Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication
Abstract
1. Introduction
- We then present a discussion of ferritin as one out of several acute-phase biomarkers (Section 4) together with the biological and the possible clinical role of the acute-phase reaction and acute-phase mediators for the initial pretreatment prognostication (Section 5), the regulation of inflammation/coagulation in AML (Section 6) and the prognostication for AML patients receiving allogeneic stem cell transplantation (Section 7).
2. The Molecular Structure and the Molecular Interactions of Ferritin
2.1. The Molecular Structure, Cellular Expression, Function and Secretion of Ferritin
- The transcription of especially FTH but also FTL is increased by proinflammatory cytokines, e.g., Interleukin 1β (IL1β), IL6 and Tumor necrosis factor α (TNFα) through increased NFκB (the nuclear factor kappa-light-chain enhancer of activated B cells) activity; this mechanism is operative in hepatic cells [12,13,14,15].
- Interferon (IFN) γ and lipopolysaccharide/TLR4 (Toll-like receptor 4) ligation in macrophages increase especially FTH, but also FTL expression through an alternative nitric-oxide-dependent mechanism that involves the degradation of IRP2 (iron-responsive element-binding protein 2) when the cellular iron level is adequate [16,17,18,19]. IRP2 regulates the cellular iron levels by binding to iron-responsive RNA elements and the iron level is thereby increased by modulated translation as well as the stability of mRNAs involved in iron homeostasis.
- Post-transcriptional regulation is mediated through the iron-responsive elements of ferritin-encoding RNAs; the final effect is then an adjustment especially of FTL, but also FTH levels to the intracellular iron level [18].
2.2. The Cellular Ferritin Receptors for Extracellular Ferritin and Their Expression by AML Cells
3. Microenvironmental Effects of Extracellular Ferritin Release: Possible Effects on AML-Associated Angiogenesis and Immunoregulation in the Bone Marrow
3.1. The AML Cells and Their Bone Marrow Microenvironment
3.2. Ferritin and AML-Supporting Nonleukemic Cells in the Bone Marrow Microenvironment: Ferritin Effects on Immunocompetent Cells
- Ferritin seems to inhibit mitogenic T cell activation [60].
- The binding of ferritin H to the CXCR4 chemokine receptor can inhibit ligand-induced downstream ERK1/2 activation [49].
- Ferritin seems to alter the profile/distribution of circulating T cells and to suppress T helper cell activity [63].
- Ferritin may also inhibit the function of tumoricidal monocytes/macrophages [64].
- Ferritin seems to have immunosuppressive effects through the modulation of dendritic cell functions and thereby the activation of Treg cells [65].
- Experimental animal studies suggest that even distant disease (i.e., spinal cord injury) can alter iron metabolism in normal cells, including the ferritin function and iron metabolism in macrophages [66].
3.3. Ferritin and AML-Supporting Nonleukemic Cells in the Bone Marrow Microenvironment: Ferritin Effects on Endothelial Cells and Bone Marrow Angiogenesis
- Mitochondrial ferritin can also be upregulated by cellular stress; it then has an antiapoptotic effect with the prevention of tight junction loss by reducing iron dysregulation and the accumulation of reactive oxygen species (ROS) [79].
- Toxic effects on endothelial cells can include ferritinophagy followed by ferroptosis [80]; these observations further support the hypothesis that ferritin is important for the endothelial cell response to stress, possibly including antiangiogenic therapy.
- High-molecular-weight kininogen is a coagulation cofactor that can be cleaved by serine proteases into the proangiogenic bradykinin (nine amino acids) and an antiangiogenic cleavage product [81,82,83]. Ferritin can bind to high-molecular-weight kininogen and thereby inhibit this protease cleavage [84,85]. However, the ferritin binding site is on the antiangiogenic cleavage product, and ferritin may, therefore. counteract the proangiogenic but not the antiangiogenic effect of the cleavage [86].
3.4. Effects of Ferritin on Adipocytes: Are the Effects Relevant in AML?
3.5. Effects of Iron and Iron Overload on Mesenchymal Stem Cells
- The release of several cytokines is reduced, and these modulations can have both direct effects on the AML cells and indirect effects via immunocompetent or endothelial cells (angiogenesis and vascular stem cell niches).
- Reduced osteoblastic differentiation can influence the support of endosteal stem cell niches.
- The ability to support normal hematopoiesis is reduced and this observation supports the hypothesis that leukemic hematopoiesis may also be affected.
4. The Acute-Phase Reaction in AML: A General Description of Acute-Phase Biomarkers (Including Ferritin) That Have a Clinical Relevance in Human AML
4.1. General Aspects of the Acute-Phase Reaction: Altered Systemic Levels of Several Molecular Markers Including Ferritin as Well as Altered Levels of Circulating Normal Blood Cells
4.2. Serum Ferritin Levels: Hematological Malignancy Is an Uncommon Cause of Hyperferritinemia
4.3. CRP in the Acute-Phase Reaction: Increased Levels Not Only During Infections, but Also in Inflammation, Malignancies and Aging
4.4. Decreased Albumin in the Acute-Phase Reaction: Inflammation, Malnutrition and Malignancy Can All Be Associated with Hypoalbuminemia
4.5. Fibrinogen Levels in Cancer Patients Can Be Modulated Both by Inflammation and by Coagulopathy
4.6. Effects of Inflammation on the Systemic Levels of Other Biomarkers of Iron Metabolism: Do These Levels Have Any Clinical Relevance in AML?
5. The Pretreatment Levels of Ferritin and Other Acute-Phase Biomarkers in Patients with Newly Diagnosed AML: Associations with Prognosis After Intensive Therapy
5.1. Serum Ferritin Levels in Newly Diagnosed AML Is Associated with Prognosis
5.2. Serum CRP, Albumin and Fibrinogen Levels in Patients with Newly Diagnosed AML
5.3. Possible Mechanisms Behind the Association Between High Pretreatment Ferritin Levels, Increased Acute-Phase Biomarkers and Adverse Prognosis After Intensive AML Chemotherapy
- Age: Ferritin did not show any significant association with the patients’ ages in any study; this was true also for fibrinogen and FAC ratio studies, whereas an association with age was seen in CRP–albumin studies. The association between age and acute-phase biomarkers other than ferritin may be caused by the previous presence of inflammaging that can be observed in elderly patients, i.e., signs of inflammation often associated with reduced physical functions [52]. Inflammation may thereby contribute to the association between CRP–albumin markers and patient age at the time of first AML diagnosis, but if this is true, inflammaging seems less important for the increased ferritin level in AML.
- Sex: All three ferritin studies showed a significantly higher frequency of males among patients with high ferritin [154,156,157]; an opposite observation was made in one study of the CRP–albumin ratio [165], but for the other studies, the ratio did not differ [161,162,163,164,166]. Further studies are needed to explain these differences.
- Associations between ferritin and other acute-phase biomarkers: Despite differences between various acute-phase biomarkers with regard to associations with age and sex, several studies described significant correlations between the systemic ferritin levels and the other acute-phase biomarkers. These observations are consistent with the hypothesis that the prognostic impact of ferritin in AML is partly caused by its association with inflammation and an acute-phase reaction.
- Secondary AML: Another difference between various acute-phase markers was their association with secondary AML; such an association was only observed for some CRP–albumin studies, but not for other markers. The association between secondary AML and CRP–albumin may be caused by inflammation/inflammatory complications that can be seen in patients with myelodysplastic syndromes (MDS) [170,171,172,173,174]. Furthermore, the absence of any association between the ferritin level and secondary AML suggest that the contribution of an iron overload due to previous transfusions, as would be expected for patients with previous MDS or cancer therapy, is less important for the increased ferritin levels at the time of first AML diagnosis. An association between high ferritin levels and secondary AML was not associated even in the study by Ihlow et al. [154] that included a relatively large number of patients with secondary AML. Thus, MDS-associated inflammation and previous transfusions are possibly less important for the prognostic impact of ferritin in AML.
- Leukemia burden: the associations between leukemization/marrow blast levels suggest that the AML burden contributes to the prognostic impact of various acute-phase biomarkers.
- Differentiation: AML cell differentiation/FAB classification does not show strong associations with acute-phase biomarkers.
- Genetic abnormalities: One study investigated FLT3 and NPM1 mutations and described a decreased frequency of NPM1 mutations in patients with high ferritin [157]. A relatively high frequency of Flt3-ITD was also observed in patients with a high CFA ratio [168]. The associations between cytogenetic/molecular genetic abnormalities may reflect differences with regard to the AML induction of inflammation [161,164,167,169].
- Performance status: One albumin study investigated the performance status and described a significant inverse correlation between a high ECOG (Eastern Cooperative Oncology Group) score and albumin level. This observation may also at least partly reflect an effect of inflammaging on ferritin levels [52,175].
5.4. Systemic Levels of Hepcidin in AML
6. Inflammation in Patients with Recently Diagnosed AML: Acute-Phase Reaction/Inflammation, Hemophagocytosis and Coagulopathy
6.1. Hemophagocytic Lymphohistiocytosis in AML
- Increased serum CRP levels (median 116 versus 19 mg/L, p = 0.0005);
- Increased frequency of hepatomegaly (7/32 versus 0/22, p = 0.0335);
- Respiratory symptoms (19/32 versus 2/22, p = 0.0002);
- Prolonged prothrombin time (median 84 versus 66.5 s, p = 0.0013);
- Decreased albumin levels (median 27 versus 33 g/L, 0.0005);
- Increased frequency of serum liver transaminases exceeding five times the upper normal limit (7/32 versus 0/22, p = 0.0335), increased serum alkaline phosphatase (median 427 versus 214 IU/L, p = 0.0005) and increased γGT (median 213 versus 60 IU/mL, p = 0.0001).
6.2. Pretreatment Coagulopathy in AML: Risk of Thrombosis and the Acute-Phase Reaction
7. The Acute-Phase Reaction in Patients Receiving Allogeneic Stem Cell Transplantation
7.1. The Prognostic Impact of High Pretransplant Serum Ferritin Levels
- Strong prognostic impact of pretransplant ferritin: Associations between high pretransplant ferritin levels and adverse prognosis were observed in all these studies. For example, one study [213] including 590 patients described more than 50% 5-year overall survival for patients with ferritin < 930 μg/L, whereas patients above this cut-off showed 37% survival.
- High ferritin has a general effect in allotransplantation: the association between high ferritin and adverse prognosis has been observed in clinical studies (i) including patients with various hematological malignancies and both intermediate/standard-risk and high-risk disease, (ii) when using both family and matched unrelated donors and (iii) patients receiving various conditioning regimens.
- High ferritin associated with increased relapse risk: six of the nine studies investigating the relapse risk described associations between high ferritin and an increased risk.
- High ferritin is associated with increased nonrelapse mortality: Increased nonrelapse mortality was described for seven of the eight studies summarized in Table 5. There is an increased risk of severe infections [204,207,209,215,217,220]; the causes of death differ between patients [217], but severe GVHD seems less important [218].
- The adverse prognosis for ferritin levels exceeding 700–1000 μg/L: Seven of these eleven studies used a ferritin cut-off between 700 and 1500 μg/L and the most common single cut-off was 1000 μg/L which was used in four of the studies. A significant prognostic impact has not been observed when using a cut-off of 2500 μg/L [203,209].
- High ferritin is only partly caused by an increased iron overload: The pretransplant ferritin levels of 198 de novo AML patients were correlated with the number of previous erythrocyte transfusions; patients with ferritin levels > 1000 μg/L then had received significantly higher numbers of pretransplant erythrocyte transfusions [208]. A similar association was also observed in another study [219]. On the other hand, a meta-analysis showed an adverse prognostic impact by ferritin > 1000 μg/L, but not by an increased iron overload as detected by magnetic resonance imaging.
- The acute-phase reaction may contribute to high ferritin: although the adverse prognostic impact of high pretransplant ferritin seems independent of pretransplant CRP [210] and albumin levels [213], a (possibly minor) contribution of inflammation to the increased ferritin levels seems to be present [209,211].
7.2. Possible Therapeutic Interventions for Allotransplant Recipients with High Serum Ferritin Levels
7.3. Pretransplant Serum CRP, Albumin and Hepcidin Levels: Associations with Survival, Performance Status, Nutrition and Pretransplant Ferritin Levels
- A previous study of the pretransplant CRP–albumin ratio in haploidentical allotransplant recipients used a cut-off of 0.087 and a high ratio was significantly associated with lower overall survival also for haploidentical stem cell transplantation [230].
- Another study suggested that the pretransplant CRP–albumin ratio showed a stronger prognostic impact than the CRP level alone; the impact of this ratio was also observed in the multivariate analysis [231]. Previous studies have described that both CRP and albumin levels are correlated with the ferritin levels and a high ratio is also correlated with several clinical parameters (Table 5). This study showed that a high CRP–albumin ratio was also correlated with a poor performance status.
- One would expect the albumin levels also to be influenced by the nutritional status. A previous study of allotransplanted AML patients showed that pretransplant weight loss during induction chemotherapy as well as the pretransplant total serum protein level were both associated with reduced overall survival and an increased risk of AML relapse [232]. This prognostic impact was independent of the karyotype and these authors also described decreased leptin levels in patients with pretransplant weight loss. Furthermore, leptin inhibits the proliferation of primary AML cells for a subset of patients [233], but it is not known whether there are altered leptin levels in patients with weight loss or contribute to the increased relapse risk in such patients.
- Hepcidin levels are also increased during the acute-phase reaction [148] and high pretransplant levels seem to be associated with an increased risk of bacterial and fungal infections post-transplant [180] (see also Section 5.4).
7.4. Altered Nontransferrin-Bound Iron in Allogeneic Stem Cell Transplantation: An Additional Parameter Reflecting Iron Overload and/or Abnormal Iron Metabolism
8. The Scientific Basis for Targeting Iron Metabolism/Ferroptosis as a Therapeutic Strategy in Human AML: The Role of Ferritin in Regulation of Ferroptosis
8.1. Iron Metabolism in AML Cells: The Importance of Mitochondrial Ferritin
8.2. The Cellular Basis for Targeting of Ferroptosis in AML: Regulation of Ferroptotic Cell Death by Iron Metabolism, ROS Production and Lipid Metabolism in AML Cells
8.3. The Clinical Basis for Targeting of Ferroptosis in AML: The Association Between AML Cell Expression of Ferroptosis-Associated Genes and Patient Survival
- CD8+ T cells can mediate anti-cancer activity through the induction of ferroptosis [261].
- A recent study defined a prognostic signature based on six ferroptosis-associated genes that were expressed in AML bone marrow and the signature was significantly associated with the marrow infiltration of CD8+ T cells [262]. This signature could be used to classify AML patients into high- and low-risk subsets and the signature could possibly also be used to refine the ELN risk classification in AML.
- Another study generated an alternative eight-gene ferroptosis signature that also could be used to stratify patients into high- and low-risk subsets [263].
- A third study used the expression of only two ferroptosis-associated genes (DNAJB6 and HSPB1) for the prognostication of AML patients [264].
- Finally, a previous study identified 20 genes whose expression showed statistically significant associations with patient survival [265]. Ferritin light and heavy chains were both included among these 20 initial genes, but not among the final 12 genes used for the construction of their prognostic AML model and classification into either high- or low-risk patient subsets with regard to survival. In vitro studies showed that their high-risk subset was characterized by decreased AML cell susceptibility to several antileukemic drugs. The correlation analyses showed that the identified high-risk AML cells had a higher expression of several immune checkpoint molecules and increased the bone marrow infiltration of M2 macrophages, whereas γδ-T cells were decreased. These results further support the hypothesis that the regulation of iron metabolism is important also for the immune-mediated support of AML leukemogenesis and chemosensitivity.
- The antileukemic agent erastin increased AML cell line sensitivity to cytarabine and daunorubicin; this drug also induced a mixed pattern of programmed cell death of AML cells with signs of both ferroptosis, apoptosis, necroptosis and autophagic death [266]. It is not known whether this mixed pattern is due to heterogeneity within the hierarchically organized AML cell population with regard to maturity/differentiation or genetic abnormalities.
9. Possible Strategies for Targeting Iron Metabolism or Regulation of Ferroptosis in Human AML
9.1. Targeting of Iron Metabolism by Iron Chelation
- A large nationwide Korean study included 5395 patients with acute leukemia; the patients were allotransplanted during the period 2003–2015, 65% of the patients had AML, 75% received peripheral blood stem cell grafts and 75% received myeloablative conditioning [218]. The overall early cumulative incidence rate of transplant-related mortality was less than 10% and patients who had received pretransplant iron-chelating therapy had a lower early post-transplant mortality. The study showed that the independent adverse factors for early transplant-related mortality were aged above 40 years, a longer duration from diagnosis to transplantation (median duration 8.8 months for all patients), previous transplantation(s) (6.1% of the patients), cord blood grafts and no pretransplant iron chelation (p-value at 50 and 100 days being p < 0.001). This large study thus suggests that pretransplant iron overload due to erythrocyte transfusions had a prognostic impact in these AML patients. However, it should be emphasized that the interval from diagnosis to transplantation was relatively long compared with the present practice.
- Another study investigated the use of iron chelation immediately before stem cell transplantation during myeloablative busulfan-based conditioning therapy and this treatment with deferazirox reduced the labile plasma iron without causing severe toxicity in any of the 25 patients [267].
