The Astroglia Syncytial Theory of Consciousness
Abstract
1. Introduction
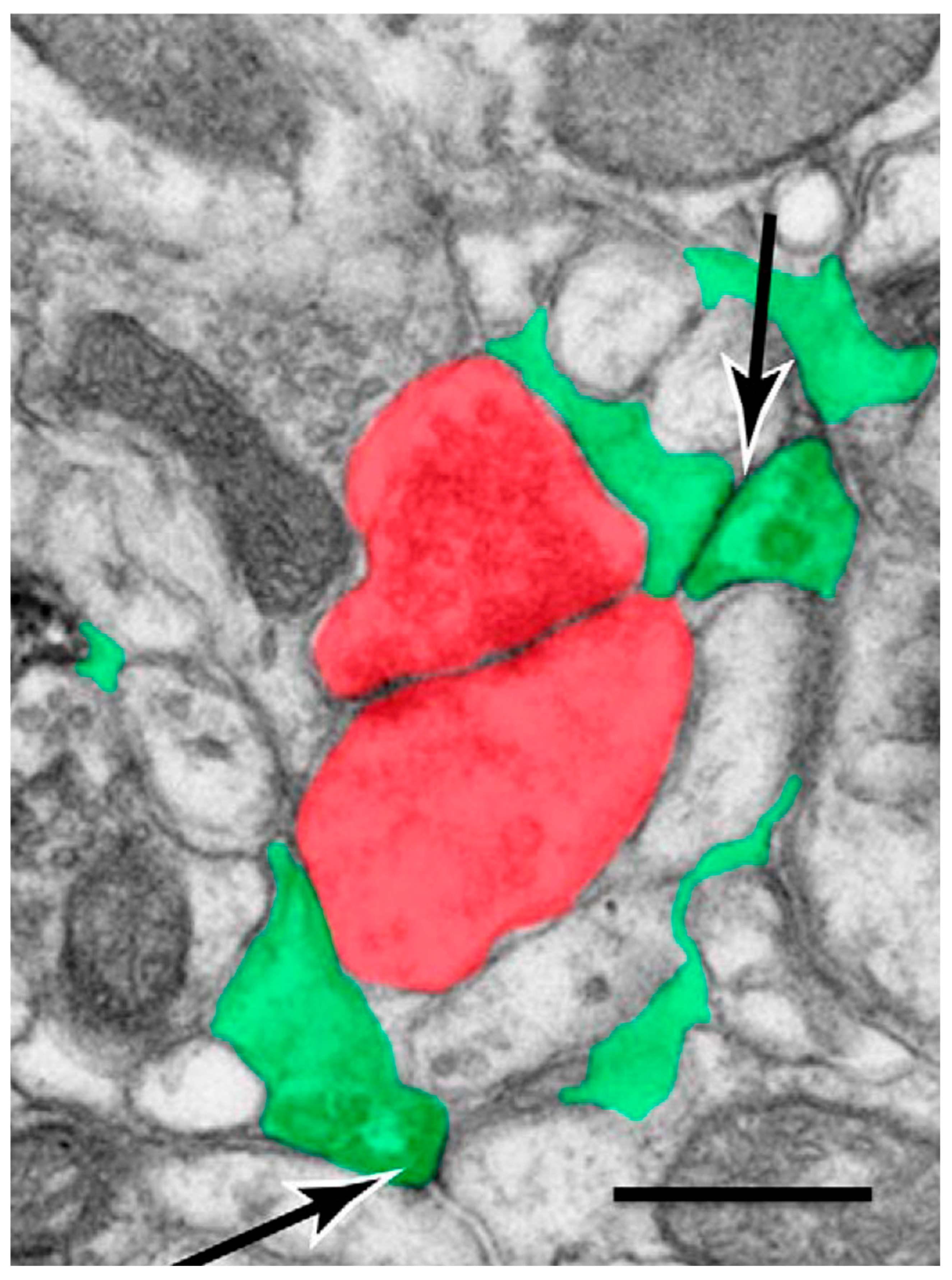
2. Astrocyte Domains, Microdomains, and Synaptic Interactions
2.1. Astrocyte-to-Neuron Signaling
2.2. Neuron-to-Astrocyte Signaling
3. Astrocyte Domains Integrate Synaptic Activity
3.1. Calcium Excitability
3.2. Electrical Activation
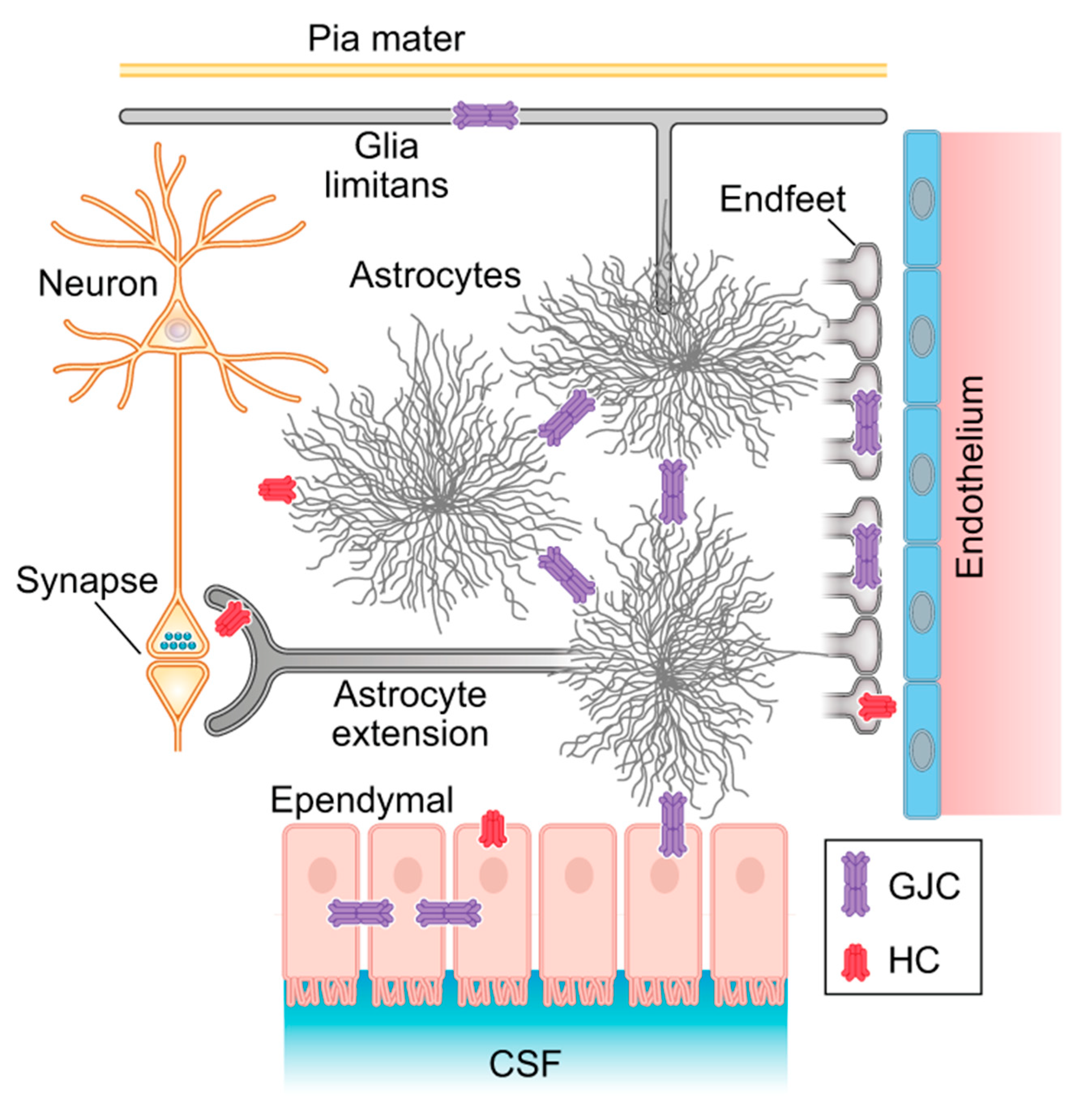
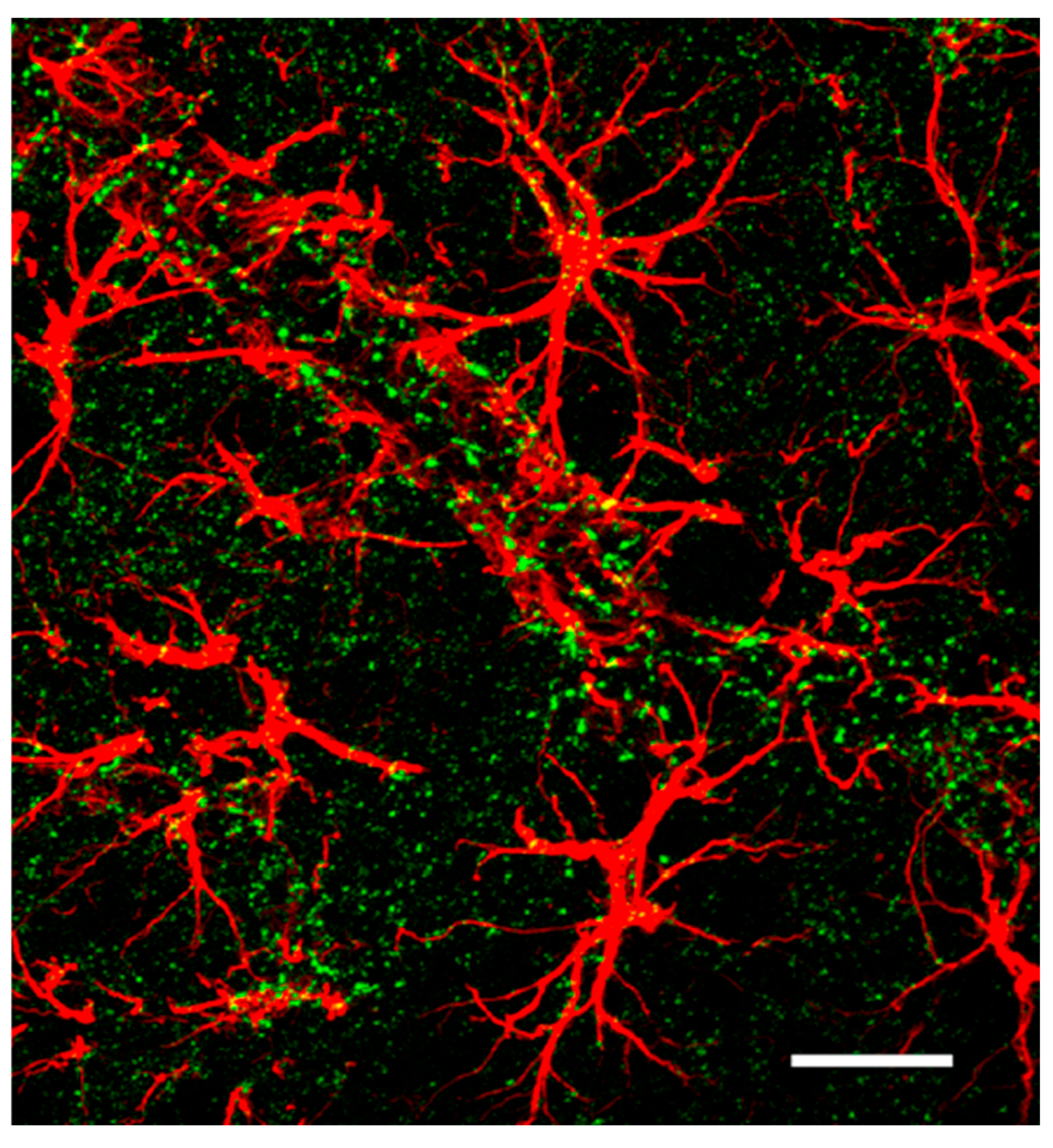
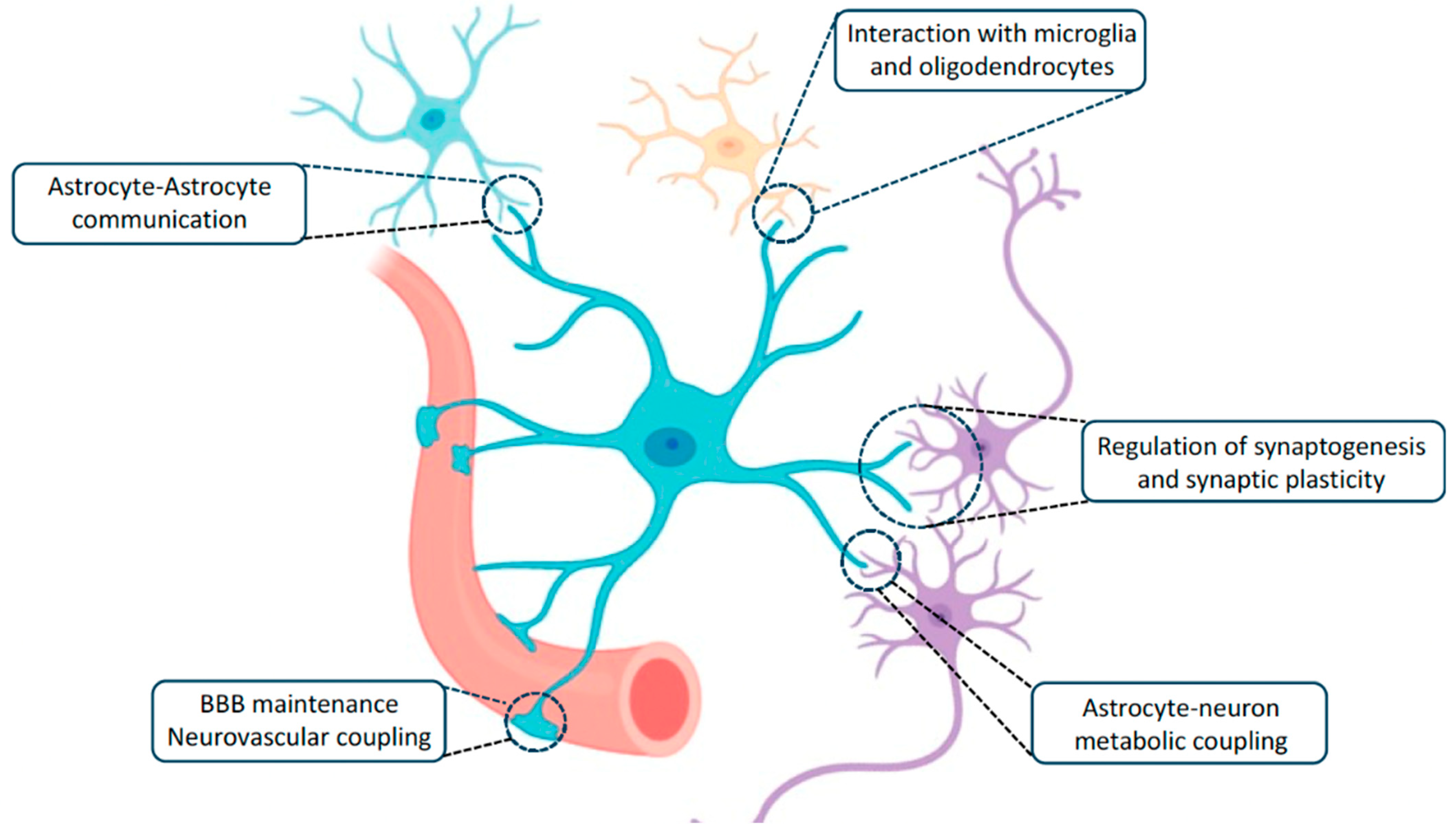
4. The Astroglia Syncytium Integrates Multiple Neural Networks Throughout the Brain
Spatial Distribution of Astrocyte Domains
5. The Panglial Syncytium Integrates the Entirety of Brain Information
5.1. Myelin Plasticity
5.2. The Corpus Callosum and Consciousness
5.3. Neurovascular Coupling
5.4. Metabolic Pathways and Human Brain Evolution
6. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Agid, Y.; Magistretti, P. Glial Man: A Revolution in Neuroscience, 1st ed.; Oxford University Press: Oxford, UK, 2021; ISBN 978-0-19-884767-0. [Google Scholar]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Pannasch, U.; Freche, D.; Dallérac, G.; Ghézali, G.; Escartin, C.; Ezan, P.; Cohen Salmon, M.; Benchenane, K.; Abudara, V.; Dufour, A.; et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat. Neurosci. 2014, 17, 549–558. [Google Scholar] [CrossRef]
- Peters, A.; Palay, S.L.; Webster, H.d.F. The Fine Structure of the Nervous System, 3rd ed.; Oxford University Press: Oxford, UK, 1991; ISBN 0-19-506571-9. [Google Scholar]
- Ventura, R.; Harris, K.M. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 1999, 19, 6897–6906. [Google Scholar] [CrossRef]
- Chao, T.I.; Rickmann, M.; Wolff, J.R. The synapse-astrocyte boundary: An anatomical basis for an integrative role of glia in synaptic transmission. In The Tripartite Synapse: Glia in Synaptic Transmission; Volterra, A., Magistretti, P., Haydon, P., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 3–23. ISBN 0-19-850854-9. [Google Scholar]
- Aten, S.; Kiyoshi, C.M.; Arzola, E.P.; Patterson, J.A.; Taylor, A.T.; Du, Y.; Guiher, A.M.; Philip, M.; Camacho, E.G.; Mediratta, D.; et al. Ultrastructural view of astrocyte arborization, astrocyte-astrocyte and astrocyte-synapse contacts, intracellular vesicle-like structures, and mitochondrial network. Prog. Neurobiol. 2022, 213, 102264. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R. The neocortex: An overview of its evolutionary development, structural organization and synaptology. Anat. Embryol. 1994, 190, 307–337. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.; Carmignoto, G. Calcium oscillations encoding neuron-to-astrocyte communication. J. Physiol.-Paris 2002, 96, 193–198. [Google Scholar] [CrossRef]
- Lin, S.C.; Bergles, D.E. Synaptic signaling between neurons and glia. Glia 2004, 47, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Araque, A. Glial calcium signaling and neuron-glia communication. Cell Calcium 2005, 38, 375–382. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef]
