Widespread Changes in the Immunoreactivity of Bioactive Peptide T14 After Manipulating the Activity of Cortical Projection Neurons
Abstract
1. Introduction
2. Results
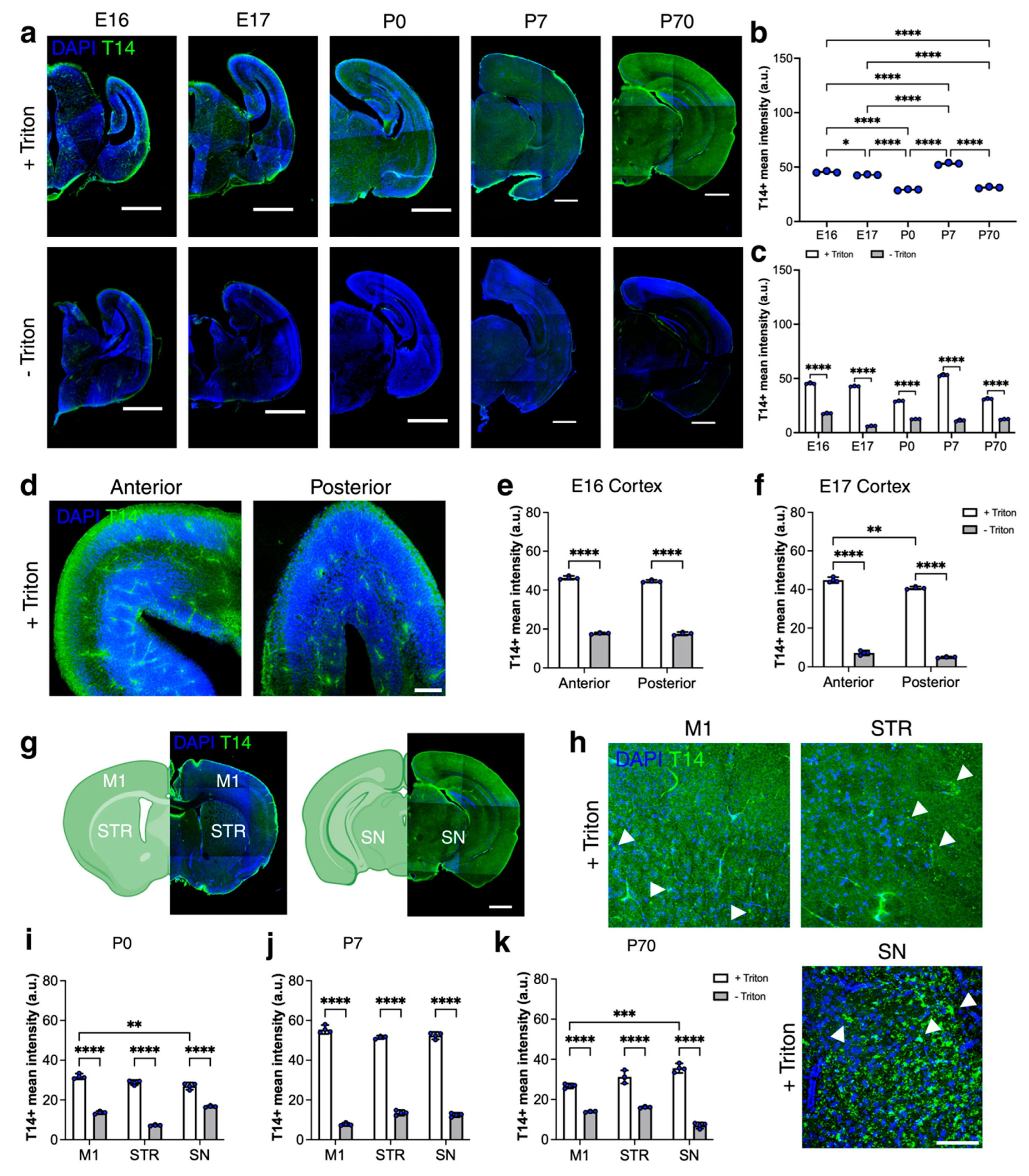
2.1. Intracellular Immunoreactivity of T14 Peptide in Developing and Adult Brains
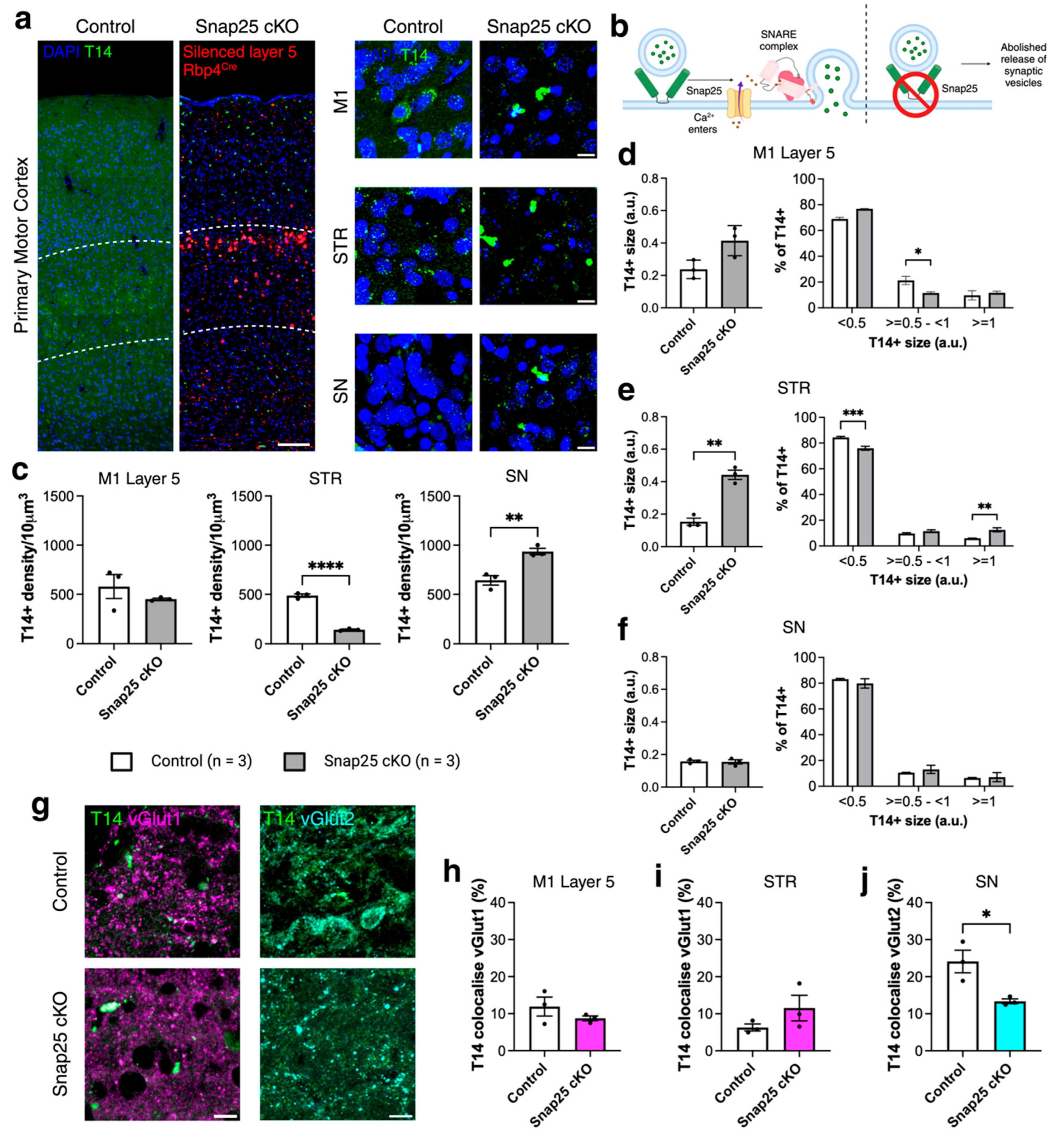
2.2. Abolishing Regulated Release of Synaptic Vesicles from a Subpopulation of Layer 5 Projection Neurons Changes the Distribution of T14 Immunoreactivity (488)
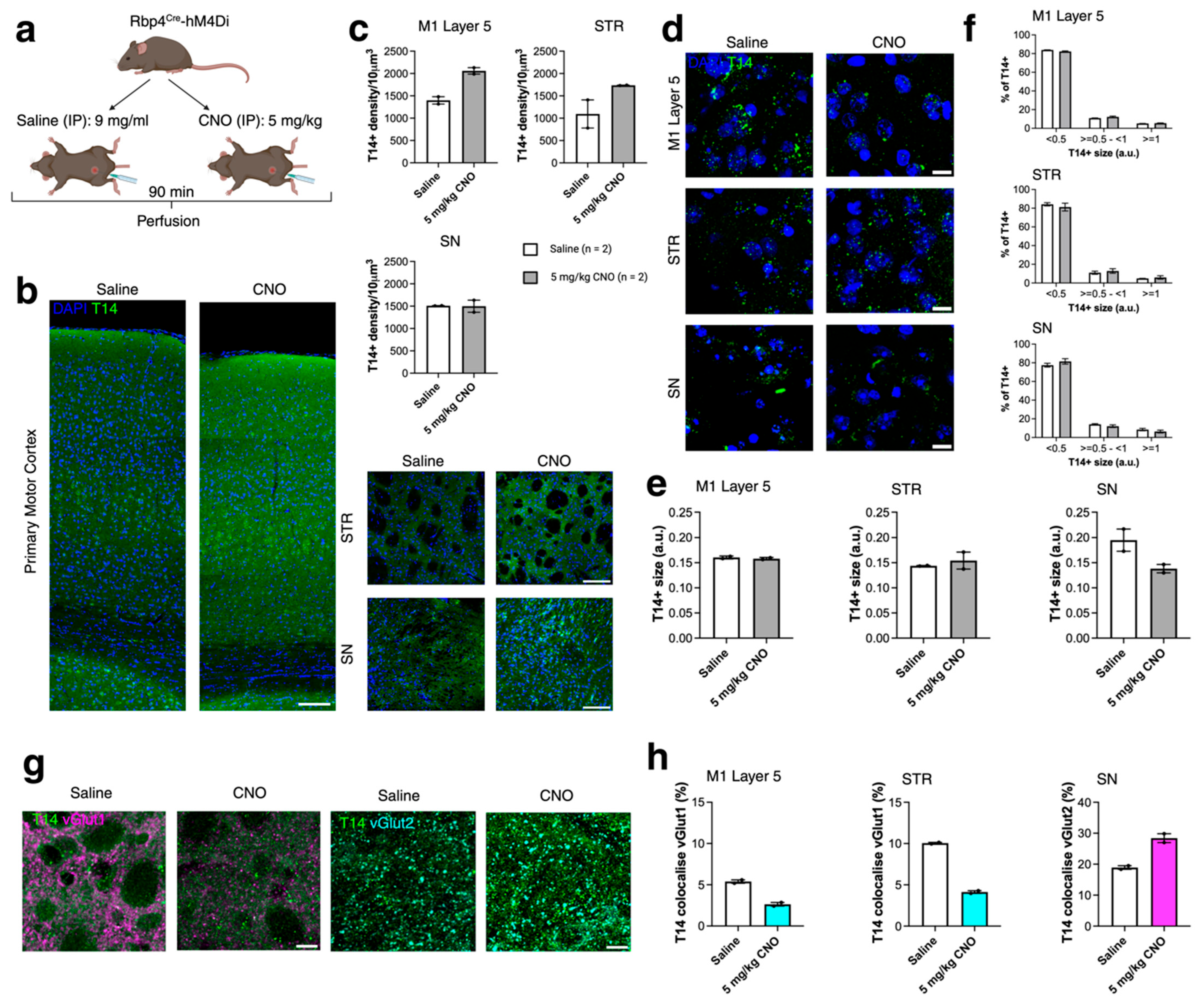
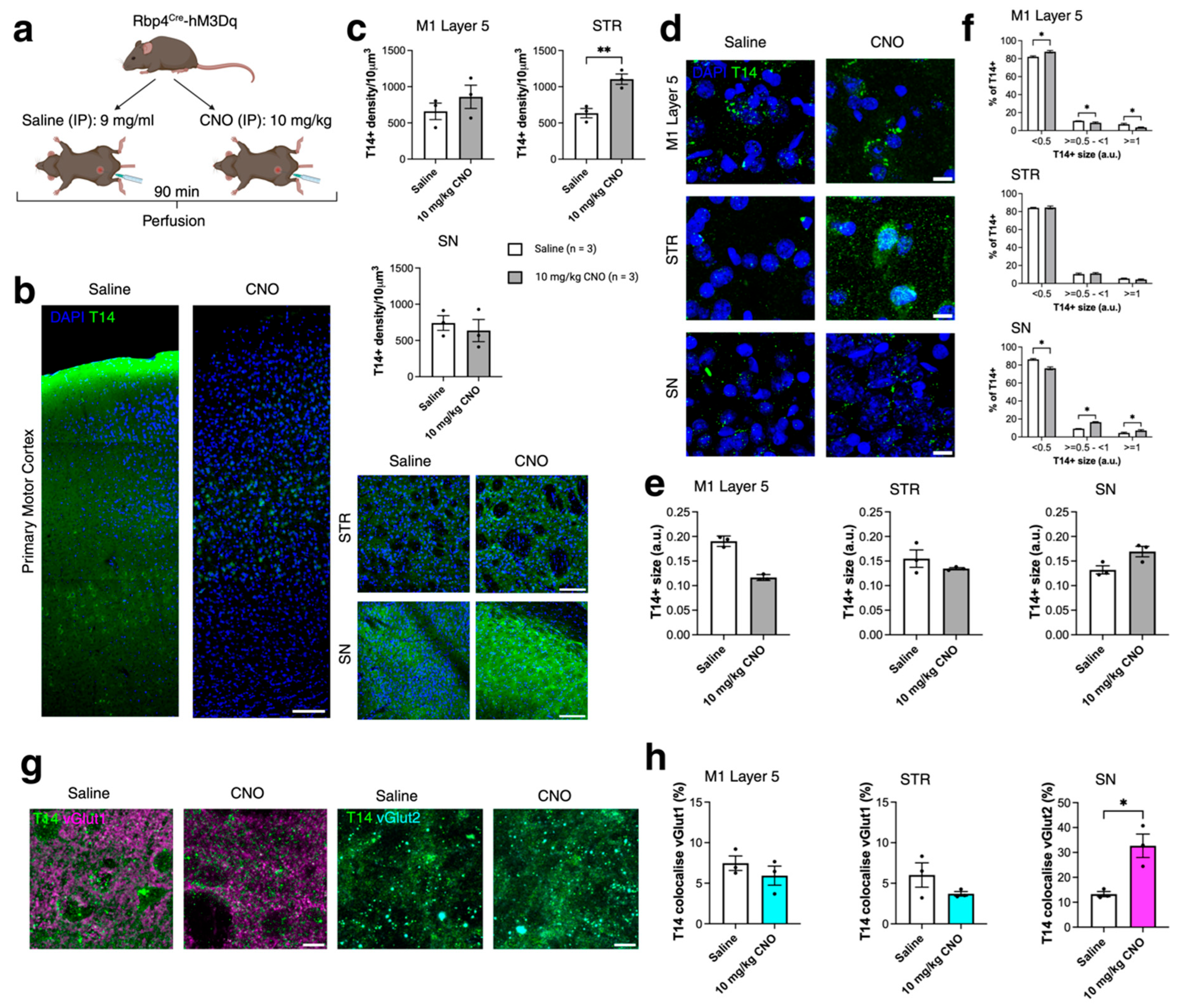
2.3. Acute Chemogenetic Manipulation Affects the Distribution of T14 Immunoreactivity and Presynaptic Compartments (430)
3. Discussion
3.1. T14 Immunoreactivity
3.2. T14 Immunoreactivity After Chronic and Acute Manipulations of Neuronal Activity
4. Materials and Methods
4.1. Breeding and Maintenance
4.2. Tissue Collection
4.3. CNO
4.4. Immunohistochemistry and Imaging
4.5. Imaging
4.5.1. Laser-Scanning Confocal Microscopy
4.5.2. Image Processing and Analysis
4.6. Statistics
5. Conclusions
Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranglani, S.; Hasan, S.; Komorowska, J.; Medina, N.M.; Mahfooz, K.; Ashton, A.; Garcia-Ratés, S.; Greenfield, S. A Novel Peptide Driving Neurodegeneration Appears Exclusively Linked to the α7 Nicotinic Acetylcholine Receptor. Mol. Neurobiol. 2024, 61, 8206–8218. [Google Scholar] [CrossRef] [PubMed]
