Overexpression of BnaXTH22 Improving Resistance to Aluminum Toxicity in Rapeseed (Brassica napus L.)
Abstract
1. Introduction
2. Result
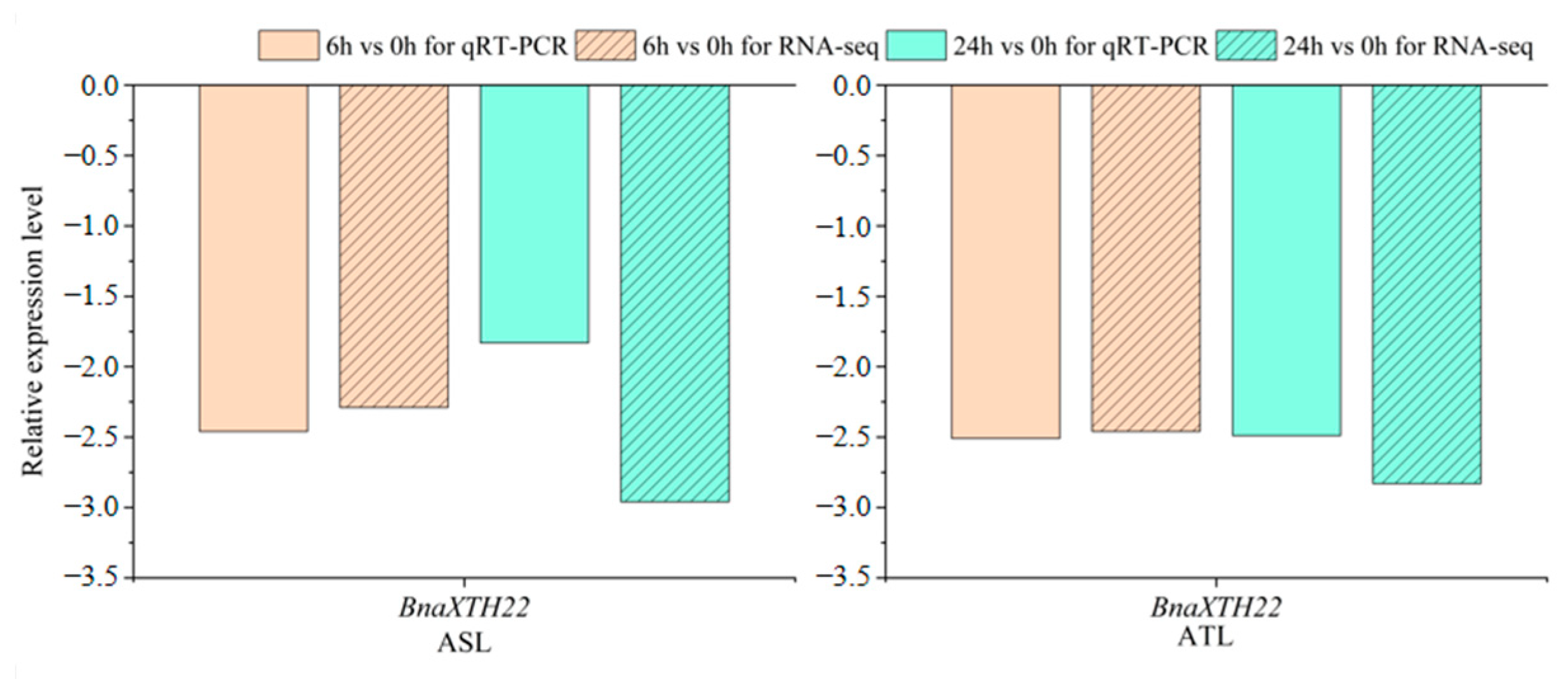
2.1. Analysis of the Expression Pattern of the BnaXTH22 Under Al Toxicity Stress
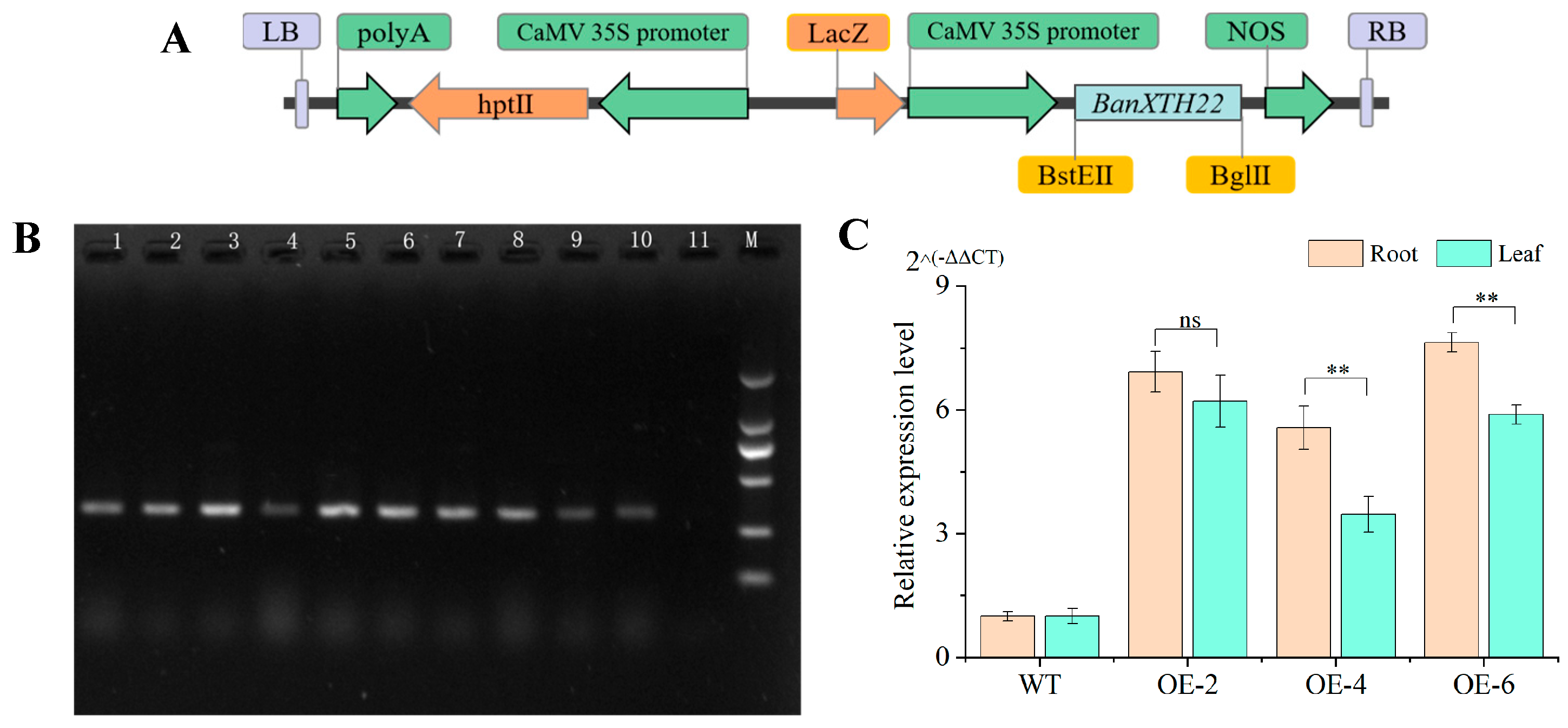
2.2. Generation of Transgenic Plants and Molecular Identification
2.3. Phenotype Characterization of Overexpressing BnaXTH22
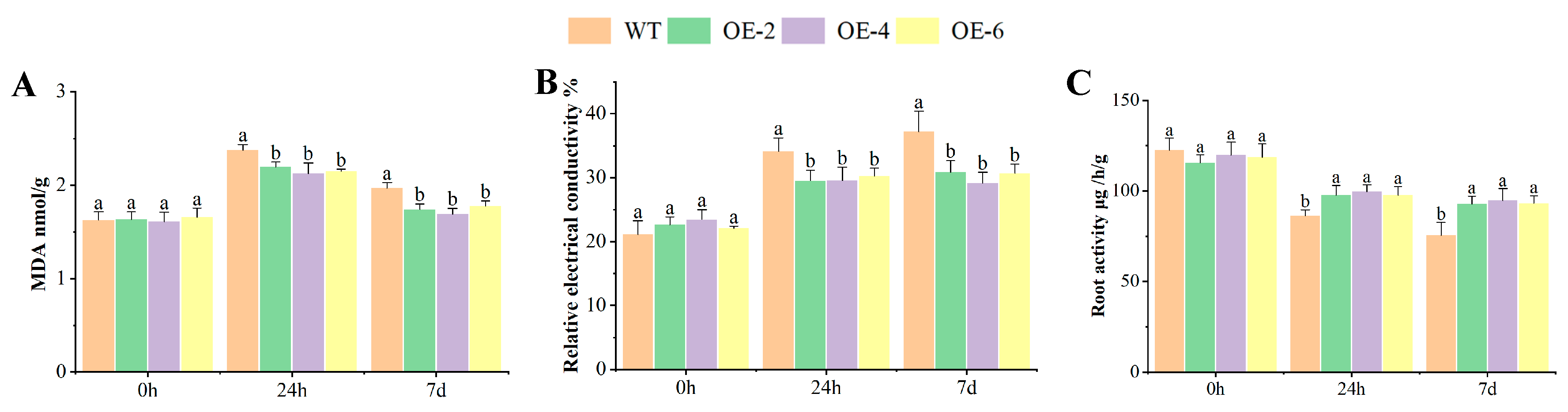
2.4. MDA, REC, and RA of OEs Response to Al Toxicity Stress
2.5. Transcriptome Analysis of Overexpressing BnaXTH22
2.6. Al Toxicity Response Related Genes with BnaXTH22 Overexpression
3. Discussion
4. Materials and Methods
4.1. Validation of Gene by qRT-PCR
4.2. Generation of Transgenic Westar Plants
