Antioxidant and Cytotoxic Evaluation of Aqueous Extracts from Hymenochaetaceae Fungi Associated with Endemic Chilean Sclerophyll Forest Trees
Abstract
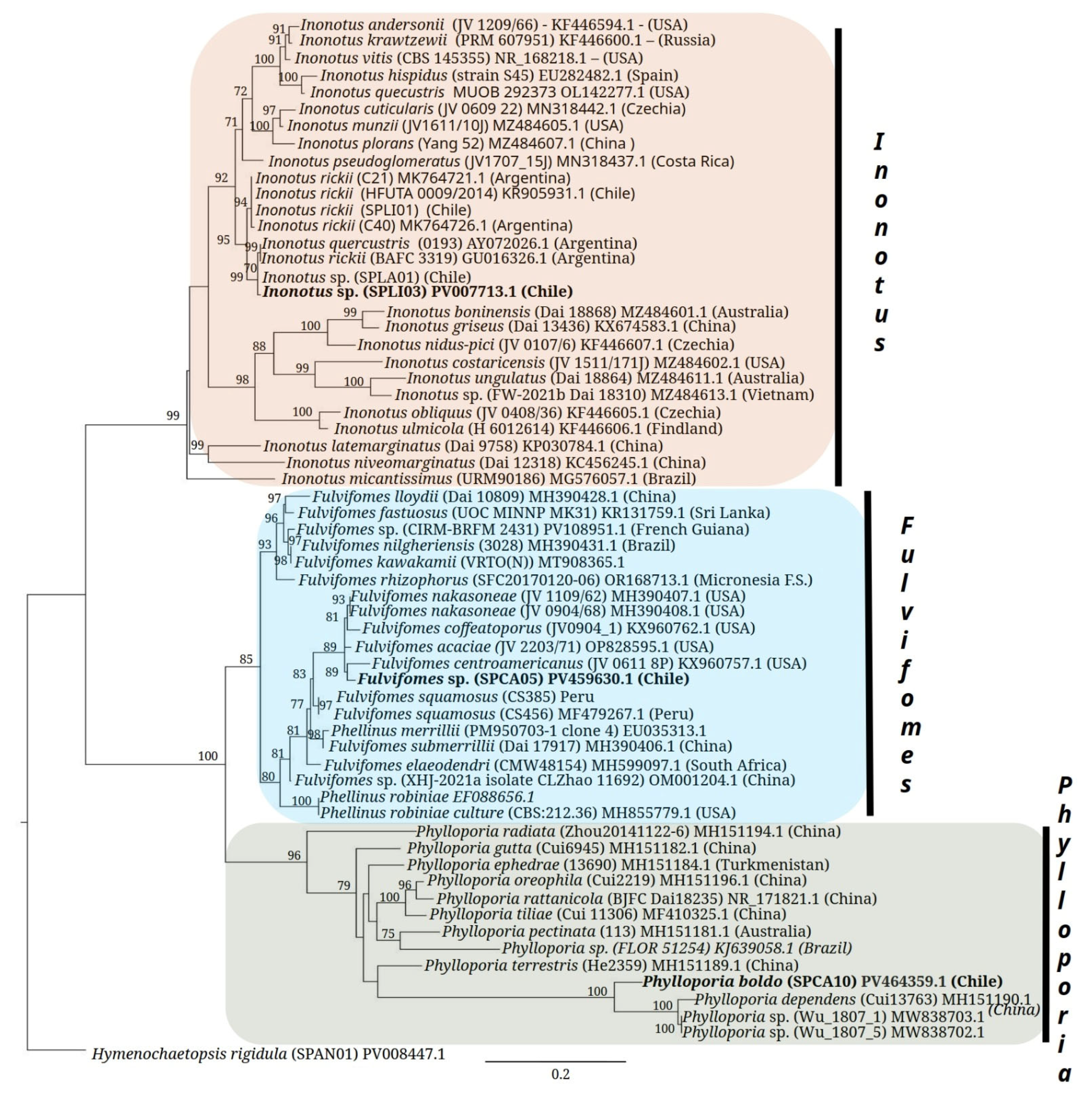
1. Introduction
2. Results and Discussion
2.1. Characterization of Aqueous Extract
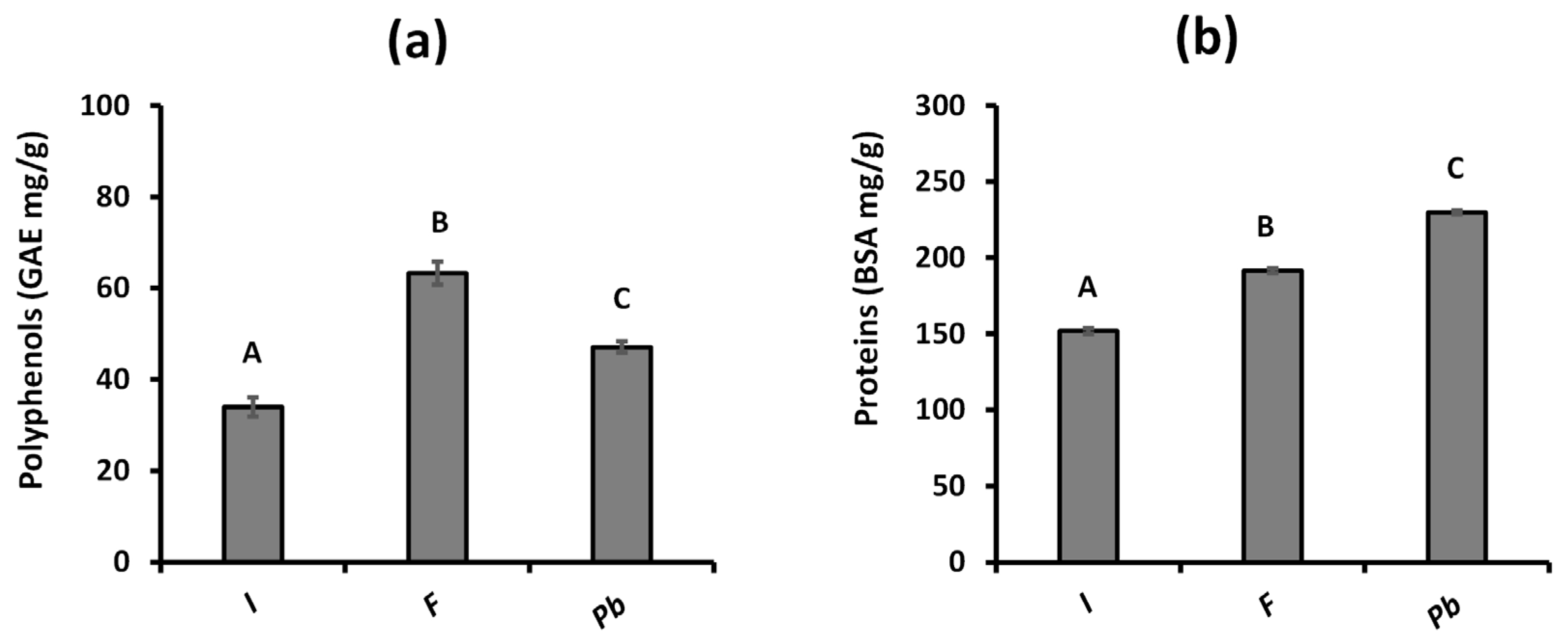
2.1.1. Determination of Total Polyphenols and Proteins
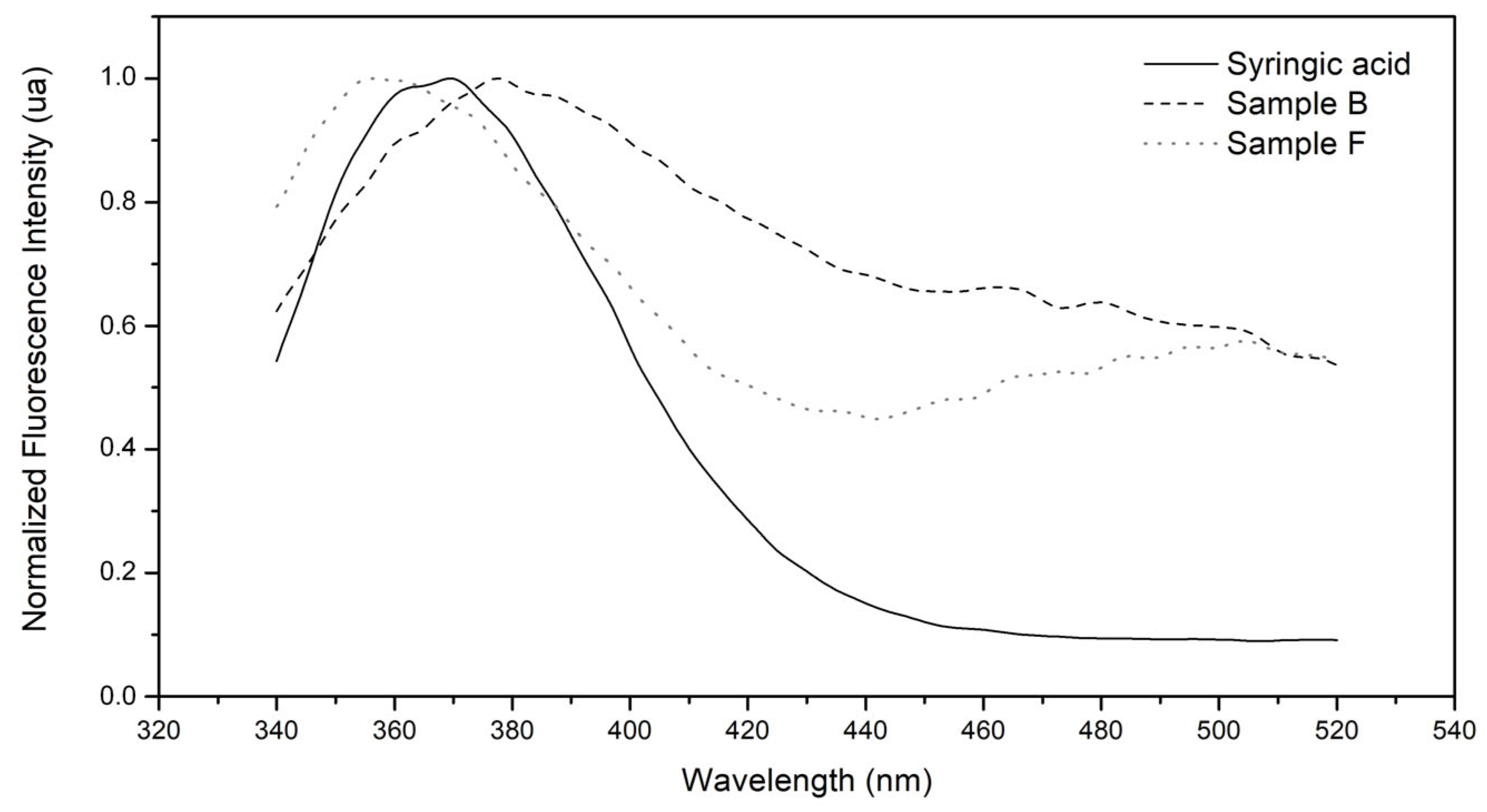
2.1.2. Identification of Phenolic Compounds
2.2. Antioxidant Activity
2.2.1. DPPH Scavenging Assay
2.2.2. ABTS Scavenging Assay
2.2.3. Hydroxyl Radical Scavenging Assay
2.3. Cytotoxicity
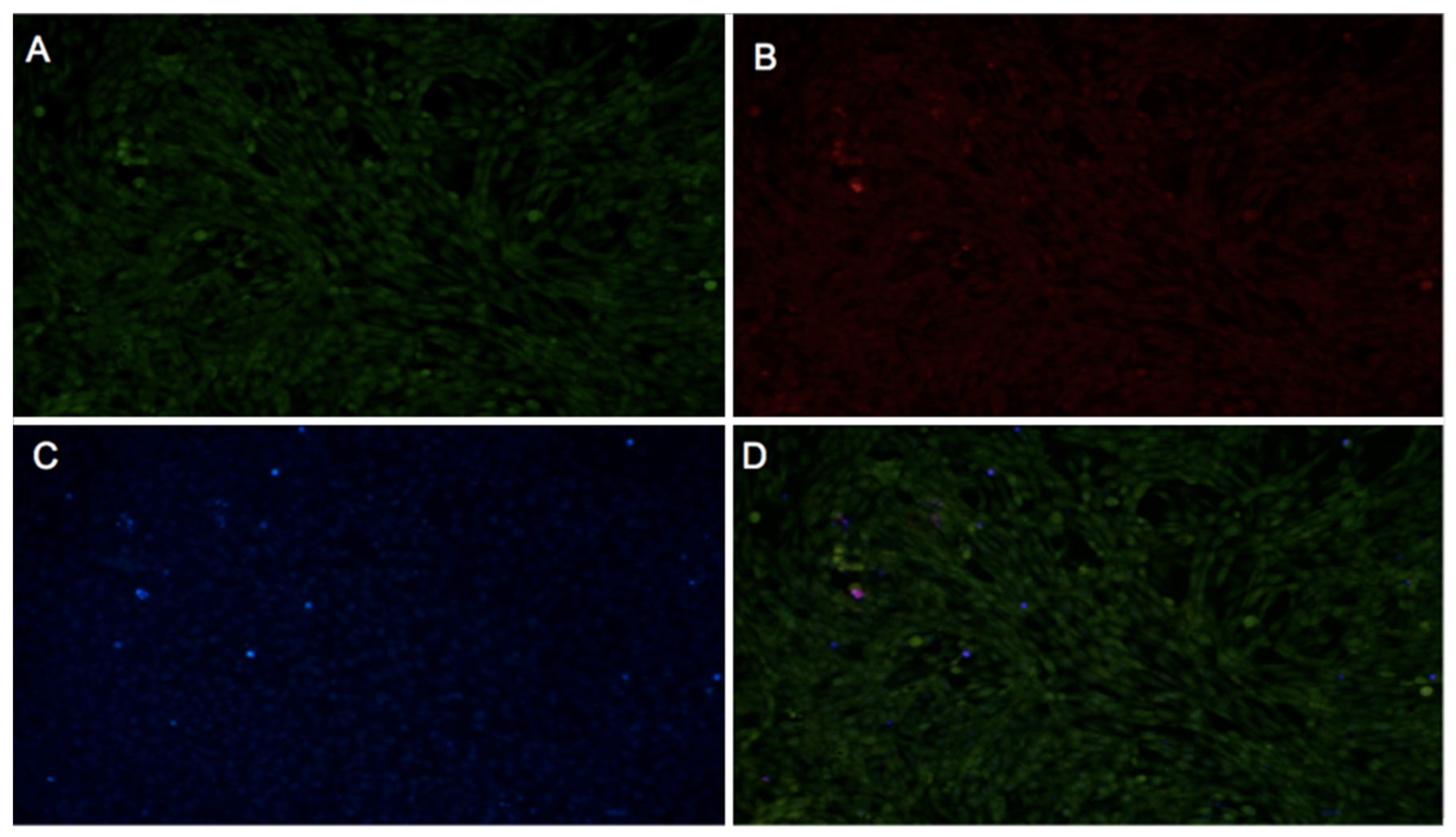
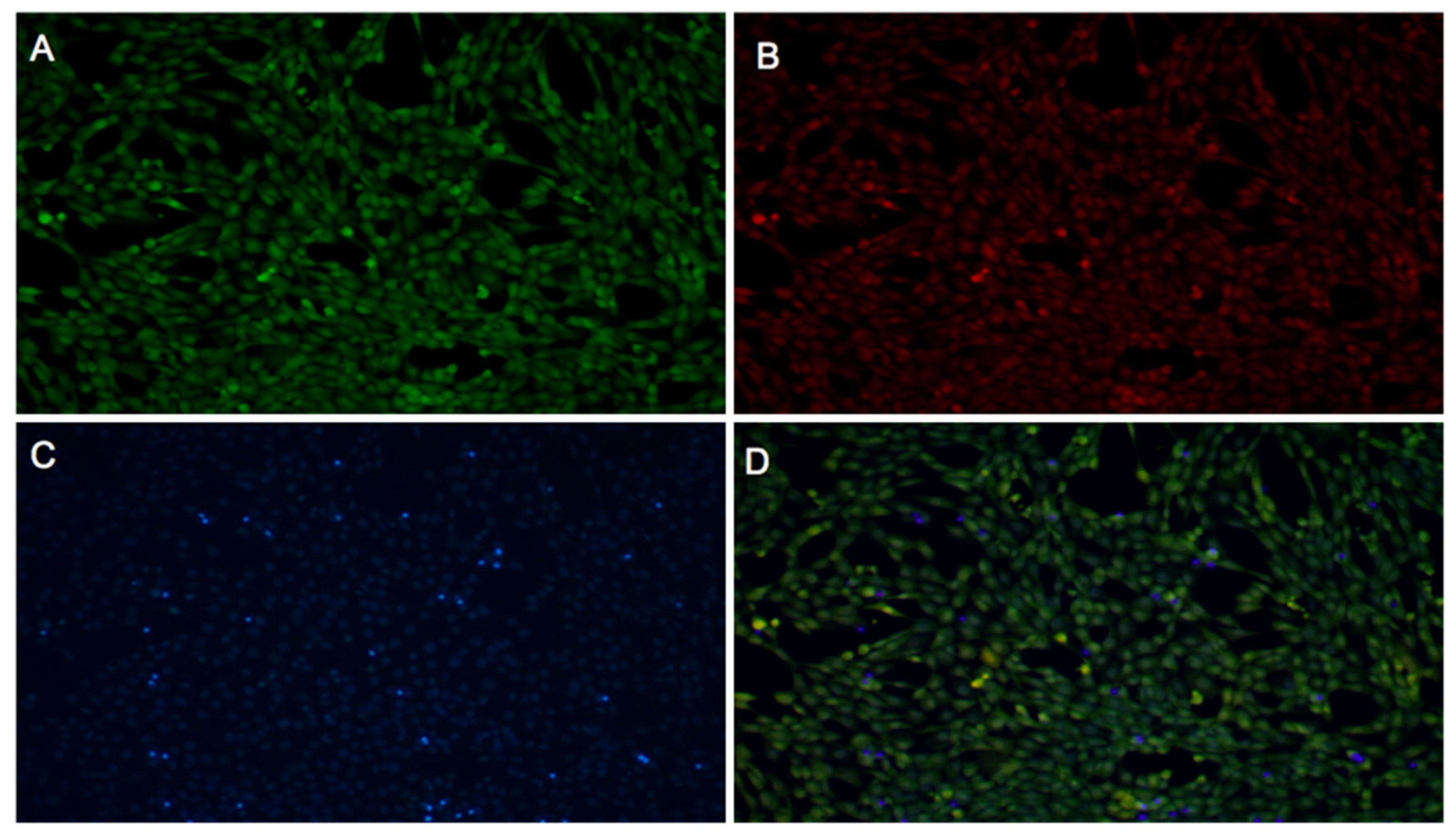
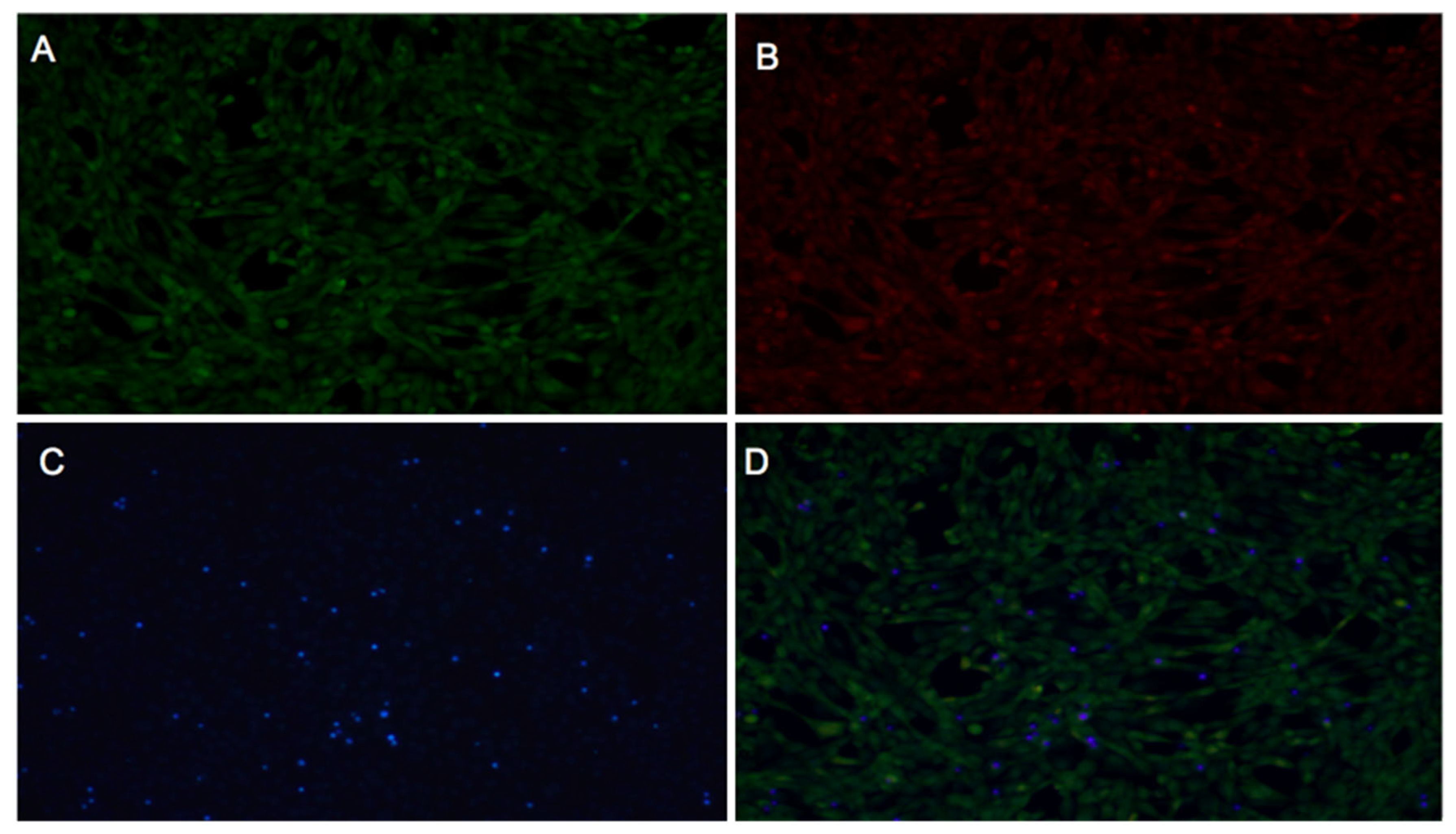
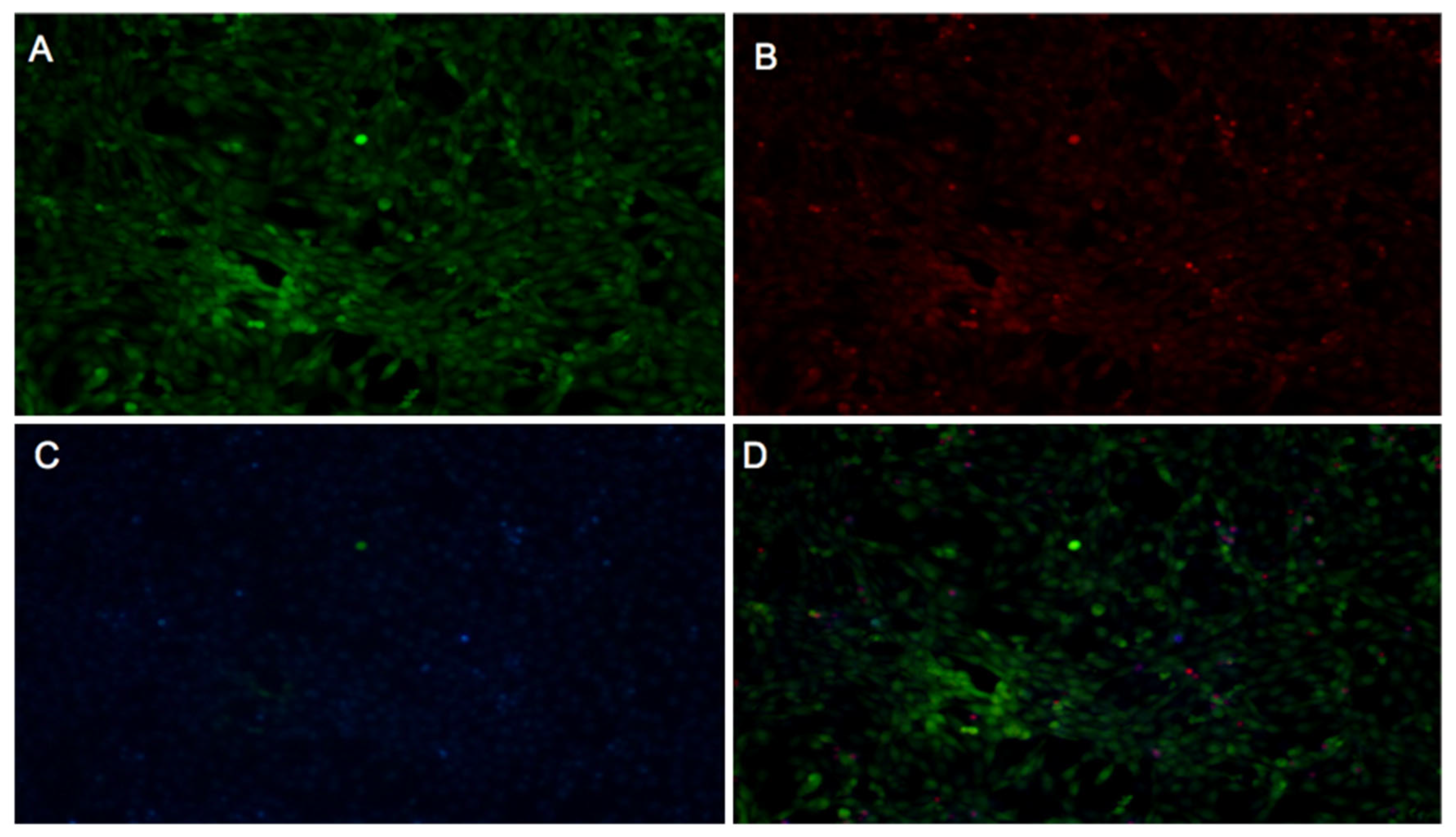
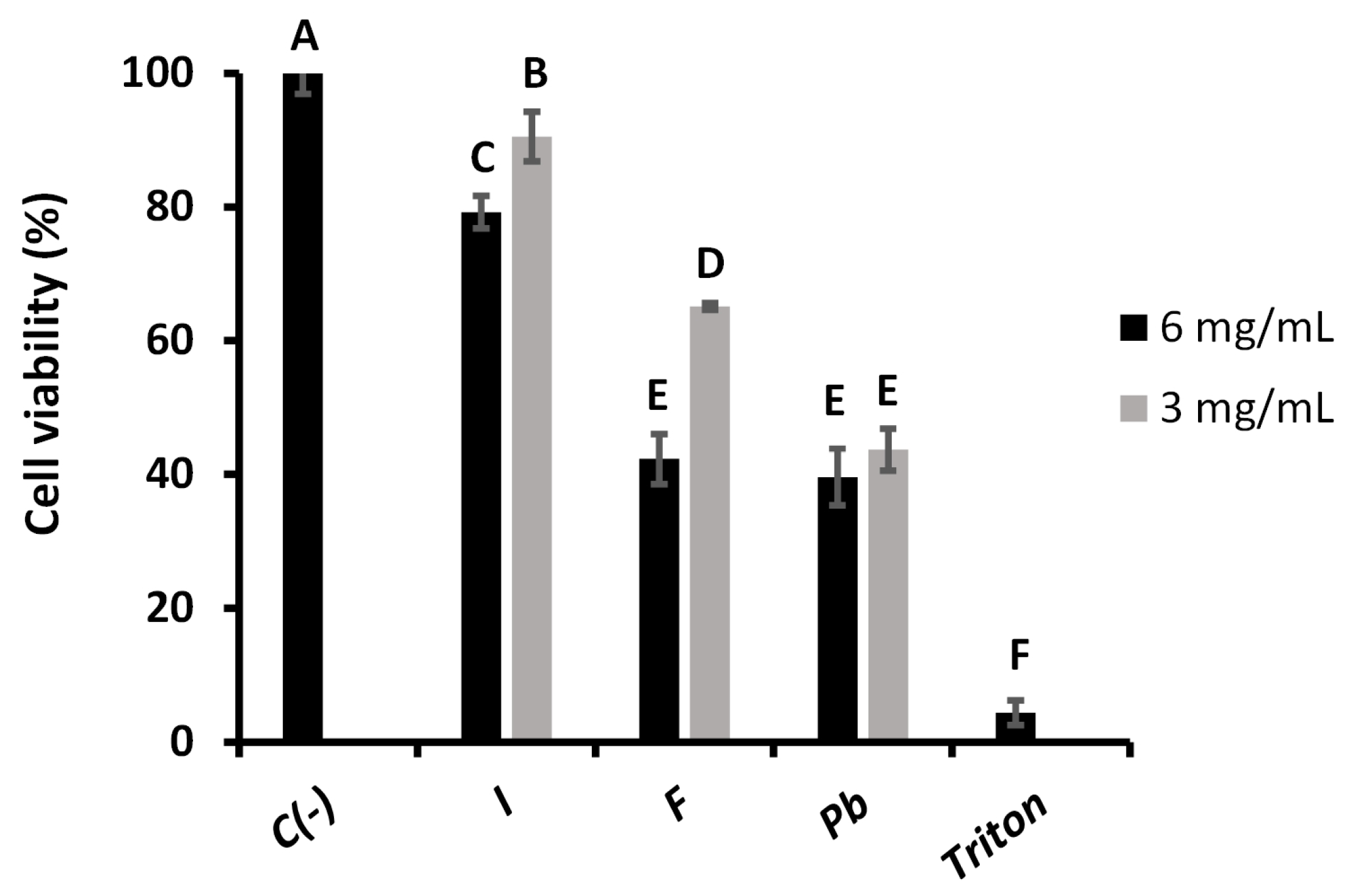
2.3.1. Fibroblast Viability
2.3.2. WST-1 Cell Proliferation Assay
3. Materials and Methods
3.1. Materials and Chemical
3.2. Preparation of Fungal Aqueous Extracts
3.3. Characterization of Aqueous Extracts
3.3.1. Determination of Total Phenolic Compounds
3.3.2. Determination of Total Protein
3.3.3. Identification of Phenolic Compounds
3.4. Antioxidant Activity
3.4.1. DPPH Scavenging Assay
3.4.2. ABTS Scavenging Assay
3.4.3. Hydroxyl Radical Scavenging Assay
3.5. Cytotoxicity
3.5.1. Fibroblast Viability
3.5.2. WST-1 Cell Proliferation Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein Oxidation—Formation Mechanisms, Detection and Relevance as Biomarkers in Human Diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef] [PubMed]