9.2. Possible Strategies for Modulation of Iron Metabolism: Are They Relevant for AML?
9.3. Modulation of Iron Metabolism and Regulation of Ferroptosis in AML Cells by Histone Deacetylase Inhibitors: Increased Intracellular Labile Iron Pool
9.4. AML Targeting by Iron Oxide Nanoparticles: Induction of Ferroptosis in Response to Increased ROS Levels
9.5. Other Possible Strategies for Targeting of Iron Metabolism or Induction of Ferroptosis in AML
- Targeting of AMPK: Dihydroartesimin induces the induction of AMPK phosphorylation and thereby the inhibition of mTOR/p70S6k signaling in AML cells; these events induce autophagy, increase ferritin degradation, increase the unstable iron pool and ROS accumulation and, finally, cause ferroptosis [292,293]. Furthermore, the pollen-derived flavonoid Typhaneoside increases intracellular and mitochondrial ROS levels and it seems to have an antiproliferative effect in AML cells that is mediated through AMPK activation followed by triggering of autophagy, ferritinophagy/ferritin degradation, ROS accumulation and ferroptosis [294]. Finally, animal studies suggest that this therapeutic strategy has a limited general toxicity [294].
- NFκB targeting: The NF-E2-related factor 2 (NRF2) shows increased NFκB-driven constitutive expression in primary AML cells compared with normal CD34+ bone marrow cells [295,296]. NRF2 expression is particularly high in AML cells with genetic abnormalities associated with adverse prognosis [297]. Several downstream NFR2 targets are directly involved in the regulation of ferroptosis, including glutathione peroxidase 2 (GPX4) that is a key regulator of ferroptosis, is upregulated in human AML and particularly high levels are associated with adverse prognosis [298,299]. NRF2 is thereby a regulator of antioxidant responses and is thus important for AML cell survival [295,296,297,298,299]. High NRF2 expression is associated with resistance to both conventional cytotoxic drugs (e.g., cytarabine) [296,297] and the BCL2 inhibitor venetoclax [298]. Finally, the antileukemic effect of NFκB inhibition may, therefore, at least partly be mediated by antagonizing this NRF2 effect [296,300].
- Retinoids: The all-trans retinoic acid derivative ATPR can inhibit NRF2; this agent is known to have antileukemic effects through increased lipid peroxidation and increased lipid ROS production [295]. The effect of ATPR on iron metabolism was also associated with monocytic differentiation [295]; the sensitivity to oxidative stress could thereby be increased and autophagy was promoted [301]. ATPR also increases ROS levels and this is possibly due to ferritin degradation and/or modulation of other components of the cellular iron metabolism [302].
- Fatty acid metabolism: A recent study described altered fatty acid metabolism during ferroptosis in human AML cell lines [303]. Twelve fatty acids were significantly altered in AML cells during ferroptosis, including dihomo-γ-linoleic acid, arachidonic acid and docosahexaenoic acid. Exposure to exogenous dihomo-γ-linoleic acid could then induce ferroptosis and this proferroptotic effect was dependent on the enzyme acyl-CoA synthetase family member 4. Taken together, these observations suggest that targeting fatty acid metabolism should be further explored as a possible antileukemic and proferroptotic strategy in AML.
- Lipid peroxidation: Imetelstat is a telomerase inhibitor that mediates antileukemic effects in patient xenograft models by proferroptotic effects [304]. This antileukemic activity is seen especially in AML cells with mutant NRAS and oxidative stress-associated gene expression signatures and this activity was mediated through the increased formation of polyunsaturated fatty acid-containing phospholipids leading to increased levels of lipid peroxidation and thereby oxidative stress [304]. This example also supports the hypothesis that targeting fatty acid metabolism can have a proferroptotic effect.
- Glutathione inhibition: This strategy can induce ferroptosis in AML cells through the induction of lipid peroxidation [304,305]. The inhibition of glutathione peroxidase 4 (GPX4, see the NFR2 chapter above) can induce ferroptosis in AML cell lines with the characteristic mitochondrial lipid peroxidation, and additional degradation of the mitochondrial electron transport chain enhanced the antileukemic effect of GPX4 inhibition [306]. Mitochondrial functions including energy metabolism are thus important/involved in the regulation of ferroptosis. Finally, inhibition of GPX4 can induce ferroptosis, and inhibition of GPX4 together with inhibition of the upstream NRF2 have synergistic effects [299].
- Erastin: The agent erastin seems to increase AML cell line sensitivity to cytarabine and daunorubicin, and this agent induces a mixed pattern of programmed cell death in AML cells with signs of ferroptosis as well as other forms of programmed cell death [266]. The molecular mechanisms behind this erastin effects are not known, but may involve activation of c-JUN N-terminal kinase and p38.
- Intracellular signaling: Various intracellular signaling pathways are important for the induction of ferroptosis, including the RAS-MAPK8(JNK)/P38 pathway in erastin-induced ferroptosis [307]. Erastin is then able to increase the susceptibility to cytarabine and doxorubicine. The ferroptosis-inducing effect of AMPK activation suggests that the status of the PI3K-Akt-mTOR pathway is also important for induction of ferroptosis [308,309].
- Honokiol: This is a natural small molecule that can induce ferroptosis in AML cells through the increased expression of the Heme oxygenase 1 enzyme [310]. However, this agent seems to have multiple effects in AML cells, including the modulation of STAT3 signaling, induction of proteasomal protein degradation, modulation of gene expression and causing cell-cycle arrest [311,312,313].
9.6. Targeting of the Chemokine Network
9.7. The Question of Patient Heterogeneisty and the Heterogeneity of the Hierarchically Organized AML Cell Population
- Patients are heterogeneous with regard to ferritin levels as well as expression of the two iron-regulatory genes ACO1 and IREB2 in their leukemic cells [316].
- AMPK is an important regulator of PI3K signaling through its modulation of the downstream AKT-mTOR activation and thereby also the regulation of autophagy and ferroptosis [292,293], but the constitutive as well as the insulin-induced activation of the PI3K-AKT-mTOR pathway differs between AML patients [317].
- AML patients are heterogeneous with regard to pretreatment hyperferritinemia at the time of first diagnosis (see Section 5.1 and Section 5.3) and the development of iron overload during the initial induction/consolidation treatment [192].
10. Discussion
10.1. Ferritin in Human AML
- Both ferritin and other acute-phase biomarkers are associated with prognosis/survival in AML; this is true both for ferritin levels at the time of first diagnosis and the levels before allogeneic stem cell transplantation. The acute-phase reaction will, therefore, represent a biological context for the ferritin-associated prognostic impact in many patients, but despite the association between ferritin and other acute-phase markers several studies have observed an independent prognostic impact of ferritin.
- Ferritin can reflect iron overload, and this may then represent an additional biological mechanism behind the prognostic impact in allotransplant recipients compared with newly diagnosed patients. The iron overload may influence the iron metabolism in the AML cells, bone marrow stromal cells and/or immunocompetent cells.
- Direct ferritin effects on the AML cells seem to involve regulation of iron metabolism, intracellular signaling and regulation of cell survival/programmed cell death (Section 2.2).
- Indirect ferritin effects mediated via neighboring cells in the AML-supporting bone marrow microenvironment may include induction of M2-polarization of macrophages or modulation of endothelial cells/angiogenesis (Section 3.2 and Section 3.3).
- Experimental studies suggest that ferritin has immunosuppressive effects that involve both the innate immunity by suppression of myelopoiesis (neutrophils, monocytes, dendritic cells) and the adaptive immune system through direct and indirect effects on the function of and balance between various T cell subsets (Section 3.2). Such effects may influence both antileukemic immune reactivity (e.g., after allogeneic stem cell transplantation) and AML supporting immunocompetent cells.
- Ferritin may be important for AML cell susceptibility to targeted therapies through its role in regulation of different steps in ferroptotic programmed cell death.
10.2. What Is the Optimal Acute-Phase Biomarker to Be Used in Human AML?
10.3. Therapeutic Targeting of Iron Metabolism/Ferroptosis: Toxicity Versus Efficiency
10.4. Ferritin for Cargo Delivery
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Salem, M.; Delwel, R.; Touw, I.; Mahmoud, L.; Löwenberg, B. Human AML colony growth in serum-free culture. Leuk. Res. 1988, 12, 157–165. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Frostad, S.; Foss, B. In vitro culture of acute myelogenous leukemia blasts: A comparison of four different culture media. J. Hematother. 1999, 8, 63–73. [Google Scholar] [CrossRef]
- Brenner, A.K.; Tvedt, T.H.; Nepstad, I.; Rye, K.P.; Hagen, K.M.; Reikvam, H.; Bruserud, Ø. Patients with acute myeloid leukemia can be subclassified based on the constitutive cytokine release of the leukemic cells; the possible clinical relevance and the importance of cellular iron metabolism. Expert Opin. Ther. Targets 2017, 21, 357–369. [Google Scholar] [CrossRef]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and biology of ferritin. Metallomics 2021, 13, mfab021. [Google Scholar] [CrossRef]
- Sudarev, V.V.; Dolotova, S.M.; Bukhalovich, S.M.; Bazhenov, S.V.; Ryzhykau, Y.L.; Uversky, V.N.; Bondarev, N.A.; Osipov, S.D.; Mikhailov, A.E.; Kuklina, D.D.; et al. Ferritin self-assembly, structure, function, and biotechnological applications. Int. J. Biol. Macromol. 2023, 224, 319–343. [Google Scholar] [CrossRef]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422. [Google Scholar] [CrossRef]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- Jia, J.; Wang, M.; Meng, J.; Ma, Y.; Wang, Y.; Miao, N.; Teng, J.; Zhu, D.; Shi, H.; Sun, Y.; et al. Ferritin triggers neutrophil extracellular trap-mediated cytokine storm through MSR1 contributing to adult-onset Still’s disease pathogenesis. Nat. Commun. 2022, 13, 6804. [Google Scholar] [CrossRef]
- Sato, D.; Ohtomo, H.; Yamada, Y.; Hikima, T.; Kurobe, A.; Fujiwara, K.; Ikeguchi, M. Ferritin Assembly Revisited: A Time-Resolved Small-Angle X-ray Scattering Study. Biochemistry 2016, 55, 287–293. [Google Scholar] [CrossRef]
- Rogers, J.T. Ferritin translation by interleukin-1 and interleukin-6: The role of sequences upstream of the start codons of the heavy and light subunit genes. Blood 1996, 87, 2525–2537. [Google Scholar] [CrossRef]
- Hirayama, M.; Kohgo, Y.; Kondo, H.; Shintani, N.; Fujikawa, K.; Sasaki, K.; Kato, J.; Niitsu, Y. Regulation of iron metabolism in HepG2 cells: A possible role for cytokines in the hepatic deposition of iron. Hepatology 1993, 18, 874–880. [Google Scholar] [CrossRef]
- Miller, L.L.; Miller, S.C.; Torti, S.V.; Tsuji, Y.; Torti, F.M. Iron-independent induction of ferritin H chain by tumor necrosis factor. Proc. Natl. Acad. Sci. USA 1991, 88, 4946–4950. [Google Scholar] [CrossRef]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar] [CrossRef]
- Kim, S.; Ponka, P. Effects of interferon-gamma and lipopolysaccharide on macrophage iron metabolism are mediated by nitric oxide-induced degradation of iron regulatory protein 2. J. Biol. Chem. 2000, 275, 6220–6226. [Google Scholar] [CrossRef]
- Ghosh, S.; Hevi, S.; Chuck, S.L. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood 2004, 103, 2369–2376. [Google Scholar] [CrossRef]
- Torti, F.M.; Torti, S.V. Regulation of ferritin genes and protein. Blood 2002, 99, 3505–3516. [Google Scholar] [CrossRef]
- Leggett, B.A.; Fletcher, L.M.; Ramm, G.A.; Powell, L.W.; Halliday, J.W. Differential regulation of ferritin H and L subunit mRNA during inflammation and long-term iron overload. J. Gastroenterol. Hepatol. 1993, 8, 21–27. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Truman-Rosentsvit, M.; Berenbaum, D.; Spektor, L.; Cohen, L.A.; Belizowsky-Moshe, S.; Lifshitz, L.; Ma, M.; Li, W.; Kesselman, E.; Abutbul-Ionita, I.; et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 2018, 131, 342–352. [Google Scholar] [CrossRef]
- Cohen, L.A.; Gutierrez, L.; Weiss, A.; Leichtmann-Bardoogo, Y.; Zhang, D.L.; Crooks, D.R.; Sougrat, R.; Morgenstern, A.; Galy, B.; Hentze, M.W.; et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010, 116, 1574–1584. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60. [Google Scholar] [CrossRef]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef]
- Rashid, K.A.; Hevi, S.; Chen, Y.; Le Cahérec, F.; Chuck, S.L. A proteomic approach identifies proteins in hepatocytes that bind nascent apolipoprotein B. J. Biol. Chem. 2002, 277, 22010–22017. [Google Scholar] [CrossRef]
- Santambrogio, P.; Massover, W.H. Rabbit serum alpha-2-macroglobulin binds to liver ferritin: Association causes a heterogeneity of ferritin molecules. Br. J. Haematol. 1989, 71, 281–290. [Google Scholar] [CrossRef]
- Orino, K.; Yamamoto, S.; Watanabe, K. Fibrinogen as a ferritin-binding protein in horse plasma. J. Vet. Med. Sci. 1993, 55, 785–787. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, C.; Wu, Y.; Guo, L.; Kong, D.; Zhang, Z.; Wang, Y.; Zheng, E.; Liu, Y.; He, Y. Interactions of ferritin with scavenger receptor class A members. J. Biol. Chem. 2020, 295, 15727–15741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Paragas, N.; Ned, R.M.; Qiu, A.; Viltard, M.; Leete, T.; Drexler, I.R.; Chen, X.; Sanna-Cherchi, S.; Mohammed, F.; et al. SCARA5 is a ferritin receptor mediating non-transferrin iron delivery. Dev. Cell 2009, 16, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, M.; Urakawa, N.; Nakamura, T.; Nishio, M.; Watajima, T.; Kuroda, D.; Komori, T.; Kakeji, Y.; Semba, S.; Yokozaki, H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1112–1119. [Google Scholar] [CrossRef]