- Fellin, T. Communication between neurons and astrocytes: Relevance to the modulation of synaptic and network activity. J. Neurochem. 2009, 108, 533–544. [Google Scholar] [CrossRef]
- Volterra, A. Astrocytes: Modulation of Synaptic Function and Network Activity. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 481–493. ISBN 978-0-19-979459-1. [Google Scholar]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Haydon, P.G. Astrocyte Modulation of Mammalian Synapses: Circuits and Behaviors. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 494–503. ISBN 978-0-19-979459-1. [Google Scholar]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 24, 232105. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Randall, J.; Janett, E.; Nikonenko, I.; König, S.; Jones, E.V.; Flores, C.E.; Murai, K.K.; Bochet, C.G.; Holtmaat, A.; et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 2014, 24, 1679–1688. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Araque, A. Astrocytes process synaptic information. Neuron Glia Biol. 2008, 4, 3–10. [Google Scholar] [CrossRef]
- Schipke, C.G.; Haas, B.; Kettenmann, H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex. Cereb. Cortex 2008, 18, 2450–2459. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Pannasch, U.; Rouach, N. Emerging role for astroglial networks in information processing: From synapse to behavior. Trends Neurosci. 2013, 36, 405–417. [Google Scholar] [CrossRef]
- Robertson, J.M. Astrocyte domains and the three-dimensional and seamless expression of consciousness and explicit memories. Med. Hypotheses 2013, 81, 1017–1024. [Google Scholar] [CrossRef]
- Ghézali, G.; Dallérac, G.; Rouach, N. Perisynaptic astroglial processes: Dynamic processors of neuronal information. Brain Struct. Funct. 2016, 221, 2427–2442. [Google Scholar] [CrossRef]
- Deemyad, T.; Lüthi, J.; Spruston, N. Astrocytes Integrate and Drive Action Potential Firing in Inhibitory Subnetworks. Nat. Commun. 2018, 9, 4336. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.M. The Gliocentric Brain. Int. J. Mol. Sci. 2018, 19, 3033. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, R.W. Naturally occurring cell death during neural development. Trends Neursci. 1985, 8, 487–493. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of synapse number by glia. Science 2001, 291, 657–661. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.A.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Paul Bornstein, P.; Barres, B.A. Thrombospondins Are Astrocyte-Secreted Proteins that Promote CNS Synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef]
- Caceres, M.; Suwyn, C.; Maddox, M.; Thomas, J.W.; Preuss, T.M. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb. Cortex 2007, 17, 2312–2321. [Google Scholar] [CrossRef]
