The Effect of Zinc Oxide Nanoparticles on the Quantitative and Qualitative Traits of Scutellaria baicalensis Georgi in In Vitro Culture
Abstract
1. Introduction
2. Results
2.1. Analysis of Morphological Characteristics
2.2. Evaluation of Mineral Content in Scutellaria Baicalensis Plant Material
2.3. Assessment of Total Phenolic Content in Extracts
2.4. Assessment of Total Antioxidant Capacity in Extracts
2.5. Determination of Photosynthetic Pigments Content
3. Discussion
4. Materials and Methods
4.1. Zinc Oxide Nanoparticles
4.2. Plant Material
4.3. Preparation of Extracts
4.4. Determination of Total Phenolic Content (TPC)
4.5. Determination of Total Antioxidant Capacity
4.6. Determination of Mineral Composition
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bochoráková, H.; Paulová, H.; Slanina, J.; Musil, P.; Táborská, E. Main flavonoids in the root of Scutellaria baicalensis cultivated in Europe and their comparative antiradical properties. Phytother. Res. 2003, 17, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, J.; Cui, M.Y.; Liu, J.; Fang, Y.M.; Yan, M.X.; Qiu, W.Q.; Shang, H.W.; Xu, Z.C.; Yidiresi, R.; et al. The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant 2019, 12, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Spyrka, K.; Brela, J. Scutellaria baicalensis as new hydrogel formulation with antioxdant propertie. Forum Leczenia Ran 2023, 4, 9–12. (In Polish) [Google Scholar] [CrossRef]
- Cai, J.; Hu, Q.; He, Z.; Chen, X.; Wang, J.; Yin, X.; Ma, X.; Zeng, J. Scutellaria baicalensis Georgi and their natural flavonoid compounds in the treatment of ovarian cancer: A review. Molecules 2023, 28, 5082. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Lin, F.; Ding, Y.K.; Dai, S.; Liang, Y.X.; Zhang, Y.S.; Li, J.; Chen, H.W. Pharmacological properties of total flavonoids in Scutellaria baicalensis for the treatment of cardiovascular diseases. Phytomedicine 2022, 107, 154458. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhang, J.; Wu, J. Scutellaria baicalensis Georgi is a promising candidate for the treatment of autoimmune diseases. Front. Pharmacol. 2022, 13, 946030. [Google Scholar] [CrossRef]
