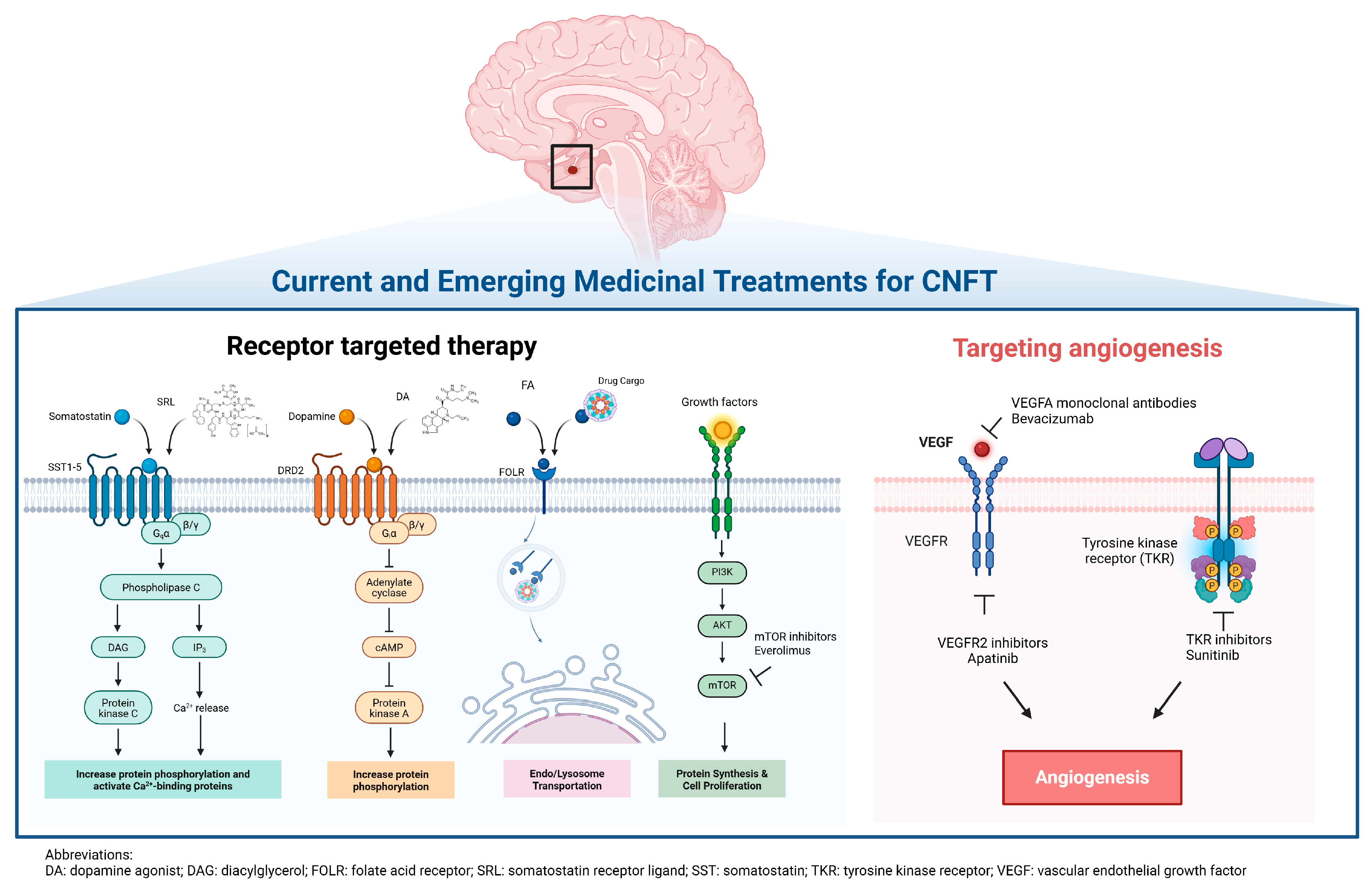
3.3.1. Somatostatin Receptor Ligands
| First Author (Year) Reference | Number of Treated Patients with CNFTs | Medication | Dose | Duration of Therapy (Mths) | Number of Patients (%), [Median Change (IQR)] Exhibiting Tumor Shrinkage; Measurement Method | Number of Patients (%) Exhibiting Stable Tumor Size | Number of Patients (%), [Median Change (IQR)] Exhibiting Tumor Growth; Measurement Method |
|---|---|---|---|---|---|---|---|
| Katznelson (1992) [31] | 6 | Octreotide SC | 50 µg BID–250 µg TID | 2 | 2, [insuff]; V (%) | 4 | 0, [NA]; V (%) |
| Gasperi (1993) [39] | 8 | Octreotide SC | 100 µg TID | 3–6 | 3, [−5 (insuff)]; D (mm) | 1 | 4, [1.5 (1–2)]; D (mm) |
| Merola (1993) [40] | 19 | Octreotide SC | 150–300 µg QD | 1–12 | 15, [−12 (−6–−30)]; A (mm2) | 0 | 4, [5 (2–14)]; A (mm2) |
| Plockinger (1994) [42] | 14 | Octreotide SC | 300–1500 µg QD | 3 | 2, [−76.5 (insuff)]; V (%) | 12 | 0, [NA]; V (%) |
| Warnet (1997) [43] | 7 with imaging before and after treatment | Octreotide SC | 100 µg QD–200 µg TID | 2 | 3, [−38 (insuff)]; V (%) | 1 | 3, [48 (insuff)]; V (%) |
| Colao (1999) [41] | 9 | Octreotide SC | 50 µg TID–600 µg QD | 6–12 | 3, [insuff]; V (%) | insuff | insuff, [insuff]; V (%) |
| Fusco (2012) [5] | 26 | Octreotide LAR | 20 mg Q1M | 37 ± 18 | 0, [NA]; D (mm) | 21 | 5, [insuff]; D (mm) |
| Boertien (2024) [45] | Lanreotide (n = 22) Placebo (n = 22) | Lanreotide LAR SC | 120 mg Q28D | 18 | Lanreotide 3/22 (14%), [insuff]; D (mm), V (mm3) Placebo 0/22 (0%), [NA]; D (mm), V (mm3) | Lanreotide 8/22 (36%), [insuff]; D (mm), V (mm3) Placebo 14/22 (64%), [insuff]; D (mm), V (mm3) | Lanreotide 11/22 (50%), [insuff]; D (mm), V (mm3) Placebo 8/22 (36%), [insuff]; D (mm), V (mm3) |
| Total Number (%) | 111 | 31 (28%) | insuff | insuff | |||
3.3.2. Dopamine Agonist Therapy
| First Author (Year) Reference | Number of Treated Patients with CNFTs | Medication | Dose | Duration of Therapy (Mths) | Number of Patients (%), [Median Change (IQR)] Exhibiting Tumor Shrinkage; Measurement Method | Number of Patients (%) Exhibiting Stable Tumor Size | Number of Patients (%), [Median Change (IQR)] Exhibiting Tumor Growth; Measurement Method |
|---|---|---|---|---|---|---|---|
| Wollesen (1982) [53] | 11 | BCP | 15–60 mg/d | 2–33 | 9, [−32 (−21.5–−41.5)]; A (%) | 0 | 2, [14 (insuff)]; A (%) |
| Barrow (1984) [54] | 7 | BCP | 2.5–7.5 mg/d | 1.5 | 1, [−2.1 (insuff)]; A (cm2) | 5 | 1, [0.1 (insuff)]; A (cm2) |
| Pullan (1985) [55] | 5 | BCP | 15–37.5 mg/d | 3–27 | 1, [insuff]; NA | 4 | 0 [NA]; NA |
| Verde (1985) [56] | 20 | BCP | 7.5–20 mg/d | 1–32 | 1, [insuff]; NA | 19 | 0 [NA]; NA |
| Zarate (1985) [57] | 7 | BCP | 15–22.5 mg/d | 0.5–12 | 0, [NA]; NA | 7 | 0 [NA]; NA |
| van Schaarde-nburg (1989) [58] | 25 (11/25 had imaging) | BCP | 5–22.5 mg/d | 1–73 | 2, [insuff]; NA | 7 | 2 [insuff]; NA |
| Ferone (1998) [60] | 6 | QUI | 300–600 µg/d | 6–12 | 2, [insuff]; NA | 4 | 0 [NA]; NA |
| Colao (2000) [65] | 10 | QUI CBG | 600 µg/d (QUI) 3 mg/w (CBG) | 12 | 2, [insuff]; V (%) | 8 | 0 [NA]; V (%) |
| Nobels (2000) [59] | 10 | QUI | 300 µg/d | 36–93 | 2, [−1.8 (insuff)]; V (cm3) [−37.3% (insuff)]; V (%) | 0 | 8, [2.3 cm3 (1.1–3.5)]; V (cm3) [31.3% (13.1–54.8)]; V (%) |
| Lohmann (2001) [61] | 13 | CBG | 0.25–1.0 mg/w | 12 | 7, [−15 (−12–−18)]; V (%) | 5 | 1, [25 (insuff)]; V (%) |
| Pivonello (2004) [50] | 9 | CBG | 1–3 mg/w | 12 | 5, [−49.3 (−38.9–−57.2)]; V (%) | 0 | 4, [8.7 (6.2–29.5)]; V (%) |
| Greenman (2005) [62] | Preventative (I): n = 20 Secondary (II): n = 13 Control I (CI): n = 47 Control II (CII): n = 38 | BCP QUI CBG | 10 mg QD (BCP) 300 mg QD (QUI) 1.5 mg/week (CBG) | 40 ± 48 | I: 9/20 (45%), [insuff]; D (mm) II: 2/13 (15%), [insuff]; D (mm) CI: 0 (0%), [NA]; D (mm) CII: 0 (0%), [NA]; D (mm) | I: 9/20 (45%), [insuff]; D (mm) II: 6/13 (46%), [insuff]; D (mm) CI: 18/47 (38%), [insuff]; D (mm) CII: 18/38 (47%), [insuff]; D (mm) | I: 2/20 (10%), [insuff]; D (mm) II: 5/13 (39%), [insuff]; D (mm) CI: 29/47 (62%), [insuff]; D (mm) CII: 20/38 (53%), [insuff]; D (mm) |
| Garcia (2013) [64] | 19 | CBG | 2 mg/w | 6 | 9, [−26.7 (−16.7–−43.4); V (%) | 6 | 4, [14.8 (12.5–74.4)]; V (%) |
| Vieira Neto (2015) [66] | 9 | CBG | 3 mg/w | 6 | 8, [−29.2 (−18.9–−39.5)]; V (%) by 3D [−17.3 (−9.6–−30.9)]; V (%) by Di Chiro and Nelson | 0 | 1, [2.6 (insuff)]; V (%) by 3D [0.2 (insuff)]; V (%) by Di Chiro and Nelson |