- Huang, J.; Lv, C.; Zhao, B.; Ji, Z.; Gao, Z. SCARA5 inhibits oral squamous cell carcinoma via inactivating the STAT3 and PI3K/AKT signaling pathways. Open Med. 2023, 18, 20230627. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, N.; Zhang, F.; Dong, C. Overexpression of SCARA5 inhibits tumor proliferation and invasion in osteosarcoma via suppression of the FAK signaling pathway. Mol. Med. Rep. 2016, 13, 2885–2891. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Zheng, D.L.; Qin, F.S.; Cheng, N.; Chen, H.; Wan, B.B.; Wang, Y.P.; Xiao, H.S.; Han, Z.G. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J. Clin. Investig. 2010, 120, 223–241. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Chen, L.; Tan, J. SCARA5 suppresses the proliferation and migration, and promotes the apoptosis of human retinoblastoma cells by inhibiting the PI3K/AKT pathway. Mol. Med. Rep. 2021, 23, 202. [Google Scholar] [CrossRef]
- You, K.; Su, F.; Liu, L.; Lv, X.; Zhang, J.; Zhang, Y.; Liu, B. SCARA5 plays a critical role in the progression and metastasis of breast cancer by inactivating the ERK1/2, STAT3, and AKT signaling pathways. Mol. Cell. Biochem. 2017, 435, 47–58. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, F.; Shang, G.; Yin, C. SCARA5 in bone marrow stromal cell-derived exosomes inhibits colorectal cancer progression by inactivating the PI3K/Akt pathway. Genomics 2023, 115, 110636. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Valença, A.; López-Luppo, M.; Pires, V.M.; Catita, J.; Nacher, V.; Navarro, M.; Carretero, A.; Rodriguez-Baeza, A.; et al. L-ferritin binding to SCARA5: A new iron traffic pathway potentially implicated in retinopathy. PLoS ONE 2014, 9, e106974. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, R.; Xiao, T.; Xiong, L.; Wu, J.; Li, J.; Feng, G.; Song, G.; Liu, K. SCARA5 induced ferroptosis to effect ESCC proliferation and metastasis by combining with Ferritin light chain. BMC Cancer 2022, 22, 1304. [Google Scholar] [CrossRef] [PubMed]
- Gudgeon, J.; Marín-Rubio, J.L.; Trost, M. The role of macrophage scavenger receptor 1 (MSR1) in inflammatory disorders and cancer. Front. Immunol. 2022, 13, 1012002. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Miyata-Takata, T.; Tanaka, R.; Komohara, Y.; Takata, K. Myeloid sarcoma incidentally found in lymph nodes dissected for advanced gastric cancer. J. Clin. Exp. Hematop. 2023, 63, 139–142. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. Role of macrophage scavenger receptor MSR1 in the progression of non-alcoholic steatohepatitis. Front. Immunol. 2022, 13, 1050984. [Google Scholar] [CrossRef]
- Brauneck, F.; Fischer, B.; Witt, M.; Muschhammer, J.; Oelrich, J.; da Costa Avelar, P.H.; Tsoka, S.; Bullinger, L.; Seubert, E.; Smit, D.J.; et al. TIGIT blockade repolarizes AML-associated TIGIT+ M2 macrophages to an M1 phenotype and increases CD47-mediated phagocytosis. J. Immunother. Cancer 2022, 10, e004794. [Google Scholar] [CrossRef]
- Béguin, E.P.; Przeradzka, M.A.; Janssen, E.F.J.; Meems, H.; Sedek, M.; van der Zwaan, C.; Mertens, K.; van den Biggelaar, M.; Meijer, A.B.; Mourik, M.J. Endocytosis by macrophages: Interplay of macrophage scavenger receptor-1 and LDL receptor-related protein-1. Haematologica 2020, 105, e133–e137. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Li, L.; Chung, D.H.; Allen, C.D.; Torti, S.V.; Torti, F.M.; Cyster, J.G.; Chen, C.Y.; Brodsky, F.M.; Niemi, E.C.; et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J. Exp. Med. 2005, 202, 955–965. [Google Scholar] [CrossRef]
- Chiou, B.; Lucassen, E.; Sather, M.; Kallianpur, A.; Connor, J. Semaphorin4A and H-ferritin utilize TIM-1 on human oligodendrocytes: A novel neuro-immune axis. Glia 2018, 66, 1317–1330. [Google Scholar] [CrossRef]
- Li, R.; Luo, C.; Mines, M.; Zhang, J.; Fan, G.H. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J. Biol. Chem. 2006, 281, 37616–37627. [Google Scholar] [CrossRef]
- Yazdani, Z.; Mousavi, Z.; Moradabadi, A.; Hassanshahi, G. Significance of CXCL12/CXCR4 Ligand/Receptor Axis in Various Aspects of Acute Myeloid Leukemia. Cancer Manag. Res. 2020, 12, 2155–2165. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, H.J.; Konopleva, M. Targeting the CXCL12/CXCR4 axis in acute myeloid leukemia: From bench to bedside. Korean J. Intern. Med. 2017, 32, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Vo, A.K.; Rekvam, H. Hematopoiesis, Inflammation and Aging-The Biological Background and Clinical Impact of Anemia and Increased C-Reactive Protein Levels on Elderly Individuals. J. Clin. Med. 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, X.; Chen, Q.; Kantawong, F.; Wan, S.; Liu, J.; Li, H.; Zhou, J.; Lu, B.; Wu, J. Identification of a Mitochondria-Related Gene Signature to Predict the Prognosis in AML. Front. Oncol. 2022, 12, 823831. [Google Scholar] [CrossRef]
- Tong, X.; Zhou, F. Integrated bioinformatic analysis of mitochondrial metabolism-related genes in acute myeloid leukemia. Front. Immunol. 2023, 14, 1120670. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Williams, D.E.; Geissler, K.; Hangoc, G.; Cooper, S.; Bicknell, D.C.; Levi, S.; Arosio, P. Suppressive effects in vivo of purified recombinant human H-subunit (acidic) ferritin on murine myelopoiesis. Blood 1989, 73, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Lu, L.; Bicknell, D.C.; Williams, D.E.; Cooper, S.; Levi, S.; Salfeld, J.; Arosio, P. The influence of purified recombinant human heavy-subunit and light-subunit ferritins on colony formation in vitro by granulocyte-macrophage and erythroid progenitor cells. Blood 1986, 68, 1257–1263. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Cooper, S.; Levi, S.; Arosio, P. Mutated recombinant human heavy-chain ferritins and myelosuppression in vitro and in vivo: A link between ferritin ferroxidase activity and biological function. Proc. Natl. Acad. Sci. USA 1991, 88, 770–774. [Google Scholar] [CrossRef]
- Dezza, L.; Cazzola, M.; Bergamaschi, G.; Stella, C.C.; Pedrazzoli, P.; Recalde, H.R. Effects of recombinant human H-subunit and L-subunit ferritins on in vitro growth of human granulocyte-monocyte progenitors. Br. J. Haematol. 1988, 68, 367–372. [Google Scholar] [CrossRef]
- Fargion, S.; Fracanzani, A.L.; Brando, B.; Arosio, P.; Levi, S.; Fiorelli, G. Specific binding sites for H-ferritin on human lymphocytes: Modulation during cellular proliferation and potential implication in cell growth control. Blood 1991, 78, 1056–1061. [Google Scholar] [CrossRef]
- Harada, T.; Baba, M.; Torii, I.; Morikawa, S. Ferritin selectively suppresses delayed-type hypersensitivity responses at induction or effector phase. Cell. Immunol. 1987, 109, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.P.; Franco, A.V.; Arosio, P.; Hersey, P. Immunosuppressive effects of melanoma-derived heavy-chain ferritin are dependent on stimulation of IL-10 production. Int. J. Cancer 2001, 92, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F.; Powell, L.W.; Halliday, J.W. Iron status and cellular immune competence. Blood Rev. 1988, 2, 43–49. [Google Scholar] [CrossRef]
- Walker, E.M., Jr.; Walker, S.M. Effects of iron overload on the immune system. Ann. Clin. Lab. Sci. 2000, 30, 354–365. [Google Scholar]
- Gray, C.P.; Arosio, P.; Hersey, P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood 2002, 99, 3326–3334. [Google Scholar] [CrossRef]
- Blissett, A.R.; Deng, B.; Wei, P.; Walsh, K.J.; Ollander, B.; Sifford, J.; Sauerbeck, A.D.; McComb, D.W.; McTigue, D.M.; Agarwal, G. Sub-cellular In-situ Characterization of Ferritin(iron) in a Rodent Model of Spinal Cord Injury. Sci. Rep. 2018, 8, 3567. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Wei, Y.; Li, X.; Jiang, W.; Xu, Y.; Li, L.; Guo, R.; Chen, D.; Gao, P.; et al. Ferritin light chain promotes the reprogramming of glioma immune microenvironment and facilitates glioma progression. Theranostics 2023, 13, 3794–3813. [Google Scholar] [CrossRef]
- Miari, K.E.; Guzman, M.L.; Wheadon, H.; Williams, M.T.S. Macrophages in Acute Myeloid Leukaemia: Significant Players in Therapy Resistance and Patient Outcomes. Front. Cell Dev. Biol. 2021, 9, 692800. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Ehninger, G.; Hamann, W.; Pawelec, G. Secretion of IL-2, IL-3, IL-4, IL-6 and GM-CSF by CD4+ and CD8+ TCR alpha beta+ T-cell clones derived early after allogeneic bone marrow transplantation. Scand. J. Immunol. 1993, 38, 65–74. [Google Scholar] [CrossRef]
- Al-Matary, Y.S.; Botezatu, L.; Opalka, B.; Hönes, J.M.; Lams, R.F.; Thivakaran, A.; Schütte, J.; Köster, R.; Lennartz, K.; Schroeder, T.; et al. Acute myeloid leukemia cells polarize macrophages towards a leukemia supporting state in a Growth factor independence 1 dependent manner. Haematologica 2016, 101, 1216–1227. [Google Scholar] [CrossRef]
- Xu, Z.J.; Gu, Y.; Wang, C.Z.; Jin, Y.; Wen, X.M.; Ma, J.C.; Tang, L.J.; Mao, Z.W.; Qian, J.; Lin, J. The M2 macrophage marker CD206: A novel prognostic indicator for acute myeloid leukemia. Oncoimmunology 2019, 9, 1683347. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, W.; Wang, R.; Yang, F.; Wang, L.; Chen, S.; Ru, Y.; Cheng, T.; Zheng, G. Repolarizing heterogeneous leukemia-associated macrophages with more M1 characteristics eliminates their pro-leukemic effects. Oncoimmunology 2017, 7, e1412910. [Google Scholar] [CrossRef] [PubMed]
- Weinhäuser, I.; Pereira-Martins, D.A.; Almeida, L.Y.; Hilberink, J.R.; Silveira, D.R.A.; Quek, L.; Ortiz, C.; Araujo, C.L.; Bianco, T.M.; Lucena-Araujo, A.; et al. M2 macrophages drive leukemic transformation by imposing resistance to phagocytosis and improving mitochondrial metabolism. Sci. Adv. 2023, 9, eadf8522. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lü, M.; Cao, F.; Wu, G.; Gao, F.; Pang, H.; Li, Y.; Zhang, Y.; Xing, H.; Liang, C.; et al. Single-cell map of diverse immune phenotypes in the acute myeloid leukemia microenvironment. Biomark. Res. 2021, 9, 15. [Google Scholar] [CrossRef]
- van Galen, P.; Hovestadt, V.; Wadsworth Ii, M.H.; Hughes, T.K.; Griffin, G.K.; Battaglia, S.; Verga, J.A.; Stephansky, J.; Pastika, T.J.; Lombardi Story, J. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019, 176, 1265–1281. [Google Scholar] [CrossRef]
- Spertini, C.; Bénéchet, A.P.; Birch, F.; Bellotti, A.; Román-Trufero, M.; Arber, C.; Auner, H.W.; Mitchell, R.A.; Spertini, O.; Smirnova, T. Macrophage migration inhibitory factor blockade reprograms macrophages and disrupts prosurvival signaling in acute myeloid leukemia. Cell Death Discov. 2024, 10, 157. [Google Scholar] [CrossRef]
- Oberle, S.; Polte, T.; Abate, A.; Podhaisky, H.P.; Schröder, H. Aspirin increases ferritin synthesis in endothelial cells: A novel antioxidant pathway. Circ. Res. 1998, 82, 1016–1020. [Google Scholar] [CrossRef]
- Juckett, M.B.; Balla, J.; Balla, G.; Jessurun, J.; Jacob, H.S.; Vercellotti, G.M. Ferritin protects endothelial cells from oxidized low density lipoprotein in vitro. Am. J. Pathol. 1995, 147, 782–789. [Google Scholar]
- Wang, P.; Ren, Q.; Shi, M.; Liu, Y.; Bai, H.; Chang, Y.Z. Overexpression of Mitochondrial Ferritin Enhances Blood-Brain Barrier Integrity Following Ischemic Stroke in Mice by Maintaining Iron Homeostasis in Endothelial Cells. Antioxidants 2022, 11, 1257. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Sainz, I.M.; Pixley, R.A.; Colman, R.W. Fifty years of research on the plasma kallikrein-kinin system: From protein structure and function to cell biology and in-vivo pathophysiology. Thromb. Haemost. 2007, 98, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.W. Regulation of angiogenesis by the kallikrein-kinin system. Curr. Pharm. Des. 2006, 12, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Colman, R.W. Two faces of high-molecular-weight kininogen (HK) in angiogenesis: Bradykinin turns it on and cleaved HK (HKa) turns it off. J. Thromb. Haemost. 2005, 3, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, N.; Torti, S.V.; Torti, F.M. Ferritin binds to light chain of human H-kininogen and inhibits kallikrein-mediated bradykinin release. Biochem. J. 2002, 365, 279–286. [Google Scholar] [CrossRef]
- Coffman, L.G.; Brown, J.C.; Johnson, D.A.; Parthasarathy, N.; D’Agostino, R.B., Jr.; Lively, M.O.; Hua, X.; Tilley, S.L.; Muller-Esterl, W.; Willingham, M.C.; et al. Cleavage of high-molecular-weight kininogen by elastase and tryptase is inhibited by ferritin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L505–L515. [Google Scholar] [CrossRef]
- Coffman, L.G.; Parsonage, D.; D’Agostino, R., Jr.; Torti, F.M.; Torti, S.V. Regulatory effects of ferritin on angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 570–575. [Google Scholar] [CrossRef]
- Restelli, C.; Ruella, M.; Paruzzo, L.; Tarella, C.; Pelicci, P.G.; Colombo, E. Recent Advances in Immune-Based Therapies for Acute Myeloid Leukemia. Blood Cancer Discov. 2024, 5, 234–248. [Google Scholar] [CrossRef]
- Knorr, D.A.; Goldberg, A.D.; Stein, E.M.; Tallman, M.S. Immunotherapy for acute myeloid leukemia: From allogeneic stem cell transplant to novel therapeutics. Leuk. Lymphoma 2019, 60, 3350–3362. [Google Scholar] [CrossRef]
- Hao, F.; Sholy, C.; Wang, C.; Cao, M.; Kang, X. The Role of T Cell Immunotherapy in Acute Myeloid Leukemia. Cells 2021, 10, 3376. [Google Scholar] [CrossRef]
- Ayala, F.; Dewar, R.; Kieran, M.; Kalluri, R. Contribution of bone microenvironmentto leukemogenesis and leukemia progression. Leukemia 2009, 23, 2233–2241. [Google Scholar] [CrossRef]
- Padró, T.; Ruiz, S.; Bieker, R.; Bürger, H.; Steins, M.; Kienast, J.; Büchner, T.; Berdel, W.E.; Mesters, R.M. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood 2000, 95, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.T.; Hou, H.A.; Liu, C.Y.; Chen, B.B.; Tang, J.L.; Chen, H.Y.; Wei, S.Y.; Yao, M.; Huang, S.Y.; Chou, W.C.; et al. Bone marrow angiogenesis magnetic resonance imaging in patients with acute myeloid leukemia: Peak enhancement ratio is an independent predictor for overall survival. Blood 2009, 113, 3161–3167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, B.; Guo, S.; Zhao, J.; Wang, X.; Zhou, B. Adipose knockout of H-ferritin improves energy metabolism in mice. Mol. Metab. 2024, 80, 101871. [Google Scholar] [CrossRef]
- Suzuki, T.; Komatsu, T.; Shibata, H.; Tanioka, A.; Vargas, D.; Kawabata-Iwakawa, R.; Miura, F.; Masuda, S.; Hayashi, M.; Tanimura-Inagaki, K.; et al. Crucial role of iron in epigenetic rewriting during adipocyte differentiation mediated by JMJD1A and TET2 activity. Nucleic Acids Res. 2023, 51, 6120–6142. [Google Scholar] [CrossRef]
