Auxin Metabolite Balance During Haploid and Zygotic Oat Embryo Development—Quantitative and Localization Studies
Abstract
1. Introduction
2. Results
2.1. Auxin Metabolites During Oat Embryo Development
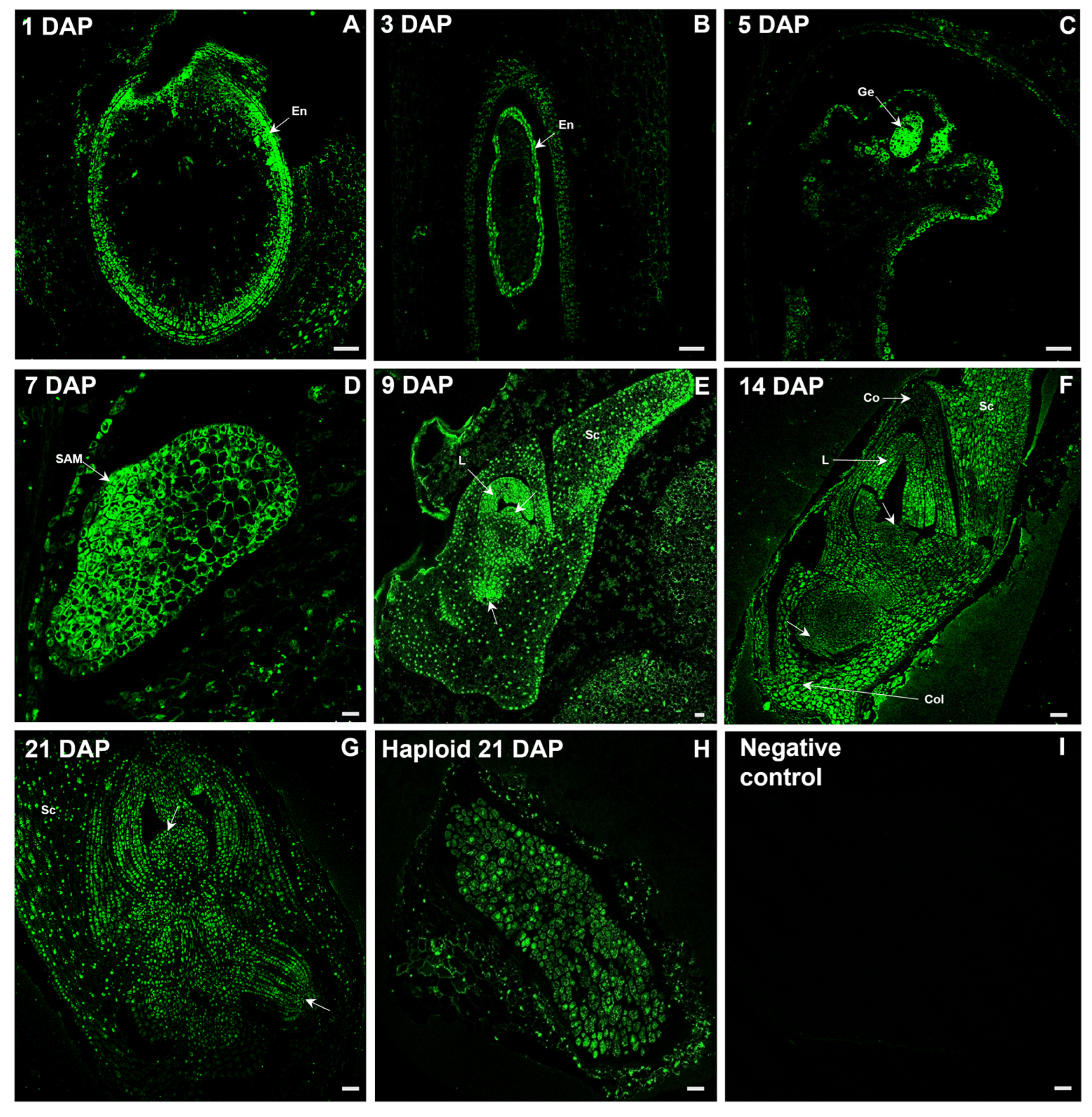
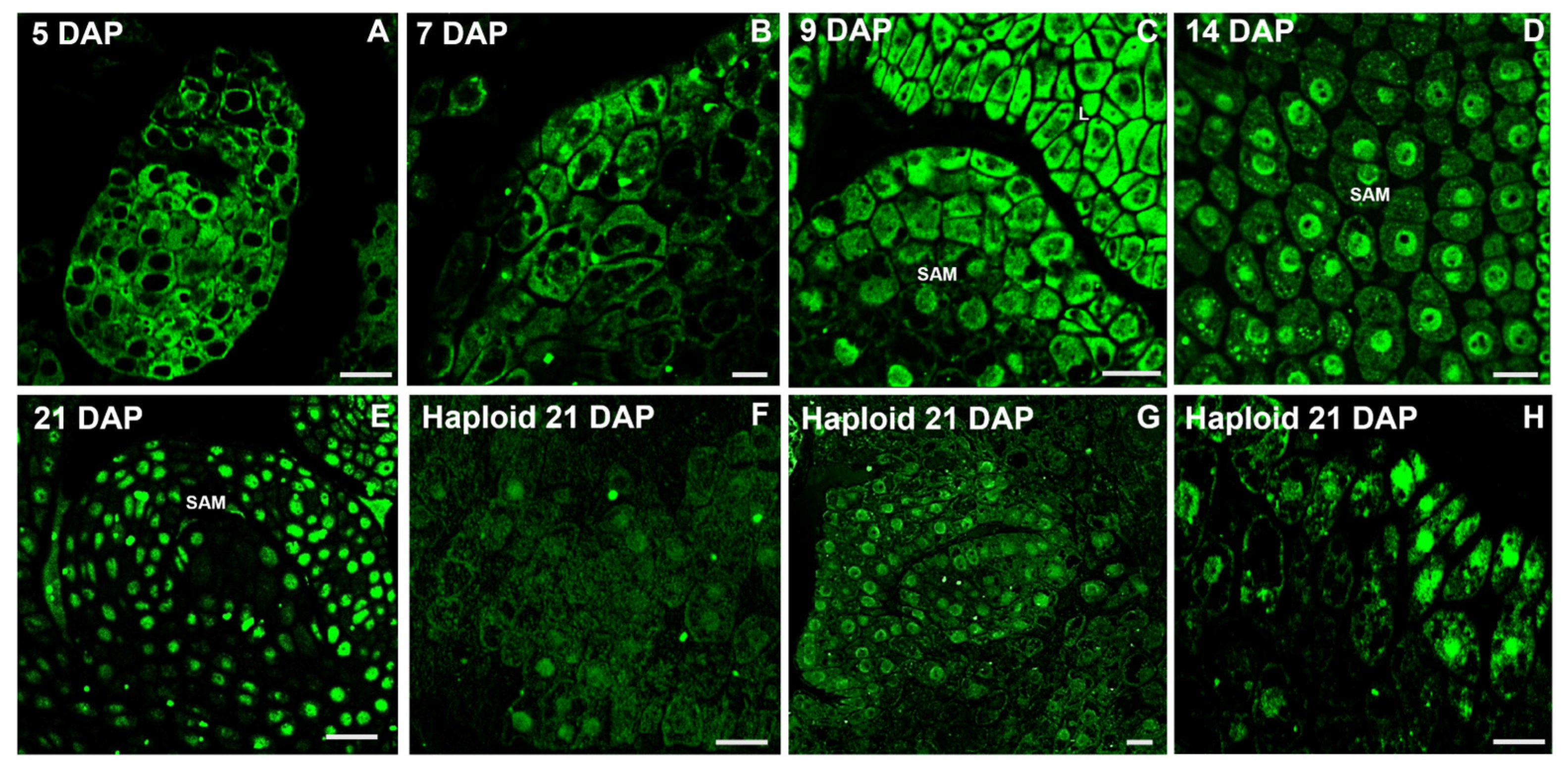
2.2. Distribution of IAA During Oat Embryo Development
3. Discussion
4. Materials and Methods
4.1. Obtaining Oat Embryos
4.2. Quantitation of Auxins by HPLC-MS/MS
4.3. Immunohistochemical Localization of IAA by Confocal Laser Microscopy
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, D.; Chang, S.; Li, X.; Qi, Y. Advances in the Study of Auxin Early Response Genes: Aux/IAA, GH3, and SAUR. Crop J. 2024, 12, 964–978. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in Action: Signalling, Transport and the Control of Plant Growth and Development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Fischer, C.; Neuhaus, G. Influence of Auxin on the Establishment of Bilateral Symmetry in Monocots. Plant J. 1996, 9, 659–669. [Google Scholar] [CrossRef]
- Hadfi, K.; Speth, V.; Neuhaus, G. Auxin-Induced Developmental Patterns in Brassica juncea Embryos. Development 1998, 125, 879–887. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-Dependent Auxin Gradients Establish the Apical-Basal Axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Mateo-Bonmatí, E.; Ljung, K. Auxin Metabolism in Plants. Cold Spring Harb. Perspect. Med. 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Zhao, Y. The Role of Local Biosynthesis of Auxin and Cytokinin in Plant Development. Curr. Opin. Plant Biol. 2008, 11, 16–22. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Auxin Conjugates: Their Role for Plant Development and in the Evolution of Land Plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Hayashi, K.-I.; Arai, K.; Aoi, Y.; Tanaka, Y.; Hira, H.; Guo, R.; Hu, Y.; Ge, C.; Zhao, Y.; Kasahara, H.; et al. The Main Oxidative Inactivation Pathway of the Plant Hormone Auxin. Nat. Commun. 2021, 12, 6752. [Google Scholar] [CrossRef]