- Eroglu, C.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Ozkan, E.; Chakraborty, C.; Mulinyawe, S.B.; Annis, D.S.; Huberman, A.D.; et al. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–392. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of Excitatory CNS Synaptogenesis by Astrocyte-Secreted Proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes Mediate Synapse Elimination through MEGF10 and MERTK Pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Allen, N.J.; Bennett, M.L.; Foo, L.C. Astrocyte glypicans 4 and 6 promote formation of excitatory synapse via GluA1 AMPA receptors. Nature 2012, 486, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J. Glial Control of Synaptogenesis. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 388–401. ISBN 978-0-19-979459-1. [Google Scholar]
- Corty, M.M.; Freeman, M.R. Cell biology in neuroscience: Architects in neural circuit design: Glia control neuron numbers and connectivity. J. Cell Biol. 2013, 203, 395–405. [Google Scholar] [CrossRef]
- Allen, N.J. Role of glia in developmental synapse formation. Curr. Opin. Neurobiol. 2013, 23, 1027–1033. [Google Scholar] [CrossRef]
- Chung, W.S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Eroglu, C. The interplay between neurons and glia in synapse development and plasticity. Curr. Opin. Neurobiol. 2017, 42, 1–8. [Google Scholar] [CrossRef]
- Baldwin, K.T.; Eroglu, C. Molecular mechanisms of astrocyte-induced synaptogenesis. Curr. Opin. Neurobiol. 2017, 45, 113–120. [Google Scholar] [CrossRef]
- Rouach, N.; Glowinski, J.; Giaume, C. Activity-dependent neuronal control of gap junctional communication in astrocytes. J. Cell Biol. 2000, 149, 1513–1526. [Google Scholar] [CrossRef]
- Ransom, B.R.; Giaume, C. Gap Junctions and Hemichannels. In The Cognitive Neurosciences III, 3rd ed.; Gazzaniga, M.S., Ed.; Chief, MIT Press: Cambridge, MA, USA, 2004; pp. 292–305. ISBN 0-262-07254-8. [Google Scholar]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef]
- Theis, M.; Giaume, C. Connexin-based intercellular communication and astrocyte heterogeneity. Brain Res. 2012, 1487, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ransom, B.; Giaume, C. Gap Junctions and Hemichannels. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 292–305. ISBN 978-0-19-979459-1. [Google Scholar]
- Giaume, C.; Naus, C.C.; Sáez, J.C.; Leybaert, L. Glial connexins and pannexins in the healthy and diseased brain. Physiol. Rev. 2022, 101, 93–145. [Google Scholar] [CrossRef]
- Fields, R.D.; Araque, A.; Johansen-Berg, H.; Lim, S.-S.; Lynch, G.; Nave, K.-A.; Nedergaard, M.; Perez, R.; Sejnowski, T.; Wake, H. Glial Biology in Learning and Cognition. Neurosci. 2014, 20, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Mederos, S.; Sánchez-Puelles, C.; Esparza, J.; Valero, M.; Ponomarenko, A.; Perea, G. GABAergic Signaling to Astrocytes in the Prefrontal Cortex Sustains Goal Directed Behaviors. Nat. Neurosci. 2021, 24, 82–92. [Google Scholar] [CrossRef]