| Greenman (2016) [6] | Preventative (P) (n = 55) Remedial (R) (n = 24) Control (C) (n = 60) | CBG BCP | 10 mg QD (BCP) 2 mg/w (CBG) | 105.6 ± 78 | P: 21/55 (38%), [insuff]; D (mm) R: 7/24 (29%), [insuff]; D (mm) C: 0/60 (0%), [NA]; D (mm) | P: 27/55 (49%), [insuff]; D (mm) R: 7/24 (29%), [insuff]; D (mm) C: 28/60 (47%), [insuff]; D (mm) | P: 7/55 (13%), [insuff]; D (mm) R: 10/24 (42%), [insuff]; D (mm) C: 32/60 (53%), [insuff]; D (mm) |
| Batista (2019) [63] | Medical Therapy (MT) (n = 59) Control (C) (n = 57) | CBG | 0.5 mg/d | 24 | MT: 17/59 (29%), [insuff]; V (%) C: 6/57 (11%), [insuff]; V (%) | MT: 39/59 (66%), [insuff]; V (%) C: 42/57 (74%), [insuff]; V (%) | MT: 3/59 (5%), [insuff]; V (%) C: 9/57 (16%), [insuff]; V (%) |
| Iglesias (2022) [67] | Medical Therapy (CAB) (n = 22) Observation (OBS) (n = 40) | CBG | 0.5–1.5 mg/w | 13 (10.5–17) | CAB: 3/22 (14%), [insuff]; D (mm) OBS: 2/40 (5%), [insuff]; D (mm) | CAB: 17/22 (77%), [insuff]; D (mm) OBS: 32/40 (80%), [insuff]; D (mm) | CAB: 2/22 (9%), [insuff]; D (mm) OBS: 6/40 (15%), [insuff]; D (mm) |
| Ayalon-Dangur (2024) [68] | 25 | CBG | ≥ 1 mg/w | 24 | 5, [6 (−2–−75)]; D (mm) | 12 | 8, [4 (2.5–8)]; D (mm) |
| Total Number (%) | 355 | 113 (33%) | 182 (51%) | 60 (17%) | |||
3.3.3. GnRH Analog Therapy
3.3.4. Peptide Receptor Radionuclide Therapy and Temozolomide
3.4. Emerging Experimental Novel Treatment Modalities of CNFPT
3.4.1. Immune Checkpoint Inhibitor Therapy
3.4.2. Folate Receptor-Mediated Drug Targeting
3.4.3. mTOR Inhibitors
3.4.4. Targeting Tumor Angiogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNFPTs | Clinically nonfunctioning pituitary tumors |
| NFPAs | Nonfunctioning pituitary adenomas |
| RT | Radiation therapy |
| SSTR | Somatostatin receptor |
| SF-1 | Steroidogenic factor 1 |
| FSH | Follicular stimulating hormone |
| LH | Luteinizing hormone |
| ACTH | Adrenocorticotropic hormone |
| POMC | Proopiomelanocortin |
| GH | Growth hormone |
| IGF-1 | Insulin like growth factor-1 (IGF-1) |
| TSH | Thyroid stimulating hormone |
| GATA | GATA binding protein |
| ERα | Estrogen receptor alpha |
| PRL | Prolactin |
| SYP | Synaptophysin |
| GTR | Gross total resection |
| DR | Dopamine receptor |
| GnRH | Gonadotrophin-releasing hormone |
| VEGF | Vascular endothelial growth factor |
| SRL | Somatostatin receptor ligand |
| IQR | Interquartile range |
| FR | Folate receptors |
| LAR | Long-acting release |
| BID | Twice daily |
| TID | Thrice daily |
References
- Ntali, G.; Wass, J.A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 2018, 21, 111–118. [Google Scholar] [CrossRef]
- Molitch, M.E. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; White, W.L.; Spetzler, R.F.; Xu, B. A prospective study of nonfunctioning pituitary adenomas: Presentation, management, and clinical outcome. J. Neuro-Oncol. 2011, 102, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, E.; Ferraroni, M.; Castrignanò, T.; Menicatti, L.; Anagni, M.; Reimondo, G.; Del Monte, P.; Bernasconi, D.; Loli, P.; Faustini-Fustini, M.; et al. Non-functioning pituitary adenoma database: A useful resource to improve the clinical management of pituitary tumors. Eur. J. Endocrinol. 2006, 155, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Giampietro, A.; Bianchi, A.; Cimino, V.; Lugli, F.; Piacentini, S.; Lorusso, M.; Tofani, A.; Perotti, G.; Lauriola, L.; et al. Treatment with octreotide LAR in clinically non-functioning pituitary adenoma: Results from a case-control study. Pituitary 2012, 15, 571–578. [Google Scholar] [CrossRef]
- Greenman, Y.; Cooper, O.; Yaish, I.; Robenshtok, E.; Sagiv, N.; Jonas-Kimchi, T.; Yuan, X.; Gertych, A.; Shimon, I.; Ram, Z.; et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur. J. Endocrinol. 2016, 175, 63–72. [Google Scholar] [CrossRef]
- Osborn, A.G.; Louis, D.N.; Poussaint, T.Y.; Linscott, L.L.; Salzman, K.L. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: What Neuroradiologists Need to Know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Larkin, S.; Ansorge, O. Pathology and pathogenesis of pituitary adenomas and other sellar lesions. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2017. [Google Scholar]
- Mete, O.; Cintosun, A.; Pressman, I.; Asa, S.L. Epidemiology and biomarker profile of pituitary adenohypophysial tumors. Mod. Pathol. 2018, 31, 900–909. [Google Scholar] [CrossRef]
- Nielsen, S.; Mellemkjaer, S.; Rasmussen, L.M.; Ledet, T.; Olsen, N.; Bojsen-Møller, M.; Astrup, J.; Weeke, J.; Jørgensen, J.O. Expression of somatostatin receptors on human pituitary adenomas in vivo and ex vivo. J. Endocrinol. Investig. 2001, 24, 430–437. [Google Scholar] [CrossRef]
