Rutin Attenuates Oxidative Stress Responses and Hepatocyte Metabolomics in β-Hydroxybutyric Acid-Induced Hepatocyte Injury in Calves
Abstract
1. Introduction
2. Results
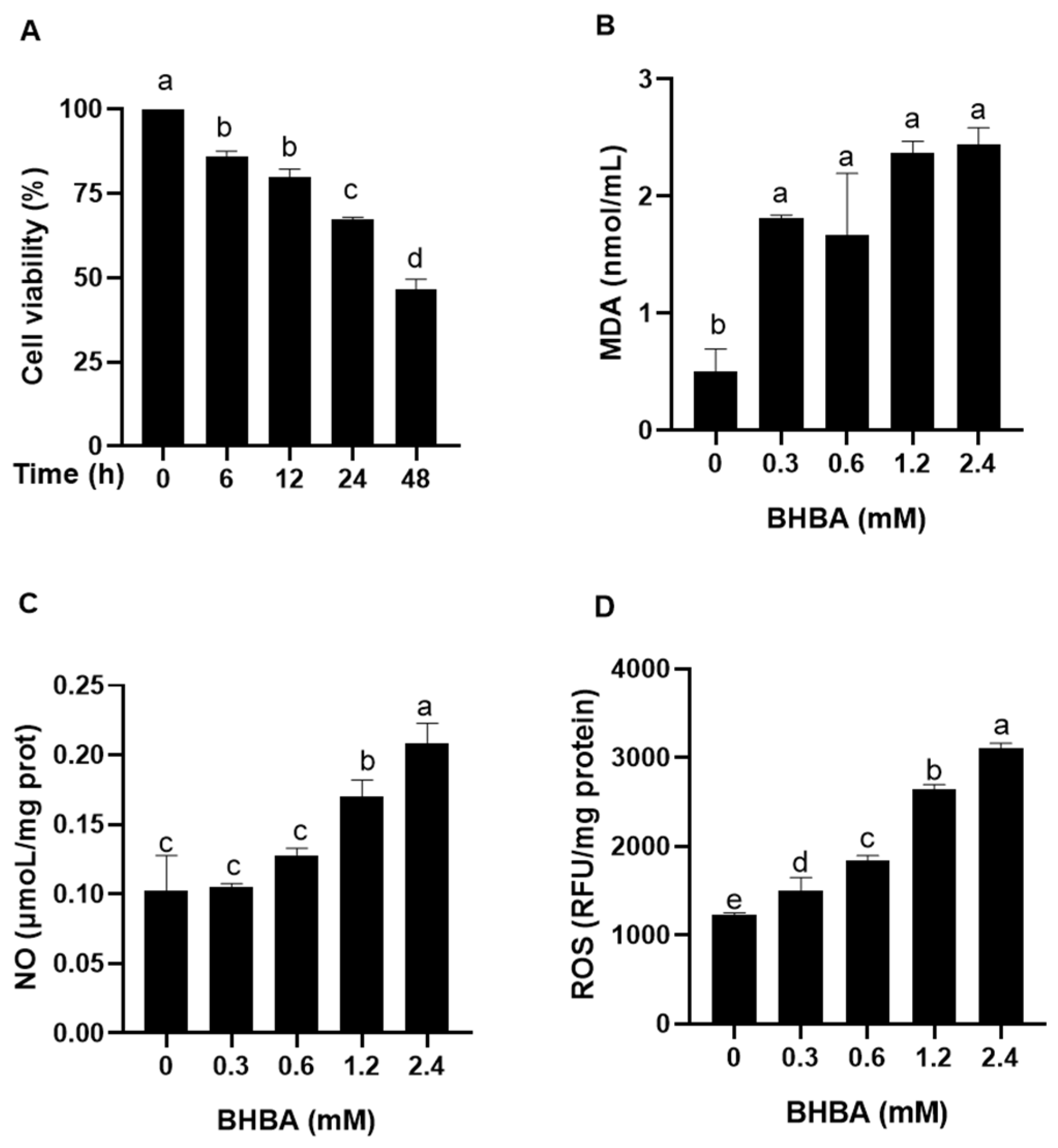
2.1. Effect of BHBA on Oxidative Stress Injury in Calf Hepatocytes
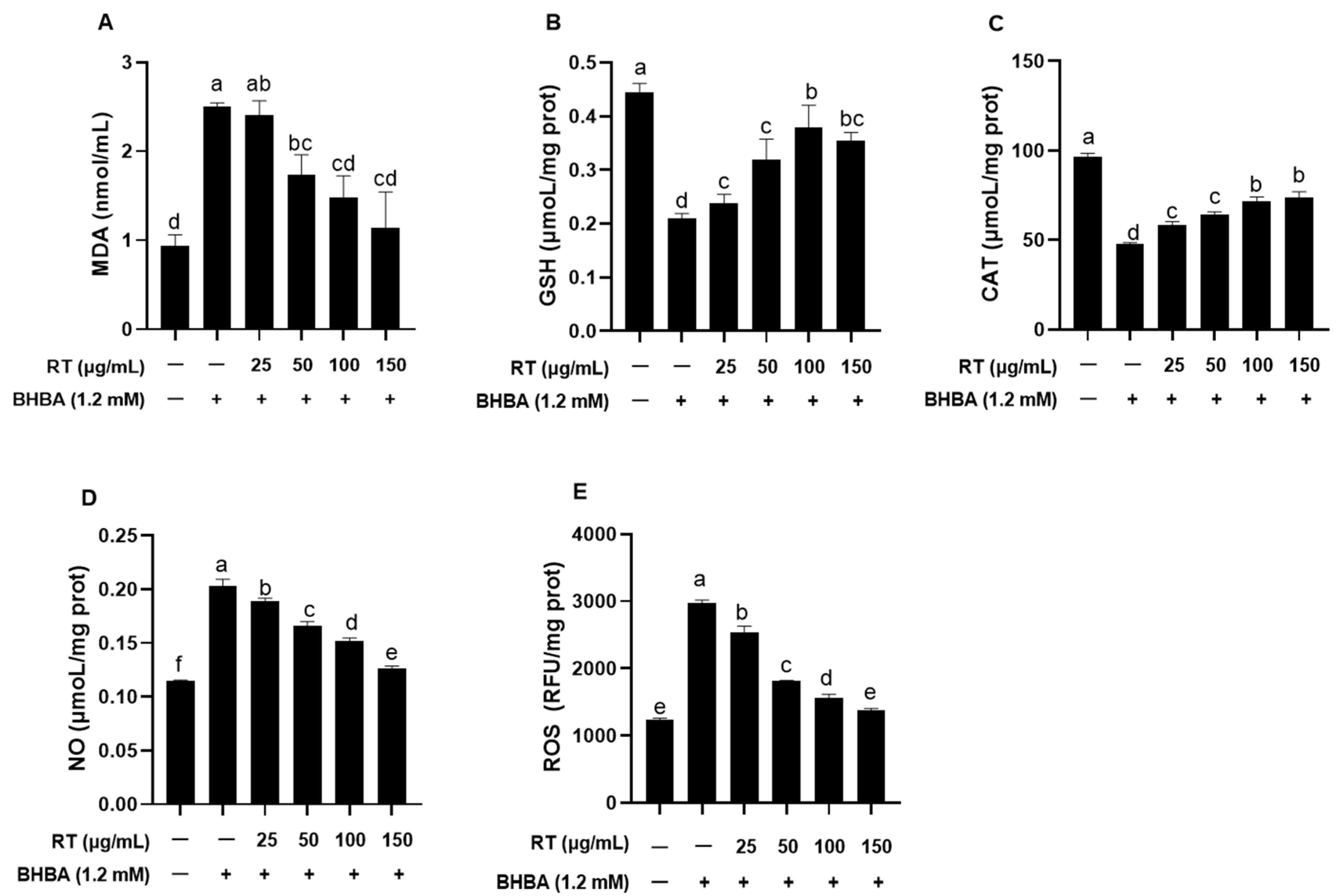
2.2. Effect of RT on BHBA-Induced Oxidative Stress in Hepatocytes
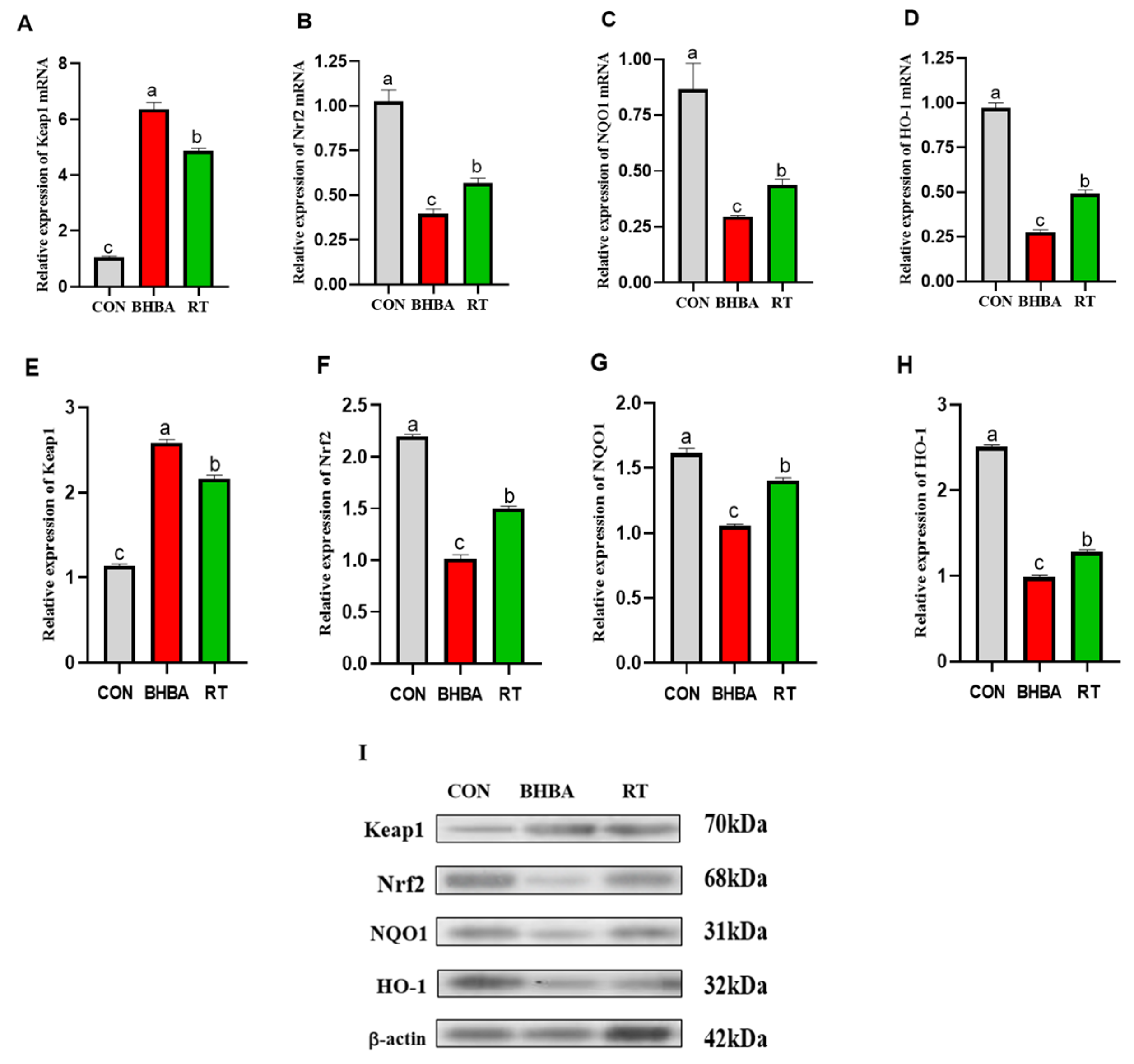
2.3. Effect of RT on Keap1-Nrf2 Signaling Pathway in Hepatocytes
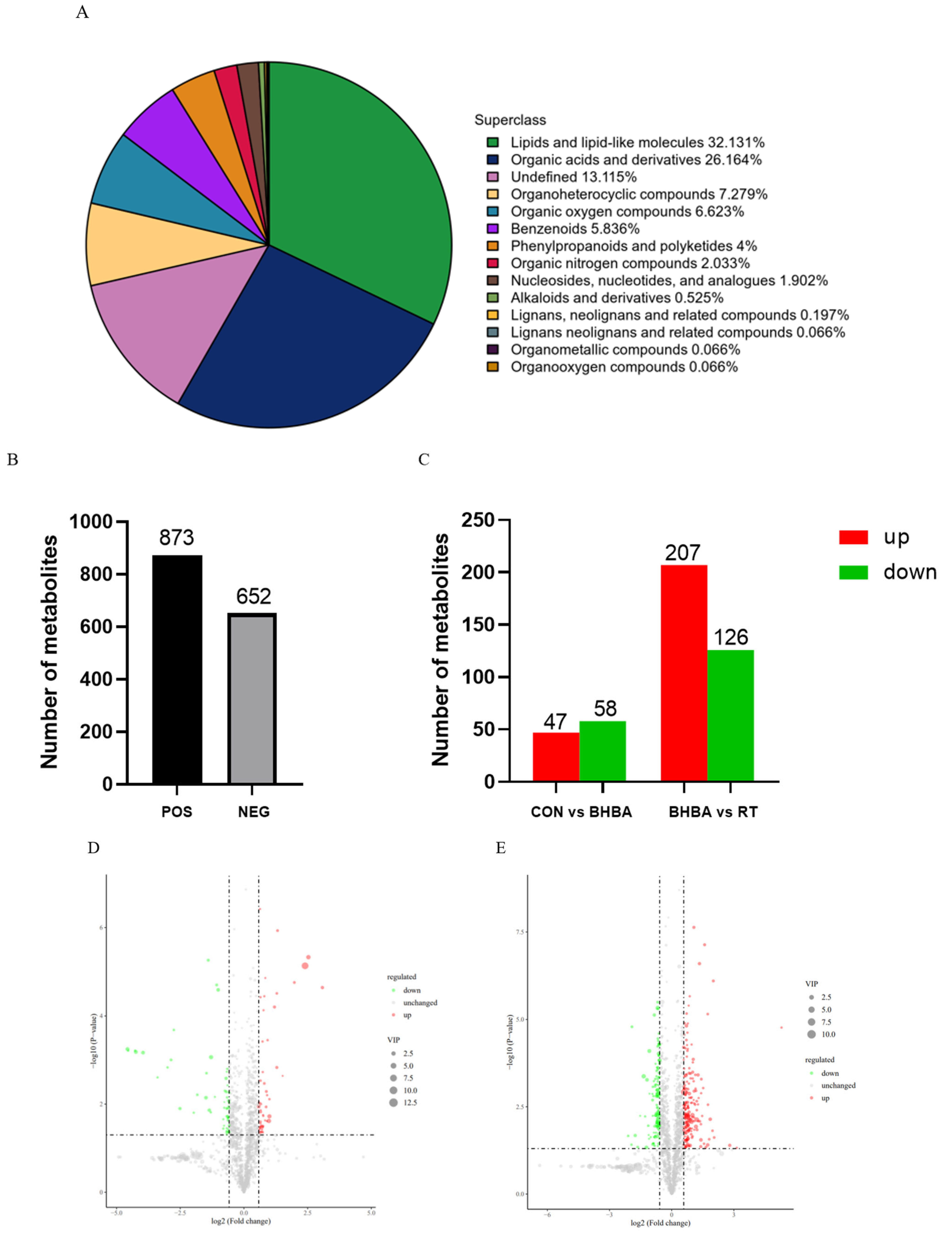
2.4. Metabolome Analysis
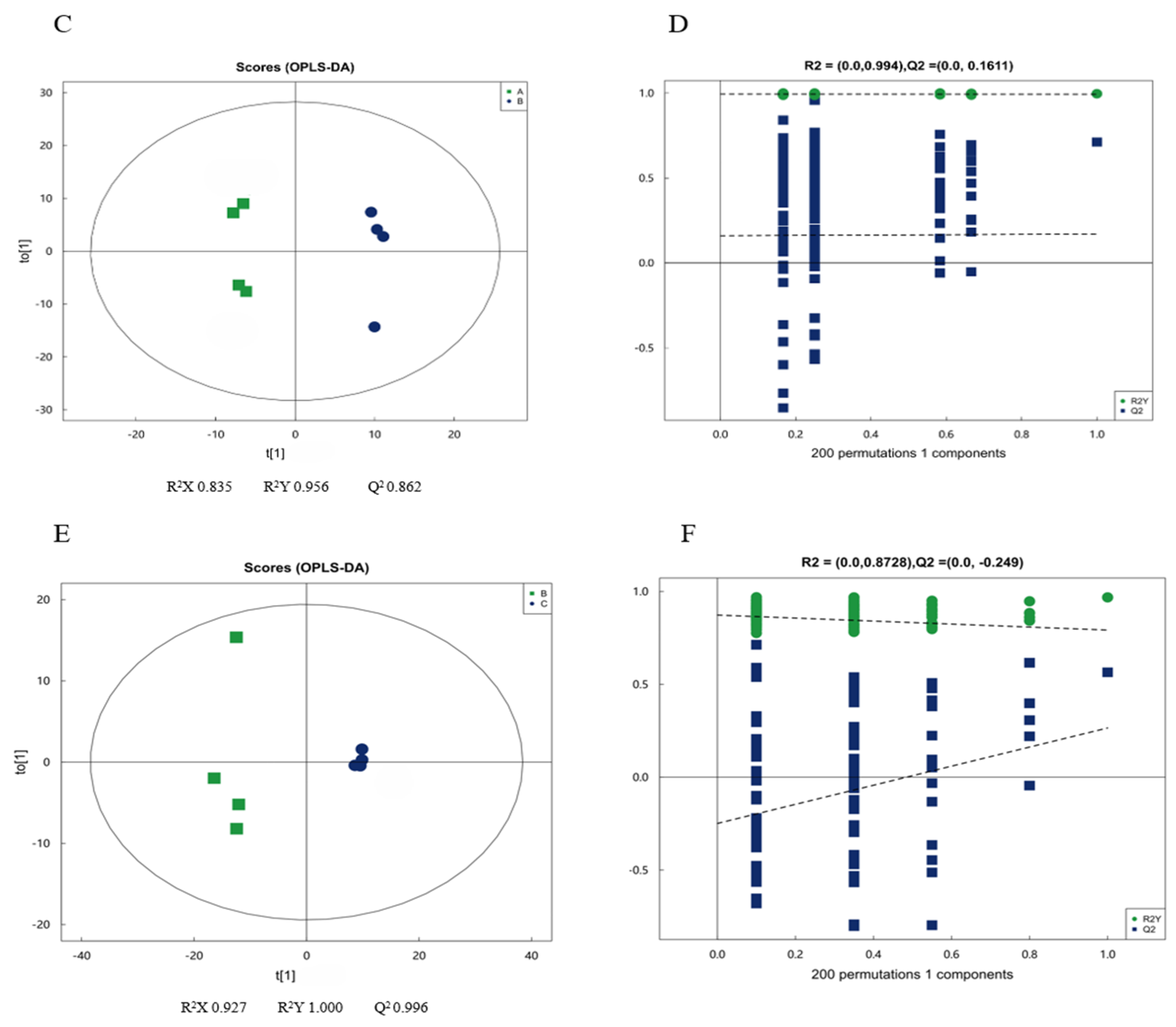
2.4.1. Multivariate Statistical Analysis
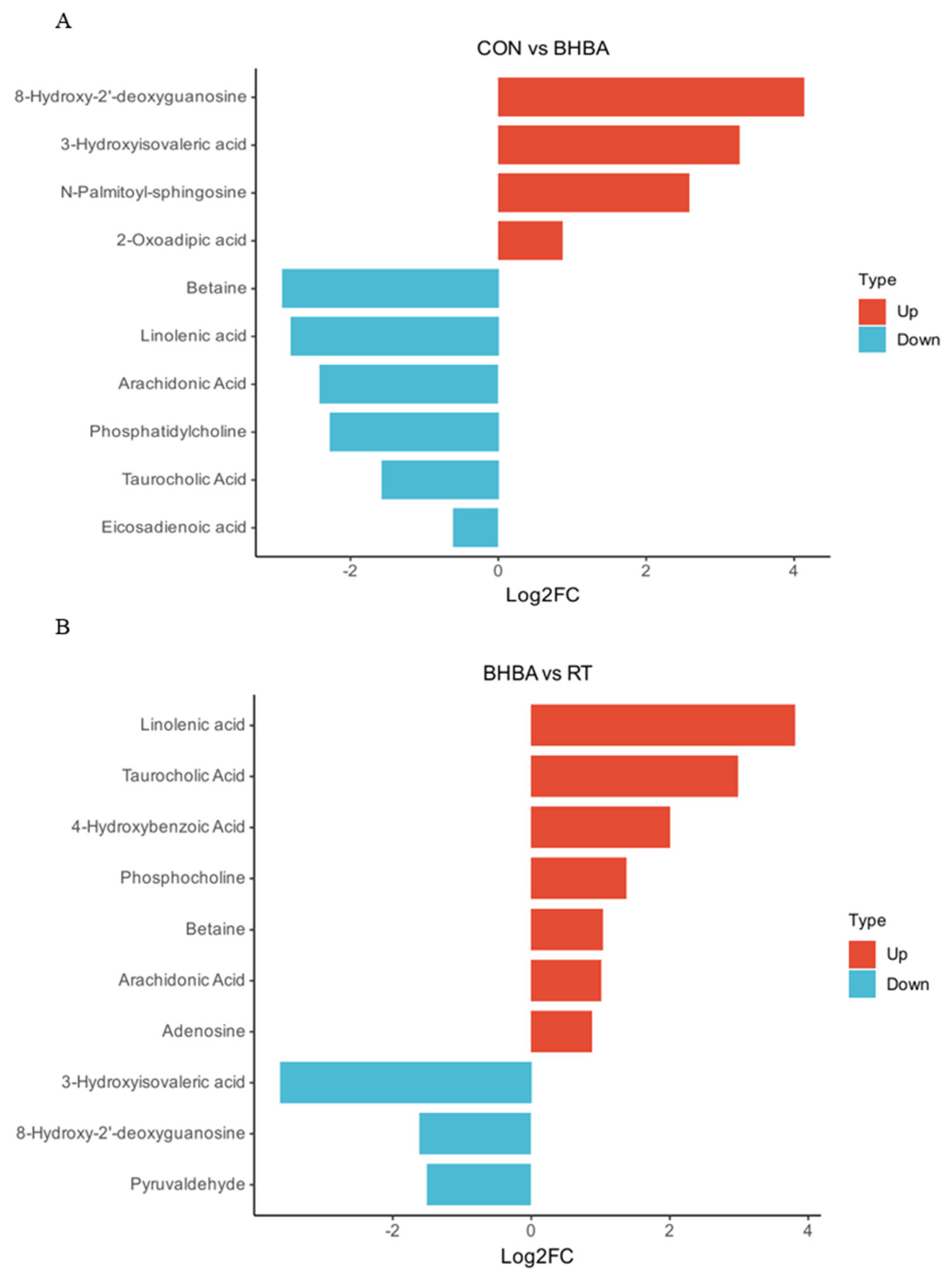
2.4.2. Screening and Identification of Differential Metabolites
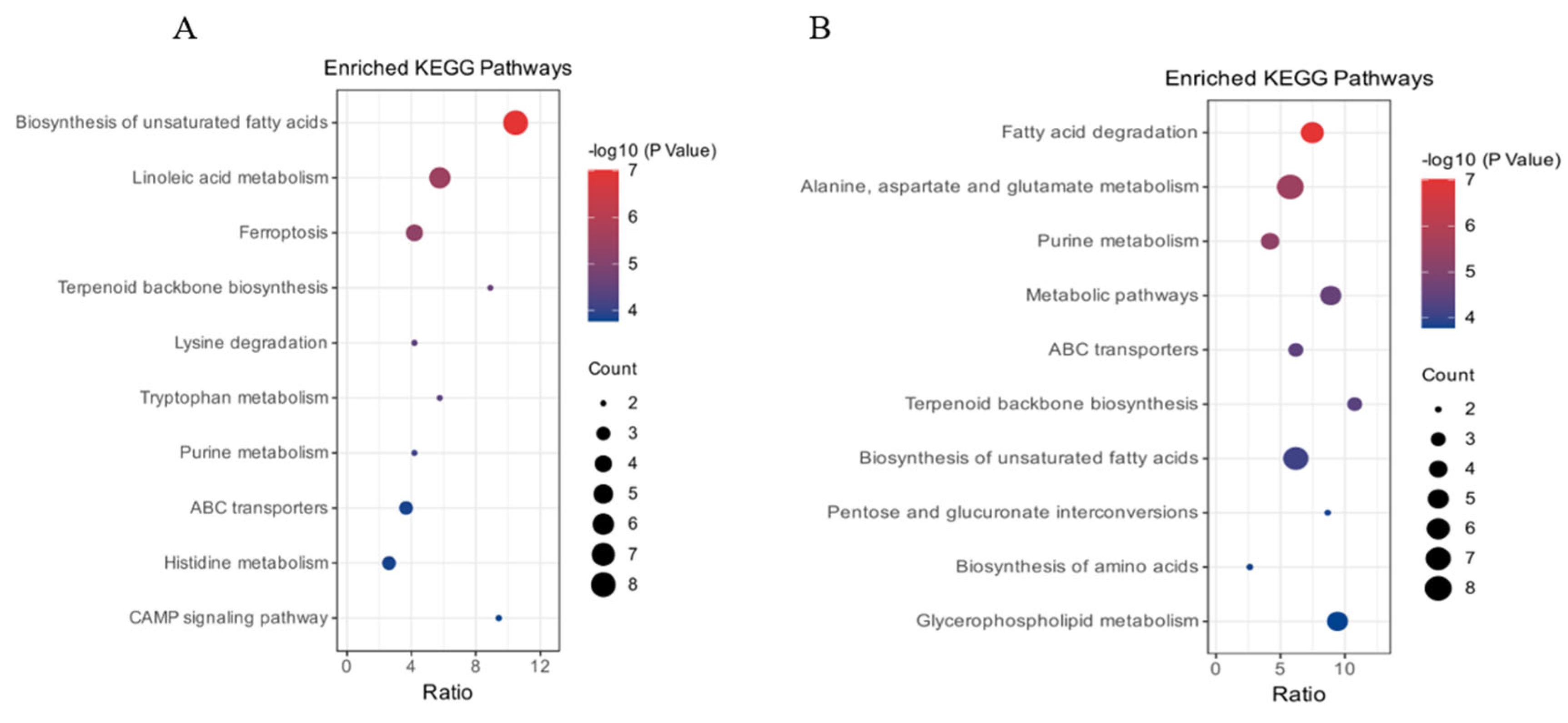
2.4.3. Differential Metabolite Pathway Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Primary Calf Hepatocytes
4.2. Cell Grouping and Treatment
4.3. Cell Viability Assay
4.4. Measurement of Oxidative Stress Indicators
4.5. RNA Extraction and Real-Time PCR
4.6. Western Blotting
4.7. Metabolomics Data Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Kok, A.; Burgers, E.E.A.; Bruckmaier, R.M.; Goselink, R.M.A.; Gross, J.J.; Kemp, B.; Lam, T.J.G.M.; Minuti, A.; Saccenti, E.; et al. Time profiles of energy balance in dairy cows in association with metabolic status, inflammatory status, and disease. J. Dairy Sci. 2024, 11, 9960–9977. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.E.; Barrientos-Blanco, M.A. Invited review: Ketone biology-the shifting paradigm of ketones and ketosis in the dairy cow. J. Dairy Sci. 2024, 107, 3367–3388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 5, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.D.; Chen, M.; Meng, M.; Ma, N.; Xie, W.; Shen, X.Z.; Li, Z.X.; Chang, G.J. Subclinical ketosis leads to lipid metabolism disorder by down-regulating the expression of Acetyl-CoA acetyltransferase 2 (ACAT2) in dairy cows. J. Dairy Sci. 2023, 12, 9892–9909. [Google Scholar] [CrossRef]