- Kofuji, P.; Araque, A. Astrocytes and Behavior. Annu. Rev. Neurosci. 2021, 44, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Hastings, N.; Yu, Y.-L.; Huang, B.; Middya, S.; Inaoka, M.; Erkamp, N.A.; Roger, J.; Mason, R.J.; Carnicer-Lombarte, A.; Rahman, S.; et al. Electrophysiological In Vitro Study of Long-Range Signal Transmission by Astrocytic Networks. Adv. Sci. 2023, 10, 2301756. [Google Scholar] [CrossRef]
- Escalada, P.; Ezkurdia, A.; Ramírez, M.J.; Solas, M. Essential Role of Astrocytes in Learning and Memory. Int. J. Mol. Sci. 2024, 25, 1899. [Google Scholar] [CrossRef]
- Fields, R.D. The Other Brain; Simon & Schuster: New York, NY, USA, 2009; ISBN 978-0-7432-9141-5. [Google Scholar]
- Visser, J.; Milior, G.; Breton, R.; Ezan, P.; Ribot, J.; Rouach, N. Astroglial networks control visual responses of superior collicular neurons and sensory-motor behavior. Cell Rep. 2024, 43, 114504. [Google Scholar] [CrossRef]
- Sattin, D.; Magnani, F.G.; Bartesaghi, L.; Caputo, M.; Fittipaldo, A.V.; Cacciatore, M.; Picozzi, M.; Leonardi, M. Theoretical Models of Consciousness: A Scoping Review. Brain Sci. 2021, 11, 535. [Google Scholar] [CrossRef]
- Zeki, S. The visual image in mind and brain. Sci. Am. 1992, 267, 69–76. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997, 17, 7817–7830. [Google Scholar] [CrossRef]
- Carmignoto, G. Reciprocal communication systems between astrocytes and neurons. Prog. Neurobiol. 2000, 62, 561–581. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Communication between astrocytes and neurons: A complex language. J. Physiol. -Paris 2002, 96, 199–207. [Google Scholar] [CrossRef]
- Gebicke-Haerter, P.J. The computational power of the human brain. Front. Cell. Neurosci. 2023, 17, 1220030. [Google Scholar] [CrossRef] [PubMed]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Shigetomi, E.; Kracun, S.; Khakh, B.S. Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters. Neuron Glia Biol. 2010, 6, 183–191. [Google Scholar] [CrossRef]
- Kettenmann, H.; Filippov, V. Signalling between neurons and Bergmann glial cells. In The Tripartite Synapse: Glia in Synaptic Transmission; Volterra, A., Magistretti, P., Haydon, P., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 139–150. ISBN 0-19-850854-9. [Google Scholar]
- Bergles, D.E.; Edwards, R.H. The role of glutamate transporters in synaptic transmission. In Structural and Functional Organization of the Synapse, 1st ed.; Hell, J.W., Ehlers, M.D., Eds.; Springer Science + Business Media LLC: New York, NY, USA, 2008; pp. 23–62. ISBN 9780387772318. [Google Scholar]