- Zhang, D.; Hugo, W.; Bergsneider, M.; Wang, M.B.; Kim, W.; Vinters, H.V.; Heaney, A.P. Single-cell RNA sequencing in silent corticotroph tumors confirms impaired POMC processing and provides new insights into their invasive behavior. Eur. J. Endocrinol. 2022, 187, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Langlois, F.; Woltjer, R.; Cetas, J.S.; Fleseriu, M. Silent somatotroph pituitary adenomas: An update. Pituitary 2018, 21, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Chinezu, L.; Vasiljevic, A.; Trouillas, J.; Lapoirie, M.; Jouanneau, E.; Raverot, G. Silent somatotroph tumour revisited from a study of 80 patients with and without acromegaly and a review of the literature. Eur. J. Endocrinol. 2017, 176, 195–201. [Google Scholar] [CrossRef]
- Bano, G. Somatotroph adenomas: Histological subtypes and predicted response to treatment. Int. J. Endocr. Oncol. 2020, 7, IJE29. [Google Scholar] [CrossRef]
- Glynn, N.; Hannon, A.M.; Farrell, M.; Brett, F.; Javadpour, M.; Agha, A. Variable Thyroid-Stimulating Hormone Dynamics in ‘Silent’ Thyrotroph Adenomas. AACE Clin. Case Rep. 2016, 2, e155–e160. [Google Scholar] [CrossRef]
- Manojlovic-Gacic, E.; Engström, B.E.; Casar-Borota, O. Histopathological classification of non-functioning pituitary neuroendocrine tumors. Pituitary 2018, 21, 119–129. [Google Scholar] [CrossRef]
- Kuzu, F.; Bayraktaroğlu, T.; Zor, F.; Gün, B.; Salihoğlu, Y.S.; Kalaycı, M. A thyrotropin-secreting macroadenoma with positive growth hormone and prolactin immunostaining: A case report and literature review. Niger. J. Clin. Pract. 2015, 18, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.; Roncaroli, F.; Grossman, A.B.; Korbonits, M. Clinical and Pathological Aspects of Silent Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 2473–2489. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Drummond, J.B.; Ribeiro-Oliveira, A., Jr.; Soares, B.S. Non-Functioning Pituitary Adenomas. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Huang, W.; Molitch, M.E. Management of nonfunctioning pituitary adenomas (NFAs): Observation. Pituitary 2018, 21, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Fernández-Balsells, M.M.; Barwise, A.; Gallegos-Orozco, J.F.; Paul, A.; Lane, M.A.; Lampropulos, J.F.; Natividad, I.; Perestelo-Pérez, L.; Ponce de León-Lovatón, P.G.; et al. Outcomes of surgical treatment for nonfunctioning pituitary adenomas: A systematic review and meta-analysis. Clin. Endocrinol. 2010, 73, 777–791. [Google Scholar] [CrossRef]
- Reddy, R.; Cudlip, S.; Byrne, J.V.; Karavitaki, N.; Wass, J.A. Can we ever stop imaging in surgically treated and radiotherapy-naive patients with non-functioning pituitary adenoma? Eur. J. Endocrinol. 2011, 165, 739–744. [Google Scholar] [CrossRef]
- Øystese, K.A.; Evang, J.A.; Bollerslev, J. Non-functioning pituitary adenomas: Growth and aggressiveness. Endocrine 2016, 53, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Scaringi, C.; Enrici, R.M. Stereotactic radiotherapy and radiosurgery for non-functioning and secreting pituitary adenomas. Rep. Pr. Pract. Oncol. Radiother. 2016, 21, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Pereira, A.M.; Romijn, J.A. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J. Clin. Endocrinol. Metab. 2008, 93, 3717–3726. [Google Scholar] [CrossRef]
- Lucas, J.W.; Bodach, M.E.; Tumialan, L.M.; Oyesiku, N.M.; Patil, C.G.; Litvack, Z.; Aghi, M.K.; Zada, G. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Primary Management of Patients with Nonfunctioning Pituitary Adenomas. Neurosurgery 2016, 79, E533–E535. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Piccin, D.; Vignali, C.; Tagliati, F.; Ambrosio, M.R.; Bondanelli, M.; Cimino, V.; Bianchi, A.; Schmid, H.A.; Scanarini, M.; et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr. Relat. Cancer 2007, 14, 91–102. [Google Scholar] [CrossRef]
- Katznelson, L.; Oppenheim, D.S.; Coughlin, J.F.; Kliman, B.; Schoenfeld, D.A.; Klibanski, A. Chronic somatostatin analog administration in patients with alpha-subunit-secreting pituitary tumors. J. Clin. Endocrinol. Metab. 1992, 75, 1318–1325. [Google Scholar] [PubMed]
- Taboada, G.F.; Luque, R.M.; Bastos, W.; Guimarães, R.F.; Marcondes, J.B.; Chimelli, L.M.; Fontes, R.; Mata, P.J.; Filho, P.N.; Carvalho, D.P.; et al. Quantitative analysis of somatostatin receptor subtype (SSTR1–5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur. J. Endocrinol. 2007, 156, 65–74. [Google Scholar] [CrossRef]