- Deng, Q.H.; Liu, G.W.; Liu, L.; Zhang, Y.M.; Yin, L.H.; Shi, X.X.; Wang, J.G.; Yuan, X.; Sun, G.Q.; Li, Y.; et al. BHBA influences bovine hepatic lipid metabolism via AMPK signaling pathway. J. Cell. Biochem. 2015, 116, 6. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Zhao, C.; Yang, Y.; Xiong, C.; Zhang, D.; Feng, S.; Wu, J.; Wang, X. Rutin Supplementation Reduces Oxidative Stress, Inflammation and Apoptosis of Mammary Gland in Sheep During the Transition Period. Front. Vet. Sci. 2022, 9, 907299. [Google Scholar] [CrossRef]
- Naderi, M.; Seyedabadi, M.; Talebpour, A.F.; Akbari, S.; Shaki, F. Rutin mitigates perfluorooctanoic acid-induced liver injury via modulation of oxidative stress, apoptosis, and inflammation. Iran. J. Basic Med. Sci. 2023, 26, 1291–1297. [Google Scholar]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2019, 8, 199–213. [Google Scholar] [CrossRef]
- Ma, X.; Ren, X.; Zhang, X.; Griffin, N.; Liu, H.; Wang, L. Rutin ameliorates perfluorooctanoic acid-induced testicular injury in mice by reducing oxidative stress and improving lipid metabolism. Drug Chem. Toxicol. 2023, 46, 1223–1234. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An emerging anticancer flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Hu, H.; Franceschini, S.; Lemal, P.; Grelet, C.; Chen, Y.; Atashi, H.; Wijnrocx, K.; Soyeurt, H.; Gengler, N. Exploring the relationship between predicted negative energy balance and its biomarkers of Holstein cows in first-parity early lactation. J. Dairy Sci. 2025, 108, 5433–5447. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Kang, S.; Jung, M.; Kim, S.B.; Hwang, S.; Lee, J.; Kim, D.; Choi, K.C.; Park, J. Changes in haematological and serum biochemical parameter concentrations from the day of calving to ketosis onset in Holstein dairy cows during the postpartum period. Ir. Vet. J. 