- Festa, M.; Ricciardelli, G.; Mele, G.; Pietropaolo, C.; Ruffo, A.; Colonna, A. Overexpression of H ferritin and up-regulation of iron regulatory protein genes during differentiation of 3T3-L1 pre-adipocytes. J. Biol. Chem. 2000, 275, 36708–36712. [Google Scholar] [CrossRef]
- Ladikou, E.E.; Sivaloganathan, H.; Pepper, A.; Chevassut, T. Acute Myeloid Leukaemia in Its Niche: The Bone Marrow Microenvironment in Acute Myeloid Leukaemia. Curr. Oncol. Rep. 2020, 22, 27. [Google Scholar] [CrossRef]
- Brenner, A.K.; Nepstad, I.; Bruserud, Ø. Mesenchymal Stem Cells Support Survival and Proliferation of Primary Human Acute Myeloid Leukemia Cells through Heterogeneous Molecular Mechanisms. Front. Immunol. 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Aasebø, E.; Brenner, A.K.; Hernandez-Valladares, M.; Birkeland, E.; Mjaavatten, O.; Reikvam, H.; Selheim, F.; Berven, F.S.; Bruserud, Ø. Patient Heterogeneity in Acute Myeloid Leukemia: Leukemic Cell Communication by Release of Soluble Mediators and Its Effects on Mesenchymal Stem Cells. Diseases 2021, 9, 74. [Google Scholar] [CrossRef]
- Reikvam, H.; Brenner, A.K.; Hagen, K.M.; Liseth, K.; Skrede, S.; Hatfield, K.J.; Bruserud, Ø. The cytokine-mediated crosstalk between primary human acute myeloid cells and mesenchymal stem cells alters the local cytokine network and the global gene expression profile of the mesenchymal cells. Stem Cell Res. 2015, 15, 530–541. [Google Scholar] [CrossRef]
- Mehta, K.J. Iron-Related Genes and Proteins in Mesenchymal Stem Cell Detection and Therapy. Stem Cell Rev. Rep. 2023, 19, 1773–1784. [Google Scholar] [CrossRef]
- Mehta, K.J. Role of iron and iron-related proteins in mesenchymal stem cells: Cellular and clinical aspects. J. Cell. Physiol. 2021, 236, 7266–7289. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Caldarelli, I.; Speranza, M.C.; Scianguetta, S.; Tramontano, A.; Bencivenga, D.; Stampone, E.; Negri, A.; Nobili, B.; Locatelli, F.; et al. Iron overload enhances human mesenchymal stromal cell growth and hampers matrix calcification. Biochim. Biophys. Acta 2016, 1860, 1211–1223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, W.; Zhao, M.; Li, D.; Chai, X.; Cao, X.; Meng, J.; Chen, J.; Xiao, X.; Li, Q.; et al. Effects of iron overload on the bone marrow microenvironment in mice. PLoS ONE 2015, 10, e0120219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhao, Y.; Guo, J.; Zhao, S.; Fei, C.; Xiao, C.; Wu, D.; Wu, L.; Li, X.; Chang, C. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells from myelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis. 2018, 9, 515. [Google Scholar] [CrossRef]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Okabe, H.; Suzuki, T.; Uehara, E.; Ueda, M.; Nagai, T.; Ozawa, K. The bone marrow hematopoietic microenvironment is impaired in iron-overloaded mice. Eur. J. Haematol. 2014, 93, 118–128. [Google Scholar] [CrossRef]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef]
- Hatfield, K.; Ryningen, A.; Corbascio, M.; Bruserud, O. Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts. Int. J. Cancer 2006, 119, 2313–2321. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Ryningen, A.; Wergeland, L.; Glenjen, N.I.; Gjertsen, B.T. Osteoblasts increase proliferation and release of pro-angiogenic interleukin 8 by native human acute myelogenous leukemia blasts. Haematologica 2004, 89, 391–402. [Google Scholar]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Trautwein, C.; Böker, K.; Manns, M.P. Hepatocyte and immune system: Acute phase reaction as a contribution to early defence mechanisms. Gut 1994, 35, 1163–1166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korkmaz, H.I.; Krijnen, P.A.J.; Ulrich, M.M.W.; de Jong, E.; van Zuijlen, P.P.M.; Niessen, H.W.M. The role of complement in the acute phase response after burns. Burns 2017, 43, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Aarstad, H.H.; Tvedt, T.H.A. Combined C-Reactive Protein and Novel Inflammatory Parameters as a Predictor in Cancer-What Can We Learn from the Hematological Experience? Cancers 2020, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Tvedt, T.H.; Skaarud, K.J.; Tjønnfjord, G.E.; Gedde-Dahl, T.; Iversen, P.O.; Bruserud, Ø. The Systemic Metabolic Profile Early after Allogeneic Stem Cell Transplantation: Effects of Adequate Energy Support Administered through Enteral Feeding Tube. Biol. Blood Marrow Transpl. 2020, 26, 380–391. [Google Scholar] [CrossRef]
- Tvedt, T.H.; Reikvam, H.; Bruserud, Ø. Nutrition in Allogeneic Stem Cell Transplantion—Clinical Guidelines and Immunobiological Aspects. Curr. Pharm. Biotechnol. 2016, 17, 92–104. [Google Scholar] [CrossRef]
- Gruys, E.; Toussaint, M.J.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef]
- Meyron-Holtz, E.G.; Moshe-Belizowski, S.; Cohen, L.A. A possible role for secreted ferritin in tissue iron distribution. J. Neural. Transm. 2011, 118, 337–347. [Google Scholar] [CrossRef]
- Fauter, M.; Mainbourg, S.; El Jammal, T.; Guerber, A.; Zaepfel, S.; Henry, T.; Gerfaud-Valentin, M.; Sève, P.; Jamilloux, Y. Extreme Hyperferritinemia: Causes and Prognosis. J. Clin. Med. 2022, 11, 5438. [Google Scholar] [CrossRef]
- Belfeki, N.; Strazzulla, A.; Picque, M.; Diamantis, S. Extreme hyperferritinemia: Etiological spectrum and impact on prognosis. Reumatismo 2020, 71, 199–202. [Google Scholar] [CrossRef]
- Koperdanova, M.; Cullis, J.O. Interpreting raised serum ferritin levels. BMJ 2015, 351, h3692. [Google Scholar] [CrossRef]
- Schram, A.M.; Campigotto, F.; Mullally, A.; Fogerty, A.; Massarotti, E.; Neuberg, D.; Berliner, N. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015, 125, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef]
- Sun, K.; Li, C.; Liao, S.; Yao, X.; Ouyang, Y.; Liu, Y.; Wang, Z.; Li, Z.; Yao, F. Ferritinophagy, a form of autophagic ferroptosis: New insights into cancer treatment. Front. Pharmacol. 2022, 13, 1043344. [Google Scholar] [CrossRef]
- Liu, Y.C.; Gong, Y.T.; Sun, Q.Y.; Wang, B.; Yan, Y.; Chen, Y.X.; Zhang, L.J.; Zhang, W.D.; Luan, X. Ferritinophagy induced ferroptosis in the management of cancer. Cell. Oncol. 2024, 47, 19–35. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Selheim, F.; Hernandez-Valladares, M.; Reikvam, H. Monocytic Differentiation in Acute Myeloid Leukemia Cells: Diagnostic Criteria, Biological Heterogeneity, Mitochondrial Metabolism, Resistance to and Induction by Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 6356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, C.; Sun, Q.; Li, Y.; Zhou, C.; Sun, C. Susceptibility of acute myeloid leukemia cells to ferroptosis and evasion strategies. Front. Mol. Biosci. 2023, 10, 1275774. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y.; Zhu, Y.; Guo, Y.; Liu, B. Basic mechanisms and novel potential therapeutic targets for ferroptosis in acute myeloid leukemia. Ann. Hematol. 2023, 102, 1985–1999. [Google Scholar] [CrossRef] [PubMed]
- Bisoendial, R.J.; Kastelein, J.J.; Levels, J.H.; Zwaginga, J.J.; Van den Bogaard, B.; Reitsma, P.H.; Meijers, J.C.; Hartman, D.; Levi, M.; Stroes, E.S. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ. Res. 2005, 96, 714–716. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Mosevoll, K.A.; Bruserud, Ø.; Reikvam, H.; Wendelbo, Ø. The Regulation of Neutrophil Migration in Patients with Sepsis: The Complexity of the Molecular Mechanisms and Their Modulation in Sepsis and the Heterogeneity of Sepsis Patients. Cells 2023, 12, 1003. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, K.J.; Evensen, L.; Reikvam, H.; Lorens, J.B.; Bruserud, Ø. Soluble mediators released by acute myeloid leukemia cells increase capillary-like networks. Eur. J. Haematol. 2012, 89, 478–490. [Google Scholar] [CrossRef]
- Hatfield, K.; Øyan, A.M.; Ersvaer, E.; Kalland, K.H.; Lassalle, P.; Gjertsen, B.T.; Bruserud, Ø. Primary human acute myeloid leukaemia cells increase the proliferation of microvascular endothelial cells through the release of soluble mediators. Br. J. Haematol. 2009, 144, 53–68. [Google Scholar] [CrossRef]
- Kupsa, T.; Vanek, J.; Pavel, Z.; Jebavy, L.; Horacek, J.M. Serum levels of soluble adhesion molecules in newly diagnosed acute myeloid leukemia and in complete remission suggest endothelial cell activation by myeloblasts. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2017, 161, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kupsa, T.; Vanek, J.; Zak, P.; Jebavy, L.; Horacek, J.M. Serum levels of selected cytokines and soluble adhesion molecules in acute myeloid leukemia: Soluble receptor for interleukin-2 predicts overall survival. Cytokine 2020, 128, 155005. [Google Scholar] [CrossRef] [PubMed]
- Kupsa, T.; Vanek, J.; Vasatova, M.; Karesova, I.; Zak, P.; Jebavy, L.; Horacek, J.M. Evaluation of cytokines and soluble adhesion molecules in patients with newly diagnosed acute myeloid leukemia: The role of TNF-alpha and FLT3-ITD. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2016, 160, 94–99. [Google Scholar] [CrossRef]
- Passaro, D.; Di Tullio, A.; Abarrategi, A.; Rouault-Pierre, K.; Foster, K.; Ariza-McNaughton, L.; Montaner, B.; Chakravarty, P.; Bhaw, L.; Diana, G.; et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell 2017, 32, 324–341. [Google Scholar] [CrossRef]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pr. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C. Humoral Innate Immunity and Acute-Phase Proteins. N. Engl. J. Med. 2023, 388, 439–452. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Mosevoll, K.A.; Johansen, S.; Wendelbo, Ø.; Nepstad, I.; Bruserud, Ø.; Reikvam, H. Cytokines, Adhesion Molecules, and Matrix Metalloproteases as Predisposing, Diagnostic, and Prognostic Factors in Venous Thrombosis. Front. Med. 2018, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Flick, M.J.; Du, X.; Witte, D.P.; Jirousková, M.; Soloviev, D.A.; Busuttil, S.J.; Plow, E.F.; Degen, J.L. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Investig. 2004, 113, 1596–1606. [Google Scholar] [CrossRef]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef]
- Wolberg, A.S. Fibrinogen and fibrin: Synthesis, structure, and function in health and disease. J. Thromb. Haemost. 2023, 21, 3005–3015. [Google Scholar] [CrossRef]
- Redman, C.M.; Xia, H. Fibrinogen biosynthesis. Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann. N. Y. Acad. Sci. 2001, 936, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Selheim, F.; Aasebø, E.; Reikvam, H.; Bruserud, Ø.; Hernandez-Valladares, M. Proteomic Comparison of Acute Myeloid Leukemia Cells and Normal CD34+ Bone Marrow Cells: Studies of Leukemia Cell Differentiation and Regulation of Iron Metabolism/Ferroptosis. Proteomes 2025, 13, 11. [Google Scholar] [CrossRef]
- Weber, S.; Parmon, A.; Kurrle, N.; Schnütgen, F.; Serve, H. The Clinical Significance of Iron Overload and Iron Metabolism in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Front. Immunol. 2021, 11, 627662. [Google Scholar] [CrossRef]
- White, G.P.; Worwood, M.; Parry, D.H.; Jacobs, A. Ferritin synthesis in normal and leukaemic leukocytes. Nature 1974, 250, 584–586. [Google Scholar] [CrossRef]
- Worwood, M.; Summers, M.; Miller, F.; Jacobs, A.; Whittaker, J.A. Ferritin in blood cells from normal subjects and patients with leukaemia. Br. J. Haematol. 1974, 28, 27–35. [Google Scholar] [CrossRef]
- Matzner, Y.; Konijn, A.M.; Hershko, C. Serum ferritin in hematologic malignancies. Am. J. Hematol. 1980, 9, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Aulbert, E.; Schmidt, C.G. Ferritin—A tumor marker in myeloid leukemia. Cancer Detect. Prev. 1985, 8, 297–302. [Google Scholar]
- Sadighi, S.; Sahinoglu, E.; Haider Kubba, A.; Patel, J.; Hosseini, S.F.; Shafiee, M.A.; Qureshi, B. Impact of Serum Ferritin and Iron Overload on Acute Myeloid Leukemia Outcomes: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2024, 25, 2951–2962. [Google Scholar] [CrossRef]
- Ihlow, J.; Gross, S.; Sick, A.; Schneider, T.; Flörcken, A.; Burmeister, T.; Türkmen, S.; Arnold, R.; Dörken, B.; Westermann, J. AML: High serum ferritin at initial diagnosis has a negative impact on long-term survival. Leuk. Lymphoma 2019, 60, 69–77. [Google Scholar] [CrossRef]
- Ihlow, J.; Gross, S.; Neuendorff, N.R.; Busack, L.; Herneth, A.; Singh, A.; Schwarz, M.; Flörcken, A.; Anagnostopoulos, I.; Türkmen, S.; et al. Clinical outcome of older adults with acute myeloid Leukemia: An analysis of a large tertiary referral Center over two decades. J. Geriatr. Oncol. 2021, 12, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Andou, T.; Tanaka, M.; Ito, S.; Miyazaki, T.; Ishii, Y.; Ogusa, E.; Koharazawa, H.; Takahashi, H.; Motohashi, K.; et al. Clinical Significance of Serum Ferritin at Diagnosis in Patients With Acute Myeloid Leukemia: A YACHT Multicenter Retrospective Study. Clin. Lymphoma Myeloma Leuk. 2018, 18, 415–421. [Google Scholar] [CrossRef]
- Lebon, D.; Vergez, F.; Bertoli, S.; Harrivel, V.; De Botton, S.; Micol, J.B.; Marolleau, J.P.; Récher, C. Hyperferritinemia at diagnosis predicts relapse and overall survival in younger AML patients with intermediate-risk cytogenetics. Leuk. Res. 2015, 39, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Q.; Cheng, Y.; Wang, M.; Tong, J.; Tang, R.; Pan, Y.; Yang, J. Can serum ferritin serve as a biomarker for the prognosis of gynecological malignant tumors? A retrospective cohort study. Cancer Biomark. 2024, 39, 127–136. [Google Scholar] [CrossRef]
- Recalcati, S.; Gammella, E.; Cairo, G. Dysregulation of iron metabolism in cancer stem cells. Free Radic. Biol. Med. 2019, 133, 216–220. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, B.; Yang, Y.; Lin, Z.; Liu, Y. Overexpression of ferritin light chain as a poor prognostic factor for breast cancer. Mol. Biol. Rep. 2023, 50, 8097–8109. [Google Scholar] [CrossRef]
- Mendes, F.R.; da Silva, W.F.; da Costa Bandeira de Melo, R.; Silveira, D.R.A.; Velloso, E.D.R.P.; Rocha, V.; Rego, E.M. Predictive factors associated with induction-related death in acute myeloid leukemia in a resource-constrained setting. Ann. Hematol. 2022, 101, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Desai, A.; Ge, B.; Li, W.; Jin, X.; Bai, H.; Yu, K.; Ye, H. Prognostic value of hypoalbuminemia at diagnosis in de novo non-M3 acute myeloid leukemia. Leuk. Lymphoma 2020, 61, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Doucette, K.; Percival, M.E.; Williams, L.; Kandahari, A.; Taylor, A.; Wang, S.; Ahn, J.; Karp, J.E.; Lai, C. Hypoalbuminemia as a prognostic biomarker for higher mortality and treatment complications in acute myeloid leukemia. Hematol. Oncol. 2021, 39, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Shi, M.; Song, J.; Niu, X.; Niu, J.; Wei, S.; Li, D.; Bai, Y.; Sun, K. The Prognostic Significance of C-Reactive Protein to Albumin Ratio in Newly Diagnosed Acute Myeloid Leukaemia Patients. Cancer Manag. Res. 2022, 14, 303–316. [Google Scholar] [CrossRef]