- Friml, J. Subcellular Trafficking of PIN Auxin Efflux Carriers in Auxin Transport. Eur. J. Cell Biol. 2010, 89, 231–235. [Google Scholar] [CrossRef]
- Hammes, U.Z.; Murphy, A.S.; Schwechheimer, C. Auxin Transporters—A Biochemical View. Cold Spring Harb. Perspect. Biol. 2022, 14, a039875. [Google Scholar] [CrossRef] [PubMed]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratge-Faillie, C.; Novak, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 Is a Vacuolar Auxin Transport Facilitator Required for Auxin Homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef] [PubMed]
- Calderón Villalobos, L.I.A.; Lee, S.; De Oliveira, C.; Ivetac, A.; Brandt, W.; Armitage, L.; Sheard, L.B.; Tan, X.; Parry, G.; Mao, H.; et al. A Combinatorial TIR1/AFB-Aux/IAA Co-Receptor System for Differential Sensing of Auxin. Nat. Chem. Biol. 2012, 8, 477–485. [Google Scholar] [CrossRef]
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of Auxin Perception by the TIR1 Ubiquitin Ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef]
- Galli, M.; Khakhar, A.; Lu, Z.; Chen, Z.; Sen, S.; Joshi, T.; Nemhauser, J.L.; Schmitz, R.J.; Gallavotti, A. The DNA Binding Landscape of the Maize AUXIN RESPONSE FACTOR Family. Nat. Commun. 2018, 9, 4526. [Google Scholar] [CrossRef]
- Capron, A.; Chatfield, S.; Provart, N.; Berleth, T. Embryogenesis: Pattern Formation from a Single Cell. Arab. Book. 2009, 7, e0126. [Google Scholar] [CrossRef]
- Vernoud, V.; Hajduch, M.; Khaled, A.G.A.; Depege, N.; Rogowsky, P. Maize Embryogenesis. Maydica 2005, 50, 469–483. [Google Scholar]
- Locascio, A.; Roig-Villanova, I.; Bernardi, J.; Varotto, S. Current Perspectives on the Hormonal Control of Seed Development in Arabidopsis and Maize: A Focus on Auxin. Front. Plant Sci. 2014, 5, 412. [Google Scholar] [CrossRef]