4.3. Morphological and Physiological Parameter Under Al Stress
4.4. RNA-Seq Under Al Tolerance and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Alvarado, F.S.; Botero-Rozo, D.; Araque, L.; Bayona, C.; Herrera-Corzo, M.; Montoya, C.; Ayala-Díaz, I.; Romero, H.M. Molecular network of the oil palm root response to aluminum stress. BMC Plant Biol. 2023, 23, 346. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, R.; Shu, K.; Lv, W.; Wang, S.; Wang, C. Aluminum stress signaling, response, and adaptive mechanisms in plants. Plant Signal. Behav. Plant Signal. Behav. 2022, 17, 2057060. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, P.; Wu, L.; Han, D.; Wu, Y.; Zheng, W.; Zhou, Q.; Xiao, X. Combined BSA-seq and RNA-seq analysis to identify candidate genes associated with aluminum toxicity in rapeseed (Brassica napus L.). Int. J. Mol. Sci. 2024, 25, 11190. [Google Scholar] [CrossRef]
- von Uexküll, H.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shen, R.F. Towards sustainable use of acidic soils: Deciphering aluminum-resistant mechanisms in plants. Fundam. Res. 2023, 14, 41. [Google Scholar] [CrossRef]
- Gao, H.H.; Ye, S.; Wang, Q.; Wang, L.Y.; Wang, R.L.; Chen, L.Y.; Tang, Z.L.; Li, J.N.; Zhou, Q.Y.; Cui, C. Screening and comprehensive evaluation of aluminum-toxicity tolerance during seed germination in Brassca napus. Acta Agron. Sin. 2019, 45, 1416–1430. Available online: https://zwxb.chinacrops.org/EN/10.3724/SP.J.1006.2019.84169 (accessed on 13 June 2025).