- Berglund, H.; Jönsson, M.T.; Penttilä, R.; Vanha-Majamaa, I. The Effects of Burning and Dead-Wood Creation on the Diversity of Pioneer Wood-Inhabiting Fungi in Managed Boreal Spruce Forests. For. Ecol. Manag. 2011, 261, 1293–1305. [Google Scholar] [CrossRef]
- Guerra, F.; Badilla, L.; Cautín, R.; Castro, M. In Vitro Propagation of Peumus Boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile. Horticulturae 2023, 9, 1032. [Google Scholar] [CrossRef]
- Otero, C.; Klagges, C.; Morales, B.; Sotomayor, P.; Escobar, J.; Fuentes, J.A.; Moreno, A.A.; Llancalahuen, F.M.; Arratia-Perez, R.; Gordillo-Fuenzalida, F.; et al. Anti-Inflammatory Chilean Endemic Plants. Pharmaceutics 2023, 15, 897. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhang, A.; Zhang, W.; Cui, G.; Wang, S.; Duan, J. Antioxidative Properties of Crude Polysaccharides from Inonotus obliquus. Int. J. Mol. Sci. 2012, 13, 9194–9206. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Lee, I.-K.; Seok, S.-J.; Lee, H.-J.; Kim, Y.-H.; Yun, B.-S. Antioxidant Polyphenols from the Mycelial Culture of the Medicinal Fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef]
- Huang, S.; Ding, S.; Fan, L. Antioxidant Activities of Five Polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef]