- Kruglova, N.N.; Titova, G.E.; Zinatullina, A.E. Critical Stages of Cereal Embryogenesis: Theoretical and Practical Significance. Russ. J. Dev. Biol. 2022, 53, 405–420. [Google Scholar] [CrossRef]
- Shen, K.; Qu, M.; Zhao, P. The Roads to Haploid Embryogenesis. Plants 2023, 12, 243. [Google Scholar] [CrossRef]
- Loskutov, I.G. Advances in Cereal Crops Breeding. Plants 2021, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef] [PubMed]
- Juzoń, K.; Warchoł, M.; Dziurka, K.; Czyczyło-Mysza, I.M.; Marcińska, I.; Skrzypek, E. The Effect of 2,4-Dichlorophenoxyacetic Acid on the Production of Oat (Avena sativa L.) Doubled Haploid Lines through Wide Hybridization. PeerJ 2022, 10, e12854. [Google Scholar] [CrossRef]
- Rines, H.W.; Dahleen, L.S. Haploid Oat Plants Produced by Application of Maize Pollen to Emasculated Oat Florets. Crop Sci. 1990, 30, 1073–1078. [Google Scholar] [CrossRef]
- Sidhu, P.K.; Howes, N.K.; Aung, T.; Zwer, P.K.; Davies, P.A. Factors Affecting Oat Haploid Production Following Oat × Maize Hybridization. Plant Breed. 2006, 125, 243–247. [Google Scholar] [CrossRef]
- Skrzypek, E.; Warchoł, M.; Czyczyło-Mysza, I.; Juzoń, K.; Dziurka, K.; Marcińska, I. Oat Doubled Haploid Production Through Wide Hybridization with Maize. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2021; Volume 2287, pp. 323–332. [Google Scholar]
- Dziurka, K.; Dziurka, M.; Warchoł, M.; Czyczyło-Mysza, I.; Marcińska, I.; Noga, A.; Kapłoniak, K.; Skrzypek, E. Endogenous Phytohormone Profile during Oat (Avena sativa L.) Haploid Embryo Development. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 221–229. [Google Scholar] [CrossRef]