2025, 78, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.B.; Fu, S.X.; Wu, C.C.; Wang, X.X.; Yu, G.J.; Long, M.; Wang, Z.; Liu, G.W. Alterations of fatty acid β-oxidation capability in the liver of ketotic cows. J. Dairy Sci. 2012, 95, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhao, H.; Gao, M.; Hu, H.; Li, D. Rutin Ameliorates BHBA-Induced Inflammation and lipid accumulation in calf hepatocytes through NF-κB signaling pathway. Curr. Issues Mol. Biol. 2025, 47, 274. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Chen, X.; Yan, Y.; Li, L.; Zhao, W. Transcriptome analysis reveals that nefa and β-hydroxybutyrate induce oxidative stress and inflammatory response in bovine mammary epithelial cells. Metabolites 2022, 12, 1060. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hajimohammadi, A.; Nasrian, A.; Nikzad, M.; Rowshan-Ghasrodashti, A.; Nazifi, S.; Naeini, A.T. Oxidative stress biomarkers and metabolic parameters in healthy holstein dairy cows and cows with left displacement abomasum during the transitional period. Vet. Med. Sci. 2025, 11, e70142. [Google Scholar] [CrossRef]
- Mohsin, M.A.; Zhou, X.; Huiru, Y.; Shen, W.; He, B.; Sobiech, P.; Pierzchała, M.; Ogłuszka, M.; Starzyński, R.; Kalra, G.; et al. Effect of β-hydroxybutyrate acid on gene expression levels of antioxidant biomarkers and growth hormone-related genes in liver cell culture. J. Vet. Res. 2024, 68, 313–324. [Google Scholar] [CrossRef]
- Saafan, S.M.; Mohamed, S.A.; Noreldin, A.E.; El Tedawy, F.A.; Elewa, Y.H.A.; Fadly, R.S.; Al Jaouni, S.K.; El-Far, A.H.; Alsenosy, A.A. Rutin attenuates D-galactose-induced oxidative stress in rats’ brain and liver: Molecular docking and experimental approaches. Food Funct. 2023, 14, 5728–5751. [Google Scholar] [CrossRef]
