Analysis of the Expression Patterns of Tumor Necrosis Factor Alpha Signaling Pathways and Regulatory MicroRNAs in Astrocytic Tumors
Abstract
1. Introduction
2. Results
2.1. Transcriptomic Profiling of TNF-α Pathway Genes
2.2. miRNA-Mediated Regulation of TNF-α Pathway Genes
2.3. Methylation Status of TNF-α Pathway Components
2.4. Protein Quantification by ELISA
2.5. Protein Expression Validation by Western Blot and Immunohistochemistry
2.6. Functional Network Analysis of Selected Proteins: Insights from Protein–Protein Interaction (PPI) Analysis
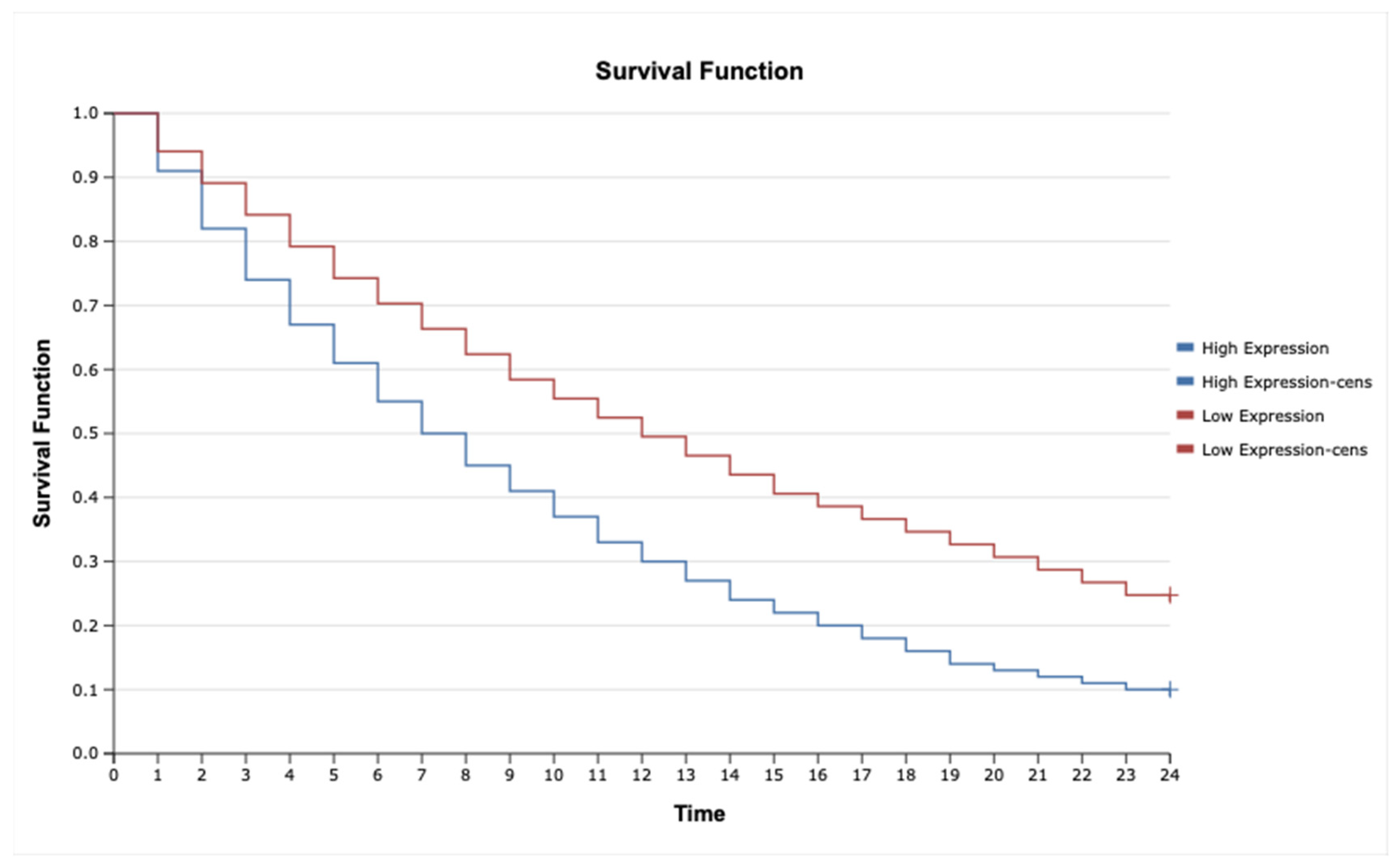
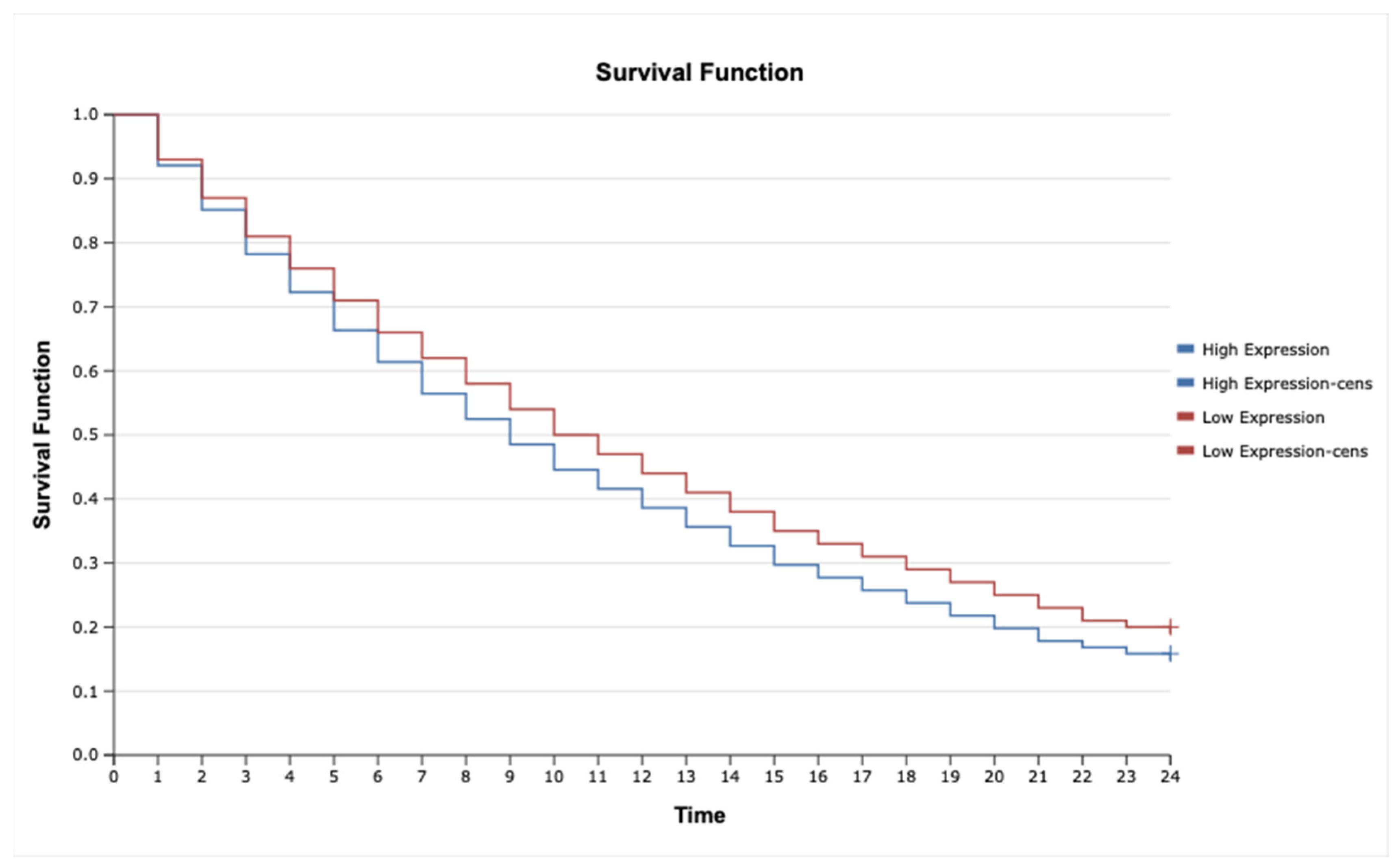
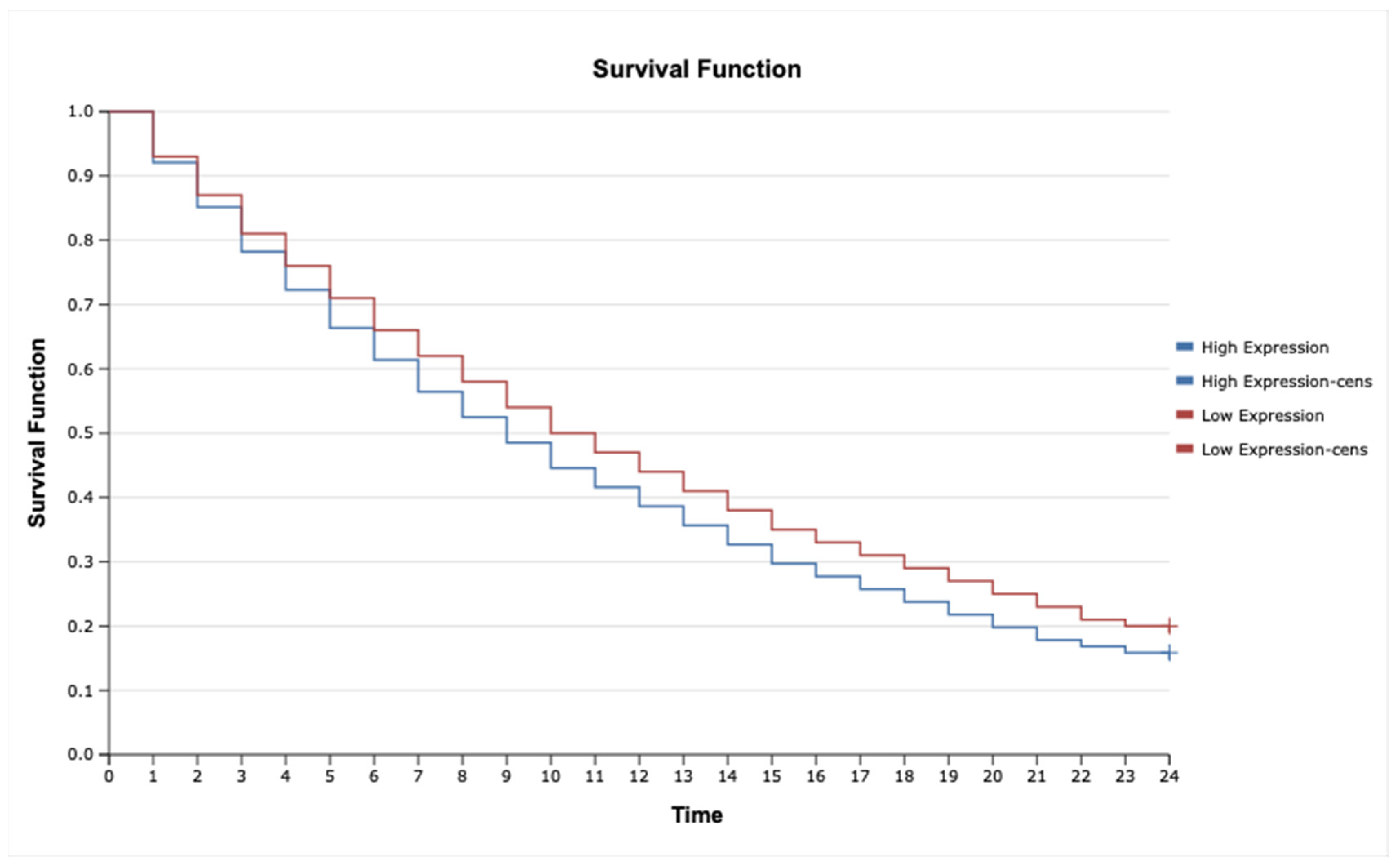
2.7. Kaplan–Meier Survival Analysis and Cox Proportional Hazards Model for TNF-α, IL-1β, TNFRSF1A, TNFRSF1B, MAP3K8, and MAP2K7 in Astrocytic Tumors
3. Discussion
4. Materials and Methods
4.1. Characterization of Participants
4.2. Extraction of Total Ribonucleic Acid (RNA)
4.3. Microarray Analysis
4.4. Microarray Profiling of TNF-α-Related miRNAs
4.5. Validation of Gene Expression Using Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.6. Methylation Analysis of Genes
4.7. Enzyme-Linked Immunosorbent Assay (ELISA) Quantification of Selected Proteins in G2, G3, G4 Astrocytic Tumor Samples
4.8. Western Blot for Protein Quantification of TNF-α and IL-1β
4.9. Immunohistochemical (IHC) Analysis of TNF-α and IL1β in Astrocytic Brain Tumor Samples
4.10. Functional Network Analysis of Protein–Protein Interactions (PPI)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-1β | Interleukin-1 Beta |
| TNFRSF1A | Tumor Necrosis Factor Receptor Superfamily Member 1A |
| TNFRSF1B | Tumor Necrosis Factor Receptor Superfamily Member 1B |
| MAP3K8 | Mitogen-Activated Protein Kinase Kinase Kinase 8 |
| MAP2K7 | Mitogen-Activated Protein Kinase Kinase 7 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| JNK | c-Jun N-terminal Kinase |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IHC | Immunohistochemistry |
| MSP | Methylation-Specific Polymerase Chain Reaction |
| PPI | Protein–Protein Interaction |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| ACTB | Beta Actin |
| miRNA | MicroRNA |
| ceRNA | Competing Endogenous RNA |
| IDH | Isocitrate Dehydrogenase |
| MGMT | O6-Methylguanine-DNA Methyltransferase |
| EGFR | Epidermal Growth Factor Receptor |
| GBM | Glioblastoma Multiforme |
| G2 | Grade II Astrocytoma |
| G3 | Grade III Astrocytoma |
| G4 | Grade IV Astrocytoma |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| SD | Standard Deviation |
| SP ZOZ | Samodzielny Publiczny Zakład Opieki Zdrowotnej |
References
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Kipanyula, M.J.; Etet, P.F.S.; Vecchio, L.; Farahna, M.; Nukenine, E.N.; Kamdje, A.H.N. Signaling Pathways Bridging Microbial-Triggered Inflammation and Cancer. Cell. Signal. 2013, 25, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, D.; Zhao, S.; Shao, Y.; Xia, Y.; Ni, D.; Qiu, X.; Zhang, J.; Chen, J.; Meng, F.; et al. Immunotherapy of Malignant Glioma by Noninvasive Administration of TLR9 Agonist CpG Nano-Immunoadjuvant. Adv. Sci. 2022, 9, 2103689. