Auxin Orchestrates Germ Cell Specification in Arabidopsis
Abstract
1. Introduction
2. Auxin Homeostasis: Biosynthesis, Polar Transport, Signal Transduction, Metabolism, and Conjugation
2.1. Auxin Biosynthesis: Key Genes and Pathways
2.2. Auxin Transport: Polar Distribution and Accumulation
2.3. Auxin Signal Transduction: From Perception to Transcription
2.4. Dynamic Equilibrium of Auxin: Integration of Biosynthesis, Transport, Signal Transduction, Metabolism, and Conjugation
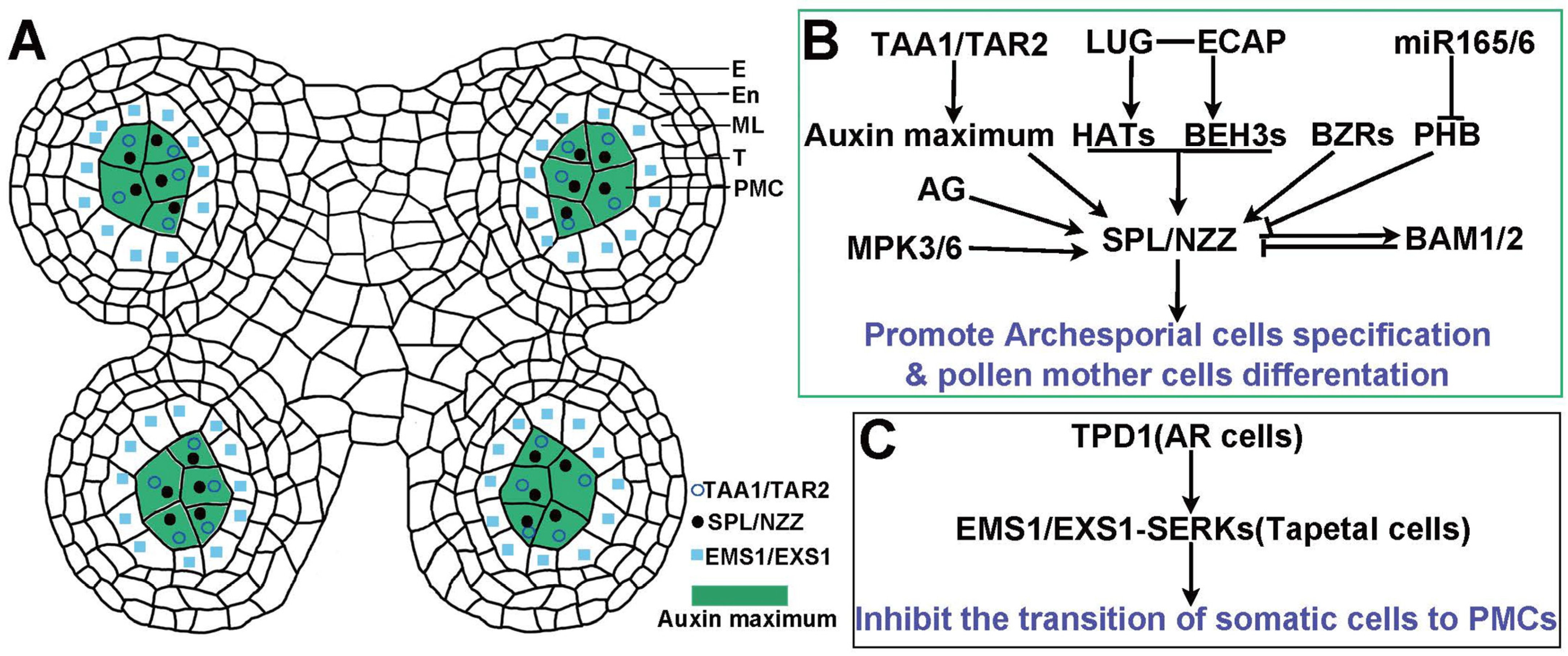
3. Auxin Orchestrates the Specification of Germ Cells in the Anther
3.1. Auxin Biosynthesis and Specification of Male Germ Cells
3.2. Auxin-Mediated Regulation of SPL/NZZ in Male Germ Cells Specification
3.2.1. Transcriptional Regulation and Feedback Mechanisms Between Auxin and SPL/NZZ
3.2.2. Transcriptional Regulation of SPL/NZZ
3.3. Synergistic Regulations Between Auxin and TPD1-EMS1 Signaling Pathways
4. Auxin Determines Female Germline Cell Specification
4.1. Dynamic Distribution of Auxin and Specification of Megaspore Mother Cell
4.2. Auxin Signaling Pathways in Female Germline Specification
4.2.1. ARFs Function in Female Germline Specification
4.2.2. ARFs and SPL/NZZ Interaction in Megasporocyte Formation
4.3. Auxin-Mediated Regulation of SPL/NZZ in Female Germline Cell Specification
5. A Proposed Theoretical Framework for Auxin-Mediated Germline Specification: Future Research Directions and Perspectives
5.1. Integrating Auxin Regulatory Networks: The Proposed Framework
5.1.1. Biosynthesis and Local Production
5.1.2. Polar Transport and Dynamic Distribution
5.1.3. Signal Transduction and Feedback Regulation
5.2. Identified Gaps and Future Research Directions
5.2.1. Spatiotemporal Dynamics of Auxin
5.2.2. Role of Auxin Signaling
5.2.3. Feedback Mechanisms Involving SPL/NZZ
5.2.4. Conservation Across Species
5.2.5. Interplay with Other Hormonal Pathways
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gossmann, T.I.; Saleh, D.; Schmid, M.W.; Spence, M.A.; Schmid, K.J. Transcriptomes of Plant Gametophytes Have a Higher Proportion of Rapidly Evolving and Young Genes than Sporophytes. Mol. Biol. Evol. 2016, 33, 1669–1678. [Google Scholar]
- Zhao, F.; Zheng, Y.F.; Zeng, T.; Sun, R.; Yang, J.Y.; Li, Y.; Ren, D.T.; Ma, H.; Xu, Z.H.; Bai, S.N. Phosphorylation of SPOROCYTELESS/NOZZLE by the MPK3/6 Kinase Is Required for Anther Development. Plant Physiol. 2017, 173, 2265–2277. [Google Scholar] [PubMed]
- Yang, W.C.; Ye, D.; Xu, J.; Sundaresan, V. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 1999, 13, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Ma, H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol. 2005, 56, 393–434. [Google Scholar]
- Yang, W.-C.; Shi, D.-Q.; Chen, Y.-H. Female Gametophyte Development in Flowering Plants. Annu. Rev. Plant Biol. 2010, 61, 89–108. [Google Scholar] [PubMed]
- Liu, F.; Li, J.P.; Li, L.S.; Liu, Q.; Li, S.W.; Song, M.L.; Li, S.; Zhang, Y. The canonical α-SNAP is essential for gametophytic development in Arabidopsis. PLoS Genet. 2021, 17, e1009505. [Google Scholar]
- Raz, E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003, 4, 690–700. [Google Scholar]
- Issigonis, M.; Redkar, A.B.; Rozario, T.; Khan, U.W.; Mejia-Sanchez, R.; Lapan, S.W.; Reddien, P.W.; Newmark, P.A. A Krüppel-like factor is required for development and regeneration of germline and yolk cells from somatic stem cells in planarians. PLoS Biol. 2022, 20, e3001472. [Google Scholar]