- Aqueveque, P.; Anke, T.; Saéz, K.; Silva, M.; Becerra, J. Antimicrobial Activity of Submerged Cultures of Chilean Basidiomycetes. Planta Med. 2010, 76, 1787–1791. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus Obliquus—From Folk Medicine to Clinical Use. J. Tradit. Complement. Med. 2021, 11, 293–302. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Lei, Z.; Yang, Y.; Sugiura, N. Comparative Study of Antioxidant Activity and Antiproliferative Effect of Hot Water and Ethanol Extracts from the Mushroom Inonotus obliquus. J. Biosci. Bioeng. 2009, 107, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rony, K.A.; Ajith, T.A.; Mathew, J.; Janardhanan, K.K. The Medicinal Cracked-Cap Polypore Mushroom Phellinus rimosus (Higher Basidiomycetes) Attenuates Alloxan-Induced Hyperglycemia and Oxidative Stress in Rats. Int. J. Med. Mushrooms 2013, 15, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.; Adhikari, A.; Nanayakkara, C.; De Silva, E.D.; Wijesundera, R.; Soysa, P. Cytotoxic Effects of Ergone, a Compound Isolated from Fulviformes fastuosus. BMC Complement. Altern. Med. 2016, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, V.A. Actividad Anticancerígena y Antioxidante In Vitro de Polisacáridos Ácidos Obtenidos Desde Hongos Pertenecientes a La Familia Hymenochaetaceae Asociados a Bosque Nativo de Chile. Ph.D. Thesis, Universidad de Concepción, Concepción, Chile, 2022. [Google Scholar]