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L. Insights into the Biology and Therapeutic Implications of TNF and Regulatory T Cells. Nat. Rev. Rheumatol. 2021, 17, 487–504. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF Activity and T Cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The Dual Role of Tumor Necrosis Factor-Alpha (TNF-α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Galozzi, P.; Bindoli, S.; Doria, A.; Sfriso, P. The Revisited Role of Interleukin-1 Alpha and Beta in Autoimmune and Inflammatory Disorders and in Comorbidities. Autoimmun. Rev. 2021, 20, 102785. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Yokose, K.; Sato, S.; Asano, T.; Yashiro, M.; Kobayashi, H.; Watanabe, H.; Suzuki, E.; Sato, C.; Kozuru, H.; Yatsuhashi, H. TNF-α Potentiates Uric Acid-Induced Interleukin-1 β (IL-1 β) Secretion in Human Neutrophils. Mod. Rheumatol. 2018, 28, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of microRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Sharma, A.R.; Sharma, G.; Lee, S.; Chakraborty, C. miRNA-Regulated Key Components of Cytokine Signaling Pathways and Inflammation in Rheumatoid Arthritis. Med. Res. Rev. 2016, 36, 425–439. [Google Scholar] [CrossRef]
- Oyama, R.; Jinnin, M.; Kakimoto, A.; Kanemaru, H.; Ichihara, A.; Fujisawa, A.; Honda, N.; Masuguchi, S.; Fukushima, S.; Maruo, K. Circulating microRNA Associated with TNF-α Signaling Pathway in Patients with Plaque Psoriasis. J. Dermatol. Sci. 2011, 61, 209–211. [Google Scholar] [CrossRef]
- Banerjee, S.; Xu, W.; Doctor, A.; Driss, A.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E.; Chowdhury, I. TNFα-Induced Altered miRNA Expression Links to NF-κB Signaling Pathway in Endometriosis. Inflammation 2023, 46, 2055–2070. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, K.; Kim, E.E.; Kim, S.-I.; Park, S.-H. Brain Tumor Classification by Methylation Profile. J. Korean Med. Sci. 2023, 38, e356. [Google Scholar] [CrossRef]
- Cano-Valdez, A.M.; Sevilla-Lizcano, D.B. Pathological Classification of Brain Tumors. In Principles of Neuro-Oncology; Monroy-Sosa, A., Chakravarthi, S.S., De La Garza-Salazar, J.G., Meneses Garcia, A., Kassam, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 75–105. ISBN 978-3-030-54878-0. [Google Scholar]
- Pienkowski, T.; Kowalczyk, T.; Kretowski, A.; Ciborowski, M. A Review of Gliomas-Related Proteins. Characteristics of Potential Biomarkers. Am. J. Cancer Res. 2021, 11, 3425. [Google Scholar]
- Machnik, G.; Bułdak, Ł.; Zapletal-Pudełko, K.; Grabarek, B.O.; Staszkiewicz, R.; Sobański, D.; Okopień, B. The Impact of Wound-Healing Assay, Phorbol Myristate Acetate (PMA) Stimulation and siRNA-Mediated FURIN Gene Silencing on Endogenous Retroviral ERVW-1 Expression Level in U87-MG Astrocytoma Cells. Adv. Med. Sci. 2024, 69, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hambardzumyan, D. Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the Molecular Genetics of Gliomas—Implications for Classification and Therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Bale, T.A.; Rosenblum, M.K. The 2021 WHO Classification of Tumors of the Central Nervous System: An Update on Pediatric Low-grade Gliomas and Glioneuronal Tumors. Brain Pathol. 