- Sprunck, S.; Groß-Hardt, R. Nuclear behavior, cell polarity, and cell specification in the female gametophyte. Sex. Plant Reprod. 2011, 24, 123–136. [Google Scholar] [CrossRef]
- Zhao, Y. Essential Roles of Local Auxin Biosynthesis in Plant Development and in Adaptation to Environmental Changes. Annu. Rev. Plant Biol. 2018, 69, 417–435. [Google Scholar]
- Schmidt, A.; Schmid, M.W.; Grossniklaus, U. Plant germline formation: Common concepts and developmental flexibility in sexual and asexual reproduction. Development 2015, 142, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, D.; Ye, S.; Chen, W.; Li, G.; Xu, Z.; Bai, S.; Zhao, F. Auxin guides germ-cell specification in Arabidopsis anthers. Proc. Natl. Acad. Sci. USA 2021, 118, e2101492118. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, L.; Malik, S.; Gentile, B.R.; Xiong, V.; Arazi, T.; Owen, H.A.; Friml, J.; Zhao, D. Specification of female germline by microRNA orchestrated auxin signaling in Arabidopsis. Nat. Commun. 2022, 13, 6960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Wu, H.-M.; Xie, D.-J.; Tang, Z.-S.; Shi, D.-Q.; Yang, W.-C. PINOID regulates floral organ development by modulating auxin transport and interacts with MADS16 in rice. Plant Biotechnol. J. 2020, 18, 1778–1795. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef]
- Bennett, T.A.; Liu, M.M.; Aoyama, T.; Bierfreund, N.M.; Braun, M.; Coudert, Y.; Dennis, R.J.; O’Connor, D.; Wang, X.Y.; White, C.D.; et al. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol. 2014, 24, 2776–2785. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Alandete-Saez, M.; Bowman, J.L.; Sundaresan, V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science 2009, 324, 1684–1689. [Google Scholar] [CrossRef]
- Su, Z.; Wang, N.; Hou, Z.; Li, B.; Li, D.; Liu, Y.; Cai, H.; Qin, Y.; Chen, X. Regulation of Female Germline Specification via Small RNA Mobility in Arabidopsis. Plant Cell 2020, 32, 2842–2854. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [PubMed]
- Rizzardi, K.; Landberg, K.; Nilsson, L.; Ljung, K.; Sundås-Larsson, A. TFL2/LHP1 is involved in auxin biosynthesis through positive regulation of YUCCA genes. Plant J. 2011, 65, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.-Y.; Doležal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-Mediated Auxin Biosynthesis Is Essential for Hormone Crosstalk and Plant Development. Cell 2008, 133, 177–191. [Google Scholar]
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998, 282, 2226–2230. [Google Scholar]
- Yang, Z.; Xia, J.; Hong, J.; Zhang, C.; Wei, H.; Ying, W.; Sun, C.; Sun, L.; Mao, Y.; Gao, Y.; et al. Structural insights into auxin recognition and efflux by Arabidopsis PIN1. Nature 2022, 609, 611–615. [Google Scholar]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar]
- Luo, P.; Li, T.T.; Shi, W.M.; Ma, Q.; Di, D.W. The Roles of GRETCHEN HAGEN3 (GH3)-Dependent Auxin Conjugation in the Regulation of Plant Development and Stress Adaptation. Plants 2023, 12, 4111. [Google Scholar] [CrossRef]
- Baster, P.; Robert, S.; Kleine-Vehn, J.; Vanneste, S.; Kania, U.; Grunewald, W.; De Rybel, B.; Beeckman, T.; Friml, J. SCFTIR1/AFB-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 2013, 32, 260–274. [Google Scholar]