- Ozma, M.A.; Khodadadi, E.; Pakdel, F.; Kamounah, F.S.; Yousefi, M.; Yousefi, B.; Asgharzadeh, M.; Ganbarov, K.; Kafil, H.S. Baicalin, a natural antimicrobial and antibiofilm agent. J. Herb. Med. 2021, 27, 100432. [Google Scholar] [CrossRef]
- Song, J.W.; Long, J.Y.; Xie, L.; Zhang, L.L.; Xie, Q.X.; Chen, H.J.; Deng, M.; Li, X.F. Applications; phytochemistry; pharmacological effects; pharmacokinetics; toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef]
- Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The use of Chinese skullcap (Scutellaria baicalensis) and its extracts for sustainable animal production. Animals 2021, 11, 1039. [Google Scholar] [CrossRef]
- Li, Y.; Xie, X.; Wang, C.; Pei, X.; Fang, C.; Yan, R.; Zhong, Y. Antibacterial and UV resistant functions of cotton fabrics: In- situ free radical polymerization of AgNPs using Baicalin. Ind. Crops Prod. 2024, 222, 119818. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Schmidt, A.; Mizielińska, M. Mixtures of Scutellaria baicalensis and Glycyrrhiza L. extracts as antibacterial and antiviral agents in active coatings. Coatings 2021, 11, 1438. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. In vitro cultures of Scutellaria alpina as a source of pharmacologically active metabolites. Acta Physiol. Plant. 2016, 38, 7. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. Study on the chemical composition and antioxidant activity of extracts from shoot culture and regenerated plants of Scutellaria altissima L. Acta Physiol. Plant. 2015, 37, 1736. [Google Scholar] [CrossRef]
- Gawroński, J.; Dyduch-Siemińska, M. Potential of in vitro culture of Scutellaria baicalensis in the formation of genetic variation confirmed by ScoT Markers. Genes 2022, 13, 2114. [Google Scholar] [CrossRef]