- Ratés, S.G.; García-Ayllón, M.; Falgàs, N.; Brangman, S.A.; Esiri, M.M.; Coen, C.W.; Greenfield, S.A. Evidence for a novel neuronal mechanism driving Alzheimer’s disease, upstream of amyloid. Alzheimer’s Dement. 2024, 20, 5027–5034. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, S.; Greenfield, S. When a trophic process turns toxic: Alzheimer’s disease as an aberrant recapitulation of a developmental mechanism. Int. J. Biochem. Cell Biol. 2022, 149, 106260. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.A.; Cole, G.M.; Coen, C.W.; Frautschy, S.; Singh, R.P.; Mekkittikul, M.; Garcia-Ratés, S.; Morrill, P.; Hollings, O.; Passmore, M.; et al. A novel process driving Alzheimer’s disease validated in a mouse model: Therapeutic potential. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12274. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.A.; Ferrati, G.; Coen, C.W.; Vadisiute, A.; Molnár, Z.; Garcia-Rates, S.; Frautschy, S.; Cole, G.M. Characterization of a Bioactive Peptide T14 in the Human and Rodent Substantia Nigra: Implications for Neurodegenerative Disease. Int. J. Mol. Sci. 2022, 23, 13119. [Google Scholar] [CrossRef]
- Korrell, K.V.; Disser, J.; Parley, K.; Vadisiute, A.; Requena-Komuro, M.; Fodder, H.; Pollart, C.; Knott, G.; Molnár, Z.; Hoerder-Suabedissen, A. Differential effect on myelination through abolition of activity-dependent synaptic vesicle release or reduction of overall electrical activity of selected cortical projections in the mouse. J. Anat. 2019, 235, 452–467. [Google Scholar] [CrossRef]
- Vadisiute, A.; Meijer, E.; Szabó, F.; Hoerder-Suabedissen, A.; Kawashita, E.; Hayashi, S.; Molnár, Z. The role of snare proteins in cortical development. Dev. Neurobiol. 2022, 82, 457–475. [Google Scholar] [CrossRef]