- Zhao, H.; Zhang, M.; Liu, Q.; Wang, X.; Zhao, R.; Geng, Y.; Wong, T.; Li, S.; Wang, X. A Comprehensive Screening Shows That Ergothioneine Is the Most Abundant Antioxidant in the Wild Macrofungus Phylloporia ribis Ryvarden. J. Environ. Sci. Health Part C 2018, 36, 98–111. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Parniakov, O.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Pulsed Electric Field Assisted Pressure Extraction and Solvent Extraction from Mushroom (Agaricus bisporus). Food Bioprocess. Technol. 2014, 7, 174–183. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Wickramasinghe, M.A.; Nadeeshani, H.; Sewwandi, S.M.; Rathnayake, I.; Kananke, T.C.; Liyanage, R. Comparison of Nutritional Composition, Bioactivities, and FTIR-ATR Microstructural Properties of Commercially Grown Four Mushroom Species in Sri Lanka; Agaricus bisporus, Pleurotus ostreatus, Calocybe sp. (MK-White), Ganoderma lucidum. Food Prod. Process. Nutr. 2023, 5, 43. [Google Scholar] [CrossRef]
- Prasad, R.; Varshney, V.K.; Harsh, N.S.K.; Kumar, M. Antioxidant Capacity and Total Phenolics Content of the Fruiting Bodies and Submerged Cultured Mycelia of Sixteen Higher Basidiomycetes Mushrooms from India. Int. J. Med. Mushrooms 2015, 17, 933–941. [Google Scholar] [CrossRef]
- Ketnawa, S.; Rawdkuen, S. Properties of Texturized Vegetable Proteins from Edible Mushrooms by Using Single-Screw Extruder. Foods 2023, 12, 1269. [Google Scholar] [CrossRef]