- Javed, R.; Yucesan, B.; Zia, M.; Ekrem, G. Elicitation of secondary metabolites in callus cultures of Stevia rebaudiana Bertoni grown under ZnO and CuO nanoparticles stress. Sugar Tech. 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Zafar, H.; Ali, A.A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: Growth dynamics and antioxidative response. Front. Plant Sci. 2016, 7, 535. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Karimi, N.; Behbahani, M.; Dini, G.; Razmjou, A. Enhancing the secondary metabolite and anticancer activity of Echinacea purpurea callus extracts by treatment with biosynthesized ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045009. [Google Scholar] [CrossRef]
- Taheri, M.; Mousavi, M.; Mortazavi, S.M.H. Different reactions of olive explants in response to zinc oxide nanoparticles and zinc sulfate under in vitro conditions. Int. J. Hortic. Sci. 2024, 11, 107–124. [Google Scholar] [CrossRef]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; Johannessen, B.; Glover, C.J.; Kappen, P.; Kopittke, P.M. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ. Sci. Technol. 2013, 47, 13822–13830. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Alenezi, N.A.; Al-Qurainy, F.; Tarroum, M.; Nadeem, M.; Khan, S.; Salih, A.M.; Shaikhaldein, H.O.; Alfarraj, N.S.; Gaafar, A.-R.Z.; Al-Hashimi, A.; et al. Zinc oxide nanoparticles (ZnO NPs); biosynthesis; characterization and evaluation of their impact to improve shoot growth and to reduce salt toxicity on Salvia officinalis in vitro cultivated. Processes 2022, 10, 1273. [Google Scholar] [CrossRef]
- El-Mahdy, M.T.; Elazab, D.S. Impact of zinc oxide nanoparticles on pomegranate growth under in vitro conditions. Russ. J. Plant Physiol. 2020, 67, 162–167. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) Plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Subba, B.; Rai, G.B.; Bhandary, R.; Parajuli, P.; Thapa, N.; Kandel, D.R.; Mulmi, S.; Shrestha, S.; Malla, S. Antifungal activity of zinc oxide nanoparticles (ZnO NPs) on Fusarium equiseti phytopathogen isolated from tomato plant in Nepal. Helyon 2024, 10, e40198. [Google Scholar] [CrossRef]