- Gabalec, F.; Drastikova, M.; Cesak, T.; Netuka, D.; Masopust, V.; Machac, J.; Marek, J.; Cap, J.; Beranek, M. Dopamine 2 and somatostatin 1-5 receptors coexpression in clinically non-functioning pituitary adenomas. Physiol. Res. 2015, 64, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Neto, L.V.; Machado Ede, O.; Luque, R.M.; Taboada, G.F.; Marcondes, J.B.; Chimelli, L.M.; Quintella, L.P.; Niemeyer, P., Jr.; de Carvalho, D.P.; Kineman, R.D.; et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J. Clin. Endocrinol. Metab. 2009, 94, 1931–1937. [Google Scholar] [CrossRef]
- Tateno, T.; Kato, M.; Tani, Y.; Oyama, K.; Yamada, S.; Hirata, Y. Differential expression of somatostatin and dopamine receptor subtype genes in adrenocorticotropin (ACTH)-secreting pituitary tumors and silent corticotroph adenomas. Endocr. J. 2009, 56, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Di Somma, C.; Pivonello, R.; Faggiano, A.; Lombardi, G.; Savastano, S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr. Relat. Cancer 2008, 15, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J. Gonadotroph cell adenomas of the pituitary. Endocr. Rev. 1985, 6, 552–563. [Google Scholar] [CrossRef]
- Bevan, J.S. Clinical review: The antitumoral effects of somatostatin analog therapy in acromegaly. J. Clin. Endocrinol. Metab. 2005, 90, 1856–1863. [Google Scholar] [CrossRef]
- Gasperi, M.; Petrini, L.; Pilosu, R.; Nardi, M.; Marcello, A.; Mastio, F.; Bartalena, L.; Martino, E. Octreotide treatment does not affect the size of most non-functioning pituitary adenomas. J. Endocrinol. Investig. 1993, 16, 541–543. [Google Scholar] [CrossRef]
- Merola, B.; Colao, A.; Ferone, D.; Selleri, A.; Di Sarno, A.; Marzullo, P.; Biondi, B.; Spaziante, R.; Rossi, E.; Lombardi, G. Effects of a chronic treatment with octreotide in patients with functionless pituitary adenomas. Horm. Res. 1993, 40, 149–155. [Google Scholar] [CrossRef]
- Colao, A.; Lastoria, S.; Ferone, D.; Varrella, P.; Marzullo, P.; Pivonello, R.; Cerbone, G.; Acampa, W.; Salvatore, M.; Lombardi, G. The pituitary uptake of 111In-DTPA-D-Phe1-octreotide in the normal pituitary and in pituitary adenomas. J. Endocrinol. Investig. 1999, 22, 176–183. [Google Scholar] [CrossRef]
- Plöckinger, U.; Reichel, M.; Fett, U.; Saeger, W.; Quabbe, H.J. Preoperative octreotide treatment of growth hormone-secreting and clinically nonfunctioning pituitary macroadenomas: Effect on tumor volume and lack of correlation with immunohistochemistry and somatostatin receptor scintigraphy. J. Clin. Endocrinol. Metab. 1994, 79, 1416–1423. [Google Scholar]
- Warnet, A.; Harris, A.G.; Renard, E.; Martin, D.; James-Deidier, A.; Chaumet-Riffaud, P. A prospective multicenter trial of octreotide in 24 patients with visual defects caused by nonfunctioning and gonadotropin-secreting pituitary adenomas. French Multicenter Octreotide Study Group. Neurosurgery 1997, 41, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Boertien, T.M.; Drent, M.L.; Booij, J.; Majoie, C.; Stokkel, M.P.M.; Hoogmoed, J.; Pereira, A.; Biermasz, N.R.; Simsek, S.; Groote Veldman, R.; et al. The GALANT trial: Study protocol of a randomised placebo-controlled trial in patients with a 68Ga-DOTATATE PET-positive, clinically non-functioning pituitary macroadenoma on the effect of lan reotide on tumour size. BMJ Open 2020, 10, e038250. [Google Scholar] [CrossRef] [PubMed]
- Boertien, T.M.; Drent, M.L.; Booij, J.; Majoie, C.B.L.M.; Stokkel, M.P.M.; Hoogmoed, J.; Pereira, A.M.; Biermasz, N.R.; Simsek, S.; Veldman, R.G.; et al. Lanreotide versus placebo for tumour reduction in patients with a 68Ga-DOTATATE PET-positive, clinically non-functioning pituitary macroadenoma (GALANT study): A randomised, multicentre, phase 3 trial with blinded outcome assessment. Lancet Reg. Health Eur. 2024, 42, 100923. [Google Scholar] [CrossRef]
- Gulde, S.; Wiedemann, T.; Schillmaier, M.; Valença, I.; Lupp, A.; Steiger, K.; Yen, H.Y.; Bäuerle, S.; Notni, J.; Luque, R.; et al. Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative In Vivo Model of Nonfunctioning Pituitary Tumors. Cancers 2021, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Di Sarno, A.; Landi, M.L.; Cirillo, S.; Sarnacchiaro, F.; Facciolli, G.; Pivonello, R.; Cataldi, M.; Merola, B.; Annunziato, L.; et al. Long-term and low-dose treatment with cabergoline induces macroprolactinoma shrinkage. J. Clin. Endocrinol. Metab. 1997, 82, 3574–3579. [Google Scholar] [CrossRef]