- Arizono, M.; Bannai, H.; Nakamura, K.; Niwa, F.; Enomoto, M.; Matsura, T.; Miyamoto, A.; Sherwood, M.W.; Nakamura, T.; Mikoshiba, K. Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation. Sci. Signal. 2012, 5, r27. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.H.; Morland, C.; Ormel, L.; Rinholm, J.E.; Larsson, M.; Wold, J.F.H.; Røe, A.T.; Stranna, M.; Santello, M.; Bouvier, D.; et al. Immunogold detection of Lglutamate and D-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb. Cortex 2012, 22, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Stobart, J.L.; Ferrari, K.D.; Barrett, M.J.P.; Stobart, M.J.; Looser, Z.J.; Saab, A.S.; Weber, B. Long-term In Vivo Calcium Imaging of Astrocytes Reveals Distinct Cellular Compartment Responses to Sensory Stimulation. Cereb. Cortex 2018, 28, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Stobart, J.L.; Ferrari, K.D.; Barrett, M.J.P.; Glück, C.; Stobart, M.J.; Zuend, M.; Weber, B. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 2018, 98, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef]
- Bezzi, P.; Gundersen, V.; Galbete, J.; Seifert, G.; Steinhäuser, C.; Pilati, E.; Volterra, A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 2004, 7, 613–620. [Google Scholar] [CrossRef]
- Sakers, K.; Lake, A.M.; Khazanchi, R.; Ouwenga, R.; Vasek, M.J.; Dani, A.; Dougherty, J.D. Astrocytes Locally Translate Transcripts in Their Peripheral Processes. Proc. Natl. Acad. Sci. USA 2017, 114, E3830–E3838. [Google Scholar] [CrossRef]
- Santello, M.; Calì, C.; Bezzi, P. Gliotransmission and the tripartite synapse. Adv. Exp. Med. Biol. 2012, 970, 307–331. [Google Scholar] [CrossRef]
- Petrelli, F.; Bezzi, P. Novel insights into gliotransmitters. Curr. Opin. Pharmacol. 2016, 26, 138–145. [Google Scholar] [CrossRef]
- Kettenmann, H.; Zorec, R. Release of Gliotransmitters and Transmitter Receptors in Astrocytes. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 197–211. ISBN 978-0-19-979459-1. [Google Scholar]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Quantal Release of Transmitter: Not Only from Neurons but from Astrocytes as Well? In Neuroglia, 2nd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 190–201. ISBN 0-19-515222-0. [Google Scholar]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Oliet, S.H.; Mothet, J.P. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience 2009, 158, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Henneberger, C.; Bard, L.; Rusakov, D.A. D-Serine: A key to synaptic plasticity? Int. J. Biochem. Cell. Biol. 2012, 44, 587–590. [Google Scholar] [CrossRef][Green Version]
- Panatier, A.; Gentles, S.J.; Bourque, C.W.; Oliet, S.H.R. Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus. J. Physiol. 2006, 573, 711–721. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef] [PubMed]
- Hillen, A.E.J.; Burbach, J.P.H.; Hol, E.M. Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog. Neurobiol. 2018, 165–167, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Pannasch, U.; Vargová, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Syková, E.; Rouach, N. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef]
- Franze, K.; Grosche, J.; Skatchkov, S.N.; Schinkinger, S.; Foja, C.; Schild, D.; Uckermann, O.; Travis, K.; Reichenbach, A.; Guck, J. Müller cells are living optical fibers in the vertebrate retina. Proc. Natl. Acad. Sci. USA 2007, 104, 8287–8292. [Google Scholar] [CrossRef]