- Dziurka, K.; Dziurka, M.; Muszyńska, E.; Czyczyło-Mysza, I.; Warchoł, M.; Juzoń, K.; Laskoś, K.; Skrzypek, E. Anatomical and Hormonal Factors Determining the Development of Haploid and Zygotic Embryos of Oat (Avena sativa L.). Sci. Rep. 2022, 12, 548. [Google Scholar] [CrossRef]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local Auxin Biosynthesis Is a Key Regulator of Plant Development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle’, T.J. Aux/LAA Proteins Repress Expression of Reporter Genes Containing Natural and Highly Active Synthetic Auxin Response Elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar]
- Pařízková, B.; Pernisová, M.; Novák, O. What Has Been Seen Cannot Be Unseen—Detecting Auxin In Vivo. Int. J. Mol. Sci. 2017, 18, 2736. [Google Scholar] [CrossRef]
- Forestan, C.; Meda, S.; Varotto, S. ZmPIN1-Mediated Auxin Transport Is Related to Cellular Differentiation during Maize Embryogenesis and Endosperm Development. Plant Physiol. 2010, 152, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.R.; Carman, J.G.; Banowetz, G.M. Hormones in Wheat Kernels during Embryony. J. Plant Physiol. 2002, 159, 379–386. [Google Scholar] [CrossRef]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin Regulates SCF TIR1-Dependent Degradation of AUX/IAA Proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef]
- Skalický, V.; Vojtková, T.; Pěnčík, A.; Vrána, J.; Juzoń, K.; Koláčková, V.; Sedlářová, M.; Kubeš, M.F.; Novák, O. Auxin Metabolite Profiling in Isolated and Intact Plant Nuclei. Int. J. Mol. Sci. 2021, 22, 12369. [Google Scholar] [CrossRef] [PubMed]
- Porco, S.; Pěnčík, A.; Rasheda, A.; Vo, U.; Casanova-Sáez, R.; Bishopp, A.; Golebiowska, A.; Bhosale, R.; Swarupa, R.; Swarup, K.; et al. Dioxygenase-Encoding AtDAO1 Gene Controls IAA Oxidation and Homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 11016–11021. [Google Scholar] [CrossRef]
- Rampey, R.A.; LeClere, S.; Kowalczyk, M.; Ljung, K.; Sandberg, G.; Bartel, B. A Family of Auxin-Conjugate Hydrolases That Contributes to Free Indole-3-Acetic Acid Levels during Arabidopsis Germination. Plant Physiol. 2004, 135, 978–988. [Google Scholar] [CrossRef]