2022, 32, e13060. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical Implications of the 2021 Edition of the WHO Classification of Central Nervous System Tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Sokolenko, A.P.; Imyanitov, E.N. Molecular Diagnostics in Clinical Oncology. Front. Mol. Biosci. 2018, 5, 76. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Radke, J.; Koch, A.; Pritsch, F.; Schumann, E.; Misch, M.; Hempt, C.; Lenz, K.; Löbel, F.; Paschereit, F.; Heppner, F.L.; et al. Predictive MGMT Status in a Homogeneous Cohort of IDH Wildtype Glioblastoma Patients. Acta Neuropathol. Commun. 2019, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Labreche, K.; Kinnersley, B.; Berzero, G.; Di Stefano, A.L.; Rahimian, A.; Detrait, I.; Marie, Y.; Grenier-Boley, B.; Hoang-Xuan, K.; Delattre, J.-Y.; et al. Diffuse Gliomas Classified by 1p/19q Co-Deletion, TERT Promoter and IDH Mutation Status Are Associated with Specific Genetic Risk Loci. Acta Neuropathol. 2018, 135, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Boroń, D.; Zmarzły, N.; Wierzbik-Strońska, M.; Rosińczuk, J.; Mieszczański, P.; Grabarek, B.O. Recent Multiomics Approaches in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 1237. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tao, J.; Liu, T.; Liu, Y.; Qiu, J.; Su, D.; Wang, R.; Luo, W.; Cao, Z.; Weng, G.; et al. Unbiasedly Decoding the Tumor Microenvironment with Single-Cell Multiomics Analysis in Pancreatic Cancer. Mol. Cancer 2024, 23, 140. [Google Scholar] [CrossRef]
- Li, L.; Bai, L.; Lin, H.; Dong, L.; Zhang, R.; Cheng, X.; Liu, Z.; Ouyang, Y.; Ding, K. Multiomics Analysis of Tumor Mutational Burden across Cancer Types. Comput. Struct. Biotechnol. J. 2021, 19, 5637–5646. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Michels, N.; van Aart, C.; Morisse, J.; Mullee, A.; Huybrechts, I. Chronic Inflammation towards Cancer Incidence: A Systematic Review and Meta-Analysis of Epidemiological Studies. Crit. Rev. Oncol./Hematol. 2021, 157, 103177. [Google Scholar] [CrossRef]
- Basheer, A.S.; Abas, F.; Othman, I.; Naidu, R. Role of Inflammatory Mediators, Macrophages, and Neutrophils in Glioma Maintenance and Progression: Mechanistic Understanding and Potential Therapeutic Applications. Cancers 2021, 13, 4226. [Google Scholar] [CrossRef]
- Ham, S.W.; Jeon, H.-Y.; Jin, X.; Kim, E.-J.; Kim, J.-K.; Shin, Y.J.; Lee, Y.; Kim, S.H.; Lee, S.Y.; Seo, S. TP53 Gain-of-Function Mutation Promotes Inflammation in Glioblastoma. Cell Death Differ. 2019, 26, 409–425. [Google Scholar] [CrossRef]