- Senjo, H.; Onozawa, M.; Hidaka, D.; Yokoyama, S.; Yamamoto, S.; Tsutsumi, Y.; Haseyama, Y.; Nagashima, T.; Mori, A.; Ota, S.; et al. High CRP-albumin ratio predicts poor prognosis in transplant ineligible elderly patients with newly diagnosed acute myeloid leukemia. Sci. Rep. 2022, 12, 8885. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, Q.; Li, Y.; Wu, L.; Zhang, S.; Yu, K. Plasma fibrinogen levels correlate with prognosis and treatment outcome in patients with non-M3 acute myeloid leukemia. Leuk. Lymphoma 2019, 60, 1503–1511. [Google Scholar] [CrossRef]
- Berger, M.D.; Heini, A.D.; Seipel, K.; Mueller, B.; Angelillo-Scherrer, A.; Pabst, T. Increased fibrinogen levels at diagnosis are associated with adverse outcome in patients with acute myeloid leukemia. Hematol. Oncol. 2017, 35, 789–796. [Google Scholar] [CrossRef]
- Heini, A.D.; Hugo, R.; Berger, M.D.; Novak, U.; Bacher, U.; Pabst, T. Simple acute phase protein score to predict long-term survival in patients with acute myeloid leukemia. Hematol. Oncol. 2020, 38, 74–81. [Google Scholar] [CrossRef]
- Sakuma, T.; Fujisawa, S.; Tanaka, M.; Hagihara, M.; Fujita, H.; Fujimaki, K.; Katsuki, K.; Akimoto, M.; Tanaka, M.; Matsumura, A.; et al. Prognostic significance of the CFA ratio for newly diagnosed acute myeloid leukemia: A multicenter retrospective study. Hematol. Oncol. 2024, 42, e3228. [Google Scholar] [CrossRef]
- Grignano, E.; Jachiet, V.; Fenaux, P.; Ades, L.; Fain, O.; Mekinian, A. Autoimmune manifestations associated with myelodysplastic syndromes. Ann. Hematol. 2018, 97, 2015–2023. [Google Scholar] [CrossRef]
- Li, H.; Hu, F.; Gale, R.P.; Sekeres, M.A.; Liang, Y. Myelodysplastic syndromes. Nat. Rev. Dis. Primers 2022, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Kanagal-Shamanna, R.; Beck, D.B.; Calvo, K.R. Clonal Hematopoiesis, Inflammation, and Hematologic Malignancy. Annu. Rev. Pathol. 2024, 19, 479–506. [Google Scholar] [CrossRef] [PubMed]
- Weeks, L.D.; Marinac, C.R.; Redd, R.; Abel, G.; Lin, A.; Agrawal, M.; Stone, R.M.; Schrag, D.; Ebert, B.L. Age-related diseases of inflammation in myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2022, 139, 1246–1250. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Starczynowski, D.T. Innate immune pathways and inflammation in hematopoietic aging, clonal hematopoiesis, and MDS. J. Exp. Med. 2021, 218, e20201544. [Google Scholar] [CrossRef]
- Loh, K.P.; Tooze, J.A.; Nicklas, B.J.; Kritchevsky, S.B.; Williamson, J.D.; Ellis, L.R.; Powell, B.L.; Pardee, T.S.; Goyal, N.G.; Klepin, H.D. Inflammatory biomarkers, geriatric assessment, and treatment outcomes in acute myeloid leukemia. J. Geriatr. Oncol. 2020, 11, 410–416. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Eisfeld, A.K.; Westerman, M.; Krahl, R.; Leiblein, S.; Liebert, U.G.; Hehme, M.; Teupser, D.; Niederwieser, D.; Al-Ali, H.K. Highly Elevated Serum Hepcidin in Patients with Acute Myeloid Leukemia prior to and after Allogeneic Hematopoietic Cell Transplantation: Does This Protect from Excessive Parenchymal Iron Loading? Adv. Hematol. 2011, 2011, 491058. [Google Scholar] [CrossRef][Green Version]
- Cheng, P.P.; Sun, Z.Z.; Jiang, F.; Tang, Y.T.; Jiao, X.Y. Hepcidin expression in patients with acute leukaemia. Eur. J. Clin. Investig. 2012, 42, 517–525. [Google Scholar] [CrossRef]
- Słomka, A.; Łęcka, M.; Styczyński, J. Hepcidin in Children and Adults with Acute Leukemia or Undergoing Hematopoietic Cell Transplantation: A Systematic Review. Cancers 2022, 14, 4936. [Google Scholar] [CrossRef]
- Hines, M.R.; von Bahr Greenwood, T.; Beutel, G.; Beutel, K.; Hays, J.A.; Horne, A.; Janka, G.; Jordan, M.B.; van Laar, J.A.M.; Lachmann, G.; et al. Consensus-Based Guidelines for the Recognition, Diagnosis, and Management of Hemophagocytic Lymphohistiocytosis in Critically Ill Children and Adults. Crit. Care Med. 2022, 50, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, J.; Wells, D.A.; Anderson, M.K.; Halford, Z. A Review of Current and Emerging Therapeutic Options for Hemophagocytic Lymphohistiocytosis. Ann. Pharmacother. 2023, 57, 867–879. [Google Scholar] [CrossRef]
- Bilston, L.; Croden, J.; Taparia, M.; Karkhaneh, M.; Grossman, J.; Sun, H.L. Validation of the HScore and the HLH-2004 diagnostic criteria for the diagnosis of hemophagocytic lymphohistiocytosis in a multicenter cohort. Eur. J. Haematol. 2022, 109, 129–137. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Bullinger, L.; Garcia-Vidal, C.; Herbrecht, R.; Maertens, J.; Menna, P.; Pagano, L.; Thiebaut-Bertrand, A.; Calandra, T. Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Leukemia 2022, 36, 1215–1226. [Google Scholar]
- Peseski, A.M.; McClean, M.; Green, S.D.; Beeler, C.; Konig, H. Management of fever and neutropenia in the adult patient with acute myeloid leukemia. Expert Rev. Anti. Infect. Ther. 2021, 19, 359–378. [Google Scholar] [CrossRef]
- Kochanek, M.; Schalk, E.; von Bergwelt-Baildon, M.; Beutel, G.; Buchheidt, D.; Hentrich, M.; Henze, L.; Kiehl, M.; Liebregts, T.; von Lilienfeld-Toal, M.; et al. Management of sepsis in neutropenic cancer patients: 2018 guidelines from the Infectious Diseases Working Party (AGIHO) and Intensive Care Working Party (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2019, 98, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Qin, X.; Liu, W. Platelet-Acute Leukemia Interactions. Clin. Chim. Acta 2022, 536, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Bigliardi, S.; Morselli, M.; Potenza, L.; Fantuzzi, V.; Faglioni, L.; Nasillo, V.; Messerotti, A.; Paolini, A.; Luppi, M. An unusual case of splenomegaly and increased lactate dehydrogenase heralding acute myeloid leukemia with eosinophilia and RUNX1-MECOM fusion transcripts. Leuk. Res. Rep. 2014, 3, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, B.P.; Menias, C.O.; Lubner, M.G.; Mellnick, V.M.; Pickhardt, P.J. Splenomegaly: A Combined Clinical and Radiologic Approach to the Differential Diagnosis. Gastroenterol. Clin. N. Am. 2018, 47, 643–666. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Qi, J.; Miao, W.; Fang, K.; Ruan, C.; Wu, D.; Han, Y. The prognostic value of plasma fibrinogen level in patients with acute myeloid leukemia: A systematic review and meta-analysis. Leuk. Lymphoma 2020, 61, 2682–2691. [Google Scholar] [CrossRef]
- Barr, R.D.; Gomez-Almaguer, D.; Jaime-Perez, J.C.; Ruiz-Argüelles, G.J. Importance of Nutrition in the Treatment of Leukemia in Children and Adolescents. Arch. Med. Res. 2016, 47, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Delavigne, K.; Bérard, E.; Bertoli, S.; Corre, J.; Duchayne, E.; Demur, C.; Mansat-De Mas, V.; Borel, C.; Picard, M.; Alvarez, M.; et al. Hemophagocytic syndrome in patients with acute myeloid leukemia undergoing intensive chemotherapy. Haematologica 2014, 99, 474–480. [Google Scholar] [CrossRef]
- Knaak, C.; Nyvlt, P.; Schuster, F.S.; Spies, C.; Heeren, P.; Schenk, T.; Balzer, F.; La Rosée, P.; Janka, G.; Brunkhorst, F.M.; et al. Hemophagocytic lymphohistiocytosis in critically ill patients: Diagnostic reliability of HLH-2004 criteria and HScore. Crit. Care 2020, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Batu, E.D.; Erden, A.; Seyhoğlu, E.; Kilic, L.; Büyükasık, Y.; Karadag, O.; Bilginer, Y.; Bilgen, S.A.; Akdogan, A.; Kiraz, S.; et al. Assessment of the HScore for reactive haemophagocytic syndrome in patients with rheumatic diseases. Scand. J. Rheumatol. 2017, 46, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Valade, S.; Monseau, G.; Mariotte, E.; Darmon, M. Diagnostic Performance of Hemophagocytic Lymphohistiocytosis Criteria and HScore in Critically Ill Patients With Severe Hemophagocytic Syndrome. Crit. Care Med. 2021, 49, e874–e879. [Google Scholar] [CrossRef]
- Jongdee, P.; Julamanee, J.; Rattarittamrong, E.; Mukura, S.; Wanitpongpun, C.; Deoisares, R.; Surawong, A.; Chajuwan, T.; Chanswangphuwana, C. Prognostic Factors of Adult Hemophagocytic Lymphohistiocytosis and Clinical Utility of HLH-2004 Diagnostic Criteria and HScore: A Real-World Multicenter Study from Thailand. Acta Haematol. 2024, 147, 447–456. [Google Scholar] [CrossRef]
- Bakhtiari, K.; Meijers, J.C.; de Jonge, E.; Levi, M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit. Care Med. 2004, 32, 2416–2421. [Google Scholar] [CrossRef]
- Uchiumi, H.; Matsushima, T.; Yamane, A.; Doki, N.; Irisawa, H.; Saitoh, T.; Sakura, T.; Jimbo, T.; Handa, H.; Tsukamoto, N.; et al. Prevalence and clinical characteristics of acute myeloid leukemia associated with disseminated intravascular coagulation. Int. J. Hematol. 2007, 86, 137–142. [Google Scholar] [CrossRef]
- Libourel, E.J.; Klerk, C.P.W.; van Norden, Y.; de Maat, M.P.M.; Kruip, M.J.; Sonneveld, P.; Löwenberg, B.; Leebeek, F.W.G. Disseminated intravascular coagulation at diagnosis is a strong predictor for thrombosis in acute myeloid leukemia. Blood 2016, 128, 1854–1861. [Google Scholar] [CrossRef]
- Tobelem, G.; Jacquillat, C.; Chastang, C.; Auclerc, M.F.; Lechevallier, T.; Weil, M.; Daniel, M.T.; Flandrin, G.; Harrousseau, J.L.; Schaison, G.; et al. Acute monoblastic leukemia: A clinical and biologic study of 74 cases. Blood 1980, 55, 71–76. [Google Scholar] [CrossRef]
- Ku, G.H.; White, R.H.; Chew, H.K.; Harvey, D.J.; Zhou, H.; Wun, T. Venous thromboembolism in patients with acute leukemia: Incidence, risk factors, and effect on survival. Blood 2009, 113, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Olivi, M.; Di Biase, F.; Lanzarone, G.; Arrigo, G.; Martella, F.; Apolito, V.; Secreto, C.; Freilone, R.; Bruno, B.; Audisio, E.; et al. Thrombosis in Acute Myeloid Leukemia: Pathogenesis, Risk Factors and Therapeutic Challenges. Curr. Treat. Options Oncol. 2023, 24, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Kim, H.T.; Virtanen, J.M.; Parkkola, R.K.; Itälä-Remes, M.A.; Majhail, N.S.; Burns, L.J.; DeFor, T.; Trottier, B.; Platzbecker, U.; et al. Iron overload in allogeneic hematopoietic cell transplantation outcome: A meta-analysis. Biol. Blood Marrow Transpl. 2014, 20, 1248–1251. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, X.; Wang, H.; Chen, Y.; Chen, L.; Wu, P.; Wang, W. Effect of pre-transplantation serum ferritin on outcomes in patients undergoing allogeneic hematopoietic stem cell transplantation: A meta-analysis. Medicine 2018, 97, e10310. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Z.; An, T.; Zhao, L. The impact of iron chelation therapy on patients with lower/intermediate IPSS MDS and the prognostic role of elevated serum ferritin in patients with MDS and AML: A meta-analysis. Medicine 2019, 98, e17406. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Dong, A.; He, M.; Lin, X.; Yao, R.; Zhu, B.; Pan, X.; Jie, J. A Meta-Analysis for Effects of Elevated Pre-Transplantation Serum Ferritin on the Outcomes inPatients Undergoing Hematopoietic Stem Cell Transplantation. Cancer Investig. 2016, 34, 340–347. [Google Scholar] [CrossRef]
- Tachibana, T.; Takasaki, H.; Tanaka, M.; Maruta, A.; Hyo, R.; Ishigatsubo, Y.; Kanamori, H. Serum ferritin and disease status at transplantation predict the outcome of allo-SCT in patients with AML or myelodysplastic syndrome. Bone Marrow Transpl. 2011, 46, 150–151. [Google Scholar] [CrossRef]
- Cho, B.S.; Jeon, Y.W.; Hahn, A.R.; Lee, T.H.; Park, S.S.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; Kim, Y.J.; Lee, S.; et al. Improved survival outcomes and restoration of graft-vs-leukemia effect by deferasirox after allogeneic stem cell transplantation in acute myeloid leukemia. Cancer Med. 2019, 8, 501–514. [Google Scholar] [CrossRef]
- Artz, A.S.; Logan, B.; Zhu, X.; Akpek, G.; Bufarull, R.M.; Gupta, V.; Lazarus, H.M.; Litzow, M.; Loren, A.; Majhail, N.S.; et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016, 101, 1426–1433. [Google Scholar] [CrossRef]
- Wahlin, A.; Wahlin, A.; Lorenz, F.; Fredriksson, M.; Remberger, M.; Wahlin, B.E.; Hägglund, H. Hyperferritinemia is associated with low incidence of graft versus host disease, high relapse rate, and impaired survival in patients with blood disorders receiving allogeneic hematopoietic stem cell grafts. Med. Oncol. 2011, 28, 552–558. [Google Scholar] [CrossRef]
- Kataoka, K.; Nannya, Y.; Hangaishi, A.; Imai, Y.; Chiba, S.; Takahashi, T.; Kurokawa, M. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2009, 15, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Tanaka, M.; Numata, A.; Takasaki, H.; Ito, S.; Ohshima, R.; Hagihara, M.; Yamazaki, E.; Tomita, N.; Fujimaki, K.; et al. Pretransplant serum ferritin has a prognostic influence on allogeneic transplant regardless of disease risk. Leuk. Lymphoma 2012, 53, 456–461. [Google Scholar] [CrossRef]
- Armand, P.; Kim, H.T.; Rhodes, J.; Sainvil, M.M.; Cutler, C.; Ho, V.T.; Koreth, J.; Alyea, E.P.; Hearsey, D.; Neufeld, E.J.; et al. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol. Blood Marrow Transpl. 2011, 17, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Pullarkat, V.; Blanchard, S.; Tegtmeier, B.; Dagis, A.; Patane, K.; Ito, J.; Forman, S.J. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2008, 42, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Peczynski, C.; van der Werf, S.; Finke, J.; Ganser, A.; Schoemans, H.; Pavlu, J.; Niittyvuopio, R.; Schroyens, W.; Kaynar, L.; et al. Association of Serum Ferritin Levels Before Start of Conditioning With Mortality After alloSCT—A Prospective, Non-interventional Study of the EBMT Transplant Complications Working Party. Front. Immunol. 2020, 11, 586. [Google Scholar] [CrossRef]
- Meyer, S.C.; O’Meara, A.; Buser, A.S.; Tichelli, A.; Passweg, J.R.; Stern, M. Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biol. Blood Marrow Transpl. 2013, 19, 440–444. [Google Scholar] [CrossRef]
- Kanda, J.; Mizumoto, C.; Ichinohe, T.; Kawabata, H.; Saito, T.; Yamashita, K.; Kondo, T.; Takakura, S.; Ichiyama, S.; Uchiyama, T.; et al. Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2011, 46, 208–216. [Google Scholar] [CrossRef]