- Du, H.; Raman, H.; Kawasaki, A.; Perera, G.; Diffey, S.; Snowdon, R.; Raman, R.; Ryan, P.R.; Ma, J.F. A genome-wide association study (GWAS) identifies multiple loci linked with the natural variation for Al3+ resistance in Brassica napus. Funct. Plant Biol. 2022, 49, 845–860. [Google Scholar] [CrossRef]
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef]
- Li, C.; Shi, H.; Xu, L.; Xing, M.; Wu, X.; Bai, Y.; Niu, M.; Gao, J.; Zhou, Q.; Cui, C. Combining transcriptomics and metabolomics to identify key response genes for aluminum toxicity in the root system of Brassica napus L. seedlings. Theor. Appl. Genet. 2023, 136, 169. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chen, S.; Feng, J.; Chen, H.; Qi, X.; Wang, H.; Deng, Y. Identification of aluminum-activated malate transporters (ALMT) family genes in hydrangea and functional characterization of HmALMT5/9/11 under aluminum stress. PeerJ 2022, 10, e13620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Lu, C.; Tang, Y.; Jiang, X.; Gai, Y. Isolation and characterization of Populus xyloglucan endotransglycosylase/hydrolase (XTH) involved in osmotic stress responses. Int. J. Biol. Macromol. 2020, 155, 1277–1287. [Google Scholar] [CrossRef]
- Van Sandt, V.S.T.; Suslov, D.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan transglucosylase activity loosens a plant cell wall. Ann. Bot. 2007, 100, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z.; et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Bi, H.; Liu, Z.; Liu, S.; Qiao, W.; Zhang, K.; Zhao, M.; Wang, D. Genome-wide analysis of wheat xyloglucan endotransglucosylase/hydrolase (XTH) gene family revealed TaXTH17 involved in abiotic stress responses. BMC Plant Biol. 2024, 24, 640. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wan, J.X.; Sun, Y.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Xyloglucan endotransglucosylase-hydrolase17 interacts with xyloglucan endotransglucosylase-hydrolase31 to confer xyloglucan endotransglucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol. 2014, 165, 1566–1574. [Google Scholar] [CrossRef]
- Luo, S.; Pan, C.; Liu, S.; Liao, G.; Li, A.; Wang, Y.; Wang, A.; Xiao, D.; He, L.-F.; Zhan, J. Identification and functional characterization of the xyloglucan endotransglucosylase/hydrolase 32 (AhXTH32) in peanut during aluminum-induced programmed cell death. Plant Physiol. Biochem. 2022, 194, 161–168. [Google Scholar] [CrossRef]
- Du, H.; Hu, X.; Yang, W.; Hu, W.; Yan, W.; Li, Y.; He, W.; Cao, M.; Zhang, X.; Luo, B.; et al. ZmXTH, a xyloglucan endotransglucosylase/hydrolase gene of maize, conferred aluminum tolerance in Arabidopsis. J. Plant Physiol. 2021, 266, 153520. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, X.; Asjad, A.; Han, D.; Zheng, W.; Xiao, G.; Huang, Y.; Zhou, Q. Integration of GWAS and transcriptome analyses to identify SNPs and candidate genes for aluminum tolerance in rapeseed (Brassica napus L.). BMC Plant Biol. 2022, 22, 130. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.; Staal, M.; Elzenga, J.T.M.; Yokoyama, R.; Nishitani, K.; Voesenek, L.A.; Pierik, R. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol. 2010, 154, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yokoyama, R. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Dong, D.; Deng, Q.; Zhang, J.; Jia, C.; Gao, M.; Wang, Y.; Zhang, L.; Zhang, N.; Guo, Y.-D. Transcription factor SlSTOP1 regulates Small Auxin-Up RNA Genes for tomato root elongation under aluminum stress. Plant Physiol. 2024, 196, 2654–2668. [Google Scholar] [CrossRef]