- Nissim, M.; Ambrosi, B.; Bernasconi, V.; Giannattasio, G.; Giovanelli, M.A.; Bassetti, M.; Vaccari, U.; Moriondo, P.; Spada, A.; Travaglini, P.; et al. Bromocriptine treatment of macroprolactinomas: Studies on the time course of tumor shrinkage and morphology. J. Endocrinol. Investig. 1982, 5, 409–415. [Google Scholar] [CrossRef]
- Bression, D.; Brandi, A.M.; Nousbaum, A.; Le Dafniet, M.; Racadot, J.; Peillon, F. Evidence of dopamine receptors in human growth hormone (GH)-secreting adenomas with concomitant study of dopamine inhibition of GH secretion in a perifusion system. J. Clin. Endocrinol. Metab. 1982, 55, 589–593. [Google Scholar] [CrossRef]
- Pivonello, R.; Matrone, C.; Filippella, M.; Cavallo, L.M.; Di Somma, C.; Cappabianca, P.; Colao, A.; Annunziato, L.; Lombardi, G. Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: Comparison with the effectiveness of cabergoline treatment. J. Clin. Endocrinol. Metab. 2004, 89, 1674–1683. [Google Scholar] [CrossRef]
- Capatina, C.; Poiana, C. Dopamine Agonists in the Management of Non-Functioning Pituitary Adenomas. Acta Endocrinol. 2021, 17, 377–382. [Google Scholar] [CrossRef]
- Cooper, O.; Greenman, Y. Dopamine Agonists for Pituitary Adenomas. Front. Endocrinol. 2018, 9, 469. [Google Scholar] [CrossRef]
- Wollesen, F.; Andersen, T.; Karle, A. Size reduction of extrasellar pituitary tumors during bromocriptine treatment. Ann. Intern. Med. 1982, 96, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Barrow, D.L.; Tindall, G.T.; Kovacs, K.; Thorner, M.O.; Horvath, E.; Hoffman, J.C., Jr. Clinical and pathological effects of bromocriptine on prolactin-secreting and other pituitary tumors. J. Neurosurg. 1984, 60, 1–7. [Google Scholar] [CrossRef]
- Pullan, P.T.; Carroll, W.M.; Chakera, T.M.; Khangure, M.S.; Vaughan, R.J. Management of extra-sellar pituitary tumours with bromocriptine: Comparison of prolactin secreting and non-functioning tumours using half-field visual evoked potentials and computerised tomography. Aust. N. Z. J. Med. 1985, 15, 203–208. [Google Scholar] [CrossRef]
- Verde, G.; Oppizzi, G.; Chiodini, P.G.; Dallabonzana, D.; Luccarelli, G.; Liuzzi, A. Effect of chronic bromocriptine administration on tumor size in patients with nonsecreting pituitary adenomas. J. Endocrinol. Investig. 1985, 8, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Zárate, A.; Morán, C.; Klériga, E.; Loyo, M.; González-Angulo, A.; Aquilar-Parada, E. Bromocriptine therapy as pre-operative adjunct of non-functional pituitary macroadenomas. Acta Endocrinol. 1985, 108, 445–450. [Google Scholar] [CrossRef]
- van Schaardenburg, D.; Roelfsema, F.; van Seters, A.P.; Vielvoye, G.J. Bromocriptine therapy for non-functioning pituitary adenoma. Clin. Endocrinol. 1989, 30, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Nobels, F.R.; de Herder, W.W.; van den Brink, W.M.; Kwekkeboom, D.J.; Hofland, L.J.; Zuyderwijk, J.; de Jong, F.H.; Lamberts, S.W. Long-term treatment with the dopamine agonist quinagolide of patients with clinically non-functioning pituitary adenoma. Eur. J. Endocrinol. 2000, 143, 615–621. [Google Scholar] [CrossRef]
- Ferone, D.; Lastoria, S.; Colao, A.; Varrella, P.; Cerbone, G.; Acampa, W.; Merola, B.; Salvatore, M.; Lombardi, G. Correlation of scintigraphic results using 123I-methoxybenzamide with hormone levels and tumor size response to quinagolide in patients with pituitary adenomas. J. Clin. Endocrinol. Metab. 1998, 83, 248–252. [Google Scholar] [CrossRef]
- Lohmann, T.; Trantakis, C.; Biesold, M.; Prothmann, S.; Guenzel, S.; Schober, R.; Paschke, R. Minor tumour shrinkage in nonfunctioning pituitary adenomas by long-term treatment with the dopamine agonist cabergoline. Pituitary 2001, 4, 173–178. [Google Scholar] [CrossRef]
- Greenman, Y.; Tordjman, K.; Osher, E.; Veshchev, I.; Shenkerman, G.; Reider, G., II.; Segev, Y.; Ouaknine, G.; Stern, N. Postoperative treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists decreases tumour remnant growth. Clin. Endocrinol. 2005, 63, 39–44. [Google Scholar] [CrossRef]