- Kong, S.G.; Jeong, S.; Lee, S.; Jeong, J.Y.; Kim, D.J.; Lee, H.S. Early transplantation-related mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia. BMC Cancer 2021, 21, 177. [Google Scholar] [CrossRef]
- Yokus, O.; Herek, C.; Cinli, T.A.; Goze, H.; Serin, I. Iron overload during the treatment of acute leukemia: Pretransplant transfusion experience. Int. J. Hematol. Oncol. 2021, 10, IJH36. [Google Scholar] [CrossRef]
- Tachibana, T.; Tanaka, M.; Takasaki, H.; Numata, A.; Ito, S.; Watanabe, R.; Hyo, R.; Ohshima, R.; Hagihara, M.; Sakai, R.; et al. Pretransplant serum ferritin is associated with bloodstream infections within 100 days of allogeneic stem cell transplantation for myeloid malignancies. Int. J. Hematol. 2011, 93, 368–374. [Google Scholar] [CrossRef]
- Pan, W.; Teng, Q.; Chen, H.; Hu, L.; Yue, X.; Qian, Z.; Dong, R.; Zhou, H.; Zhao, X.; Xiao, H.; et al. Association between the pre-transplantation serum ferritin level and outcomes of hematopoietic stem cell transplantation: A systematic review and meta-analysis. Heliyon 2024, 10, e37436. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Stanworth, S.J.; Dennis, J.A.; Trivella, M.; Roubinian, N.; Fergusson, D.A.; Triulzi, D.; Dorée, C.; Hébert, P.C. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst. Rev. 2021, 12, CD002042. [Google Scholar] [PubMed]
- Carson, J.L.; Stanworth, S.J.; Guyatt, G.; Valentine, S.; Dennis, J.; Bakhtary, S.; Cohn, C.S.; Dubon, A.; Grossman, B.J.; Gupta, G.K.; et al. Red Blood Cell Transfusion: 2023 AABB International Guidelines. JAMA 2023, 330, 1892–1902. [Google Scholar] [CrossRef]
- Byun, J.M.; Park, W.; Shin, D.Y.; Koh, Y.; Kim, I.; Yoon, S.S.; Park, J.H.; Kim, H.; Hong, J. A pilot randomized study for optimal red cell transfusion in acute myeloid leukemia patients with intensive chemotherapy. Blood Transfus. 2023, 21, 479–487. [Google Scholar]
- Tay, J.; Allan, D.S.; Chatelain, E.; Coyle, D.; Elemary, M.; Fulford, A.; Petrcich, W.; Ramsay, T.; Walker, I.; Xenocostas, A.; et al. Liberal Versus Restrictive Red Blood Cell Transfusion Thresholds in Hematopoietic Cell Transplantation: A Randomized, Open Label, Phase III, Noninferiority Trial. J. Clin. Oncol. 2020, 38, 1463–1473. [Google Scholar] [CrossRef]
- DeZern, A.E.; Williams, K.; Zahurak, M.; Hand, W.; Stephens, R.S.; King, K.E.; Frank, S.M.; Ness, P.M. Red blood cell transfusion triggers in acute leukemia: A randomized pilot study. Transfusion 2016, 56, 1750–1757. [Google Scholar] [CrossRef]
- Ballo, O.; Fleckenstein, P.; Eladly, F.; Kreisel, E.M.; Stratmann, J.; Seifried, E.; Müller, M.; Serve, H.; Bug, G.; Bonig, H.; et al. Reducing the red blood cell transfusion threshold from 8·0 g/dL to 7·0 g/dL in acute myeloid leukaemia patients undergoing induction chemotherapy reduces transfusion rates without adversely affecting patient outcome. Vox Sang. 2020, 115, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liang, W.; Chen, X.; Chen, L.; Yang, X.; Yan, Z.; Wang, W. Pretransplant C-reactive protein as a prognostic marker in allogeneic stem cell transplantation: A PRISMA-compliant meta-analysis. Medicine 2019, 98, e14474. [Google Scholar] [CrossRef]
- Tvedt, T.H.; Lie, S.A.; Reikvam, H.; Rye, K.P.; Lindås, R.; Gedde-Dahl, T.; Ahmed, A.B.; Bruserud, Ø. Pretransplant Levels of CRP and Interleukin-6 Family Cytokines; Effects on Outcome after Allogeneic Stem Cell Transplantation. Int. J. Mol. Sci. 2016, 17, 1823. [Google Scholar] [CrossRef]
- Wang, K.; Jian, X.; Xu, Z.; Wang, H. Pre-transplant CRP-albumin ratio as a biomarker in patients receiving haploidentical allogeneic hematopoietic transplantation: Developing a novel DRCI-based nomogram. Front. Immunol. 2023, 14, 1128982. [Google Scholar]
- Miyazaki, T.; Tachibana, T.; Suzuki, T.; Izumi, A.; Fujimaki, K.; Sato, S.; Tamai, Y.; Michishita, Y.; Suzuki, T.; Ishii, R.; et al. Pretransplantation Inflammatory and Nutritional Status in Elderly Allogeneic Hematopoietic Stem Cell Transplantation: Prognostic Value of C-Reactive Protein-to-Albumin Ratio. Transpl. Cell. Ther. 2024, 30, 400.e1–400.e9. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Radujkovic, A.; Stölzel, F.; Falk, C.S.; Benner, A.; Schaich, M.; Bornhäuser, M.; Ehninger, G.; Krämer, A.; Hegenbart, U.; et al. Pretransplant metabolic distress predicts relapse of acute myeloid leukemia after allogeneic stem cell transplantation. Transplantation 2015, 99, 1065–1071. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Huang, T.S.; Glenjen, N.; Gjertsen, B.T.; Foss, B. Leptin in human acute myelogenous leukemia: Studies of in vivo levels and in vitro effects on native functional leukemia blasts. Haematologica 2002, 87, 584–595. [Google Scholar]
- Cabantchik, Z.I.; Breuer, W.; Zanninelli, G.; Cianciulli, P. LPI-labile plasma iron in iron overload. Best Pract. Res. Clin. Haematol. 2005, 18, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Wermke, M.; Eckoldt, J.; Götze, K.S.; Klein, S.A.; Bug, G.; de Wreede, L.C.; Kramer, M.; Stölzel, F.; von Bonin, M.; Schetelig, J.; et al. Enhanced labile plasma iron and outcome in acute myeloid leukaemia and myelodysplastic syndrome after allogeneic haemopoietic cell transplantation (ALLIVE): A prospective, multicentre, observational trial. Lancet Haematol. 2018, 5, e201–e210. [Google Scholar] [CrossRef]
- Duca, L.; Cappellini, M.D.; Baronciani, D.; Pilo, F.; Targhetta, C.; Visani, G.; Nava, I.; Angelucci, E. Non-transferrin-bound iron and oxidative stress during allogeneic hemopoietic stem cell transplantation in patients with or without iron overload. Am. J. Hematol. 2018, 93, E250–E252. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Rouault, T.A.; Maio, N. Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J. Biol. Chem. 2017, 292, 12744–12753. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Dardi, M.; Cozzi, A.; Santambrogio, P. Mitochondrial Ferritin: Its Role in Physiological and Pathological Conditions. Cells 2021, 10, 1969. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Mancini, M.; De Santis, S.; Monaldi, C.; Cavo, M.; Soverini, S. The Role of Hypoxic Bone Marrow Microenvironment in Acute Myeloid Leukemia and Future Therapeutic Opportunities. Int. J. Mol. Sci. 2021, 22, 6857. [Google Scholar] [CrossRef]
- Yi, W.; Zhang, J.; Huang, Y.; Zhan, Q.; Zou, M.; Cheng, X.; Zhang, X.; Yin, Z.; Tao, S.; Cheng, H.; et al. Ferritin-mediated mitochondrial iron homeostasis is essential for the survival of hematopoietic stem cells and leukemic stem cells. Leukemia 2024, 38, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, P.; Liu, J.; Zhu, S.; Kroemer, G.; Klionsky, D.J.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 2019, 5, eaaw2238. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Ye, C.L.; Du, Y.; Yu, X.; Chen, Z.Y.; Wang, L.; Zheng, Y.F.; Liu, X.H. STEAP3 Affects Ferroptosis and Progression of Renal Cell Carcinoma Through the p53/xCT Pathway. Technol. Cancer Res. Treat. 2022, 21, 15330338221078728. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, Q.; Xu, Z.; Huang, J.; Chen, X.; Cai, Y.; Peng, B.; Yi, Q. DownregulatedFerroptosis-Related Gene STEAP3 as a Novel Diagnostic and Prognostic Target for Hepatocellular Carcinoma and Its Roles in Immune Regulation. Front. Cell Dev. Biol. 2021, 9, 743046. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Paubelle, E.; Bérard, E.; Saland, E.; Thomas, X.; Tavitian, S.; Larcher, M.V.; Vergez, F.; Delabesse, E.; Sarry, A.; et al. Ferritin heavy/light chain (FTH1/FTL) expression, serum ferritin levels, and their functional as well as prognostic roles in acute myeloid leukemia. Eur. J. Haematol. 2019, 102, 131–142. [Google Scholar] [CrossRef]
- Vela, D.; Vela-Gaxha, Z. Differential regulation of hepcidin in cancer and non-cancer tissues and its clinical implications. Exp. Mol. Med. 2018, 50, e436. [Google Scholar] [CrossRef]
- Khoshtabiat, L.; Mahdavi, M.; Dehghan, G.; Rashidi, M.R. Oxidative Stress-Induced Apoptosis in Chronic Myelogenous Leukemia K562 Cells by an Active Compound from the Dithio- Carbamate Family. Asian Pac. J. Cancer Prev. 2016, 17, 4267–4273. [Google Scholar]
- Kaweme, N.M.; Zhou, S.; Changwe, G.J.; Zhou, F. The significant role of redox system in myeloid leukemia: From pathogenesis to therapeutic applications. Biomark. Res. 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Rivera-Del Valle, N.; Chandra, J. Redox control of leukemia: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 1349–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Shen, Q.; Claret, F.X. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J. Leukoc. Biol. 2013, 94, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sillar, J.R.; Germon, Z.P.; DeIuliis, G.N.; Dun, M.D. The Role of Reactive Oxygen Species in Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2019, 20, 6003. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lai, Y.S.; Tsai, H.J.; Kuo, C.C.; Yen, B.L.; Yeh, S.P.; Sun, H.S.; Hung, W.C. The oncometabolite R-2-hydroxyglutarate activates NF-κB-dependent tumor-promoting stromal niche for acute myeloid leukemia cells. Sci. Rep. 2016, 6, 32428. [Google Scholar] [CrossRef]
- Pabst, T.; Kortz, L.; Fiedler, G.M.; Ceglarek, U.; Idle, J.R.; Beyoğlu, D. The plasma lipidome in acute myeloid leukemia at diagnosis in relation to clinical disease features. BBA Clin. 2017, 7, 105–114. [Google Scholar] [CrossRef]
- Godfrey, R.; Arora, D.; Bauer, R.; Stopp, S.; Müller, J.P.; Heinrich, T.; Böhmer, S.A.; Dagnell, M.; Schnetzke, U.; Scholl, S.; et al. Cell transformation by FLT3 ITD in acute myeloid leukemia involves oxidative inactivation of the tumor suppressor protein-tyrosine phosphatase DEP-1/PTPRJ. Blood 2012, 119, 4499–4511. [Google Scholar] [CrossRef]
- Reddy, M.M.; Fernandes, M.S.; Salgia, R.; Levine, R.L.; Griffin, J.D.; Sattler, M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia 2011, 25, 281–289. [Google Scholar] [CrossRef][Green Version]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Jin, P.; Xiao, X.; Shen, J.; Li, R.; Zhang, Y.; Li, X.; Xue, K.; Li, J. Identification and validation of a novel CD8+ T cell-associated prognostic model based on ferroptosis in acute myeloid leukemia. Front. Immunol. 2023, 14, 1149513. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tian, S.; Zhang, P.; Zhang, N.; Shen, Y.; Deng, J. Construction and Validation of a Novel Ferroptosis-Related Prognostic Model for Acute Myeloid Leukemia. Front. Genet. 2022, 12, 708699. [Google Scholar] [CrossRef]
- Han, C.; Zheng, J.; Li, F.; Guo, W.; Cai, C. Novel Prognostic Signature for Acute Myeloid Leukemia: Bioinformatics Analysis of Combined CNV-Driven and Ferroptosis-Related Genes. Front. Genet. 2022, 13, 849437. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, D.; Ye, X.; Jin, J. A novel ferroptosis-related gene signature can predict prognosis and influence immune microenvironment in acute myeloid leukemia. Bosn. J. Basic Med. Sci. 2022, 22, 608–628. [Google Scholar]
- Yu, Y.; Xie, Y.; Cao, L.; Yang, L.; Yang, M.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell. Oncol. 2015, 2, e1054549. [Google Scholar] [CrossRef]
- Essmann, S.; Heestermans, M.; Dadkhah, A.; Janson, D.; Wolschke, C.; Ayuk, F.; Kröger, N.M.; Langebrake, C. Iron Chelation with Deferasirox Suppresses the Appearance of Labile Plasma Iron During Conditioning Chemotherapy Prior to Allogeneic Stem Cell Transplantation. Transpl. Cell. Ther. 2023, 29, 42.e1–42.e6. [Google Scholar] [CrossRef]
- Majhail, N.S.; Lazarus, H.M.; Burns, L.J. A prospective study of iron overload management in allogeneic hematopoietic cell transplantation survivors. Biol. Blood Marrow Transpl. 2010, 16, 832–837. [Google Scholar] [CrossRef]
- Jaekel, N.; Lieder, K.; Albrecht, S.; Leismann, O.; Hubert, K.; Bug, G.; Kröger, N.; Platzbecker, U.; Stadler, M.; de Haas, K.; et al. Efficacy and safety of deferasirox in non-thalassemic patients with elevated ferritin levels after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2016, 51, 89–95. [Google Scholar] [CrossRef]
- Vallejo, C.; Batlle, M.; Vázquez, L.; Solano, C.; Sampol, A.; Duarte, R.; Hernández, D.; López, J.; Rovira, M.; Jiménez, S.; et al. Phase IV open-label study of the efficacy and safety of deferasirox after allogeneic stem cell transplantation. Haematologica 2014, 99, 1632–1637. [Google Scholar] [CrossRef]
- Tachibana, T.; Kanda, J.; Machida, S.; Saito, T.; Tanaka, M.; Najima, Y.; Koyama, S.; Miyazaki, T.; Yamamoto, E.; Takeuchi, M.; et al. Deferasirox for the treatment of iron overload after allogeneic hematopoietic cell transplantation: Multicenter phase I study (KSGCT1302). Int. J. Hematol. 2018, 107, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Taetle, R.; Honeysett, J.M.; Trowbridge, I. Effects of anti-transferrin receptor antibodies on growth of normal and malignant myeloid cells. Int. J. Cancer 1983, 32, 343–349. [Google Scholar] [CrossRef]
- Callens, C.; Moura, I.C.; Lepelletier, Y.; Coulon, S.; Renand, A.; Dussiot, M.; Ghez, D.; Benhamou, M.; Monteiro, R.C.; Bazarbachi, A.; et al. Recent advances in adult T-cell leukemia therapy: Focus on a new anti-transferrin receptor monoclonal antibody. Leukemia 2008, 22, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Crépin, R.; Goenaga, A.L.; Jullienne, B.; Bougherara, H.; Legay, C.; Benihoud, K.; Marks, J.D.; Poul, M.A. Development of human single-chain antibodies to the transferrin receptor that effectively antagonize the growth of leukemias and lymphomas. Cancer Res. 2010, 70, 5497–5506. [Google Scholar] [CrossRef] [PubMed]
- Neiveyans, M.; Melhem, R.; Arnoult, C.; Bourquard, T.; Jarlier, M.; Busson, M.; Laroche, A.; Cerutti, M.; Pugnière, M.; Ternant, D.; et al. A recycling anti-transferrin receptor-1 monoclonal antibody as an efficient therapy for erythroleukemia through target up-regulation and antibody-dependent cytotoxic effector functions. mAbs 2019, 11, 593–605. [Google Scholar] [CrossRef]
- Brooks, D.; Taylor, C.; Dos Santos, B.; Linden, H.; Houghton, A.; Hecht, T.T.; Kornfeld, S.; Taetle, R. Phase Ia trial of murine immunoglobulin A antitransferrin receptor antibody 42/6. Clin. Cancer Res. 1995, 1, 1259–1265. [Google Scholar]
- Modi, N.B.; Shames, R.; Lickliter, J.D.; Gupta, S. Pharmacokinetics, pharmacodynamics, and tolerability of an aqueous formulation of rusfertide (PTG-300), a hepcidin mimetic, in healthy volunteers: A double-blind first-in-human study. Eur. J. Haematol. 2024, 113, 340–350. [Google Scholar] [CrossRef]