- Jiang, F.; Lyi, S.M.; Sun, T.; Li, L.; Wang, T.; Liu, J. Involvement of cytokinins in STOP1-mediated resistance to proton toxicity. Stress Biol. 2022, 2, 17. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Geng, X.; He, C.; Quan, T.; Hayashi, K.; De Smet, I.; Robert, H.S.; Ding, Z.; Yang, Z. Local regulation of auxin transport in root-apex transition zone mediates aluminium-induced Arabidopsis root-growth inhibition. Plant J. 2021, 108, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xiao, X.; Huang, Q.; Zhu, H.; Feng, Y.; Li, Y.; Li, X.; Guo, Z.; Liu, J.; Wu, F.; et al. Boron supply restores aluminum-blocked auxin transport by the modulation of PIN2 trafficking in the root apical transition zone. Plant J. 2023, 114, 176–192. [Google Scholar] [CrossRef] [PubMed]
- da Silva de, J.D.; Martins, F.M.; de Azevedo, A.D. Structural changes in leaves and roots are anatomical markers of aluminum sensitivity in sunflower. Pesqui. Agropecu. Trop. 2016, 46, 383–390. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.D.; Zang, P.C.; Xu, F.S.; Wang, C.; Ding, G.D. Screening extreme varieties with aluminum tolerance and analyzing physiological mechanisms of aluminum tolerance in Brassica napus. J. Huazhong Agric. Univ. 2023, 42, 154–163. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Guo, S.; Yuan, X.; Zhao, S.; Tian, H.; Dai, S.; Kong, X.; Ding, Z. AtHB7/12 regulate root growth in response to aluminum stress. Int. J. Mol. Sci. 2020, 21, 4080. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, J.; Li, R.; Zhao, X.; Zhu, Z.; Zhang, F.; Zhou, K.; Lin, X. Sodium hydrosulfide alleviates aluminum toxicity in Brassica napus through maintaining H2S, ROS homeostasis and enhancing aluminum exclusion. Sci. Total. Environ. 2022, 858 Pt 3, 160073. [Google Scholar] [CrossRef]
- Han, D.P.; Liu, X.Y.; Wang, X.Y.; Luo, S.; Fu, D.H.; Zhou, Q.H. Effects of aluminum stress on morphology parameters of roots and physiological indexes in Brassica napus L. J. Nucl. Agric. Sci. 2019, 33, 1824–1832. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Shi, Q.-H.; Cao, H.-J.; Ma, Q.-B.; Nian, H.; Zhang, X.-X. Heterologous expression of a Glycine soja C2H2 zinc finger gene improves aluminum tolerance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2754. [Google Scholar] [CrossRef]
- Ribeiro, C.; de Marcos Lapaz, A.; de Freitas-Silva, L.; Ribeiro, K.V.G.; Yoshida, C.H.P.; Dal-Bianco, M.; Cambraia, J. Aluminum promotes changes in rice root structure and ascorbate and glutathione metabolism. Physiol. Mol. Biol. Plants 2022, 28, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.V.M.; Mattos, J.P.O.; Wertonge, G.S.; Rosa, F.C.R.; Lovato, L.R.; Valsoler, D.V.; Azevedo, T.D.; Nicoloso, F.T.; Tabaldi, L.A. Silicon as an attenuator of the toxic effects of aluminum in Schinus terebinthifolius plants. Braz. J. Biol. 2023, 83, e271301. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Cheng, J.; Zhang, Y.; Jiang, C. Boron-mediated lignin metabolism in response to aluminum toxicity in citrus (Poncirus trifoliata (L.) Raf.) root. Plant Physiol. Biochem. 2022, 185, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Magwanga, R.O.; Kirungu, J.N.; Hu, Y.; Dong, Q.; Cai, X.; Zhou, Z.; Wang, X.; Zhang, Z.; Hou, Y.; et al. Overexpression of cotton a DTX/MATE gene enhances drought, salt, and cold stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2019, 10, 299. [Google Scholar] [CrossRef]