- Batista, R.L.; Musolino, N.R.C.; Cescato, V.A.S.; da Silva, G.O.; Medeiros, R.S.S.; Herkenhoff, C.G.B.; Trarbach, E.B.; Cunha-Neto, M.B. Cabergoline in the Management of Residual Nonfunctioning Pituitary Adenoma: A Single-Center, Open-Label, 2-Year Randomized Clinical Trial. Am. J. Clin. Oncol. 2019, 42, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.C.; Naves, L.A.; Silva, A.O.; de Castro, L.F.; Casulari, L.A.; Azevedo, M.F. Short-term treatment with cabergoline can lead to tumor shrinkage in patients with nonfunctioning pituitary adenomas. Pituitary 2013, 16, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Ferone, D.; Lastoria, S.; Cerbone, G.; Di Sarno, A.; Di Somma, C.; Lucci, R.; Lombardi, G. Hormone levels and tumour size response to quinagolide and cabergoline in patients with prolactin-secreting and clinically non-functioning pituitary adenomas: Predictive value of pituitary scintigraphy with 123I-methoxybenzamide. Clin. Endocrinol. 2000, 52, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Vieira Neto, L.; Wildemberg, L.E.; Moraes, A.B.; Colli, L.M.; Kasuki, L.; Marques, N.V.; Gasparetto, E.L.; de Castro, M.; Takiya, C.M.; Gadelha, M.R. Dopamine receptor subtype 2 expression profile in nonfunctioning pituitary adenomas and in vivo response to cabergoline therapy. Clin. Endocrinol. 2015, 82, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Biagetti, B.; Araujo-Castro, M.; Alcázar, V.; Guerrero-Pérez, F.; Rivero, N.; Casteràs, A.; Gómez, C.G.; Izquierdo, B.G.; Torres, V.V.; et al. Effect of cabergoline on tumor remnant after surgery in nonfunctioning pituitary adenoma. J. Neuro-Oncol. 2022, 160, 351–359. [Google Scholar] [CrossRef]
- Ayalon-Dangur, I.; Turjeman, A.; Hirsch, D.; Robenshtok, E.; Tsvetov, G.; Gorshtein, A.; Masri, H.; Shraga-Slutzky, I.; Manisterski, Y.; Akirov, A.; et al. Cabergoline treatment for surgery-naïve non-functioning pituitary macroadenomas. Pituitary 2024, 27, 52–60. [Google Scholar] [CrossRef]
- Andersen, M.; Bjerre, P.; Schrøder, H.D.; Edal, A.; Høilund-Carlsen, P.F.; Pedersen, P.H.; Hagen, C. In vivo secretory potential and the effect of combination therapy with octreotide and cabergoline in patients with clinically non-functioning pituitary adenomas. Clin. Endocrinol. 2001, 54, 23–30. [Google Scholar] [CrossRef]
- Roman, S.H.; Goldstein, M.; Kourides, I.A.; Comite, F.; Bardin, C.W.; Krieger, D.T. The luteinizing hormone-releasing hormone (LHRH) agonist [D-Trp6-Pro9-NEt]LHRH increased rather than lowered LH and α-subunit levels in a patient with an LH-secreting pituitary tumor. J. Clin. Endocrinol. Metab. 1984, 58, 313–319. [Google Scholar] [CrossRef]
- Chapman, A.J.; MacFarlane, I.A.; Shalet, S.M.; Beardwell, C.G.; Dutton, J.; Sutton, M.L. Discordant serum α-subunit and FSH concentrations in a woman with a pituitary tumour. Clin. Endocrinol. 1984, 21, 123–129. [Google Scholar] [CrossRef]
- Colombo, P.; Ambrosi, B.; Saccomanno, K.; Bassetti, M.; Cortelazzi, D.; Faglia, G. Effects of long-term treatment with the gonadotropin-releasing hormone analog nafarelin in patients with non-functioning pituitary adenomas. Eur. J. Endocrinol. 1994, 130, 339–345. [Google Scholar] [CrossRef]
- Daneshdoost, L.; Pavlou, S.N.; Molitch, M.E.; Gennarelli, T.A.; Savino, P.J.; Sergott, R.C.; Bosley, T.M.; River, J.E.; Vale, W.W.; Snyder, P.J. Inhibition of follicle-stimulating hormone secretion from gonadotroph adenomas by repetitive administration of a gonadotropin-releasing hormone antagonist. J. Clin. Endocrinol. Metab. 1990, 71, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Klibanski, A.; Deutsch, P.J.; Jameson, J.L.; Ridgway, E.C.; Crowley, W.F.; Hsu, D.W.; Habener, J.F.; Black, P.M. Luteinizing hormone-secreting pituitary tumor: Biosynthetic characterization and clinical studies. J. Clin. Endocrinol. Metab. 1987, 64, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Klibanski, A.; Jameson, J.L.; Biller, B.M.; Crowley, W.F., Jr.; Zervas, N.T.; Rivier, J.; Vale, W.W.; Bikkal, H. Gonadotropin and alpha-subunit responses to chronic gonadotropin-releasing hormone analog administration in patients with glycoprotein hormone-secreting pituitary tumors. J. Clin. Endocrinol. Metab. 1989, 68, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, G.; Lejeune, H.; Trouillas, J.; Forest, M.G.; Claustrat, B.; Lahlou, N.; Loras, B. Gonadotropin-releasing hormone agonists are unsuccessful in reducing tumoral gonadotropin secretion in two patients with gonadotropin-secreting pituitary adenomas. J. Clin. Endocrinol. Metab. 1988, 67, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Jensen, R.T. Perspectives on the current pharmacotherapeutic strategies for management of functional neuroendocrine tumor syndromes. Expert. Opin. Pharmacother. 2021, 22, 685–693. [Google Scholar] [CrossRef]
- Maclean, J.; Aldridge, M.; Bomanji, J.; Short, S.; Fersht, N. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: Variable clinical response in preliminary evaluation. Pituitary 2014, 17, 530–538. [Google Scholar] [CrossRef]