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Petit, J.M.; Gyger, J.; Burlet-Godinot, S.; Fiumelli’, H.; Martin, J.L.; Magistretti, P.J. Genes involved in the astrocyte-neuron lactate shuttle (ANLS) are specifically regulated in cortical astrocytes following sleep deprivation in mice. Sleep 2013, 36, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.-M.; Magistretti, P.J. Regulation of Neuron-Astrocyte Metabolic Coupling Across the Sleep-Wake Cycle. Neuroscience 2016, 323, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, C.; Seifert, G.; Deitmer, W. Physiology of Astrocyte Ion Channels and Ion Transporters. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 185–196. ISBN 978-0-19-979459-1. [Google Scholar]
- Navarrete, M.; Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 2010, 68, 113–126. [Google Scholar] [CrossRef]
- Shao, Y.; Porter, J.T.; McCarthy, K.D. Neuroligand receptor heterogeneity among astroglia. Perspect. Dev. Neurobiol. 1994, 2, 205–215. [Google Scholar]
- Snyder, S. Drugs and the Brain; Scientific American Library: New York, NY, USA, 1996; ISBN 0-7167-5015-5. [Google Scholar]
- Nilsson, M.; Hansson, E.; Rönnbäck, L. Adrenergic and 5-HT2 receptors on the same astroglial cell. A microspectrofluorimetric study on cytosolic Ca2+ responses in single cells in primary culture. Dev. Brain Res. 1991, 63, 33–41. [Google Scholar] [CrossRef]
- Bekar, L.K.; He, W.; Nedergaard, M. Locus coeruleus alpha-adrenergic mediated activation of cortical astrocytes in vivo. Cereb. Cortex 2008, 18, 2789–2795. [Google Scholar] [CrossRef]
- Stone, E.A.; John, S.M. Further evidence for a glial localization of rat cortical beta adrenoceptors: Studies of in vivo cyclic AMP responses to catecholamines. Brain Res. 1991, 549, 78–82. [Google Scholar] [CrossRef]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C. Beta-adrenergic receptors: Astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J. Neurosci. 1992, 12, 781–792. [Google Scholar] [CrossRef]
- Marchaland, J.; Calì, C.; Voglmaier, S.M.; Li, H.; Regazzi, R.; Edwards, R.H.; Bezzi, P. Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic like microvesicles in astrocytes. J. Neurosci. 2008, 28, 9122–9132. [Google Scholar] [CrossRef]
- Cornell-Bell, A.H.; Finkbeiner, S.M.; Cooper, M.S.; Smith, S.J. Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling. Science 1990, 247, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.W.; Chernjavsky, A.; Smith, S.J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 1992, 8, 429–440. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V. Calcium signaling in neuroglia. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 320–332. ISBN 978-0-19-979459-1. [Google Scholar]
- McCrone, J. How Do You Persist When Your Molecules Don’t? Science & Consciousness Review June, No.1 1-3; 2004. Available online: www.scirev.org (accessed on 6 May 2025).