- Liu, S.; Mo, X.; Sun, L.; Gao, L.; Su, L.; An, Y.; Zhou, P. MsDjB4, a HSP40 chaperone in Alfalfa (Medicago sativa L.), improves Alfalfa hairy root tolerance to aluminum stress. Plants 2023, 12, 2808. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Kusunoki, K.; Saha, B.; Kobayashi, Y.; Koyama, H.; Panda, S.K. Comparative RNA-Seq analysis of the root revealed transcriptional regulation system for aluminum tolerance in contrasting indica rice of North East India. Protoplasma 2020, 258, 517–528. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, J.; Cai, Y.; Yang, S.; Liu, J.; Wang, W.; Gai, J.; Hu, Z.; Li, Y. Comparative transcriptome analysis of two contrasting soybean varieties in response to aluminum toxicity. Int. J. Mol. Sci. 2020, 21, 4316. [Google Scholar] [CrossRef]
- Brhane, H.; Haileselassie, T.; Tesfaye, K.; Ortiz, R.; Hammenhag, C.; Abreha, K.B.; Vetukuri, R.R.; Geleta, M. Finger millet RNA-seq reveals differential gene expression associated with tolerance to aluminum toxicity and provides novel genomic resources. Front. Plant Sci. 2022, 13, 1068383. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, H.B.; Zhang, X.Y.; Zhai, H.H.; Li, X.M.; Peng, J.W.; Tian, Y.; Chen, H.J. Functional identification of peach gene PpSAUR73. Sci. Agric. Sin. 2023, 56, 4072–4086. [Google Scholar] [CrossRef]
- Chen, Y.; Ling, Q.; Li, X.; Ma, Q.; Tang, S.; Yuanzhi, P.; Liu, Q.-L.; Jia, Y.; Yong, X.; Jiang, B. Transcriptome analysis during axillary bud growth in chrysanthemum (chrysanthemum × morifolium). PeerJ 2023, 11, e16436. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Wagner, C.; Nosenko, T.; Omony, J.; Steiner, B.; Nussbaumer, T.; Mayer, K.F.X.; Buerstmayr, H. Fusarium head blight resistance in European winter wheat: Insights from genome-wide transcriptome analysis. BMC Genom. 2021, 22, 470. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Rao, X.; Li, L.; Dixon, R.A. Abscisic acid regulates secondary cell-wall formation and lignin deposition in Arabidopsis thaliana through phosphorylation of NST1. Proc. Natl. Acad. Sci. USA 2021, 118, e2010911118. [Google Scholar] [CrossRef]
- Lou, H.Q.; Fan, W.; Jin, J.F.; Xu, J.M.; Chen, W.W.; Yang, J.L.; Zheng, S.J. A NAC-type transcription factor confers aluminium resistance by regulating cell wall-associated receptor kinase 1 and cell wall pectin. Plant Cell Environ. 2019, 43, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB transcription factors and its regulation in secondary cell wall formation and lignin biosynthesis during xylem development. Int. J. Mol. Sci. 2021, 22, 3560. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, Y.; Huang, J.; Liu, Y.S.; Yang, X.Z.; Jing, H.K.; Shen, R.F.; Zhu, X.F. The MYB transcription factor MYB103 acts upstream of TRICHOME BIREFRINGENCE-LIKE27 in regulating aluminum sensitivity by modulating the O-acetylation level of cell wall xyloglucan in Arabidopsis thaliana. Plant J. 2022, 111, 529–545. [Google Scholar] [CrossRef]