- Kwiecień, I.; Łukaszyk, A.; Miceli, N.; Tawiano, M.F.; Davi, F.; Kędzia, E.; Ekier, H. In vitro cultures of Scutellaria brevibracteata subsp. subvelutina as a source of bioactive phenolic metabolites. Molecules 2023, 28, 1785. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (american skullcap) grown in vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Karimkhanlooei, G.; Sharafi, A. Study of tissue culture and in vitro organogenesis of Scutellaria bornmuelleri using benzylaminopurine; lsopentenyl adenine and thidiazuron. S. Afr. J. Bot. 2021, 139, 458–469. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A. High-frequency adventitious shoot organogenesis from in vitro stem explants of Scutellaria araxensis Grossh. BioTechnologia 2022, 103, 143–151. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of biogenic ZnO nanoparticles on growth; physiological; biochemical traits and antioxidants on olive tree in vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol. Appl. Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Geremew, A.; Carson, L.; Woldesenbet, S.; Wang, H.; Reeves, S.; Brooks, N., Jr.; Saganti, P.; Weerasooriya, A. Effect of zinc oxide nanoparticles synthesized from Carya illinoinensis leaf extract on growth and antioxidant properties of mustard (Brassica juncea). Front. Plant Sci. 2023, 14, 1108186. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, E.S.; Farooq, M.; Metwali, E.M.R. Influence of nano zinc oxide on the in vitro callus growth; ex vitro tuber yield and nutrional quality of potato (Solanum tuberosum L.) cultivars under salt stress. J. Anim. Plant Sci. 2022, 32, 440–449. [Google Scholar] [CrossRef]
- Alharby, H.F.; Metwali, E.M.R.; Fuller, M.P.; Aldhebiani, A.Y. Impact of application of zinc oxide nanoparticles on callus induction; plant regeneration; element content; and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Acta Sci. Biol. Sci. 2016, 68, 723–735. [Google Scholar] [CrossRef]