- Gromeier, M.; Brown, M.C.; Zhang, G.; Lin, X.; Chen, Y.; Wei, Z.; Beaubier, N.; Yan, H.; He, Y.; Desjardins, A. Very Low Mutation Burden Is a Feature of Inflamed Recurrent Glioblastomas Responsive to Cancer Immunotherapy. Nat. Commun. 2021, 12, 352. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of Immune Responses to Cytokines at Single-Cell Resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Broekman, M.L.; Maas, S.L.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional Communication in the Microenvirons of Glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Peng, C.; Li, D. Epigenetic Underpinnings of Inflammation: A Key to Unlock the Tumor Microenvironment in Glioblastoma. Front. Immunol. 2022, 13, 869307. [Google Scholar] [CrossRef]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain Immunology and Immunotherapy in Brain Tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Collignon, J.; Eefsen, R.; Ochsenreither, S.; Zvirbule, Z.; Ivanauskas, A.; Millrud, C.R.; Skoog, P.; Losic, N.; Magnusson, S. Tumor IL1RAP Levels and Reduction in Serum Biomarkers Correlate with Response in PDAC Patients Treated with Nadunolimab, an Anti-IL1RAP Monoclonal Antibody, in Combination with Gemcitabine and Nab-Paclitaxel. Cancer Res. 2023, 83, 2172. [Google Scholar] [CrossRef]
- Oyende, Y.; Taus, L.J.; Fatatis, A. IL-1β in Neoplastic Disease and the Role of Its Tumor-Derived Form in the Progression and Treatment of Metastatic Prostate Cancer. Cancers 2025, 17, 290. [Google Scholar] [CrossRef]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the Era of Immune Checkpoint Inhibitors: Friend or Foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef]
- Walsh, M.J.; Ali, L.R.; Lenehan, P.; Kureshi, C.T.; Kureshi, R.; Dougan, M.; Knipe, D.M.; Dougan, S.K. Blockade of Innate Inflammatory Cytokines TNF α, IL-1 β, or IL-6 Overcomes Virotherapy-Induced Cancer Equilibrium to Promote Tumor Regression. Immunother. Adv. 2023, 3, ltad011. [Google Scholar] [CrossRef]
- Caronni, N.; La Terza, F.; Vittoria, F.M.; Barbiera, G.; Mezzanzanica, L.; Cuzzola, V.; Barresi, S.; Pellegatta, M.; Canevazzi, P.; Dunsmore, G. IL-1β+ Macrophages Fuel Pathogenic Inflammation in Pancreatic Cancer. Nature 2023, 623, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, R.; Sawoo, R.; Dutta, P.; Bishayi, B. Impact of Dual Neutralization of TNF-α and IL-1β along with Gentamicin Treatment on the Functions of Blood and Splenic Neutrophils and Its Role on Improvement of S. Aureus Induced Septic Arthritis. Int. Immunopharmacol. 2023, 123, 110766. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamasaki, K. Psoriasis and Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 6791. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, H.; Soroka, Y.; Zlotkin-Frušić, M.; Gilhar, A.; Milner, Y.; Tamir, S. Isothiocyanates Inhibit Psoriasis-Related Proinflammatory Factors in Human Skin. Inflamm. Res. 2012, 61, 735–742. [Google Scholar] [CrossRef]
- Mukherjee, T.; Mohanty, S.; Kaur, J.; Das, M.; Adhikary, K.; Chatterjee, P.; Maiti, R. Exploring Small-Molecule Inhibitors Targeting MAPK Pathway Components: Focus on ERK, MEK1, and MEK2 Kinases in Cancer Treatment. Chem. Biol. Lett. 2024, 11, 659. [Google Scholar] [CrossRef]
- Bai, D.; Cheng, X.; Li, Q.; Zhang, B.; Zhang, Y.; Lu, F.; Sun, T.; Hao, J. Eupatilin Inhibits Keratinocyte Proliferation and Ameliorates Imiquimod-Induced Psoriasis-like Skin Lesions in Mice via the P38 MAPK/NF-κB Signaling Pathway. Immunopharmacol. Immunotoxicol. 2023, 45, 133–139. [Google Scholar] [CrossRef]