- McCormack, A.; Dekkers, O.M.; Petersenn, S.; Popovic, V.; Trouillas, J.; Raverot, G.; Burman, P. Treatment of aggressive pituitary tumours and carcinomas: Results of a European Society of Endocrinology (ESE) survey 2016. Eur. J. Endocrinol. 2018, 178, 265–276. [Google Scholar] [CrossRef]
- Halevy, C.; Whitelaw, B.C. How effective is temozolomide for treating pituitary tumours and when should it be used? Pituitary 2017, 20, 261–266. [Google Scholar] [CrossRef]
- Lamas, C.; Cámara, R.; Fajardo, C.; Remon-Ruiz, P.; Biagetti, B.; Guerrero-Pérez, F.; Araujo-Castro, M.; Mora, M.; Hanzu, F.; Iglesias, P.; et al. Efficacy and safety of temozolomide in the treatment of aggressive pituitary neuroendocrine tumours in Spain. Front. Endocrinol. 2023, 14, 1204206. [Google Scholar] [CrossRef]
- Lopes-Pinto, M.; Lacerda-Nobre, E.; Silva, A.L.; Marques, P. Therapeutical Usefulness of PD-1/PD-L1 Inhibitors in Aggressive or Metastatic Pituitary Tumours. Cancers 2024, 16, 3033. [Google Scholar] [CrossRef]
- Wibowo, A.S.; Singh, M.; Reeder, K.M.; Carter, J.J.; Kovach, A.R.; Meng, W.; Ratnam, M.; Zhang, F.; Dann, C.E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 15180–15188. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Sarkar, A.; Feldmann, D.P.; Hoffmann, P.; Merkel, O.M. Revisiting the value of competition assays in folate receptor-mediated drug delivery. Biomaterials 2017, 138, 35–45. [Google Scholar] [CrossRef]
- Matherly, L.H.; Hou, Z. Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam. Horm. 2008, 79, 145–184. [Google Scholar] [PubMed]
- Sosnik, A. Chapter 1—From the “magic bullet” to advanced nanomaterials for active targeting in diagnostics and therapeutics. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–32. [Google Scholar]
- Evans, C.O.; Reddy, P.; Brat, D.J.; O’Neill, E.B.; Craige, B.; Stevens, V.L.; Oyesiku, N.M. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003, 63, 4218–4224. [Google Scholar]
- Evans, C.O.; Yao, C.; Laborde, D.; Oyesiku, N.M. Folate receptor expression in pituitary adenomas cellular and molecular analysis. Vitam. Horm. 2008, 79, 235–266. [Google Scholar]
- Galt, J.R.; Halkar, R.K.; Evans, C.-O.; Osman, N.A.; LaBorde, D.; Fox, T.H.; Faraj, B.A.; Kumar, K.; Wang, H.; Oyesiku, N.M. In Vivo Assay of Folate Receptors in Nonfunctional Pituitary Adenomas with 99mTc-Folate SPECT/CT. J. Nucl. Med. 2010, 51, 1716–1723. [Google Scholar] [CrossRef]
- Liu, X.; Ma, S.; Dai, C.; Cai, F.; Yao, Y.; Yang, Y.; Feng, M.; Deng, K.; Li, G.; Ma, W.; et al. Antiproliferative, antiinvasive, and proapoptotic activity of folate receptor α-targeted liposomal doxorubicin in nonfunctional pituitary adenoma cells. Endocrinology 2013, 154, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, X.; Li, Y.; Han, X.; Ren, B.; Yin, G. Targeting pituitary adenomas with folate-conjugated multiple drug decorated liposomal formulations for improved antiproliferative anticancer efficacy. J. Exp. Nanosci. 2022, 17, 14–33. [Google Scholar] [CrossRef]
- Evans, C.-O.; Halkar, R.K.; Galt, J.R.; Faraj, B.A.; Oyesiku, N.M. Molecular Targeting and Imaging of Non-Functional Pituitary Tumors: Preliminary Results. Jpn. J. Neurosurg. 2006, 15, 437–443. [Google Scholar]
- Lee, M.; Pellegata, N.S. Folate Receptor-Mediated Drug Targeting: A Possible Strategy for Nonfunctioning Pituitary Adenomas? Endocrinology 2013, 154, 1387–1389. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, Y.; Lu, M.; Zhan, X.; Zhou, T.; Li, B.; Zhan, X. Quantitative Analysis of Proteome in Non-functional Pituitary Adenomas: Clinical Relevance and Potential Benefits for the Patients. Front. Endocrinol. 2019, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, H.; Xie, W.; Guo, J.; Fang, Q.; Zhao, P.; Liu, C.; Zhu, H.; Wang, Z.; Wang, J.; et al. Genomic and transcriptomic analysis of pituitary adenomas reveals the impacts of copy number variations on gene expression and clinical prognosis among prolactin-secreting subtype. Aging 2020, 13, 1276–1293. [Google Scholar] [CrossRef] [PubMed]
- Mangili, F.; Esposito, E.; Treppiedi, D.; Catalano, R.; Marra, G.; Di Muro, G.; Barbieri, A.M.; Locatelli, M.; Lania, A.G.; Mangone, A.; et al. DRD2 Agonist Cabergoline Abolished the Escape Mechanism Induced by mTOR Inhibitor Everolimus in Tumoral Pituitary Cells. Front. Endocrinol. 2022, 13, 867822. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.C.; Minoia, M.; Filieri, C.; Tagliati, F.; Buratto, M.; Ambrosio, M.R.; Lapparelli, M.; Scanarini, M.; degli Uberti, E.C. Effect of Everolimus on Cell Viability in Nonfunctioning Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2010, 95, 968–976. [Google Scholar] [CrossRef]