- Devasia, J.; Muniswamy, B.; Mishra, M.K. Investigation of ZnO nanoparticles on in vitro cultures of coffee (Coffea arabica L.). Int. J. Nanosci. Nanotechnol. 2020, 16, 271–277. [Google Scholar]
- Shafique, N.; Jabeen, K.S.; Ahmad, S.; Irum, S.; Anwaar, N.; Ahmad, S.; Alam, M.; Ilyas, T.F.; Khan, S.Z.; Hussain, S. Green fabricated zinc oxide nanoformulated media enhanced callus induction and regeneration dynamics of Panicum virgatum L. PLoS ONE 2020, 15, e0230464. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. The effect of humic acid (HA) and zinc oxide nanoparticles (ZnO-NPs) on in vitro regeneration of date palm (Phoenix dactylifera L.) cv. Quntar. Plant Cell Tissue Organ Cult. 2021, 145, 445–456. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Matyszczuk, K. Influence of zinc oxide nanoparticles in in vitro culture and bacteria Bacillus thuringiensis in ex vitro conditions on the growth and development of blackberry (Rubus fruticosus L.). Appl. Sci. 2024, 14, 3743. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Matyszczuk, K.; Dyduch-Siemińska, M. The effect of zinc oxide nanoparticles on the growth and development of Stevia plants cultured in vitro. Acta Sci. Pol. Hortorum Cultus 2024, 23, 43–56. [Google Scholar] [CrossRef]
- Sheng, J.P.; Chen, H.R.; Shen, L. Determination of six mineral elements in roots; stems; leaves; flowers and seeds of Scutellaria baicalensis by FAAS. Spectrosc. Spectr. Anal. 2009, 29, 519–521. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Luo, X.; Zhao, D.-P.; Zhai, Z.-X.; Guo, Y.-H. Analysis of the content of mineral elements in Scutellaria baicalensis; skullcap tea and its solution. Spectrosc. Spectr. Anal. 2011, 31, 3112–3114. [Google Scholar] [CrossRef]