- Hoerder-Suabedissen, A.; Korrell, K.V.; Hayashi, S.; Jeans, A.; Ramirez, D.M.O.; Grant, E.; Christian, H.C.; Kavalali, E.T.; Wilson, M.C.; Molnár, Z. Cell-Specific Loss of SNAP25 from Cortical Projection Neurons Allows Normal Development but Causes Subsequent Neurodegeneration. Cereb. Cortex 2019, 29, 2148–2159. [Google Scholar] [CrossRef]
- Schoch, S.; Deák, F.; KönIgstorfer, A.; Mozhayeva, M.; Sara, Y.; SüdHof, T.C.; Kavalali, E.T. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 2001, 294, 1117–1122. [Google Scholar] [CrossRef]
- Verhage, M.; Maia, A.S.; Plomp, J.J.; Brussaard, A.B.; Heeroma, J.H.; Vermeer, H.; Toonen, R.F.; Hammer, R.E.; van Den, T.K.; Berg, T.K.V.D.; et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 2000, 287, 864–869. [Google Scholar] [CrossRef]
- Jurado, S.; Goswami, D.; Zhang, Y.; Molina, A.J.M.; Südhof, T.C.; Malenka, R.C. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron 2013, 77, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Washbourne, P.; Thompson, P.M.; Carta, M.; Costa, E.T.; Mathews, J.R.; Lopez-Benditó, G.; Molnár, Z.; Becher, M.W.; Valenzuela, C.F.; Partridge, L.D.; et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat. Neurosci. 2002, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Demarque, M.; Represa, A.; Becq, H.; Khalilov, I.; Ben-Ari, Y.; Aniksztejn, L. Paracrine Intercellular Communication by a Ca2+- and SNARE-Independent Release of GABA and Glutamate Prior to Synapse Formation. Neuron 2002, 36, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.P. Role of SNAREs in Membrane Fusion. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Shimojo, M.; Courchet, J.; Pieraut, S.; Torabi-Rander, N.; Sando, R.; Polleux, F.; Maximov, A. SNAREs Controlling Vesicular Release of BDNF and Development of Callosal Axons. Cell Rep. 2015, 11, 1054–1066. [Google Scholar] [CrossRef]
- Yoon, T.-Y.; Munson, M. SNARE complex assembly and disassembly. Curr. Biol. 2018, 28, R397–R401. [Google Scholar] [CrossRef]
- Hay, J.C. SNARE Complex Structure and Function. Exp. Cell Res. 2001, 271, 10–21. [Google Scholar] [CrossRef]
- Vadisiute, A.; Meijer, E.; Therpurakal, R.N.; Mueller, M.; Szabó, F.; Messore, F.; Jursenas, A.; Bredemeyer, O.; Krone, L.B.; Mann, E.; et al. Glial cells undergo rapid changes following acute chemogenetic manipulation of cortical layer 5 projection neurons. Commun. Biol. 2024, 7, 1286. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Boger, H.A.; Smith, R.J.; Li, H.; Haydon, P.G.; Kalivas, P.W. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol. Psychiatry 2015, 78, 441–451. [Google Scholar] [CrossRef]
- Herrewegen, Y.V.D.; Sanderson, T.M.; Sahu, S.; De Bundel, D.; Bortolotto, Z.A.; Smolders, I. Side-by-side comparison of the effects of Gq- and Gi-DREADD-mediated astrocyte modulation on intracellular calcium dynamics and synaptic plasticity in the hippocampal CA1. Mol. Brain 2021, 14, 144. [Google Scholar] [CrossRef]
- Rocha, S.; Ratés, S.G.; Moswete, T.; Kalleberg, K.; Villa, A.; Harcup, J.P.; Greenfield, S.A. A novel peptide ‘T14’ reflects age and photo-aging in human skin. Aging 2023, 15, 5279–5289. [Google Scholar] [CrossRef]