- Ribeiro, B.; Lopes, R.; Andrade, P.B.; Seabra, R.M.; Gonçalves, R.F.; Baptista, P.; Quelhas, I.; Valentão, P. Comparative Study of Phytochemicals and Antioxidant Potential of Wild Edible Mushroom Caps and Stipes. Food Chem. 2008, 110, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, S.; Tran, H.B.; Nishida, M.; Kaifuchi, S.; Suhara, H.; Doi, K.; Fukami, K.; Parajuli, G.P.; Shimizu, K. Antioxidative Activities of 62 Wild Mushrooms from Nepal and the Phenolic Profile of Some Selected Species. J. Nat. Med. 2016, 70, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Attri, S.; Kumar, A.; Mohana, P.; Singh, S.; Kaur, P.; Ram, E.; Dhingra, G.S.; Arora, S.; Singh, A.P. Valorization of Polypore Mushroom Phellinus Fastuosus by Analyzing Antioxidative, Antiproliferative and Apoptosis Induction Potential. Waste Biomass Valorization 2023, 14, 2659–2672. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.-A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, F.; Teng, C.; Qu, J. Optimization for the Extraction of Polyphenols from Inonotus obliquus and Its Antioxidation Activity. Prep. Bioch. Biotechnol. 2021, 51, 852–859. [Google Scholar] [CrossRef]
- Wong, S.; Leong, L.; Williamkoh, J. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Foglieni, C.; Meoni, C.; Davalli, A.M. Fluorescent Dyes for Cell Viability: An Application on Prefixed Conditions. Histochem. Cell Biol. 2001, 115, 223–229. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxidative Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef]