- LeClere, S.; Tellez, R.; Rampey, R.A.; Matsuda, S.P.T.; Bartel, B. Characterization of a Family of IAA-Amino Acid Conjugate Hydrolases from Arabidopsis. J. Biol. Chem. 2002, 277, 20446–20452. [Google Scholar] [CrossRef]
- Tang, Q.; Yu, P.; Tillmann, M.; Cohen, J.D.; Slovin, J.P. Indole-3-Acetylaspartate and Indole-3-Acetylglutamate, the IAA-Amide Conjugates in the Diploid Strawberry Achene, Are Hydrolyzed in Growing Seedlings. Planta 2019, 249, 1073–1085. [Google Scholar] [CrossRef]
- Cohen, J.D.; Strader, L.C. An Auxin Research Odyssey: 1989–2023. Plant Cell 2024, 36, 1410–1428. [Google Scholar] [CrossRef]
- Ljung, K.; Hull, A.K.; Kowalczyk, M.; Marchant, A.; Celenza, J.; Cohen, J.D.; Sandberg, G. Biosynthesis, Conjugation, Catabolism and Homeostasis of Indole-3-Acetic Acid in Arabidopsis Thaliana. Plant Mol. Biol. 2002, 50, 309–332. [Google Scholar] [CrossRef]
- Warchoł, M.; Skrzypek, E.; Nowakowska, A.; Marcińska, I.; Czyczyło-Mysza, I.; Dziurka, K.; Juzoń, K.; Cyganek, K. The Effect of Auxin and Genotype on the Production of Avena sativa L. Doubled Haploid Lines. Plant Growth Regul. 2016, 78, 155–165. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Rusaczonek, A.; Kochańska-Jeziorska, A. Exploring Apoplast Reorganization in the Nodules of Lotus corniculatus L. Growing on Old Zn–Pb Calamine Wastes. J. Plant Physiol. 2022, 268, 153561. [Google Scholar] [CrossRef] [PubMed]
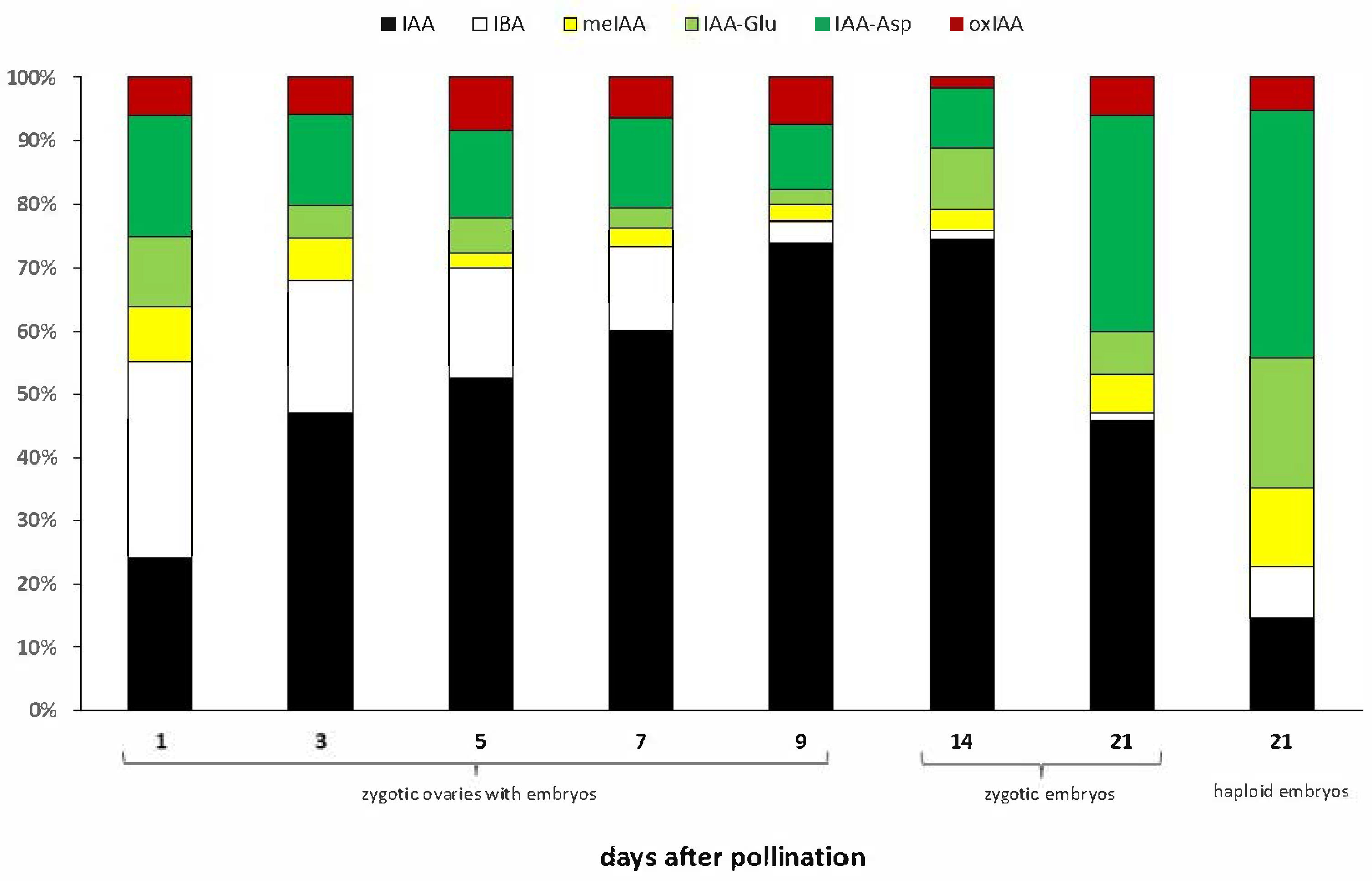
| Metabolite [nmol/g DW] | Zygotic Ovaries with Embryos | Zygotic Embryos | Haploid Embryos | |||||
|---|---|---|---|---|---|---|---|---|
| 1 DAP | 3 DAP | 5 DAP | 7 DAP | 9 DAP | 14 DAP | 21 DAP | 21 DAP | |
| IAA | 0.57 ± 0.5 b * | 1.43 ± 0.08 b | 2.09 ± 0.51 b | 2.77 ± 0.13 b | 5.97 ± 0.67 a | 5.30 ± 1.47 a | 1.70 ± 0.52 b | 0.83 ± 0.02 b |
| IBA | 0.74 ± 0.22 a | 0.63 ± 0.25 bc | 0.69 ± 0.04 bc | 0.61 ± 0.06 bc | 0.28 ± 0.03 cd | 0.10 ± 0.01 d | 0.05 ± 0.01 d | 0.46 ± 0.12 bcd |