- Garcia-Ratés, S.; Morrill, P.; Tu, H.; Pottiez, G.; Badin, A.-S.; Tormo-Garcia, C.; Heffner, C.; Coen, C.W.; Greenfield, S.A. (I) Pharmacological profiling of a novel modulator of the α7 nicotinic receptor: Blockade of a toxic acetylcholinesterase-derived peptide increased in Alzheimer brains. Neuropharmacology 2016, 105, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.-A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Hoerder-Suabedissen, A.; Kiyokage, E.; Maclachlan, C.; Toida, K.; Knott, G.; Molnár, Z. Maturation of Complex Synaptic Connections of Layer 5 Cortical Axons in the Posterior Thalamic Nucleus Requires SNAP25. Cereb. Cortex 2021, 31, 2625–2638. [Google Scholar] [CrossRef]
- Sanchez-Varo, R.; Trujillo-Estrada, L.; Sanchez-Mejias, E.; Torres, M.; Baglietto-Vargas, D.; Moreno-Gonzalez, I.; De Castro, V.; Jimenez, S.; Ruano, D.; Vizuete, M.; et al. Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol. 2012, 123, 53–70. [Google Scholar] [CrossRef]
- Masliah, E.; Hansen, L.; Alford, M.; Deteresa, R.; Mallory, M. Deficient glutamate tranport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1996, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive Involvement of Autophagy in Alzheimer Disease: An Immuno-Electron Microscopy Study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef]
- Kishi-Itakura, C.; Koyama-Honda, I.; Itakura, E.; Mizushima, N. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 2014, 127, 4984. [Google Scholar] [CrossRef]