- Albornoz, V.; Casas-Arrojo, V.; Figueroa, F.; Riquelme, C.; Hernández, V.; Rajchenberg, M.; Smith, C.T.; Campos, V.L.; Abdala-Díaz, R.T.; Becerra, J.; et al. In Vitro Cytotoxic Capacity against Tumor Cell Lines and Antioxidant Activity of Acidic Polysaccharides Isolated from the Andean Patagonian Fungus Phylloporia boldo. Nat. Prod. Res. 2023, 37, 4274–4279. [Google Scholar] [CrossRef]
- Chun, S.; Gopal, J.; Muthu, M. Antioxidant Activity of Mushroom Extracts/Polysaccharides—Their Antiviral Properties and Plausible AntiCOVID-19 Properties. Antioxidants 2021, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, L.-W.; Vlasák, J.; Dai, Y.-C. Global Diversity and Systematics of Hymenochaetaceae with Poroid Hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- Rajchenberg, M.; Pildain, M.B.; Madriaga, D.C.; De Errasti, A.; Riquelme, C.; Becerra, J. New Poroid Hymenochaetaceae (Basidiomycota, Hymenochaetales) from Chile. Mycol. Prog. 2019, 18, 865–877. [Google Scholar] [CrossRef]
- Ossa, C.G.; Larridon, I.; Peralta, G.; Asselman, P.; Pérez, F. Development of Microsatellite Markers Using Next-Generation Sequencing for the Columnar Cactus Echinopsis chiloensis (Cactaceae). Mol. Biol. Evol. 2016, 43, 1315–1320. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Hall, T.A. A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Rambaut, A.; FigTree. Tree Figure Drawing Tool. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 16 June 2025).