- Paardekooper, L.M.; Bendix, M.B.; Ottria, A.; de Haer, L.W.; Ter Beest, M.; Radstake, T.R.; Marut, W.; van den Bogaart, G. Hypoxia Potentiates Monocyte-Derived Dendritic Cells for Release of Tumor Necrosis Factor α via MAP3K8. Biosci. Rep. 2018, 38, BSR20182019. [Google Scholar] [CrossRef]
- Pattison, M.J.; Mitchell, O.; Flynn, H.R.; Chen, C.-S.; Yang, H.-T.; Ben-Addi, H.; Boeing, S.; Snijders, A.P.; Ley, S.C. TLR and TNF-R1 Activation of the MKK3/MKK6–P38α Axis in Macrophages Is Mediated by TPL-2 Kinase. Biochem. J. 2016, 473, 2845–2861. [Google Scholar] [CrossRef]
- Martinelli, E.; Morgillo, F.; Troiani, T.; Ciardiello, F. Cancer Resistance to Therapies against the EGFR-RAS-RAF Pathway: The Role of MEK. Cancer Treat. Rev. 2017, 53, 61–69. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Ren, J.; Xu, Y.; Liu, J.; Wu, S.; Zhang, R.; Cao, H.; Sun, J. MAP3K8 Is a Prognostic Biomarker and Correlated With Immune Response in Glioma. Front. Mol. Biosci. 2021, 8, 779290. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Scott, A.F.; Amberger, J.; Valle, D.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM). Hum. Mutat. 2000, 15, 57–61. [Google Scholar] [CrossRef]
- Jean-Quartier, C.; Jeanquartier, F.; Holzinger, A. Open Data for Differential Network Analysis in Glioma. Int. J. Mol. Sci. 2020, 21, 547. [Google Scholar] [CrossRef]
- Fu, J.; Imani, S.; Wu, M.-Y.; Wu, R.-C. MicroRNA-34 Family in Cancers: Role, Mechanism, and Therapeutic Potential. Cancers 2023, 15, 4723. [Google Scholar] [CrossRef] [PubMed]
- Tokumaru, Y.; Katsuta, E.; Oshi, M.; Sporn, J.C.; Yan, L.; Le, L.; Matsuhashi, N.; Futamura, M.; Akao, Y.; Yoshida, K.; et al. High Expression of miR-34a Associated with Less Aggressive Cancer Biology but Not with Survival in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 3045. [Google Scholar] [CrossRef]
- Mao, L.; Liu, S.; Hu, L.; Jia, L.; Wang, H.; Guo, M.; Chen, C.; Liu, Y.; Xu, L. miR-30 Family: A Promising Regulator in Development and Disease. BioMed Res. Int. 2018, 2018, 9623412. [Google Scholar] [CrossRef]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.R.; Junejo, K.; Steinhoff, M.; Uddin, S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells 2019, 8, 840. [Google Scholar] [CrossRef]
- Garavelli, S.; De Rosa, V.; De Candia, P. The Multifaceted Interface between Cytokines and microRNAs: An Ancient Mechanism to Regulate the Good and the Bad of Inflammation. Front. Immunol. 2018, 9, 3012. [Google Scholar] [CrossRef]
- Manjili, D.A.; Babaei, F.N.; Younesirad, T.; Ghadir, S.; Askari, H.; Daraei, A. Dysregulated Circular RNA and Long Non-Coding RNA-Mediated Regulatory Competing Endogenous RNA Networks (ceRNETs) in Ovarian and Cervical Cancers: A Non-Coding RNA-Mediated Mechanism of Chemotherapeutic Resistance with New Emerging Clinical Capacities. Arch. Biochem. Biophys. 2025, 768, 110389. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Xie, Q.; Zhang, S.; Liang, X.; Li, S.; Zhang, P. Identification of a lncRNA/circRNA-miRNA-mRNA Network in Nasopharyngeal Carcinoma by Deep Sequencing and Bioinformatics Analysis. J. Cancer 2024, 15, 1916–1928. [Google Scholar] [CrossRef]