- Guo, L.; Wang, S.; Zhang, J.; Yang, G.; Zhao, M.; Ma, W.; Zhang, X.; Li, X.; Han, B.; Chen, N.; et al. Effects of ecological factors on secondary metabolites and inorganic elements of Scutellaria baicalensis and analysis of geoherblism. Sci. China Life Sci. 2013, 56, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, X.-F.; Wu, J.; Yin, Z.-Q.; Wan, L.-Q.; Sun, H.-Y.; An, Y.-L. Geochemical characteristics and growth suitability assessment of Scutellaria baicalensis Georgi in the Earth’s critical zone of North China. J. Mt. Sci. 2022, 19, 1245–1262. [Google Scholar] [CrossRef]
- Vergun, O.; Svydenko, L.; Grygorieva, O.; Shymanska, O.; Rakhmetov, D.; Brindza, J.; Ivanišová, E. Antioxidant capacity of plant raw material of Scutellaria baicalensis Georgi. Potravin. Slovak J. Food Sci. 2019, 13, 614–621. [Google Scholar] [CrossRef]
- Lim, J.; Kim, K.; Kwon, D.Y.; Kim, J.K.; Sathasivam, R.; Park, S.U. Effects of different solvents on the extraction of phenolic and flavonoid compounds; and antioxidant activities; in Scutellaria baicalensis hairy roots. Horticulturae 2024, 10, 160. [Google Scholar] [CrossRef]
- Vaidya, B.N.; Brearley, T.A.; Joshee, N. Antioxidant capacity of fresh and dry leaf extracts of sixteen Scutellaria species. J. Med. Act. Plants 2014, 2, 42–49. [Google Scholar] [CrossRef]
- Dziurka, M.; Kubica, P.; Kwiecień, I.; Biesaga-Kościelniak, J.; Ekiert, H.; Abdelmohsen, S.A.M.; Al-Harbi, F.F.; El-Ansary, D.O.; Elansary, H.O.; Szopa, A. In vitro cultures of some medicinal plant species (Cistus × incanus; Verbena officinalis; Scutellaria lateriflora; and Scutellaria baicalensis) as a rich potential source of antioxidants-evaluation by cuprac and quencher-cuprac assays. Plants 2021, 10, 454. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and re-active oxygen species: Generation; signaling; and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Parveen, N.; Kandhol, N.; Sharma, S.; Singh, V.P.; Chauhan, D.K.; Ludwig-Müller, J.; Tripathi, D.K. Auxin crosstalk with reactive oxygen and nitrogen species in plant development and abiotic stress. Plant Cell Physiol. 2022, 63, 1814–1825. [Google Scholar] [CrossRef]