- Brown, D.; Wightman, R.; Zhang, Z.; Gomez, L.D.; Atanassov, I.; Bukowski, J.; Tryfona, T.; McQueen-Mason, S.J.; Dupree, P.; Turner, S. Arabidopsis genes IRREGULAR XYLEM (IRX15) and IRX15L encode DUF579-containing proteins that are essential for normal xylan deposition in the secondary cell wall. Plant J. 2011, 66, 401–413. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Cheng, Z.; Ke, Y.; Liu, J.; Ma, H. Evolution analysis of the fasciclin-like arabinogalactan proteins in plants shows variable fasciclin-AGP domain constitutions. Int. J. Mol. Sci. 2019, 20, 1945. [Google Scholar] [CrossRef]


| Sample | Clean Reads | Clean Bases | Proportion of Q30 | Mapped Ratio | GC Content |
|---|---|---|---|---|---|
| WT 0 h-1 | 42,143,362 | 6,044,721,779 | 0.934 | 0.910 | 0.467 |
| WT 0 h-2 | 43,114,336 | 6,185,668,673 | 0.934 | 0.906 | 0.466 |
| WT 0 h-3 | 41,970,324 | 5,993,085,324 | 0.927 | 0.905 | 0.466 |
| WT 24 h-1 | 42,655,310 | 6,122,028,343 | 0.931 | 0.913 | 0.461 |
| WT 24 h-2 | 40,637,354 | 5,813,384,648 | 0.935 | 0.904 | 0.465 |
| WT 24 h-3 | 44,551,704 | 6,365,419,321 | 0.933 | 0.903 | 0.466 |
| Total | 255,072,390 | 36,524,308,088 | |||
| OE-2 0 h-1 | 42,527,036 | 6,069,309,557 | 0.938 | 0.904 | 0.468 |
| OE-2 0 h-2 | 42,746,136 | 6,093,607,070 | 0.929 | 0.904 | 0.467 |
| OE-2 0 h-3 | 42,817,394 | 6,145,304,675 | 0.933 | 0.905 | 0.467 |
| OE-2 24 h-1 | 54,965,910 | 7,885,013,726 | 0.935 | 0.917 | 0.462 |
| OE-2 24 h-2 | 44,846,140 | 6,395,780,703 | 0.937 | 0.899 | 0.471 |
| OE-2 24 h-3 | 42,181,708 | 6,004,578,510 | 0.920 | 0.902 | 0.468 |
| Total | 270,084,324 | 38,593,594,241 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Size/bp |
|---|---|---|---|
| BnaXTH22 | CACGAGAGGTGGTTTGGTCA | GAGCCGTAGAGTCAAGCTCC | 173 |
| ACT7 | CCTCTCAACCCGAAAGCCAA | CATCACCAGAGTCGAGCACA | 148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Han, D.; Chen, M.; Yang, L.; Li, Y.; Huang, T.; Xiong, W.; Cheng, Y.; Liu, X.; Wan, C.; et al. Overexpression of BnaXTH22 Improving Resistance to Aluminum Toxicity in Rapeseed (Brassica napus L.). Int. J. Mol. Sci. 2025, 26, 5780. https://doi.org/10.3390/ijms26125780
Yu P, Han D, Chen M, Yang L, Li Y, Huang T, Xiong W, Cheng Y, Liu X, Wan C, et al. Overexpression of BnaXTH22 Improving Resistance to Aluminum Toxicity in Rapeseed (Brassica napus L.). International Journal of Molecular Sciences. 2025; 26(12):5780. https://doi.org/10.3390/ijms26125780
Chicago/Turabian StyleYu, Paolan, Depeng Han, Ming Chen, Lei Yang, Yazhen Li, Tianbao Huang, Wen Xiong, Yewei Cheng, Xiaosan Liu, Changyan Wan, and et al. 2025. "Overexpression of BnaXTH22 Improving Resistance to Aluminum Toxicity in Rapeseed (Brassica napus L.)" International Journal of Molecular Sciences 26, no. 12: 5780. https://doi.org/10.3390/ijms26125780
APA StyleYu, P., Han, D., Chen, M., Yang, L., Li, Y., Huang, T., Xiong, W., Cheng, Y., Liu, X., Wan, C., Zheng, W., & Xiao, X. (2025). Overexpression of BnaXTH22 Improving Resistance to Aluminum Toxicity in Rapeseed (Brassica napus L.). International Journal of Molecular Sciences, 26(12), 5780. https://doi.org/10.3390/ijms26125780