- Farré, P.L.; Duca, R.B.; Massillo, C.; Dalton, G.N.; Graña, K.D.; Gardner, K.; Lacunza, E.; De Siervi, A. MiR-106b-5p: A Master Regulator of Potential Biomarkers for Breast Cancer Aggressiveness and Prognosis. Int. J. Mol. Sci. 2021, 22, 11135. [Google Scholar] [CrossRef] [PubMed]
- Elguindy, M.M.; Young, J.S.; Ho, W.S.; Lu, R.O. Co-Evolution of Glioma and Immune Microenvironment. J. Immunother. Cancer 2024, 12, e009175. [Google Scholar] [CrossRef] [PubMed]
- J Dabrowski, M.; Wojtas, B. Global DNA Methylation Patterns in Human Gliomas and Their Interplay with Other Epigenetic Modifications. Int. J. Mol. Sci. 2019, 20, 3478. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Natsume, A. Overview of DNA Methylation in Adult Diffuse Gliomas. Brain Tumor Pathol. 2019, 36, 84–91. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, Y.; Wang, C.; Tong, S.; He, Y.; Lu, W.; Zhang, Z. Epigenetic Clocks and Gliomas: Unveiling the Molecular Interactions between Aging and Tumor Development. Front. Mol. Biosci. 2024, 11, 1446428. [Google Scholar] [CrossRef]
- Wei, J.; Chen, P.; Gupta, P.; Ott, M.; Zamler, D.; Kassab, C.; Bhat, K.P.; Curran, M.A.; de Groot, J.F.; Heimberger, A.B. Immune Biology of Glioma-Associated Macrophages and Microglia: Functional and Therapeutic Implications. Neuro Oncol. 2020, 22, 180–194. [Google Scholar] [CrossRef]
- Tong, N.; He, Z.; Ma, Y.; Wang, Z.; Huang, Z.; Cao, H.; Xu, L.; Zou, Y.; Wang, W.; Yi, C.; et al. Tumor Associated Macrophages, as the Dominant Immune Cells, Are an Indispensable Target for Immunologically Cold Tumor-Glioma Therapy? Front. Cell Dev. Biol. 2021, 9, 706286. [Google Scholar] [CrossRef]
- Hao, J.; Cao, Y.; Yu, H.; Zong, L.; An, R.; Xue, Y. Effect of MAP3K8 on Prognosis and Tumor-Related Inflammation in Renal Clear Cell Carcinoma. Front. Genet. 2021, 12, 674613. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Willis-Owen, S.A.G.; Wong, K.C.C.; Farrow, S.N.; Cookson, W.O.C.; Moffatt, M.F.; Zhang, Y. MAP3K8 Is a Potential Therapeutic Target in Airway Epithelial Inflammation. J. Inflamm. 2024, 21, 27. [Google Scholar] [CrossRef]
- Gianì, F.; Russo, G.; Pennisi, M.; Sciacca, L.; Frasca, F.; Pappalardo, F. Computational Modeling Reveals MAP3K8 as Mediator of Resistance to Vemurafenib in Thyroid Cancer Stem Cells. Bioinformatics 2019, 35, 2267–2275. [Google Scholar] [CrossRef]
- Kukreja, L.; Li, C.J.; Ezhilan, S.; Iyer, V.R.; Kuo, J.S. Emerging Epigenetic Therapies for Brain Tumors. Neuromol Med. 2022, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Ryu, H.-W.; Won, H.-R.; Kwon, S.H. Advances in Epigenetic Glioblastoma Therapy. Oncotarget 2017, 8, 18577–18589. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.; Shah, A.H.; Di, L.; Rivas, S.; Suter, R.K.; Eichberg, D.G.; Luther, E.; Lu, V.; Morell, A.A.; Ivan, M.E.; et al. Systematic Review of Epigenetic Therapies for Treatment of IDH-Mutant Glioma. World Neurosurg. 2022, 162, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Kulma, A.; Gutowicz, J. Up-Regulation of Key Glycolysis Proteins in Cancer Development. Open Life Sci. 2018, 13, 569–581. [Google Scholar] [CrossRef]