- Iqbal, A.; Hafeez Kamran, S.; Siddique, F.; Ishtiaq, S.; Hameed, M.; Manzoor, M. Modulatory effects of rutin and vitamin A on hyperglycemia induced glycation, oxidative stress and inflammation in high-fat-fructose diet animal model. PLoS ONE 2024, 19, e0303060. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Chay-Canul, A.J.; Miranda-Romero, L.A.; Mendoza-Martínez, G.D. Meta-analysis of flavonoids use into beef and dairy cattle diet: Performance, antioxidant status, ruminal fermentation, meat quality, and milk composition. Front. Vet. Sci. 2023, 10, 1134925. [Google Scholar] [CrossRef]
- Gao, D.; Zhuang, Y.; Liu, S.; Ma, B.; Xu, Y.; Zhang, H.; Nuermaimaiti, Y.; Chen, T.; Hou, G.; Guo, W.; et al. Multi-omics profiling of dairy cattle oxidative stress identifies hindgut-derived Phascolarctobacterium succinatutens exhibiting antioxidant activity. Npj Biofilms Microbiomes 2025, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Xue, Y.; Gao, Y.; Wang, B.; Qu, Y.; Li, S.; Li, H.; Li, Z.; Wang, B.; Luo, H. Vitamin E can ameliorate oxidative damage of ovine hepatocytes In Vitro by regulating genes expression associated with apoptosis and pyroptosis, but not ferroptosis. Molecules 2021, 26, 4520. [Google Scholar] [CrossRef] [PubMed]
- Farghali, H.; Kameniková, L.; Hynie, S.; Kmonicková, E. Silymarin effects on intracellular calcuim and cytotoxicity: A study in perfused rat hepatocytes after oxidative stress injury. Pharmacol. Res. 2020, 41, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, K.; Shen, D.; Li, C. Oxidative stress and Keap1-Nrf2 pathway involvement in bisphenol a-induced liver damage in rats. Toxics 2024, 12, 864. [Google Scholar] [CrossRef]
- Hussein, R.M.; Sawy, D.M.; Kandeil, M.A.; Farghaly, H.S. Chlorogenic acid, quercetin, coenzyme Q10 and silymarin modulate Keap1-Nrf2/heme oxygenase-1 signaling in thioacetamide-induced acute liver toxicity. Life Sci. 2021, 277, 119460. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Miao, J.; Zhang, X.; Xiong, Y.; Ma, F.; Ding, J.; He, S. Bamboo leaf flavonoids ameliorate cyclic heat stress-induced oxidative damage in broiler liver through activation of Keap1-Nrf2 signaling pathway. Poult. Sci. 2025, 104, 104952. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects t butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomed. Int. J. Phytother. Phytopharm. 2019, 55, 92–104. [Google Scholar] [CrossRef]
- Spoto, B.; Politi, C.; Pizzini, P.; Parlongo, R.M.; Testa, A.; Mobrici, M.; Tripepi, G.L.; Mallamaci, F.; Zoccali, C. 8-hydroxy-2′-deoxyguanosine, a biomarker of oxidative DNA injury, in diabetic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103722. [Google Scholar] [CrossRef]
- Alak, G.; Ucar, A.; Parlak, V.; Yeltekin, A.Ç.; Taş, I.H.; Ölmez, D.; Kocaman, E.M.; Yılgın, M.; Atamanalp, M.; Yanık, T. Assessment of 8-hydroxy-2-deoxyguanosine activity, gene expression and antioxidant enzyme activity on rainbow trout (Oncorhynchus mykiss) tissues exposed to biopesticide. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2017, 203, 51–58. [Google Scholar] [CrossRef]
- Mock, D.M.; Stratton, S.L.; Horvath, T.D.; Bogusiewicz, A.; Matthews, N.I.; Henrich, C.L.; Dawson, A.M.; Spencer, H.J.; Owen, S.N.; Boysen, G.; et al. Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans. J. Nutr. 2011, 141, 1925–1930. [Google Scholar] [CrossRef]
- Kang, Y.J.; Song, W.; Lee, S.J.; Choi, S.A.; Chae, S.; Yoon, B.R.; Kim, H.Y.; Lee, J.H.; Kim, C.; Cho, J.Y.; et al. Inhibition of BCAT1-mediated cytosolic leucine metabolism regulates Th17 responses via the mTORC1-HIF1α pathway. Exp. Mol. Med. 2024, 56, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bergkamen, A.; Okun, J.G.; Spiekerkötter, U.; Lindner, M.; Haas, D.; Kohlmüller, D.; Mayatepek, E.; Schulze-Bergkamen, H.; Greenberg, C.R.; Zschocke, J.; et al. Quantitative acylcarnitine profiling in peripheral blood mononuclear cells using in vitro loading with palmitic and 2-oxoadipic acids: Biochemical confirmation of fatty acid oxidation and organic acid disorders. Pediatr. Res. 