- Pang, Z.P.; Sun, J.; Rizo, J.; Maximov, A.; Südhof, T.C. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006, 25, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.B. SNARE complexes prepare for membrane fusion. Trends Neurosci. 2005, 28, 453–455. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224 Pt 3, 213–232. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadisiute, A.; Garcia-Rates, S.; Coen, C.W.; Greenfield, S.A.; Molnár, Z. Widespread Changes in the Immunoreactivity of Bioactive Peptide T14 After Manipulating the Activity of Cortical Projection Neurons. Int. J. Mol. Sci. 2025, 26, 5786. https://doi.org/10.3390/ijms26125786
Vadisiute A, Garcia-Rates S, Coen CW, Greenfield SA, Molnár Z. Widespread Changes in the Immunoreactivity of Bioactive Peptide T14 After Manipulating the Activity of Cortical Projection Neurons. International Journal of Molecular Sciences. 2025; 26(12):5786. https://doi.org/10.3390/ijms26125786
Chicago/Turabian StyleVadisiute, Auguste, Sara Garcia-Rates, Clive W. Coen, Susan Adele Greenfield, and Zoltán Molnár. 2025. "Widespread Changes in the Immunoreactivity of Bioactive Peptide T14 After Manipulating the Activity of Cortical Projection Neurons" International Journal of Molecular Sciences 26, no. 12: 5786. https://doi.org/10.3390/ijms26125786
APA StyleVadisiute, A., Garcia-Rates, S., Coen, C. W., Greenfield, S. A., & Molnár, Z. (2025). Widespread Changes in the Immunoreactivity of Bioactive Peptide T14 After Manipulating the Activity of Cortical Projection Neurons. International Journal of Molecular Sciences, 26(12), 5786. https://doi.org/10.3390/ijms26125786