- Chen, S.; Luo, X.; Wang, W.; Chen, X.-H.; Ma, N.; Zhu, X.-Y.; Zhou, T.; Gao, Q.-J.; Zhao, D.-W. ACTN1 Promotes Cell Invasion, Migration, and EMT in Thyroid Cancer and Is Associated with Immune Infiltration. Sci. Rep. 2024, 14, 32060. [Google Scholar] [CrossRef]
- Staszkiewicz, R.; Sobański, D.; Pulka, W.; Gładysz, D.; Gadzieliński, M.; Strojny, D.; Grabarek, B.O. Variances in the Expression Profile of Circadian Clock-Related Genes in Astrocytic Brain Tumors. Cancers 2024, 16, 2335. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological Systems Database as a Model of the Real World. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective microRNA Target Sites in Mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An Online Database for Prediction of Functional microRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X. Prediction of Functional microRNA Targets by Integrative Modeling of microRNA Binding and Target Expression Data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Li, L.-C.; Dahiya, R. MethPrimer: Designing Primers for Methylation PCRs. Bioinformatics 2002, 18, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
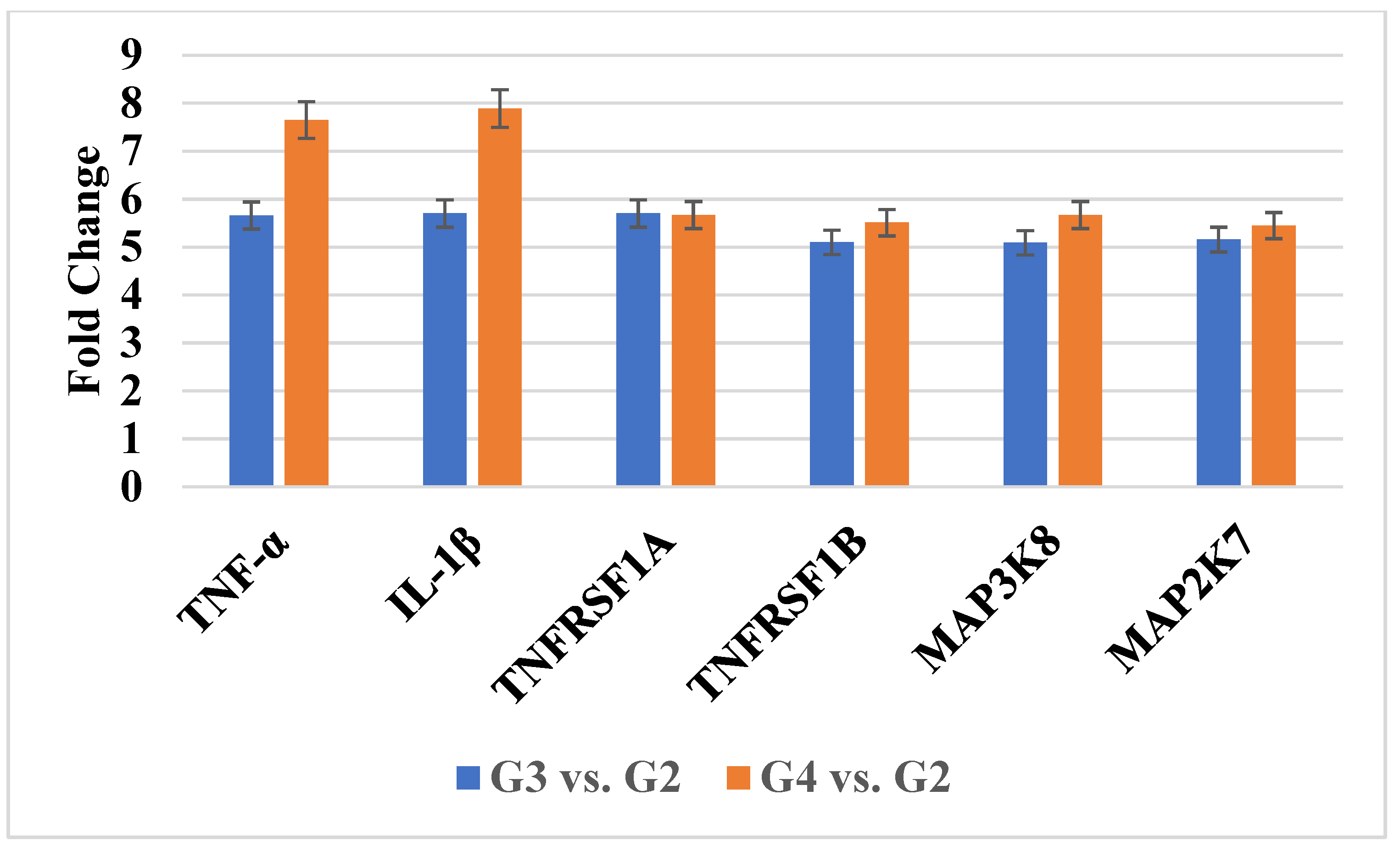
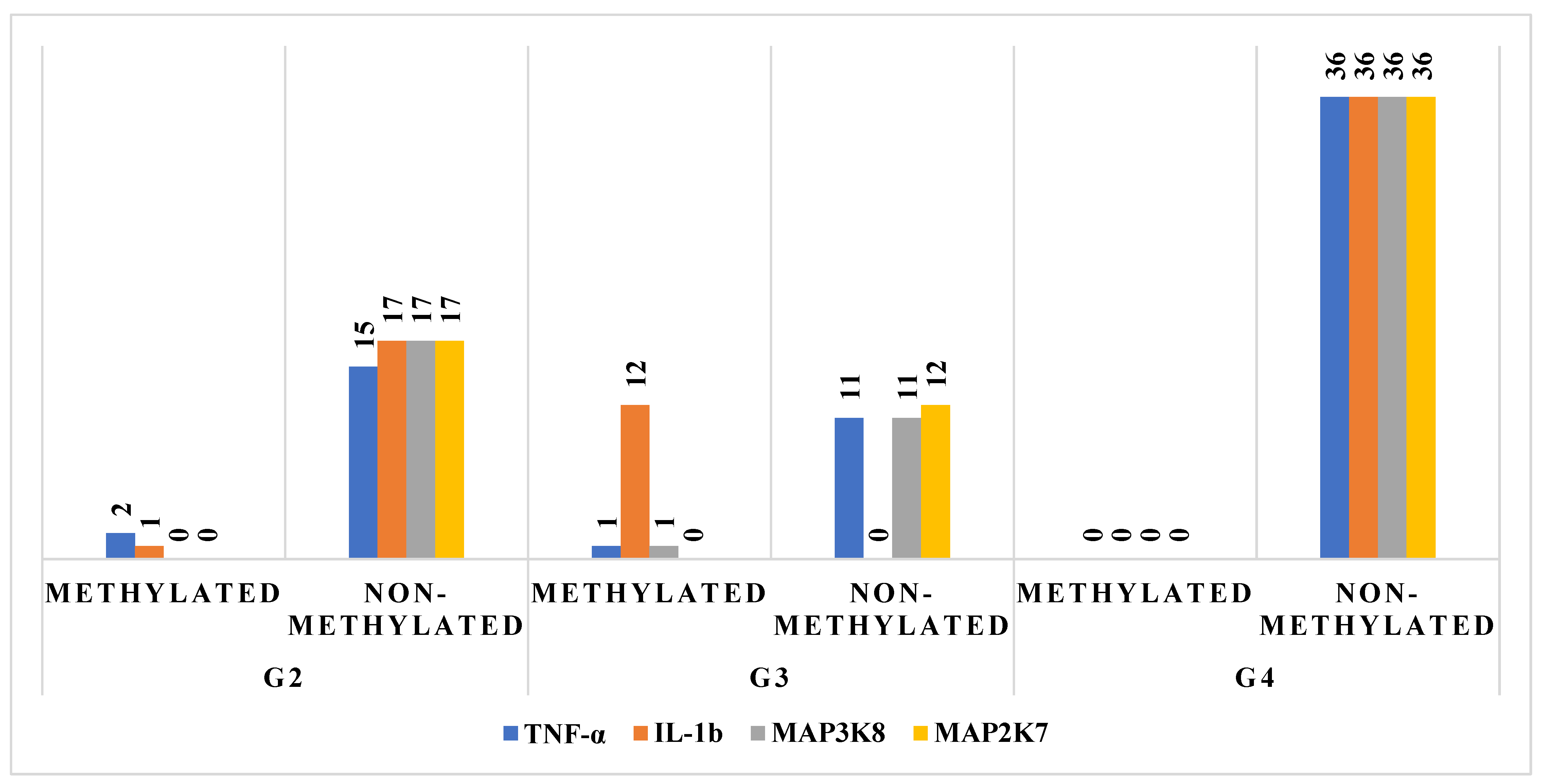
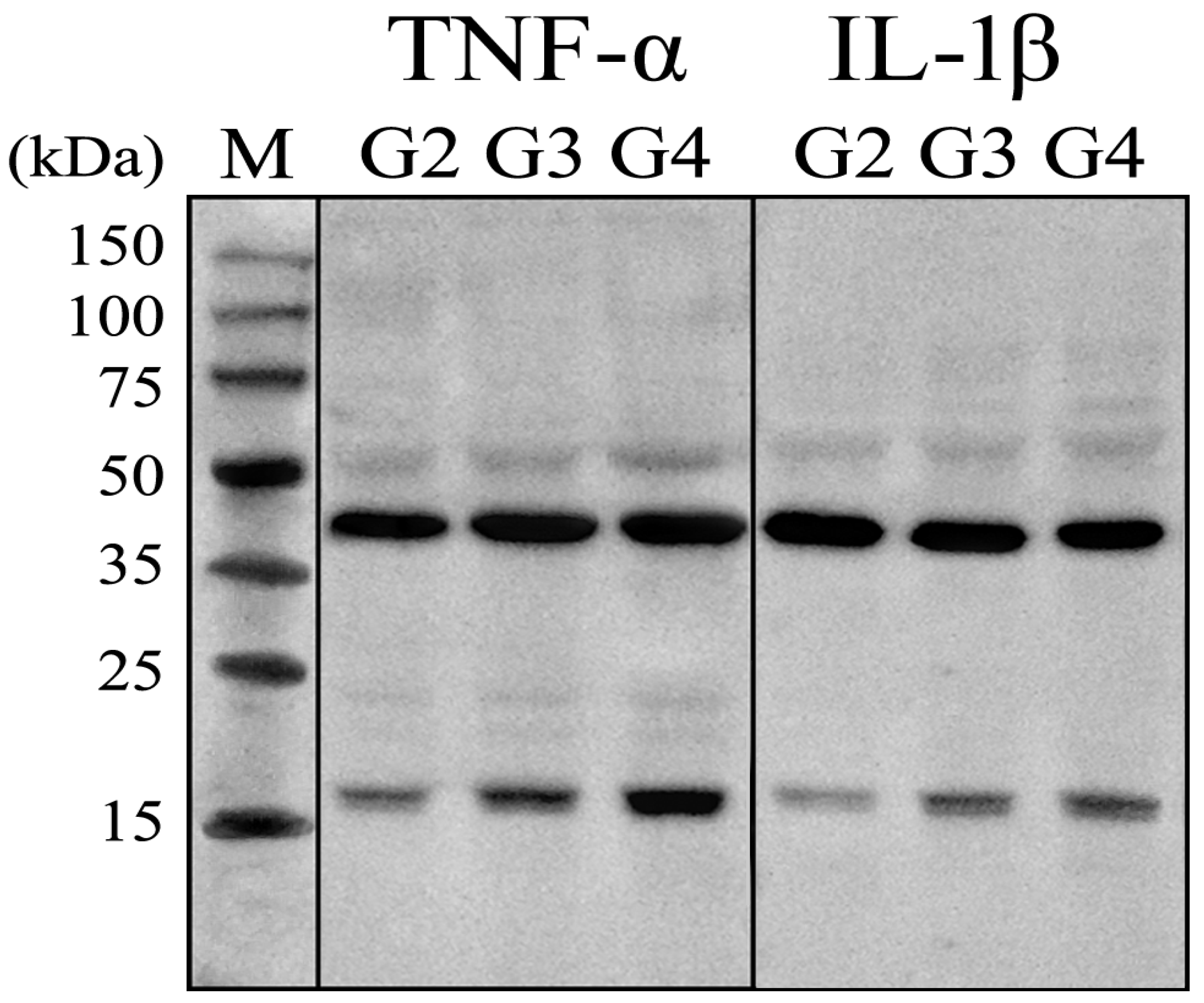
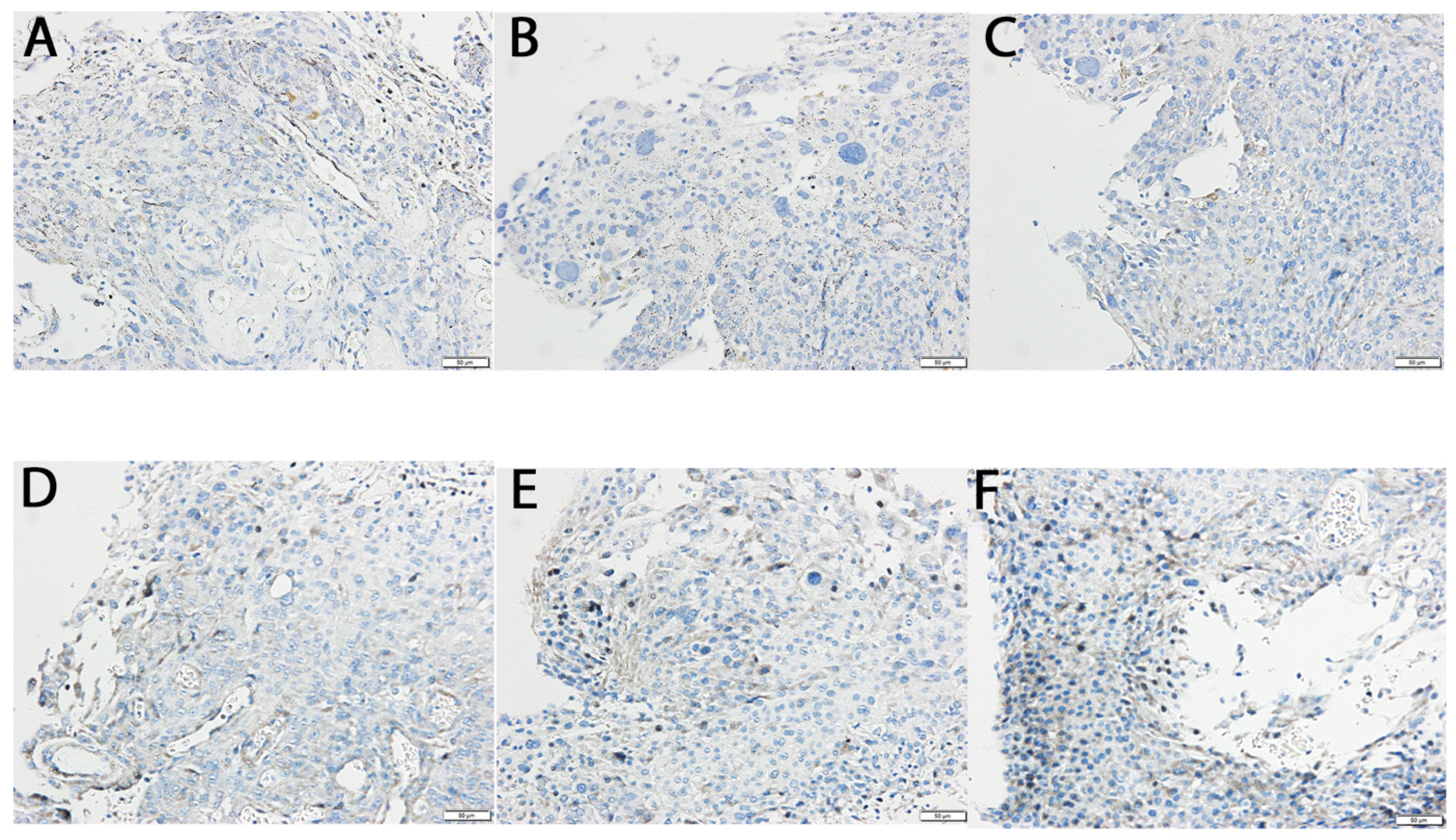




| ID | mRNA | log2FC G3 vs. G2 | log2FC G4 vs. G2 |
|---|---|---|---|
| 207113_s_at | TNF-α | 5.98 ± 0.34 | 8.19 ± 0.33 |
| 1563357_at | 5.89 ± 0.43 | 8.76 ± 0.13 | |
| 39402_at | IL-1β | 5.56 ± 0.56 | 7.23 ± 0.98 |
| 207643_s_at | TNFRSF1A | 5.09 ± 0.71 | 5.56 ± 0.77 |
| 203508_at | TNFRSF1B | 5.10 ± 0.81 | 5.51 ± 0.98 |
| 205027_s_at | MAP3K8 | 5.11 ± 0.44 | 5.87 ± 0.19 |
| 235421_at | 5.14 ± 0.67 | 5.67 ± 0.34 | |
| 209951_s_at | MAP2K7 | 5.33 ± 0.81 | 5.71 ± 0.55 |
| 209952_s_at | 5.43 ± 0.19 | 6.03 ± 0.63 | |
| 216206_x_at | 5.16 ± 0.34 | 6.01 ± 0.76 | |
| 226023_at | 5.77 ± 0.81 | 5.61 ± 0.99 | |
| 226053_at | 5.18 ± 0.18 | 5.78 ± 1.01 |
| mRNA | miRNA | Target Score | G3 vs. G2 (FC) | G4 vs. G2 (FC) |
|---|---|---|---|---|
| TNF-α | hsa-miR-34a-3p | 96 | −2.42 ± 0.71 | −2.12 ± 0.34 |
| IL-1β | hsa-miR-30a-3p | 83 | −1.72 ± 0.21 | −2.87 ± 0.21 |
| hsa-miR-30d-3p | −1.88 ± 0.19 | −2.10 ± 0.19 | ||
| hsa-miR-30e-3p | −3.45 ± 0.33 | −3.78 ± 0.78 | ||
| MAP3K8 | hsa-miR-106b-5p | 86 | +1.98 ± 0.18 | +2.01 ± 0.42 |
| MAP2K7 | hsa-miR-27b-3p | 86 | +1.71 ± 0.51 | +2.43 ± 0.10 |
| Protein | G2 | G3 | G4 |
|---|---|---|---|
| TNF-α [pg/mL] | 237.89 ± 19.12 | 376.12 ± 21.87 * | 381.91 ± 23.98 * |
| IL-1β [pg/mL] | 67.12 ± 8.19 | 89.91 ± 10.41 * | 100.12 ± 12.56 * |
| TNFRSF1A [ng/mL] | 1.23 ± 0.11 | 1.45 ± 0.09 | 1.76 ± 0.23 |
| TNFRSF1B [ng/mL] | 0.98 ± 0.12 | 1.34 ± 0.22 | 1.50 ± 0.19 |
| MAP3K8 [ng/mL] | 13.98 ± 0.98 | 18.71 ± 1.23 | 19.18 ± 1.34 |
| MAP2K7 [ng/mL] | 17.18 ± 1.78 | 18.98 ± 2.71 | 18.45 ± 2.09 |
| mRNA | RT-qPCR Amplification Primers (5′-3′) |
|---|---|
| TNF-α | Forward: AGGACCAGCTAAGAGGGAGA Reverse: CCCGGATCATGCTTTCAGTG |