2005, 58, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Leandro, J.; Dodatko, T.; Aten, J.; Nemeria, N.S.; Zhang, X.; Jordan, F.; Hendrickson, R.C.; Sanchez, R.; Yu, C.; DeVita, R.J.; et al. DHTKD1 and OGDH display substrate overlap in cultured cells and form a hybrid 2-oxo acid dehydrogenase complex In Vivo. Hum. Mol. Genet. 2020, 29, 1168–1179. [Google Scholar] [CrossRef]
- Grala, T.M.; Handley, R.R.; Phyn, C.V.C.; Roche, J.R.; Dalley, D.E. A quantitative case study assessment of changes to hepatic metabolism from nonlactating grazing dairy cows consuming a large proportion of their diet as fodder beet. J. Dairy Sci. 2019, 102, 8559–8570. [Google Scholar] [CrossRef]
- Wang, S.; Bai, H.; Liu, T.; Yang, J.; Wang, Z. Optimization of concentrations of different n-3PUFAs on antioxidant capacity in mouse hepatocytes. Lipids Health Dis. 2024, 23, 214. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Y.; Zhou, Y.; Ke, X.; Tian, Y.; Zheng, S.; Sun, Y.; Huang, Z.; Zhou, J.; Wu, L. Unraveling the liver metabolomic profile of ADB-BUTINACA-induced hepatotoxicity. Ecotoxicol. Environ. Saf. 2024, 288, 117375. [Google Scholar] [CrossRef]
- Chin, C.F.; Galam, D.; Gao, L.; Tan, B.C.; Wong, B.H.; Chua, G.L.; Loke, R.Y.; Lim, Y.C.; Wenk, M.R.; Lim, M.S.; et al. Blood-derived lysophospholipid sustains hepatic phospholipids and fat storage necessary for hepatoprotection in overnutrition. J. Clin. Investig. 2023, 133, e171267. [Google Scholar] [CrossRef]
- Li, P.; Hu, J.; Zhao, H.; Feng, J.; Chai, B. Multi-omics reveals inhibitory effect of baicalein on non-alcoholic fatty liver disease in mice. Front. Pharmacol. 2022, 13, 925349. [Google Scholar] [CrossRef]
- Song, G.; Weng, F.; Zou, B.; Zhao, J.; Jin, J.; Yan, D.; Huang, K.; Sun, X.; Liu, C.; Hu, Y.; et al. Potential therapeutic action of tauroursodeoxycholic acid against cholestatic liver injury via hepatic Fxr/Nrf2 and CHOP-DR5-caspase-8 pathway. Clin. Sci. 2023, 137, 561–577. [Google Scholar] [CrossRef]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxidative Med. Cell. Longev. 2017, 12, 6297080. [Google Scholar] [CrossRef] [PubMed]