- Regni, L.; Del Buono, D.; Micheli, M.; Facchin, S.L.; Cesarini, A.; Priolo, D.; Proietti, P. Biogenic zinc oxide nanoparticles improve in vitro growth of blueberries. Horticulturae 2024, 10, 1234. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A. Effect of extraction solvents on the phenolic content and antioxidant properties of two papaya cultivars. J. Med. Plants Res. 2013, 7, 3354–3359. [Google Scholar]
- PN-EN ISO 6869:2002; Forage. Determine the Content of Calcium; Copper; Iron; Magnesium; Manganese and Zinc-Method by Atomic Absorption Spectrometry. ISO: Warszawa, Poland, 2002; pp. 1–18. (In Polish)
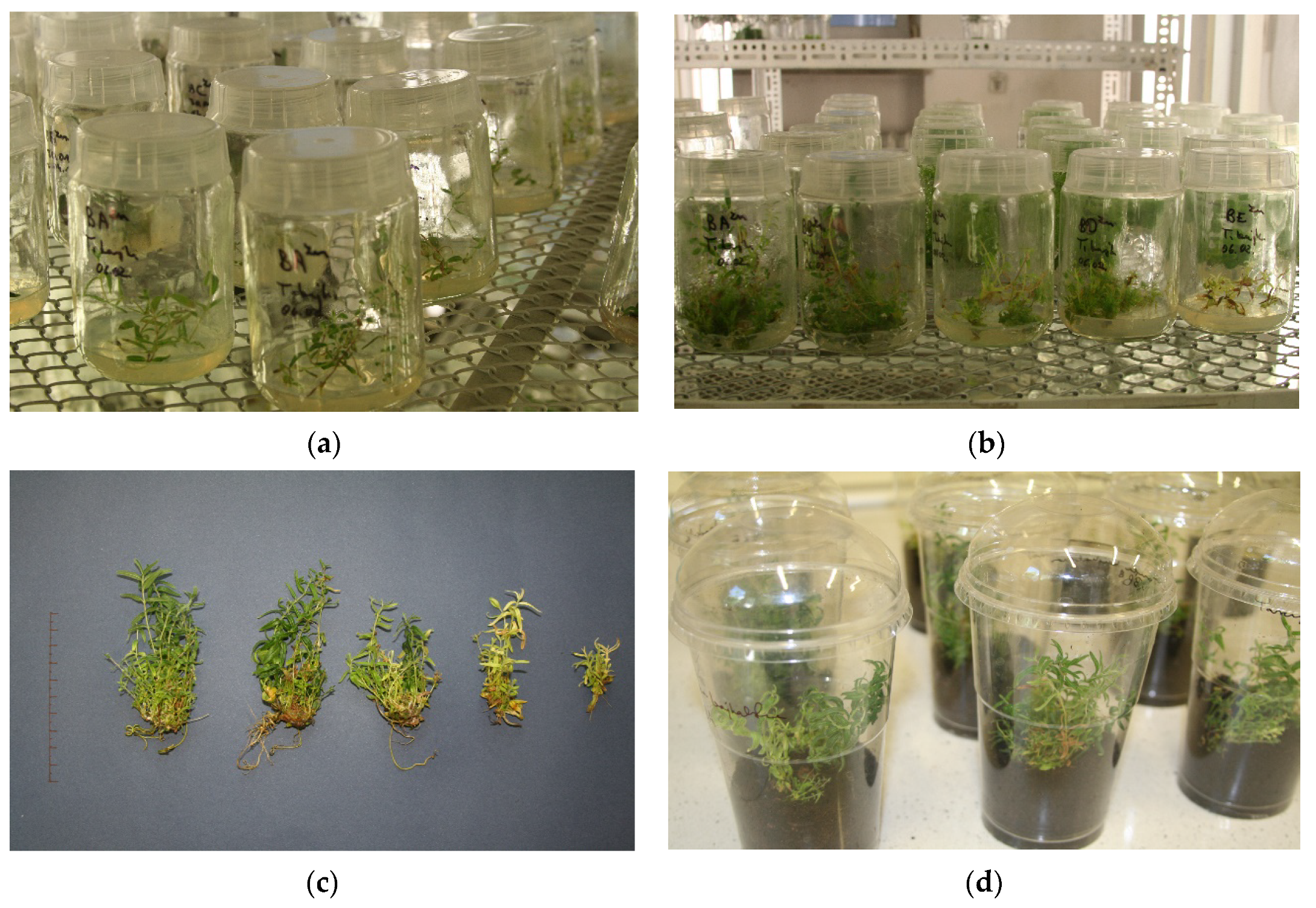
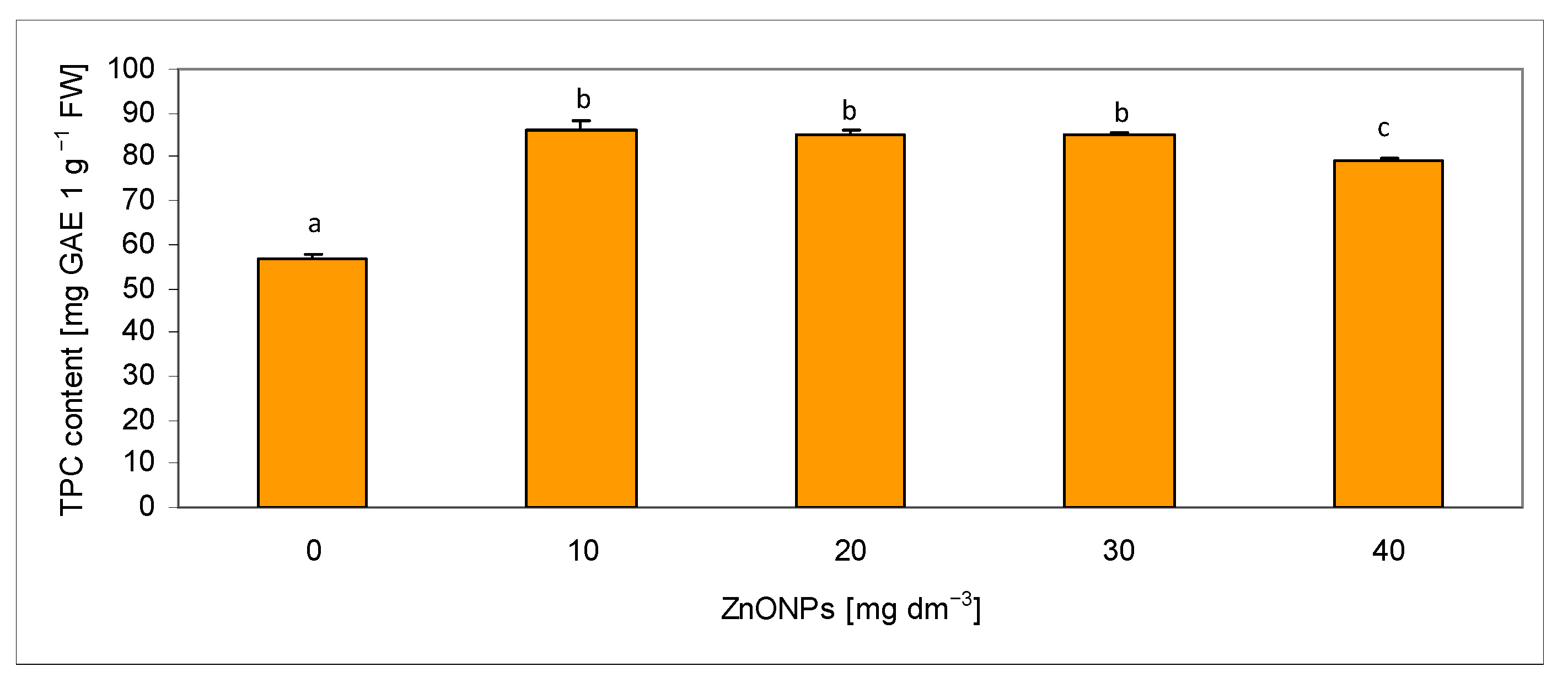
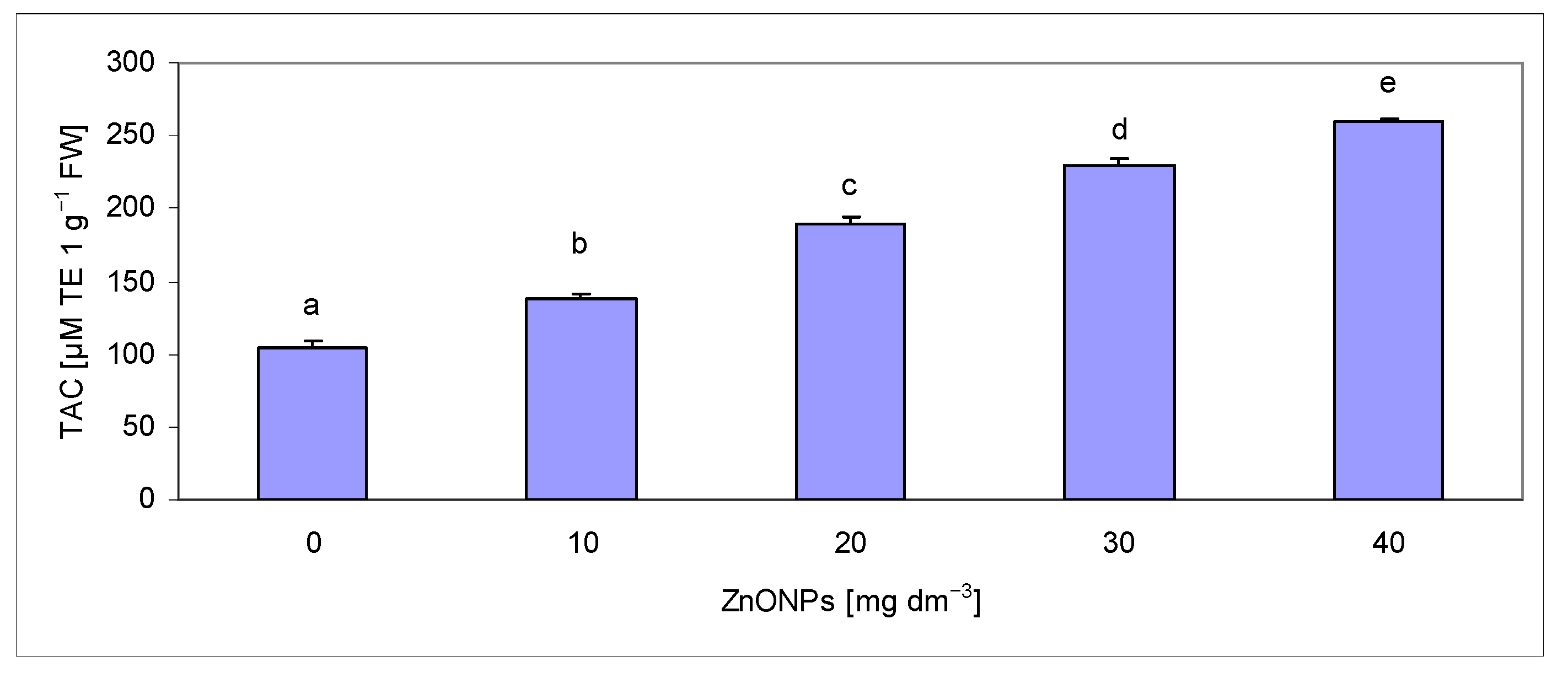
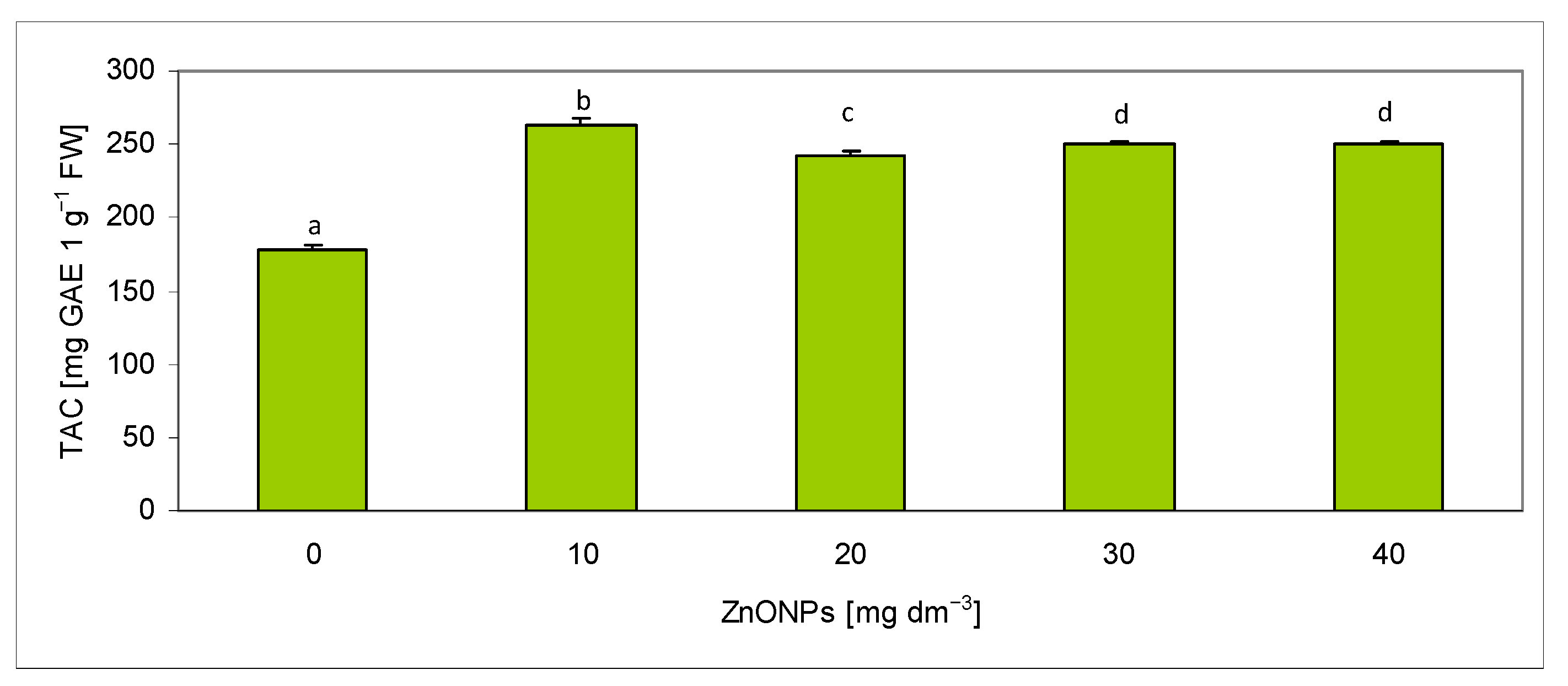

| Biometric Feature | Concentration of ZnONPs [mg dm−3] | |||||
|---|---|---|---|---|---|---|
| 0 (Control) | 10 | 20 | 30 | 40 | LSD p < 0.05 | |
| Number of shoots | 10.43 a* | 8.94 a | 8.77 ab | 5.76bc | 2.92 c | 3.585 |
| Shoot length [cm] | 8.91 a | 8.58 a | 7.63 ab | 5.83 bc | 3.05 c | 2.905 |
| Number of roots | 4.19a | 3.86ab | 2.79 bc | 1.92 bc | 1.55 c | 1.739 |
| Root length [cm] | 3.64 a | 3.06 a | 2.25 ab | 1.80 b | 0.81 b | 1.705 |
| Fresh weight of plant [g] (Increment [g]) | 0.686 a (0.657) a | 0.702 a (0.681) a | 0.658 a (0.632) a | 0.418 b (0.394) b | 0.109 b (0.092) b | 0.255 (0.232) |
| ZnONPs [mg dm−3] | Mineral Content [mg 100 g−1 DW] | ||||||
|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Fe | Mn | Zn | Cu | |
| 0 | 2330 a | 517 a | 178 a | 38.7 a | 25.4 a | 14.6 a | 0.77 a |
| 10 | 2455 b | 552 b | 195 b | 43.5 b | 25.9 a | 42.3 b | 0.75 a |
| 20 | 2520 c | 582 c | 203 c | 56.2 c | 26.0 a | 69.2 c | 0.73 ab |
| 30 | 3440 d | 507 d | 219 d | 33.1 d | 22.7 b | 211.0 d | 0.69 b |
| 40 | 2260 e | 453 e | 175 a | 32.5 d | 21.2 b | 236.0 e | 0.64 b |
| LSD p < 0.05 | 13.50 | 9.15 | 7.86 | 2.11 | 3.55 | 6.30 | 0.09 |
| ZnONPs [mg dm−3] | Mineral Content Ratio | ||||||
|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Fe | Mn | Zn | Cu | |