- Kuffler, S.W.; Nicholls, J.G.; Orkand, R.K. Physiological properties of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966, 29, 768–787. [Google Scholar] [CrossRef]
- Murphy, T.H.; Blatter, L.A.; Wier, W.G.; Baraban, J.M. Rapid communication between neurons and astrocytes in primary cortical cultures. J. Neurosci. 1993, 13, 2672–2679. [Google Scholar] [CrossRef]
- Winship, I.R.; Plaa, N.; Murphy, T.H. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J. Neurosci. 2007, 27, 6268–6272. [Google Scholar] [CrossRef] [PubMed]
- Chuquet, J.; Quilichini, P.; Nimchinsky, E.A.; Buzsáki, G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J. Neurosci. 2010, 30, 15298–15303. [Google Scholar] [CrossRef]
- Crick, F.; Koch, C. A Framework for Consciousness. In The Cognitive Neurosciences III, 3rd ed.; Gazzaniga, M.S., Ed.; MIT Press: Cambridge, MA, USA, 2004; pp. 1133–1143. ISBN 0-262-07254-8. [Google Scholar]
- Du, Y.; Ma, B.; Kiyoshi, C.M.; Alford, C.C.; Wang, W.; Zhou, M. Freshly dissociated mature hippocampal astrocytes exhibit similar passive membrane conductance and low membrane resistance as syncytial coupled astrocytes. J. Neurophysiol. 2015, 113, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Buckalew, R.; Du, Y.; Kiyoshi, C.M.; Alford, C.C.; Wang, W.; McTigue, D.M.; Enyeart, J.J.; Terman, D.; Zhou, M. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 2016, 64, 214–226. [Google Scholar] [CrossRef]
- Kiyoshi, C.M.; Zhou, M. Astrocyte syncytium: A functional reticular system in the brain. Neural Regen. Res. 2019, 14, 595–596. [Google Scholar] [CrossRef]
- Huang, M.; Du, Y.; Kiyoshi, C.M.; Wu, X.; Askwith, C.C.; McTigue, D.M.; Zhou, M. Syncytial isopotentiality: An electrical feature of spinal cord astrocyte networks. Neuroglia 2018, 1, 271–279. [Google Scholar] [CrossRef]
- De Pina-Benabou, M.H.; Srinivas, M.; Spray, D.C.; Scemes, E. Calmodulin Kinase Pathway Mediates the K -Induced Increase in Gap Junctional Communication between Mouse Spinal Cord Astrocytes. J. Neurosci. 2001, 21, 6635–6643. [Google Scholar] [CrossRef] [PubMed]
- Rouach, N.; Koulakoff, A.; Giaume, C. Neurons set the tone of gap junctional communication in astrocytic networks. Neurochem. Int. 2004, 4545, 265–272. [Google Scholar] [CrossRef]
- Mazaud, D.; Capano, A.; Rouach, N. The many ways astroglial connexins regulate neurotransmission and behavior. Glia 2021, 69, 2527–2545. [Google Scholar] [CrossRef]
- De Pittà, M.; Brunel, N. Modulation of Synaptic Plasticity by Glutamatergic Gliotransmission: A Modeling Study. Neural Plast. 2016, 2016, 7607924. [Google Scholar] [CrossRef]
- Emsley, J.G.; Macklis, J.D. Astroglial heterogeneity closely reflects the neuronal defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006, 2, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Houades, V.; Koulakoff, A.; Ezan, P.; Seif, I.; Giaume, C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J. Neurosci. 2008, 28, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Roux, L.; Benchenane, K.; Rothstein, J.D.; Bonvento, G.; Giaume, C. Plasticity of astroglial networks in olfactory glomeruli. Proc. Natl. Acad. Sci. USA 2011, 108, 18442–18446. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T.; Davidson, K.G.V.; Furman, C.S.; Dudek, F.E.; Nagy, J.I. Identification of Cells Expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in Gap Junctions of Rat Brain and Spinal Cord. Cell. Commun. Adhes. 2001, 8, 315–320. [Google Scholar] [CrossRef]
- Valiunas, V.; Polosina, Y.Y.; Miller, H.; Potapova, I.A.; Valiuniene, L.; Doronin, S.; Mathias, R.T.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; et al. Connexin specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. 2005, 568 Pt 2, 459–468. [Google Scholar] [CrossRef]
- Brink, P.R.; Valiunas, V.; Gordon, C.; Rosen, M.R.; Cohen, I.S. Can gap junctions deliver? Biochim. Biophys. Acta BBA-Biomembr. 2012, 1818, 2076–2081. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Mehler, M.F. Non-coding RNA networks underlying cognitive disorders across the lifespan. Trends Mol. Med. 2011, 17, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ladecola, C.; Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007, 10, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- James, W. The Principles of Psychology; Benton: Chicago, IL, USA, 1890; ISBN-13: 978-1543183184. [Google Scholar]
- Mountcastle, V.B. The columnar organization of the neocortex. Brain 1997, 120, 701–722. [Google Scholar] [CrossRef]
- Nagy, J.I.; Ionescu, A.V.; Lynn, B.D.; Rash, J.E. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 2003, 44, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, L.M.; Goodenough, D.A.; Paul, D.L. Functional Heterotypic Interactions Between Astrocyte and Oligodendrocyte Connexins. Glia 2011, 59, 26–34. [Google Scholar] [CrossRef]
- Elfgang, C.; Eckert, R.; Lichtenberg-Frat, H.; Butterweck, A.; Traub, O.; Klein, R.A.; Hiilser, D.E.; Willecke, K. Specific Permeability and Selective Formation of Gap Junction Channels in Connexin-transfected HeLa Cell. J. Cell Biol. 1995, 129, 805–817. [Google Scholar] [CrossRef]