- Babenko, N.A.; Shakhova, E.G. Effects of flavonoids on sphingolipid turnover in the toxin-damaged liver and liver cells. Lipids Health Dis. 2008, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Váli, L.; Stefanovits-Bányai, E.; Szentmihályi, K.; Fébel, H.; Sárdi, E.; Lugasi, A.; Kocsis, I.; Blázovics, A. Liver-protecting effects of table beet (Beta vulgaris var. rubra) during ischemia-reperfusion. Nutrition 2007, 23, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, C.; Yang, J.; Tian, Y.; You, L.; Yang, H.; Li, Y.; Liu, H.; Pan, D.; Liu, Z. Kaempferol improves exercise performance by regulating glucose uptake, mitochondrial biogenesis, and protein synthesis via PI3K/AKT and MAPK signaling pathways. Foods 2024, 13, 1068. [Google Scholar] [CrossRef]
- Leipnitz, G.; Seminotti, B.; Amaral, A.U.; de Bortoli, G.; Solano, A.; Schuck, P.F.; Wyse, A.T.; Wannmacher, C.M.; Latini, A.; Wajner, M. Induction of oxidative stress by the metabolites accumulating in 3-methylglutaconic aciduria in cerebral cortex of young rats. Life Sci. 2008, 82, 652–662. [Google Scholar] [CrossRef]
- Erdem, I.; Aktas, S.; Ogut, S. Neohesperidin dihydrochalcone ameliorates experimental colitis via anti-inflammatory, antioxidative, and antiapoptosis effects. J. Agric. Food Chem. 2024, 72, 15715–15724. [Google Scholar] [CrossRef]
- Berdowska, I.; Matusiewicz, M.; Fecka, I. A comprehensive review of metabolic dysfunction-associated steatotic liver disease: Its mechanistic development focusing on methylglyoxal and counterbalancing treatment strategies. Int. J. Mol. Sci. 2025, 26, 2394. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wei, X.; Hou, Y.; Jia, S.; Li, S.; Zhao, X. Metabolomics analysis of the effects of quercetin on hepatotoxicity induced by acrylamide exposure in rats. Free. Radic. Res. 2021, 55, 831–841. [Google Scholar] [CrossRef]