| 0 | 3026 | 671 | 231 | 50 | 33 | 19 | 1 |
| 10 | 3273 | 736 | 260 | 58 | 35 | 56 | 1 |
| 20 | 3452 | 797 | 278 | 77 | 36 | 95 | 1 |
| 30 | 4986 | 735 | 317 | 48 | 33 | 306 | 1 |
| 40 | 3531 | 708 | 273 | 51 | 33 | 369 | 1 |
| Measurements | ZnONPs Treatments | TPC | DPPH | ABTS | Chl a | Chl b | Chl a + b | Carotenoids |
|---|---|---|---|---|---|---|---|---|
| ZnONPs treatments | 1 | |||||||
| TPC | 0.631 | 1 | ||||||
| DPPH | 0.625 | 0.955 | 1 | |||||
| ABTS | 0.896 | 0.690 | 0.565 | 1 | ||||
| chl a | −0.974 | −0.756 | −0.772 | −0.856 | 1 | |||
| chl b | −0.976 | −0.744 | −0.722 | −0.909 | 0.989 | 1 | ||
| chl a + b | −0.977 | −0.754 | −0.760 | −0.872 | 0.999 | 0.994 | 1 | |
| carotenoids | −0.953 | −0.675 | −0.649 | −0.883 | 0.962 | 0.986 | 0.971 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzepiłko, A.; Prażak, R.; Święciło, A.; Gawroński, J. The Effect of Zinc Oxide Nanoparticles on the Quantitative and Qualitative Traits of Scutellaria baicalensis Georgi in In Vitro Culture. Int. J. Mol. Sci. 2025, 26, 5836. https://doi.org/10.3390/ijms26125836
Krzepiłko A, Prażak R, Święciło A, Gawroński J. The Effect of Zinc Oxide Nanoparticles on the Quantitative and Qualitative Traits of Scutellaria baicalensis Georgi in In Vitro Culture. International Journal of Molecular Sciences. 2025; 26(12):5836. https://doi.org/10.3390/ijms26125836
Chicago/Turabian StyleKrzepiłko, Anna, Roman Prażak, Agata Święciło, and Jacek Gawroński. 2025. "The Effect of Zinc Oxide Nanoparticles on the Quantitative and Qualitative Traits of Scutellaria baicalensis Georgi in In Vitro Culture" International Journal of Molecular Sciences 26, no. 12: 5836. https://doi.org/10.3390/ijms26125836
APA StyleKrzepiłko, A., Prażak, R., Święciło, A., & Gawroński, J. (2025). The Effect of Zinc Oxide Nanoparticles on the Quantitative and Qualitative Traits of Scutellaria baicalensis Georgi in In Vitro Culture. International Journal of Molecular Sciences, 26(12), 5836. https://doi.org/10.3390/ijms26125836