- Wake, H.; Lee, P.R.; Fields, R.D. Control of local protein synthesis and initial events in myelination by action potentials. Science 2011, 333, 1647–1651. [Google Scholar] [CrossRef]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.-J.; Cossell, L.; Attwell, D.; Tohyama, K. Oligodendrocyte Dynamics in the Healthy Adult CNS: Evidence for Myelin Remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef]
- Fields, R.D. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef]
- Fields, R.D. Regulation of Myelination by Functional Activity. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 573–585. ISBN 978-0-19-979459-1. [Google Scholar]
- Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008, 31, 361–370. [Google Scholar] [CrossRef]
- Xin, W.; Chan, J.R. Myelin plasticity: Sculpting circuits in learning and memory. Nat. Rev. Neurosci. 2020, 21, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Huxley, T.H. Man’s Place in Nature; McMillan: London, UK, 1863. [Google Scholar]
- Sperry, R.W. Lateral specialization in the surgically separated hemispheres. In Neuroscience Third Study Program; Schmitt, F.O., Worden, F.G., Eds.; MIT Press: Cambridge, UK, 1974; Volume 3, pp. 5–19. [Google Scholar] [CrossRef]
- Sperry, R.W. Hemisphere deconnection and unity in conscious awareness. Am. Psychol. 1968, 23, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Sperry, R. Consciousness, personal identity and the divided brain. Neuropsychologia 1984, 22, 661–673. [Google Scholar] [CrossRef]
- Paolino, A.; Fenlon, L.R.; Kozulin, P.; Haines, E.; Lim, J.W.C.; Richards, L.J.; Suarez, R. Differential timing of a conserved transcriptional network underlies divergent cortical projection routes across mammalian brain evolution. Proc. Natl. Acad. Sci. USA 2020, 117, 10554–10564. [Google Scholar] [CrossRef]
- Faissner, A. Neuron Migration and Axon Guidance. In Neuroglia, 3rd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: New York, NY, USA, 2013; pp. 402–416. ISBN 978-0-19-979459-1. [Google Scholar]
- Simard, M.; Arcuino, G.; Takano, T.; Liu, Q.S.; Nedergaard, M. Signaling at the Gliovascular Interface. J. Neurosci. 2003, 23, 9254–9262. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial Metabolic Networks Sustain Hippocampal Synaptic Transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Magistretti, P.J.; Chatton, J.Y. Astrocytes generate Na+-mediated metabolic waves. Proc. Natl. Acad. Sci. USA 2004, 101, 14937–14942. [Google Scholar] [CrossRef]
- Porras, O.H.; Ruminot, I.; Loaiza, A.; Barros, L.F. Na+-Ca2+ cosignaling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia 2008, 56, 59–68. [Google Scholar] [CrossRef]
- Ferrari, R.; Grandi, N.; Tramontano, E.; Dieci, G. Retrotransposons as Drivers of Mammalian Brain Evolution. Life 2021, 11, 376. [Google Scholar] [CrossRef]
- Wang, T.; Medynets, M.; Johnson, K.R.; Doucet-O’Hare, T.T.; DiSanza, B.; Li, W.; Xu, Y.; Bagnell, A.; Tyagi, R.; Sampson, K.; et al. Regulation of stem cell function and neuronal differentiation by HERV-K via mTOR pathway. Proc. Natl. Acad. Sci. USA 2020, 117, 17842–17853. [Google Scholar] [CrossRef] [PubMed]
- Wehle, D.T.; Bass, C.S.; Sulc, J.; Mirzaa, G.; Smith, S.E.P. Protein interaction network analysis of mTOR signaling reveals modular organization. J. Biol. Chem. 2023, 299, 105271. [Google Scholar] [CrossRef]
- Masvidal, L.; Hulea, L.; Furic, L.; Topisirovic, I.; Larsson, O. mTOR-sensitive translation: Cleared fog reveals more trees. RNA Biol. 2017, 14, 1299–1305. [Google Scholar] [CrossRef]
- Galambos, R. A glia-neural theory of brain function. Proc. Natl. Acad. Sci. USA 1961, 47, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Greenough, W.T. Behavioral experience-dependent plasticity of glial-neuronal interactions. In The Tripartite Synapse: Glia in Synaptic Transmission; Volterra, A., Magistretti, P., Haydon, P., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 248–260. ISBN 0 19 850854 9. [Google Scholar]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013, 12, 342–353. [Google Scholar] [CrossRef]
- LeDoux, J. Synaptic Self: How Our Brains Become Who We Are; Penguin Books: New York, NY, USA, 2002; ISBN 0-670-03028-7. [Google Scholar]
- Sherrington, C. The Brain and Its Mechanism; Cambridge University Press: Cambridge, UK, 1937. [Google Scholar]
- Shepherd, G.M.; Erulkar, S.D. Centenary of the synapse: From Sherrington to the molecular biology of the synapse and beyond. Trends Neurosci. 1997, 20, 385–392. [Google Scholar] [CrossRef]
- Ehlers, M.D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 2003, 6, 231–242. [Google Scholar] [CrossRef]
- Star, E.N.; Kwiatkowski, D.J.; Murthy, V.N. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat. Neurosci. 2002, 5, 239–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, J.M. The Astroglia Syncytial Theory of Consciousness. Int. J. Mol. Sci. 2025, 26, 5785. https://doi.org/10.3390/ijms26125785
Robertson JM. The Astroglia Syncytial Theory of Consciousness. International Journal of Molecular Sciences. 2025; 26(12):5785. https://doi.org/10.3390/ijms26125785
Chicago/Turabian StyleRobertson, James M. 2025. "The Astroglia Syncytial Theory of Consciousness" International Journal of Molecular Sciences 26, no. 12: 5785. https://doi.org/10.3390/ijms26125785
APA StyleRobertson, J. M. (2025). The Astroglia Syncytial Theory of Consciousness. International Journal of Molecular Sciences, 26(12), 5785. https://doi.org/10.3390/ijms26125785