| IL-1β | Forward: GGAGAATGACCTGAGCACCT Reverse: GGAGGTGGAGAGCTTTCAGT |
| TNFRSF1A | Forward: GCTGTACCAAGTGCCACAAA Reverse: CTCCACCTGACCCATTTCCT |
| TNFRSF1B | Forward: TTGTGTCTGCGTCTGTGTTG Reverse: GCTACACTGGTTTCCCCTCT |
| MAP3K8 | Forward: GGCCATTCAACCAAAGCAGA Reverse: GGTTTCTCTCCAGGGAAGCT |
| MAP2K7 | Forward: GGGACGTTCATCACCAACAC Reverse: CTTCAGGTAGTACAGCGCCT |
| ACTB | Forward: TCACCCACACTGTGCCCATCTACGA Reverse: CAGCGGAACCGCTCATTGCCAATGG |
| GAPDH | Forward: GGCCAGATCCTGTCCAAGC Reverse: GTGGGTTTCCACCATTAGCAC |
| mRNA | M/U | NCBI Reference Sequen | Primers (5′-3′) |
|---|---|---|---|
| TNF-α | M | NM_000594.4 | Forward: GAGTATTGAAAGTATGATTCGGGAC Reverse: CAAAATACAACAAACAAAAAAACGTA |
| U | Forward: GTATTGAAAGTATGATTTGGGATGT Reverse: AAAATACAACAAACAAAAAAACATA | ||
| IL-1β | M | NM_000576 | Forward: ATAGTAAGGGTTTTAGGTAGGTCGC Reverse: CGAAACGTACAATTCAATAATCGTA |
| U | Forward: AGTAAGGGTTTTAGGTAGGTTGTGT Reverse: CCAAAACATACAATTCAATAATCATA | ||
| TNFRSF1A | M | NM_001065.4 | Forward: GAGGCGTAATATAGTATGTTGGC Reverse: TCATCTAAAAAAACTAAACGCGAA |
| U | Forward: GAGGTGTAATATAGTATGTTGGTGA Reverse: ACCTCATCTAAAAAAACTAAACACAAA | ||
| TNFRSF1B | M | NM_001066.3 | Forward: GTTGAGTAAGTAGGAGGGGTGTC Reverse: ACTCCGAATAAAAAACGTAAACGTA |
| U | Forward: GTTGAGTAAGTAGGAGGGGTGTT Reverse: ACTCCAAATAAAAAACATAAACATA | ||
| MAP3K8 | M | NM_005204.4 | Forward: TCGTCGGATTTTAGTGGTTC Reverse: AAAAATTACATCTACGACCTTAACG |
| U | Forward: TGTTGGATTTTAGTGGTTTG Reverse: AAATTACATCTACAACCTTAACACT | ||
| MAP2K7 | M | NM_001297555.2 | Forward: GTATTTTGGGTAAGATGATAGTGGC Reverse: CTTAACTTTAAAATCCACCAAACGA |
| U | Forward: TATTTTGGGTAAGATGATAGTGGTG Reverse: TTAACTTTAAAATCCACCAAACAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skóra, K.; Strojny, D.; Sobański, D.; Staszkiewicz, R.; Gogol, P.; Miller, M.; Grabarek, B.O. Analysis of the Expression Patterns of Tumor Necrosis Factor Alpha Signaling Pathways and Regulatory MicroRNAs in Astrocytic Tumors. Int. J. Mol. Sci. 2025, 26, 5892. https://doi.org/10.3390/ijms26125892
Skóra K, Strojny D, Sobański D, Staszkiewicz R, Gogol P, Miller M, Grabarek BO. Analysis of the Expression Patterns of Tumor Necrosis Factor Alpha Signaling Pathways and Regulatory MicroRNAs in Astrocytic Tumors. International Journal of Molecular Sciences. 2025; 26(12):5892. https://doi.org/10.3390/ijms26125892
Chicago/Turabian StyleSkóra, Klaudia, Damian Strojny, Dawid Sobański, Rafał Staszkiewicz, Paweł Gogol, Mateusz Miller, and Beniamin Oskar Grabarek. 2025. "Analysis of the Expression Patterns of Tumor Necrosis Factor Alpha Signaling Pathways and Regulatory MicroRNAs in Astrocytic Tumors" International Journal of Molecular Sciences 26, no. 12: 5892. https://doi.org/10.3390/ijms26125892
APA StyleSkóra, K., Strojny, D., Sobański, D., Staszkiewicz, R., Gogol, P., Miller, M., & Grabarek, B. O. (2025). Analysis of the Expression Patterns of Tumor Necrosis Factor Alpha Signaling Pathways and Regulatory MicroRNAs in Astrocytic Tumors. International Journal of Molecular Sciences, 26(12), 5892. https://doi.org/10.3390/ijms26125892