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T. Effects of Non-Traditional Extraction Methods on Extracting Bioactive Compounds from Chaga Mushroom (Inonotus obliquus) Compared with Hot Water Extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Rosa, P.A.J.; Ferreira, I.F.; Aires-Barros, M.R. Chromatography-Free Recovery of Biopharmaceuticals through Aqueous Two-Phase Processing. Trends. Biotechnol. 2009, 27, 240–247. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Fryer, H.J.L.; Davis, G.E.; Manthorpe, M.; Varon, S. Lowry Protein Assay Using an Automatic Microtiter Plate Spectrophotometer. Anal. Biochem. 1986, 153, 262–266. [Google Scholar] [CrossRef]
- Elena Tarnok, M.; Aguilar, L.F.; Bravo, M.A. Nonchromatographic Analysis of Polyphenols in Red Wine Samples: Evaluation of Front-Face Fluorescence Spectroscopy and Third-Order Multivariate Calibration to Quantify Catechin and Epicatechin. Microchem. J. 2023, 195, 109506. [Google Scholar] [CrossRef]
- Nuñez, S.M.; Cárdenas, C.; Valencia, P.; Pinto, M.; Silva, J.; Pino-Cortés, E.; Almonacid, S. Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing. Foods 2023, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, M.S.; Ordoñez, R.M.; Zampini, I.C.; Sayago, J.E.; Isla, M.I. Nutritional and Antioxidant Properties of Geoffroea Decorticans, an Argentinean Fruit, and Derived Products (Flour, Arrope, Decoction and Hydroalcoholic Beverage). Food Res. Int. 2013, 54, 160–168. [Google Scholar] [CrossRef]
- Suhevic, J.; Malcervelli, D.; Torres, P.; Fratto, M.C. Determinación de La Calidad de Las Membranas Espermáticas Porcinas Mediante Triple Tinción Fluorocrómica En Semen Fresco y Refrigerado. SPERMOVA 2015, 5, 134–138. [Google Scholar] [CrossRef]
- Chávez, J.C.; Darszon, A.; Treviño, C.L.; Nishigaki, T. Quantitative Intracellular pH Determinations in Single Live Mammalian Spermatozoa Using the Ratiometric Dye SNARF-5F. Front. Cell Dev. Biol. 2020, 7, 366. [Google Scholar] [CrossRef]
- Jimeno-Romero, A.; Gwinner, F.; Müller, M.; Mariussen, E.; Soto, M.; Kohl, Y. Sea Bass Primary Cultures versus RTgill-W1 Cell Line: Influence of Cell Model on the Sensitivity to Nanoparticles. Nanomaterials 2021, 11, 3136. [Google Scholar] [CrossRef]