- Modi, N.B.; Khanna, S.; Rudraraju, S.; Valone, F. Pharmacokinetics and Pharmacodynamics of Rusfertide, a Hepcidin Mimetic, Following Subcutaneous Administration of a Lyophilized Powder Formulation in Healthy Volunteers. Drugs R&D 2024, 24, 539–552. [Google Scholar]
- Vadhan-Raj, S.; Abonour, R.; Goldman, J.W.; Smith, D.A.; Slapak, C.A.; Ilaria, R.L., Jr.; Tiu, R.V.; Wang, X.; Callies, S.; Cox, J.; et al. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J. Hematol. Oncol. 2017, 10, 73. [Google Scholar] [CrossRef]
- Zhao, N.; Nizzi, C.P.; Anderson, S.A.; Wang, J.; Ueno, A.; Tsukamoto, H.; Eisenstein, R.S.; Enns, C.A.; Zhang, A.S. Low intracellular iron increases the stability of matriptase-2. J. Biol. Chem. 2015, 290, 4432–4446. [Google Scholar] [CrossRef]
- Wahedi, M.; Wortham, A.M.; Kleven, M.D.; Zhao, N.; Jue, S.; Enns, C.A.; Zhang, A.S. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J. Biol. Chem. 2017, 292, 18354–18371. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J.; Toudjarska, I.; Sendamarai, A.K.; Racie, T.; Milstein, S.; Bettencourt, B.R.; Hettinger, J.; Bumcrot, D.; Fleming, M.D. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood 2013, 121, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.M.; Maurer, E.; Lülsdorff, V.; Wilms, A.; Furtmann, N.; Bajorath, J.; Gütschow, M.; Stirnberg, M. En Route to New Therapeutic Options for Iron Overload Diseases: Matriptase-2 as a Target for Kunitz-Type Inhibitors. ChemBioChem 2016, 17, 595–604. [Google Scholar] [CrossRef]
- Zhang, A.S.; Gao, J.; Koeberl, D.D.; Enns, C.A. The role of hepatocyte hemojuvelin in the regulation of bone morphogenic protein-6 and hepcidin expression in vivo. J. Biol. Chem. 2010, 285, 16416–16423. [Google Scholar] [CrossRef]
- Sheetz, M.; Barrington, P.; Callies, S.; Berg, P.H.; McColm, J.; Marbury, T.; Decker, B.; Dyas, G.L.; Truhlar, S.M.E.; Benschop, R.; et al. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br. J. Clin. Pharmacol. 2019, 85, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Shang, Y.; Xu, N.; Liu, B.; Ma, Y.; Li, H.; Chen, J.; Yao, Q. HDAC inhibitor enhances ferroptosis susceptibility of AML cells by stimulating iron metabolism. Cell. Signal. 2025, 127, 111583. [Google Scholar] [CrossRef]
- Hole, P.S.; Zabkiewicz, J.; Munje, C.; Newton, Z.; Pearn, L.; White, P.; Marquez, N.; Hills, R.K.; Burnett, A.K.; Tonks, A.; et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood 2013, 122, 3322–3330. [Google Scholar] [CrossRef]
- Sun, J.; Xia, F.; Zhang, S.; Zhang, B.; Guan, Y.; Hu, X.; Xue, P.; Yang, S.; Zhou, Y.; Ling, D.; et al. A Selective Nano Cell Cycle Checkpoint Inhibitor Overcomes Leukemia Chemoresistance. Small 2023, 19, e2300736. [Google Scholar] [CrossRef]
- Zhang, S.; Lou, S.; Bian, W.; Liu, J.; Wang, R.; Wang, Y.; Zhao, Y.; Zou, X.; Jin, D.; Liang, Y.; et al. Selective eradication of venetoclax-resistant monocytic acute myeloid leukemia with iron oxide nanozymes. Biochem. Biophys. Res. Commun. 2024, 719, 150117. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, W.; Tan, R.; Zhang, M.; Zhong, T.; Wang, J.; Chen, H.; Fang, X. Ferroptosis: Potential therapeutic targets and prognostic predictions for acute myeloid leukemia (Review). Oncol. Lett. 2024, 28, 574. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Benthani, F.A.; Wu, J.; Liang, D.; Bian, Z.X.; Jiang, X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020, 27, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W.; et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 2019, 131, 356–369. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Huang, Z.X.; Chen, G.Q.; Sheng, F.; Zheng, Y.S. Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy. Biochem. Biophys. Res. Commun. 2019, 516, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Bao, J.; Zhang, M.J.; Li, L.L.; Xu, X.L.; Chen, H.; Feng, Y.B.; Peng, X.Q.; Chen, F.H. Targeting ferroptosis contributes to ATPR-induced AML differentiation via ROS-autophagy-lysosomal pathway. Gene 2020, 755, 144889. [Google Scholar] [CrossRef]
- Rushworth, S.A.; Zaitseva, L.; Murray, M.Y.; Shah, N.M.; Bowles, K.M.; MacEwan, D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood 2012, 120, 5188–5198. [Google Scholar] [CrossRef]
- Hu, T.; Pan, C.; Zhang, T.; Ni, M.; Wang, W.; Zhang, S.; Chen, Y.; Wang, J.; Fang, Q. Nrf2 overexpression increases the resistance of acute myeloid leukemia to cytarabine by inhibiting replication factor C4. Cancer Gene Ther. 2022, 29, 1773–1790. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Tan, J.; Li, Y.; Yang, P.; Liu, X.; Lai, J.; Zhang, Y.; Cai, L.; Gu, Y.; et al. Inhibition of NRF2 enhances the acute myeloid leukemia cell death induced by venetoclax via the ferroptosis pathway. Cell Death Discov. 2024, 10, 35. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, S.; Qiu, K.; Chen, X.; Wu, W.; Zheng, J.; Liu, Y.; Wu, H.; Fan, S.; Nie, D.; et al. Targeting NRF2 uncovered an intrinsic susceptibility of acute myeloid leukemia cells to ferroptosis. Exp. Hematol. Oncol. 2023, 12, 47. [Google Scholar] [CrossRef]
- Di Francesco, B.; Verzella, D.; Capece, D.; Vecchiotti, D.; Di Vito Nolfi, M.; Flati, I.; Cornice, J.; Di Padova, M.; Angelucci, A.; Alesse, E.; et al. NF-κB: A Druggable Target in Acute Myeloid Leukemia. Cancers 2022, 14, 3557. [Google Scholar] [CrossRef]
- Nishizawa, H.; Yamanaka, M.; Igarashi, K. Ferroptosis: Regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 2023, 290, 1688–1704. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, M.J.; Li, L.L.; Xu, X.L.; Chen, H.; Feng, Y.B.; Li, Y.; Peng, X.Q.; Chen, F.H. ATPR triggers acute myeloid leukaemia cells differentiation and cycle arrest via the RARα/LDHB/ERK-glycolysis signalling axis. J. Cell. Mol. Med. 2020, 24, 6952–6965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, Y.; Hong, X.; Wu, W.; Lin, Y.; Lin, L.; Xue, Y.; Lin, D. Exogenous dihomo-γ-linolenic acid triggers ferroptosis via ACSL4-mediated lipid metabolic reprogramming in acute myeloid leukemia cells. Transl. Oncol. 2025, 52, 102227. [Google Scholar] [CrossRef]
- Bruedigam, C.; Porter, A.H.; Song, A.; Vroeg In de Wei, G.; Stoll, T.; Straube, J.; Cooper, L.; Cheng, G.; Kahl, V.F.S.; Sobinoff, A.P.; et al. Imetelstat-mediated alterations in fatty acid metabolism to induce ferroptosis as a therapeutic strategy for acute myeloid leukemia. Nat. Cancer 2024, 5, 47–65. [Google Scholar] [CrossRef]
- Yu, Y.; Meng, Y.; Xu, X.; Tong, T.; He, C.; Wang, L.; Wang, K.; Zhao, M.; You, X.; Zhang, W.; et al. A Ferroptosis-Inducing and Leukemic Cell-Targeting Drug Nanocarrier Formed by Redox-Responsive Cysteine Polymer for Acute Myeloid Leukemia Therapy. ACS Nano 2023, 17, 3334–3345. [Google Scholar] [CrossRef]
- Akiyama, H.; Zhao, R.; Ostermann, L.B.; Li, Z.; Tcheng, M.; Yazdani, S.J.; Moayed, A.; Pryor, M.L., 2nd; Slngh, S.; Baran, N.; et al. Mitochondrial regulation of GPX4 inhibition-mediated ferroptosis in acute myeloid leukemia. Leukemia 2024, 38, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Visnjic, D.; Dembitz, V.; Lalic, H. The Role of AMPK/mTOR Modulators in the Therapy of Acute Myeloid Leukemia. Curr. Med. Chem. 2019, 26, 2208–2229. [Google Scholar] [CrossRef]
- Du, W.; Xu, A.; Huang, Y.; Cao, J.; Zhu, H.; Yang, B.; Shao, X.; He, Q.; Ying, M. The role of autophagy in targeted therapy for acute myeloid leukemia. Autophagy 2021, 17, 2665–2679. [Google Scholar] [CrossRef]
- Lai, X.; Sun, Y.; Zhang, X.; Wang, D.; Wang, J.; Wang, H.; Zhao, Y.; Liu, X.; Xu, X.; Song, H.; et al. Honokiol Induces Ferroptosis by Upregulating HMOX1 in Acute Myeloid Leukemia Cells. Front. Pharmacol. 2022, 13, 897791. [Google Scholar] [CrossRef]
- Li, H.Y.; Ye, H.G.; Chen, C.Q.; Yin, L.H.; Wu, J.B.; He, L.C.; Gao, S.M. Honokiol induces cell cycle arrest and apoptosis via inhibiting class I histone deacetylases in acute myeloid leukemia. J. Cell. Biochem. 2015, 116, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, H.; Xing, C.; Ye, H.; Feng, J.; Wu, J.; Lu, Z.; Fang, J.; Gao, S. Honokiol induces proteasomal degradation of AML1-ETO oncoprotein via increasing ubiquitin conjugase UbcH8 expression in leukemia. Biochem. Pharmacol. 2017, 128, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Yu, Z.; Wu, J.; Yu, K.; Hong, G.; Lu, Z.; Gao, S. Honokiol Inhibits Constitutive and Inducible STAT3 Signaling via PU.1-Induced SHP1 Expression in Acute Myeloid Leukemia Cells. Tohoku J. Exp. Med. 2015, 237, 163–172. [Google Scholar] [CrossRef]
- Chen, Z.G.; Xie, Y.T.; Yang, C.; Xiao, T.; Chen, S.Y.; Wu, J.H.; Guo, Q.N.; Gao, L. M2 macrophages secrete CCL20 to regulate iron metabolism and promote daunorubicin resistance in AML cells. Life Sci. 2025, 361, 123297. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Ryningen, A.; Olsnes, A.M.; Stordrange, L.; Øyan, A.M.; Kalland, K.H.; Gjertsen, B.T. Subclassification of patients with acute myelogenous leukemia based on chemokine responsiveness and constitutive chemokine release by their leukemic cells. Haematologica 2007, 92, 332–341. [Google Scholar] [CrossRef]
- Pourcelot, E.; El Samra, G.; Mossuz, P.; Moulis, J.M. Molecular Insight into Iron Homeostasis of Acute Myeloid Leukemia Blasts. Int. J. Mol. Sci. 2023, 24, 14307. [Google Scholar] [CrossRef]
- Nepstad, I.; Hatfield, K.J.; Grønningsæter, I.S.; Aasebø, E.; Hernandez-Valladares, M.; Hagen, K.M.; Rye, K.P.; Berven, F.S.; Selheim, F.; Reikvam, H.; et al. Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct. Target. Ther. 2019, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Tsykunova, G.; Hernandez-Valladares, M.; Reikvam, H.; Tvedt, T.H.A. Therapeutic Use of Valproic Acid and All-Trans Retinoic Acid in Acute Myeloid Leukemia-Literature Review and Discussion of Possible Use in Relapse after Allogeneic Stem Cell Transplantation. Pharmaceuticals 2021, 14, 423. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Pawlowska, N.; Thomas, S.; Olin, R.; Logan, A.C.; Damon, L.E.; Martin, T.; Kang, M.; Sayre, P.H.; Boyer, W.; et al. Histone Deacetylase Inhibition with Panobinostat Combined with Intensive Induction Chemotherapy in Older Patients with Acute Myeloid Leukemia: Phase I Study Results. Clin. Cancer Res. 2019, 25, 4917–4923. [Google Scholar] [CrossRef]
- Larrue, C.; Mouche, S.; Angelino, P.; Sajot, M.; Birsen, R.; Kosmider, O.; Mckee, T.; Vergez, F.; Recher, C.; Mas, V.M.; et al. Targeting ferritinophagy impairs quiescent cancer stem cells in acute myeloid leukemia in vitro and in vivo models. Sci. Transl. Med. 2024, 16, eadk1731. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sandnes, M.; Ulvik, R.J.; Vorland, M.; Reikvam, H. Hyperferritinemia-A Clinical Overview. J. Clin. Med. 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Maywald, O.; Buchheidt, D.; Bergmann, J.; Schoch, C.; Ludwig, W.D.; Reiter, A.; Hastka, J.; Lengfelder, E.; Hehlmann, R. Spontaneous remission in adult acute myeloid leukemia in association with systemic bacterial infection-case report and review of the literature. Ann. Hematol. 2004, 83, 189–194. [Google Scholar] [PubMed]
- Zeng, Q.; Yuan, Y.; Li, P.; Chen, T. Spontaneous remission in patients with acute myeloid leukemia with t(8;21) or cutaneous myeloid sarcoma: Two case reports and a review of the literature. Intern. Med. 2013, 52, 1227–1233. [Google Scholar] [CrossRef][Green Version]
- Imataki, O.; Ishida, T.; Kida, J.I.; Uemura, M.; Fujita, H.; Kadowaki, N. Repeated spontaneous remission of acute myeloid leukemia in response to various infections: A case report. BMC Infect. Dis. 2023, 23, 215. [Google Scholar] [CrossRef]
- Reikvam, H.; Hatfield, K.; Sandnes, M.; Bruserud, Ø. Future biomarkers for acute graft-versus-host disease: Potential roles of nucleic acids, metabolites, and immune cell markers. Expert Rev. Clin. Immunol. 2024, 21, 305–321. [Google Scholar] [CrossRef]
- Yang, X.; Pu, X.; Xu, Y.; Zhao, J.; Fang, X.; Cui, J.; Deng, G.; Liu, Y.; Zhu, L.; Shao, M.; et al. A novel prognosis evaluation indicator of patients with sepsis created by integrating six microfluidic-based neutrophil chemotactic migration parameters. Talanta 2025, 281, 126801. [Google Scholar] [CrossRef]
- Moreno-Castaño, A.B.; Salas, M.Q.; Palomo, M.; Martinez-Sanchez, J.; Rovira, M.; Fernández-Avilés, F.; Martínez, C.; Cid, J.; Castro, P.; Escolar, G.; et al. Early vascular endothelial complications after hematopoietic cell transplantation: Role of the endotheliopathy in biomarkers and target therapies development. Front. Immunol. 2022, 13, 1050994. [Google Scholar] [CrossRef]
- Eftychidis, I.; Sakellari, I.; Anagnostopoulos, A.; Gavriilaki, E. Endothelial dysfunction and vascular complications after allogeneic hematopoietic cell transplantation: An expert analysis. Expert Rev. Hematol. 2021, 14, 831–840. [Google Scholar] [CrossRef]
- Grishina, O.; Schmoor, C.; Döhner, K.; Hackanson, B.; Lubrich, B.; May, A.M.; Cieslik, C.; Müller, M.J.; Lübbert, M. DECIDER: Prospective randomized multicenter phase II trial of low-dose decitabine (DAC) administered alone or in combination with the histone deacetylase inhibitor valproic acid (VPA) and all-trans retinoic acid (ATRA) in patients > 60 years with acute myeloid leukemia who are ineligible for induction chemotherapy. BMC Cancer 2015, 15, 430. [Google Scholar]
- Bresser, H.; Schmoor, C.; Grishina, O.; Pfeifer, D.; Thomas, J.; Rehman, U.U.; Crysandt, M.; Jost, E.; Thol, F.; Heuser, M.; et al. Impact of TP53 Mutation Status in Elderly AML Patients When Adding All-Trans Retinoic Acid or Valproic Acid to Decitabine. Eur. J. Haematol. 2025, 114, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.B.S.; Hafezi, N.; Sanaei, M.J.; Jafari-Raddani, F.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/AKT/mTOR signaling pathway in breast cancer: Review of clinical trials and latest advances. Cell Biochem. Funct. 2024, 42, e3998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, G.; He, J.; Yan, X.; Fan, K. Ferritin nanocage: A promising and designable multi-module platform for constructing dynamic nanoassembly-based drug nanocarrier. Adv. Drug Deliv. Rev. 2021, 176, 113892. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Gao, L.; Yan, X. Human ferritin for tumor detection and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 287–298. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Knez, M. Comparison of two endogenous delivery agents in cancer therapy: Exosomes and ferritin. Pharmacol. Res. 2016, 110, 1–9. [Google Scholar] [CrossRef]