| meIAA | 0.21 ± 0.03 bc | 0.20 ± 0.02 bc | 0.10 ± 0.02 c | 0.14 ± 0.00 bc | 0.21 ± 0.01 bc | 0.22 ± 0.04 b | 0.23 ± 0.06 b | 0.71 ± 0.04 a |
| IAA-Asp | 0.45 ± 0.11 c | 0.44 ± 0.29 c | 0.55 ± 0.20 c | 0.65 ± 0.06 bc | 0.83 ± 0.09 bc | 0.68 ± 0.12 bc | 1.26 ± 0.10 b | 2.21 ± 0.65 a |
| IAA-Glu | 0.26 ± 0.09 c | 0.15 ± 0.01 c | 0.21 ± 0.01 c | 0.14 ± 0.01 c | 0.19 ± 0.01 c | 0.69 ± 0.06 b | 0.25 ± 0.03 c | 1.16 ± 0.14 a |
| oxIAA | 0.14 ± 0.04 b | 0.18 ± 0.08 b | 0.34 ± 0.12 b | 0.30 ± 0.05 b | 0.60 ± 0.06 a | 0.12 ± 0.02 b | 0.22 ± 0.05 b | 0.30 ± 0.04 b |
| Sum of conjugates | 0.93 c | 0.79 c | 0.86 c | 0.93 c | 1.23 bc | 1.60 b | 1.74 b | 4.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziurka, K.; Dziurka, M.; Sujkowska-Rybkowska, M.; Laskoś, K.; Grela, M.; Muszyńska, E. Auxin Metabolite Balance During Haploid and Zygotic Oat Embryo Development—Quantitative and Localization Studies. Int. J. Mol. Sci. 2025, 26, 5737. https://doi.org/10.3390/ijms26125737
Dziurka K, Dziurka M, Sujkowska-Rybkowska M, Laskoś K, Grela M, Muszyńska E. Auxin Metabolite Balance During Haploid and Zygotic Oat Embryo Development—Quantitative and Localization Studies. International Journal of Molecular Sciences. 2025; 26(12):5737. https://doi.org/10.3390/ijms26125737
Chicago/Turabian StyleDziurka, Kinga, Michał Dziurka, Marzena Sujkowska-Rybkowska, Kamila Laskoś, Magdalena Grela, and Ewa Muszyńska. 2025. "Auxin Metabolite Balance During Haploid and Zygotic Oat Embryo Development—Quantitative and Localization Studies" International Journal of Molecular Sciences 26, no. 12: 5737. https://doi.org/10.3390/ijms26125737
APA StyleDziurka, K., Dziurka, M., Sujkowska-Rybkowska, M., Laskoś, K., Grela, M., & Muszyńska, E. (2025). Auxin Metabolite Balance During Haploid and Zygotic Oat Embryo Development—Quantitative and Localization Studies. International Journal of Molecular Sciences, 26(12), 5737. https://doi.org/10.3390/ijms26125737