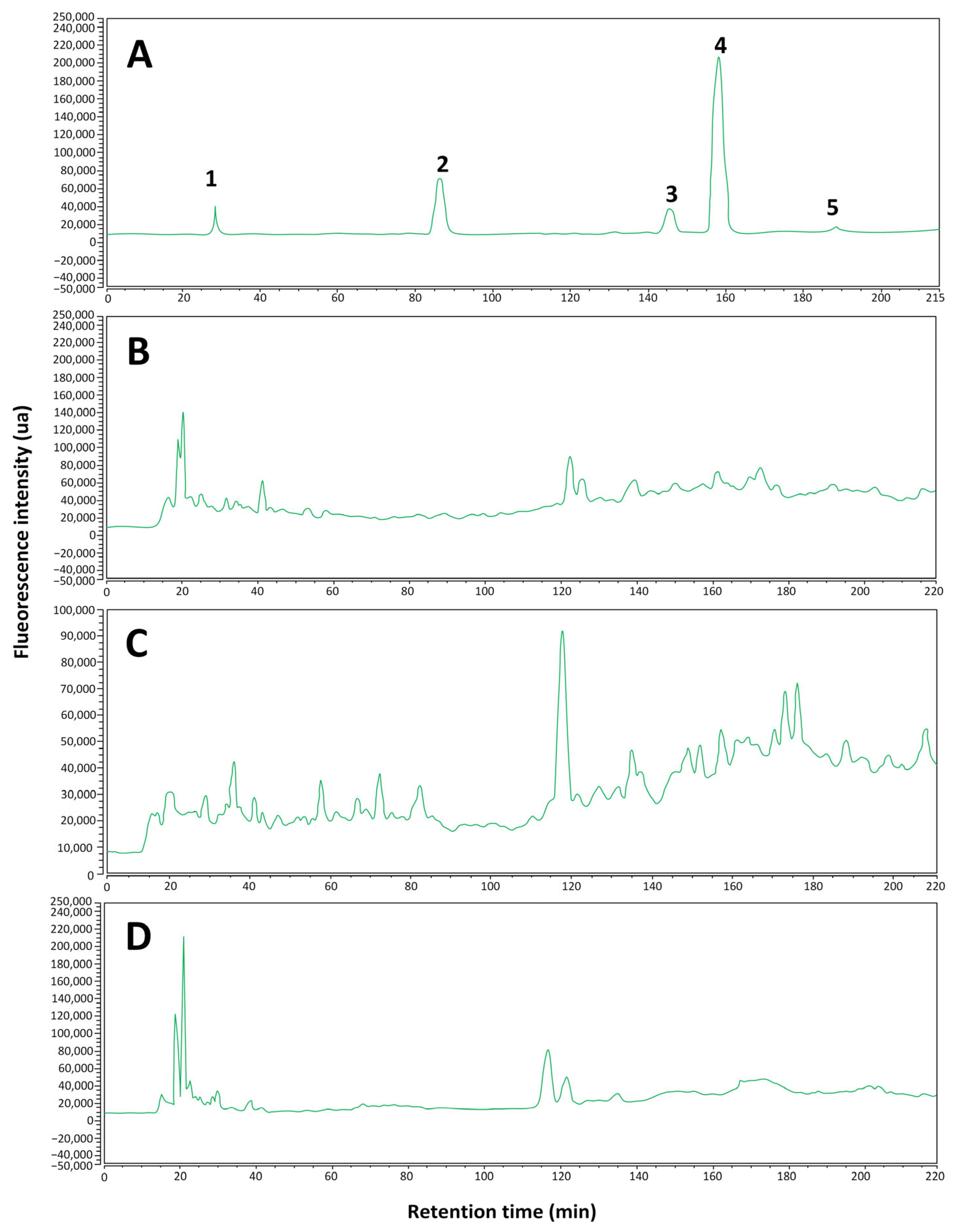
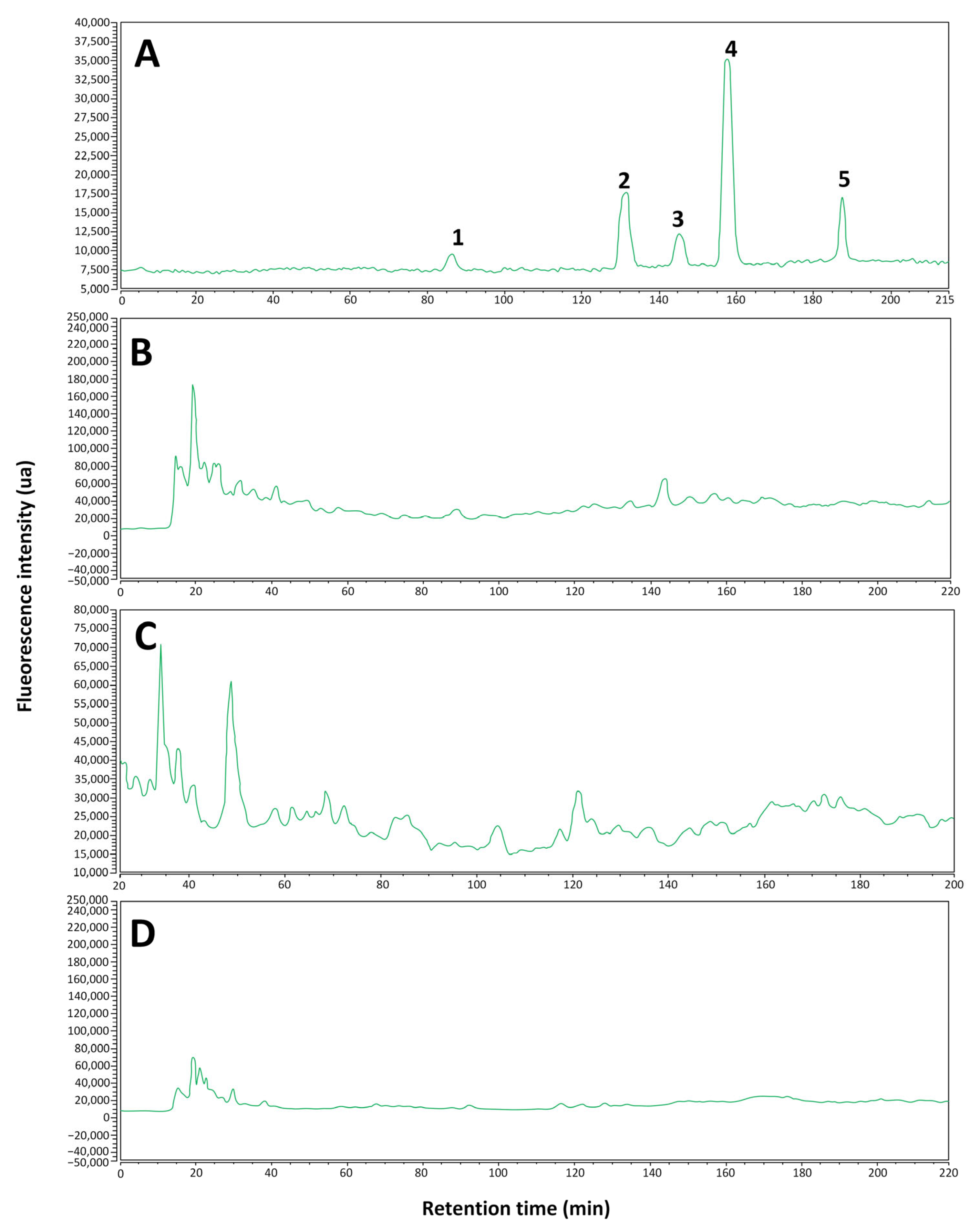



| Polyphenolic Compounds | Presence of Polyphenols in Aqueous Extract | ||
|---|---|---|---|
| Inonotus sp. | Fulvifomes sp. | P. boldo | |
| Gallic acid | no | no | no |
| Catechin | no | no | no |
| Epicatechin | no | no | no |
| Syringic acid | no | yes | yes |
| Caffeic acid | no | no | no |
| p-Coumaric acid | no | no | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuñez, S.M.; García, A.; Roman, T.; Aguilar, L.; Tarnok, M.E.; Guzmán, F.; Cárdenas, C.; Ponce, S.; Vásquez, D.; Carrasco, S.; et al. Antioxidant and Cytotoxic Evaluation of Aqueous Extracts from Hymenochaetaceae Fungi Associated with Endemic Chilean Sclerophyll Forest Trees. Int. J. Mol. Sci. 2025, 26, 5877. https://doi.org/10.3390/ijms26125877
Nuñez SM, García A, Roman T, Aguilar L, Tarnok ME, Guzmán F, Cárdenas C, Ponce S, Vásquez D, Carrasco S, et al. Antioxidant and Cytotoxic Evaluation of Aqueous Extracts from Hymenochaetaceae Fungi Associated with Endemic Chilean Sclerophyll Forest Trees. International Journal of Molecular Sciences. 2025; 26(12):5877. https://doi.org/10.3390/ijms26125877
Chicago/Turabian StyleNuñez, Suleivys M., Ahyra García, Tanya Roman, Luis Aguilar, María Elena Tarnok, Fanny Guzmán, Constanza Cárdenas, Sebastián Ponce, Dreidy Vásquez, Samuel Carrasco, and et al. 2025. "Antioxidant and Cytotoxic Evaluation of Aqueous Extracts from Hymenochaetaceae Fungi Associated with Endemic Chilean Sclerophyll Forest Trees" International Journal of Molecular Sciences 26, no. 12: 5877. https://doi.org/10.3390/ijms26125877
APA StyleNuñez, S. M., García, A., Roman, T., Aguilar, L., Tarnok, M. E., Guzmán, F., Cárdenas, C., Ponce, S., Vásquez, D., Carrasco, S., & Valín, J. L. (2025). Antioxidant and Cytotoxic Evaluation of Aqueous Extracts from Hymenochaetaceae Fungi Associated with Endemic Chilean Sclerophyll Forest Trees. International Journal of Molecular Sciences, 26(12), 5877. https://doi.org/10.3390/ijms26125877