- Cerovac, V.; Monteserin-Garcia, J.; Rubinfeld, H.; Buchfelder, M.; Losa, M.; Florio, T.; Paez-Pereda, M.; Stalla, G.K.; Theodoropoulou, M. The Somatostatin Analogue Octreotide Confers Sensitivity to Rapamycin Treatment on Pituitary Tumor Cells. Cancer Res. 2010, 70, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Way, J.S.; Zhang, X.; Sergey, M.; Bergsneider, M.; Wang, M.B.; Yong, W.H.; Heaney, A.P. Effect of Everolimus in Treatment of Aggressive Prolactin-Secreting Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 1929–1936. [Google Scholar] [CrossRef]
- Lin, A.L.; Geer, E.B.; Lala, N.; Page-Wilson, G.; Magge, R.; Young, R.J.; Tabar, V. The treatment of aggressive prolactinomas with everolimus. Pituitary 2023, 26, 474–481. [Google Scholar] [CrossRef]
- Jouanneau, E.; Wierinckx, A.; Ducray, F.; Favrel, V.; Borson-Chazot, F.; Honnorat, J.; Trouillas, J.; Raverot, G. New targeted therapies in pituitary carcinoma resistant to temozolomide. Pituitary 2012, 15, 37–43. [Google Scholar] [CrossRef]
- Alshaikh, O.M.; Asa, S.L.; Mete, O.; Ezzat, S. An Institutional Experience of Tumor Progression to Pituitary Carcinoma in a 15-Year Cohort of 1055 Consecutive Pituitary Neuroendocrine Tumors. Endocr. Pathol. 2019, 30, 118–127. [Google Scholar] [CrossRef]
- Nakano-Tateno, T.; Lau, K.J.; Wang, J.; McMahon, C.; Kawakami, Y.; Tateno, T.; Araki, T. Multimodal Non-Surgical Treatments of Aggressive Pituitary Tumors. Front. Endocrinol. 2021, 12, 118–127. [Google Scholar] [CrossRef]
- Turner, H.E.; Nagy, Z.; Gatter, K.C.; Esiri, M.M.; Harris, A.L.; Wass, J.A.H. Angiogenesis in Pituitary Adenomas and the Normal Pituitary Gland. J. Clin. Endocrinol. Metab. 2000, 85, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Akutsu, H.; Hara, T.; Yamamoto, T.; Matsumura, A. Correlations of Vascular Architecture and Angiogenesis with Pituitary Adenoma Histotype. Int. J. Endocrinol. 2014, 2014, 989574. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liang, S.; Sun, B.; Li, Y.; Kang, J. Anti-VEGF Therapy in Refractory Pituitary Adenomas and Pituitary Carcinomas: A Review. Front. Oncol. 2021, 11, 773905. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S.; Sim, H.-W.; McCormack, A.I. Exploring the Role of Novel Medical Therapies for Aggressive Pituitary Tumors: A Review of the Literature—“Are We There Yet?”. Cancers 2020, 12, 308. [Google Scholar] [CrossRef]
| Tumor Type | Subtype | Frequency (% of All CNFTs) | Lineage Specific Transcription Factor | Hormone Produced |
|---|---|---|---|---|
| Silent Gonadotroph Tumor | 40–79% | SF-1 (NR5A1), GATA2, ERa | FSHb, LHb, a-subunit | |
| Silent Corticotroph Tumor | Sparsely Granulated Corticotroph Tumor | 3–6% | Tpit (TBX19) | ACTH |
| Crooke’s Cell Adenoma | Tpit (TBX19) | ACTH | ||
| Silent Somatotroph Tumor | Sparsely Granulated Somatotroph Tumor | 2–4% | Pit-1 (POU1F1) | GH (or PRL) |
| Mammosomatotroph Tumor | Pit-1 (POU1F1), ERa | GH and PRL (same cells) | ||
| Mixed GH-PRL Tumor | Pit-1 (POU1F1), ERa | GH and PRL (different cells) | ||
| Plurihormonal Tumor | Pit-1 (POU1F1), ERa | GH, PRL, TSHb | ||
| Silent Lactotroph Tumor | Sparsely Granulated Lactotroph Tumor | 1% | Pit-1 (POU1F1), ERa | PRL |
| Mammosomatotroph Tumor | Pit-1 (POU1F1), ERa | GH and PRL (same cells) | ||
| Mixed GH-PRLTumor | Pit-1 (POU1F1), ERa | GH and PRL (different cells) | ||
| Plurihormonal Tumor | Pit-1 (POU1F1), ERa | GH, PRL, TSHb | ||
| Acidophilic Stem Cell Adenoma | Pit-1 (POU1F1), ERa | GH, PRL | ||
| Silent Thyrotroph Tumor | 1–2% | Pit-1 (POU1F1), GATA2 | TSHb | |
| Null Cell Tumor | 17% | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogar, N.; Zhang, D.; Heaney, A.P. A Bridge Too Far? Towards Medical Therapy for Clinically Nonfunctioning Pituitary Tumors. Int. J. Mol. Sci. 2025, 26, 5898. https://doi.org/10.3390/ijms26125898
Mogar N, Zhang D, Heaney AP. A Bridge Too Far? Towards Medical Therapy for Clinically Nonfunctioning Pituitary Tumors. International Journal of Molecular Sciences. 2025; 26(12):5898. https://doi.org/10.3390/ijms26125898
Chicago/Turabian StyleMogar, Nikita, Dongyun Zhang, and Anthony P. Heaney. 2025. "A Bridge Too Far? Towards Medical Therapy for Clinically Nonfunctioning Pituitary Tumors" International Journal of Molecular Sciences 26, no. 12: 5898. https://doi.org/10.3390/ijms26125898
APA StyleMogar, N., Zhang, D., & Heaney, A. P. (2025). A Bridge Too Far? Towards Medical Therapy for Clinically Nonfunctioning Pituitary Tumors. International Journal of Molecular Sciences, 26(12), 5898. https://doi.org/10.3390/ijms26125898