- Bitonto, V.; Alberti, D.; Ruiu, R.; Aime, S.; Geninatti Crich, S.; Cutrin, J.C. L-ferritin: A theranostic agent of natural origin for MRI visualization and treatment of breast cancer. J. Control Release 2020, 319, 300–310. [Google Scholar] [CrossRef]

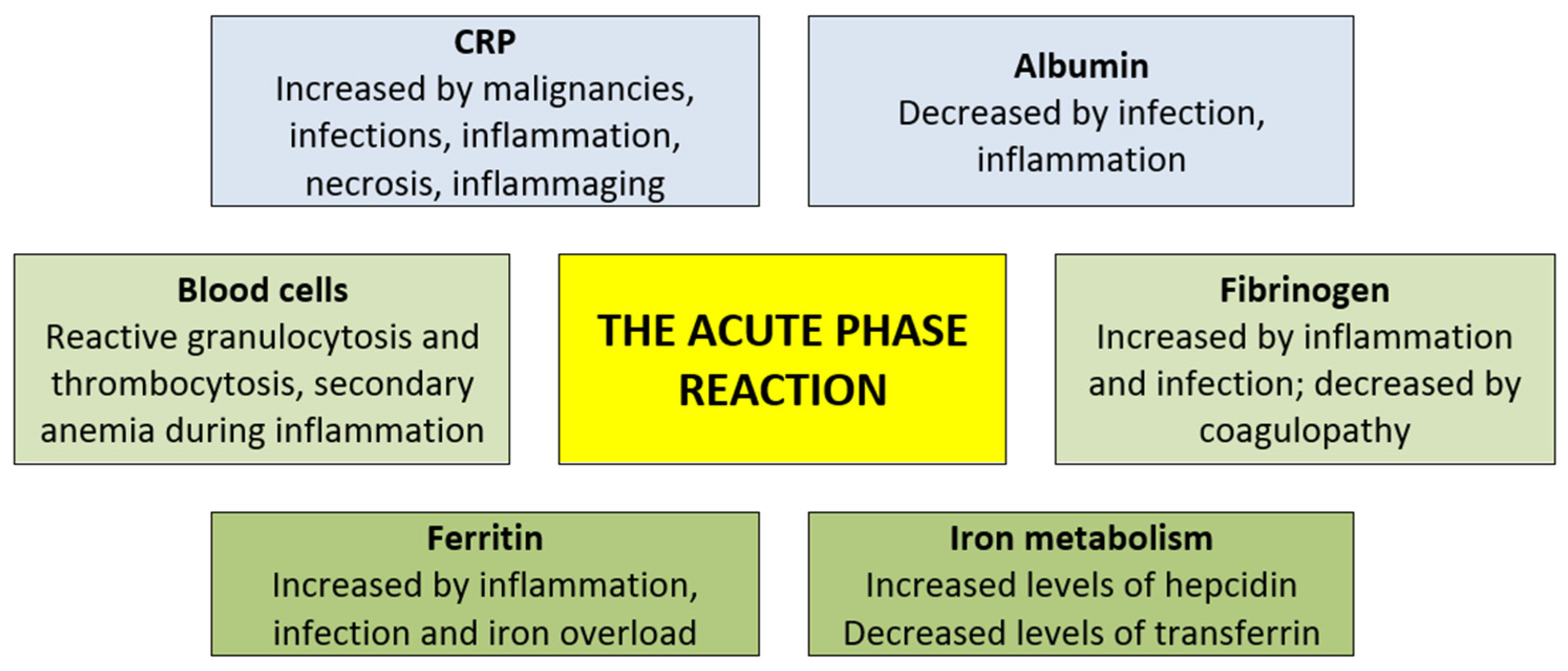
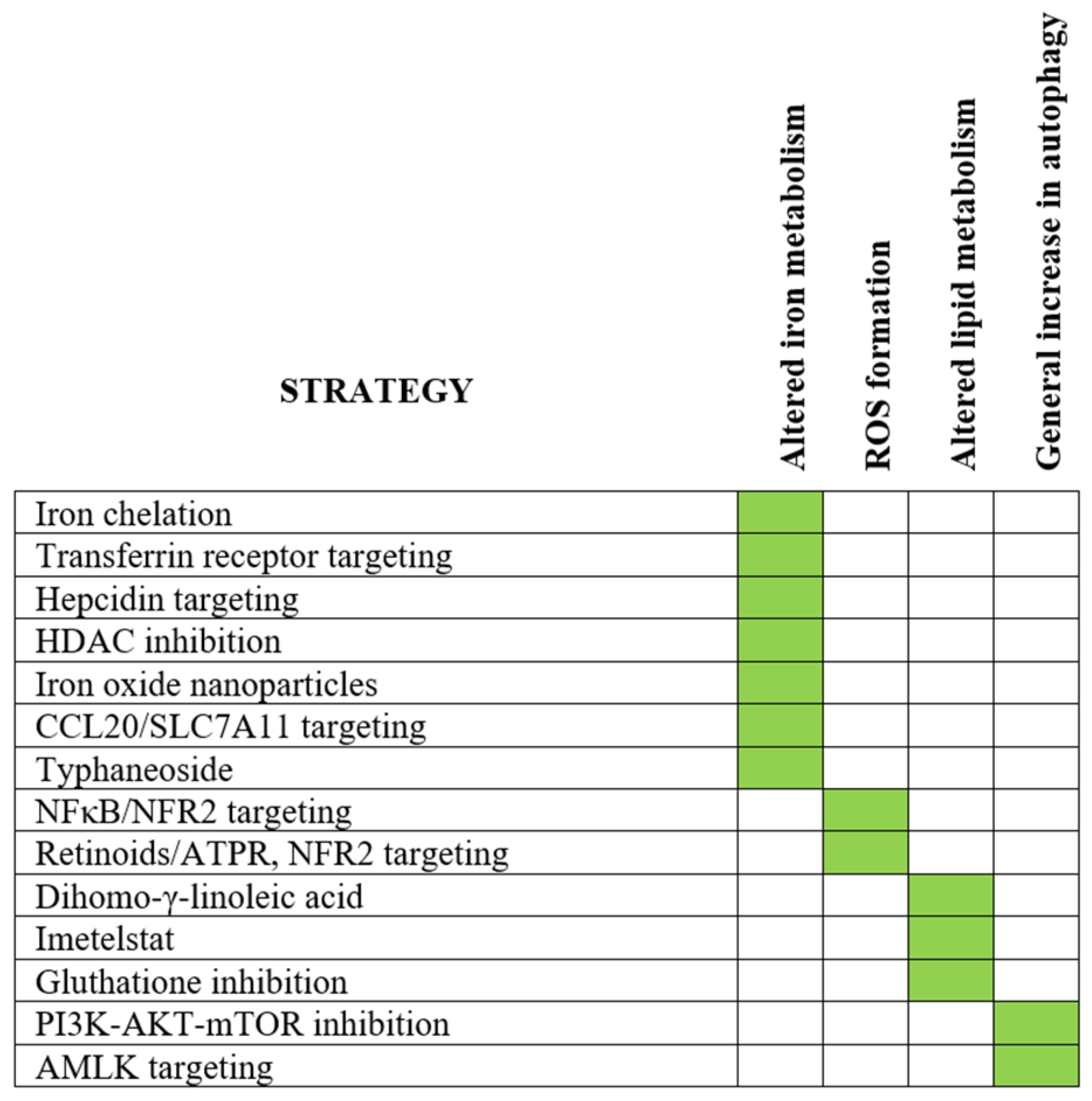
| Molecular Structure | Cellular Expression | Distribution |
|---|---|---|
| Ferritin chains/subunits: Ferritin light chain (FRL; weight 10 kDa). Ferritin heavy chain (FRH; weight 21 kDa). 55% homology between the chains Similar three-dimensional structure. Encoding genes: Ferritin light-chain chromosome 11q Ferritin heavy-chain chromosome 19q Molecular structure of ferritin: A spherical macromolecule formed by 24 subunits. The inner iron-containing nanocage has six hydrophobic and eight hydrophilic channels: the last ones are the main pores for iron uptake. | Increased transcription: (i) proinflammatory cytokines (IL1β, IL6, TNFα) via NFκB, (ii) IFNγ and TLR4 ligation through nitric oxide-dependent mechanisms involving IRP2 degradation. Most important for FTH expression. Post-transcriptional regulation: Mediated through the iron-responsive elements of ferritin-encoding RNAs; adjustment of ferritin level to intracellular iron level. More important for FTL than for FTH. Ferritinophagy: Degradation by autophagy, a part of programmed ferroptotic cell death. | Intracellular compartmentalization: 2:1 distribution between membranous compartments and cytosol. Intracellular functions Iron oxidation and storage, an antioxidant effect by reducing toxic free iron, regulation of iron metabolism and ferroptosis. Extracellular release/distribution: Secreted via the multivesicular body–exosome pathway and autophagosome-related pathways. Serum protein binding: high molecular weight kininogen, apolipoprotein B, α-2-macroglobulin and fibrinogen. Receptors: CD71, CD204, Scavenger receptors, TIM-2, CXCR4 (for details see Section 2.2). |
| Cellular ferritin uptake |
| Iron enters MSCs both through transferrin-dependent and -independent mechanisms [102]. |
| Proliferation |
| Decreased MSC proliferation in iron overload has been described [101,103], but another in vitro study described increased proliferation of in vitro-cultured MSCs exposed to iron concentrations corresponding to systemic levels observed in patients with iron overload [102]. Altered cell cycle regulation [101,102]. |
| Cell survival |
| Increased apoptosis has been described during iron overload [101]; it can be accompanied by mitochondrial fragmentation due to high ROS levels and enhanced autophagy during iron overload [104]. ATP concentrations were also decreased due to high ROS levels and reduced respiratory chain activity. Similar observations were made both for normal murine MSCs and MSCs derived from MDS patients. |
| Intracellular signaling |
| Increased mRNA and protein expression of PI3K and FOXO3 (Forkhead box protein O3) [103]. Increased AMPK activation leading to mitochondrial fragmentation [104]; increased ERK1/2 activation, but possibly decreased PI3K-AKT signaling [100]. Activation of MAP kinase pathways [102] and reduced CCR2 expression [100] have also been described. |
| Cellular metabolism |
| Increased ROS production [101]. Decreased ATP production and decreased respiratory chain activity [104]. Iron can induce upregulation of ferritin expression [105]. |
| Differentiation |
| Inhibition of osteoblastic differentiation [102], whereas effects on adipogenic and chondrogenic differentiation are absent/weaker [105]. Ferritin treatment can have an anti-osteogenic effect [105]. |
| Soluble mediator release |
| Bone marrow MSCs show decreased levels of IL10, CXCL12, IGF1, SCF, VEGF and MMP2/9 [103,106]. |
| Effects of iron overload on normal hematopoiesis |
| Murine studies suggest an increased number of myeloid progenitors, whereas the number and function of erythroid progenitors and hematopoietic stem cells were not altered [101,106]. Bone marrow transplantation to recipients with iron overload was then associated with delayed hematological reconstitution. The bone marrow is characterized by increased oxidative stress [106]. Reduced support of normal hematopoiesis [103]. |
| Systemic effects |
| Mice with iron overload show increased systemic levels of proinflammatory TNFα and IL6 together with increased systemic ROS levels and altered bone microarchitecture [107]. |
| Infectious diseases 39% |
| Bacterial 15%, fungal 5%, viral 4%, others 1% and agent not documented 14%. |
| Hemophagocytic lymphohistiocytosis (HLH) 19% |
| Together with hematological malignancies 13%, infections 6% (including 4% with viral infections), other uncommon causes of HLH including solid cancers, primary HLH, rheumatic/inflammatory disease and drugs (1.2%). |
| Hepatitis 15% (defined as aminotransferase levels ≥ 10 times upper normal limit) |
| Cancers and infections were most common; also including drug-induced, alcoholic and rheumatic/inflammatory disease. |
| Iron overload syndrome 13% |
| Cancers 11%, hemoglobinopathy 2% |
| Malignancies 6% |
| Hematological malignancies and solid cancers, 3% each |
| Others 8% |
| Cytokine release syndrome (only diagnosed for Car-T cell recipients) 3%, rheumatic/inflammatory disease 2%, acute hemolysis 1.4% and not classified 1.8% |
| Patients [Reference] | Parameter (Cut-Off) | Overall Survival | Event-Free Survival | Nonrelapse Mortality | Comment |
|---|---|---|---|---|---|
| 206 patients, median age 54 years (range 17–74 years) [161] | CRP (>150 mg/L) | ↑ | Increased 60 days induction mortality in multivariate analysis | ||
| 243 patients, median age 47 years (range 14–80 years) [162] | Albumin (<35 g/L) | ↓ | ↓ | Decreased survival in multivariate analysis | |
| 756 patients, median age 60 years (range 18–85 years) [163] | Albumin (<25, 25–35 and >35 mg/L) | ↓ | ↑ | Increased 30 and 60 days mortality; increased grade ≥ 3 toxicity | |
| 212 patients, median age 49 years (range 7–82 years) [164] | CRP:albumin ratio (1.015) | ↓ | ↓ | Ratio correlated with ferritin level. Ratio better predictor of prognosis than CRP, albumin or ferritin as single markers | |
| 188 patients, age ≥ 65 years, transplant-ineligible [165] | CRP:albumin ratio | ↓ | Decreased survival for favorable, intermediate and adverse genetic risk groups | ||
| 215 patients, median age 40 years (range 14.65 years) [166] | Fibrinogen (>3.775 g/L) | ↓ | ↓ | ||
| 375 patients, median age 53 years (range 17–77 years) [167] | Fibrinogen (>4.1 g/L) | ↓ | ↓ | ||
| 282 patients, median age 51 years (range 19–66 years) [168] | FAC ratio 1 Cut-off 3.06 | ↓ | ↓ | Over mortality and mortality after 6 months increased | |
| 328 patients, median age 49.5 years (range 15–75 years) [169] | FAC ratio 1 Cut-off 1.44 | ↓ | ↓ | The prognostic impact was seen both for patients with intermediate and adverse ELN risk classification |
| Study | Acute-Phase Parameter (Cut-Off) | Age | Secondary AML | Leukemization; Increased WBC Count the Blood | WBC Count/Bone Marrow Blasts (%) | FAB Classification | Cytogenetic Risk Classification | Remission After 1/2 Cycles | Ferritin Level | CRP Level | Albumin Level | Fibrinogen Level | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [154] | Ferritin (>750 μg/L) | − | − | nt | nt | nt | − | − | − | nt | nt | |||
| [156] | Ferritin (>400 μg/L) | − | − | + | − | − | + | nt | + | + | nt | |||
| [157] | Ferritin (>4 × upper normal limit) | − | nt | + | nt | − | − | − | + | nt | nt | |||
| [161] | CRP (>150 mg/L) | + | nt | nt | nt | + | + | nt | nt | nt | nt | |||
| [162] | Albumin (<35 g/L) | + | nt | + | + | − | − | + | nt | nt | nt | nt | ||
| [163] | Albumin (<25, 25–35 and >35 g/L) | + | + | nt | nt | nt | − | nt | nt | nt | nt | |||
| [164] | CRP–albumin ratio (1.015) | + | + | − | + | + | + | + | + | + | + | nt | ||
| [165] | CRP–albumin ratio | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | ||
| [166] | Fibrinogen (3.775 g/L) | − | nt | − | − | − | − | − | − | − | − | |||
| [167] 1 | Fibrinogen (4.1 g/L) | − | − | − | − | + | + | nt | nt | + | nt | |||
| [168] | FAC ratio (3.06) 2 | − | nt | + | + | − | nt | nt | nt | nt | nt | nt | ||
| [169] | FAC ratio (1.44) 2 | − | nt | + | + | nt | + | nt | nt | + | + | + |
| Study [Reference] | [207] | [208] | [209] |
| Time period (years) | 2000–2008 | 2007–2012 | 2008–2010 |
| Patient number | 119 | 198 | 784 |
| Median age (range) | 41 years (18–63 years) | 41 years (19–66 years) | 50 years (18–78 years) |
| Diagnosis (number) | AML (99), MDS (20) | All de novo AML | AML 626, MDS 129 |
| Related donor (number) | Related donor 54 | Sibling donor 179 | - |
| Marrow grafts (number) | 74 | 144 | 136 |
| Conditioning (number) Myeloablative/others | 92/27 | 143/55 | 566/218 |
| TBI | - | - | 177 |
| Pretransplant erythrocyte transfusions (number) | - | Low ferritin 13 (0–32) High 19 (0–56) | - |
| Median ferritin (range) | 972 μg/L (31–11 500 μg/L) | 1075 μg/L (10–7455 μg/L) | 1148 μg/L (51–14,298 μg/L) |
| Cut-off level for ferritin | 1000 μg/L | 1000 μg/L | 2500 μg/L |
| Overall disease-free survival High/low | 5-years overall survival 30%/70% | 49%/72% | No significant association |
| Relapse rate or relapse-free survival; high versus low pretransplant ferritin level | Higher relapse rate for high-ferritin patients | AML-free survival 47%/73% Relapse 35%/16% | No significant associations |
| Comment | Ferritin and disease risk were independent risk factors | No difference in acute and chronic graft versus host disease (GVHD) | Biomarker risk groups defined by ferritin, CRP and albumin were associated with transplant-related mortality |
| Study Period References | Patients | Cut-Off | Overall Survival | Relapse Mortality | Nonrelapse Mortality | Acute GVHD | Chronic GVHD | Comment |
|---|---|---|---|---|---|---|---|---|
| 1998–2005 [210] | 309 patients, including AML, ALL, MDS, CML, CLL, lymphoma, myeloma and others. | 400 μg/L | ↓ | ↑ | = | Nt | ↓ | Prognostic impact maintained in multivariate analysis, independent of CPR. Association between high ferritin and disease risk not examined. |
| 1996–2006 [211] | 264 patients including AML, CML, MDS, ALL, NHL. | 600 μg/L | ↓ | = | ↑ | = | = | (i) Effects on survival and mortality maintained in multivariate analyses. (ii) Death from infections/organ failure more common with high ferritin. (iii) The frequency of patients with high CRP/low albumin was associated with high ferritin levels. |
| 2000–2008 [212] | 159 patients with AML, MDS and ALL. | 1000 μg/L | ↓ | ↑ | ↑ | = | nt | Ferritin level and disease risk were independent risk factors; similar impact in standard- and high-risk patients. |
| 1997–2005 [213] | 590 patients with CML, AML, ALL, NHL. | 2034 μg/L | ↓ | = | ↑ | Nt | nt | The adverse ferritin effect was seen especially for AML and MDS patients. Combination with albumin < 40 g/L did not influence the impact of ferritin on outcome, i.e., the effect seems independent of the acute-phase reaction. |
| 2005–2006 [214] | 190 patients with myeloid and lymphoproliferative malignancies. | 1000 μg/L | ↓ | nt | ↑ | ↑ | nt | Only day +100 mortality was examined. Increased risk of blood-stream infections. The impact on survival seems stronger for myeloid malignancies. |
| 2014–2018 [215] | 290 patients with acute leukemia, MDS and lymphoma. | 1500 μg/L | ↓ | ↑ | ↑ | = | = | All received matched sibling donor grafts. Increased non-relapse mortality was due to severe infections. |
| 2000–2009 [216] | 290 patients, mainly with AML/MDS but also myeloma, lymphoma, CML, CMN. | 1358 μg/L | ↓ | ↑ | ↑ | High ferritin associated with reduced survival in all the periods of 0–6 months, 6–12 months, 1–2 years and 2–5 years; this was independent of erythrocyte transfusions and GVHD. | ||
| 2004–2009 [217] | 112 consecutive patients with hematological malignancies. | 700 μg/L | ↓ | nt | ↑ | nt | nt | High ferritin associated with increased risk of sepsis/septic shock/organ failure. Diverse causes of death. |
|
AML cells are at risk of developing or may have signs of cellular iron overload:
|
|
| High ROS levels in AML cells are associated with chemosensitivity [250], whereas low levels are associated with resistance [251]. The NOX family of oxidases [252] and the high mobility group box transcriptional protein [246] seem of particular importance for ROS production in AML cells. ROS modulates the function of signaling proteins through oxidation of cysteine residues and can thereby promote leukemogenesis through regulation of redox-sensitive transcription factors/enzymes/oncogenes and also promote genetic instability [253,254,255,256]. |
|
| AML cells show increased levels of free fatty acids [123]. Mitochondria are the main source of ROS in AML [255] and mitochondrial metabolism is altered in AML cells compared with normal cells [256]. Certain mutations may also be associated with susceptibility to induction of ferroptosis, e.g., IDH [256] and FLT3 mutations [257,258]. Lipid peroxide is cytotoxic and is regarded as a primary cause of ferroptosis [123,259,260]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reikvam, H.; Rolfsnes, M.G.; Rolsdorph, L.; Sandnes, M.; Selheim, F.; Hernandez-Valladares, M.; Bruserud, Ø. Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication. Int. J. Mol. Sci. 2025, 26, 5744. https://doi.org/10.3390/ijms26125744
Reikvam H, Rolfsnes MG, Rolsdorph L, Sandnes M, Selheim F, Hernandez-Valladares M, Bruserud Ø. Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication. International Journal of Molecular Sciences. 2025; 26(12):5744. https://doi.org/10.3390/ijms26125744
Chicago/Turabian StyleReikvam, Håkon, Magnus Gramstad Rolfsnes, Linn Rolsdorph, Miriam Sandnes, Frode Selheim, Maria Hernandez-Valladares, and Øystein Bruserud. 2025. "Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication" International Journal of Molecular Sciences 26, no. 12: 5744. https://doi.org/10.3390/ijms26125744
APA StyleReikvam, H., Rolfsnes, M. G., Rolsdorph, L., Sandnes, M., Selheim, F., Hernandez-Valladares, M., & Bruserud, Ø. (2025). Ferritin in Acute Myeloid Leukemia: Not Only a Marker of Inflammation and Iron Overload, but Also a Regulator of Cellular Iron Metabolism, Signaling and Communication. International Journal of Molecular Sciences, 26(12), 5744. https://doi.org/10.3390/ijms26125744