- Wang, S.; Sheng, F.; Zou, L.; Xiao, J.; Li, P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 2021, 34, 109–122. [Google Scholar] [CrossRef]

| Gene | Accession No. | Primer Sequence (5′ to 3′) | Length (bp) |
|---|---|---|---|
| ACTB | NM_280979 | F: GCAAATGCTTCTAGGCGGAC | 203 |
| R: ATGCTCGATCCAACCGACTG | |||
| Keap1 | NM_3476823 | F: AGAGAAACGAGTGGCGGATG | 159 |
| R: ACGTCCACGTTTCTGTCTCC | |||
| Nrf2 | NM_4092041 | F: AGCCTCAAAGCACCGTCCTC | 171 |
| R: TGTCAATCAAATCCATGTCCTGC | |||
| NQO1 | NM_4938920 | F: GATCGTACTGGCCCACTCAG | 106 |
| R: CCGGGGTCCTTCAGTTTACC | |||
| HO-1 | NM_3950281 | F: TGAGCTGACCCAAGAAGGTT | 135 |
| R: AGTGTAGACGGGGTTCTCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Zhao, H.; Gao, M.; Hu, H.; Li, D. Rutin Attenuates Oxidative Stress Responses and Hepatocyte Metabolomics in β-Hydroxybutyric Acid-Induced Hepatocyte Injury in Calves. Int. J. Mol. Sci. 2025, 26, 5878. https://doi.org/10.3390/ijms26125878
Yang K, Zhao H, Gao M, Hu H, Li D. Rutin Attenuates Oxidative Stress Responses and Hepatocyte Metabolomics in β-Hydroxybutyric Acid-Induced Hepatocyte Injury in Calves. International Journal of Molecular Sciences. 2025; 26(12):5878. https://doi.org/10.3390/ijms26125878
Chicago/Turabian StyleYang, Kun, Haixia Zhao, Min Gao, Honglian Hu, and Dabiao Li. 2025. "Rutin Attenuates Oxidative Stress Responses and Hepatocyte Metabolomics in β-Hydroxybutyric Acid-Induced Hepatocyte Injury in Calves" International Journal of Molecular Sciences 26, no. 12: 5878. https://doi.org/10.3390/ijms26125878
APA StyleYang, K., Zhao, H., Gao, M., Hu, H., & Li, D. (2025). Rutin Attenuates Oxidative Stress Responses and Hepatocyte Metabolomics in β-Hydroxybutyric Acid-Induced Hepatocyte Injury in Calves. International Journal of Molecular Sciences, 26(12), 5878. https://doi.org/10.3390/ijms26125878