- Qi, L.; Kwiatkowski, M.; Chen, H.; Hoermayer, L.; Sinclair, S.; Zou, M.; Del Genio, C.I.; Kubes, M.F.; Napier, R.; Jaworski, K.; et al. Adenylate cyclase activity of TIR1/AFB auxin receptors in plants. Nature 2022, 611, 133–138. [Google Scholar]
- Xu, T.; Dai, N.; Chen, J.; Nagawa, S.; Cao, M.; Li, H.; Zhou, Z.; Chen, X.; De Rycke, R.; Rakusová, H.; et al. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 2014, 343, 1025–1028. [Google Scholar]
- Friml, J.; Gallei, M.; Gelova, Z.; Johnson, A.; Mazur, E.; Monzer, A.; Rodriguez, L.; Roosjen, M.; Verstraeten, I.; Zivanovic, B.D.; et al. ABP1-TMK auxin perception for global phosphorylation and auxin canalization. Nature 2022, 609, 575–581. [Google Scholar] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [PubMed]
- Tan, S.; Luschnig, C.; Friml, J. Pho-view of Auxin: Reversible Protein Phosphorylation in Auxin Biosynthesis, Transport and Signaling. Mol. Plant 2021, 14, 151–165. [Google Scholar]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic Pathways and Functions of Indole-3-Acetic Acid in Microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef]
- Wang, B.; Chu, J.; Yu, T.; Xu, Q.; Sun, X.; Yuan, J.; Xiong, G.; Wang, G.; Wang, Y.; Li, J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 4821–4826. [Google Scholar]
- Chen, L.; Tong, J.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.W.; Xu, L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local Auxin Biosynthesis Is a Key Regulator of Plant Development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar]
- Benjamins, R.; Scheres, B. Auxin: The looping star in plant development. Annu. Rev. Plant Biol. 2008, 59, 443–465. [Google Scholar]
- Wisniewska, J.; Xu, J.; Seifertová, D.; Brewer, P.B.; Ruzicka, K.; Blilou, I.; Rouquié, D.; Benková, E.; Scheres, B.; Friml, J. Polar PIN localization directs auxin flow in plants. Science 2006, 312, 883. [Google Scholar]
- Vieten, A.; Sauer, M.; Brewer, P.B.; Friml, J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007, 12, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Petrásek, J.; Mravec, J.; Bouchard, R.; Blakeslee, J.J.; Abas, M.; Seifertová, D.; Wisniewska, J.; Tadele, Z.; Kubes, M.; Covanová, M.; et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 2006, 312, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Yang, X.; Michniewicz, M.; Weijers, D.; Quint, A.; Tietz, O.; Benjamins, R.; Ouwerkerk, P.B.; Ljung, K.; Sandberg, G.; et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 2004, 306, 862–865. [Google Scholar] [CrossRef]
- Michniewicz, M.; Zago, M.K.; Abas, L.; Weijers, D.; Schweighofer, A.; Meskiene, I.; Heisler, M.G.; Ohno, C.; Zhang, J.; Huang, F.; et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130, 1044–1056. [Google Scholar] [CrossRef]
- Robert, S.; Kleine-Vehn, J.; Barbez, E.; Sauer, M.; Paciorek, T.; Baster, P.; Vanneste, S.; Zhang, J.; Simon, S.; Čovanová, M.; et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 2010, 143, 111–121. [Google Scholar]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef]
- Kepinski, S.; Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 2003, 15, 533–543. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar]
- Kato, H.; Ishizaki, K.; Kouno, M.; Shirakawa, M.; Bowman, J.L.; Nishihama, R.; Kohchi, T. Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in Marchantia polymorpha. PLoS Genet. 2015, 11, e1005084. [Google Scholar]
- Simonini, S.; Deb, J.; Moubayidin, L.; Stephenson, P.; Valluru, M.; Freire-Ríos, A.; Sorefan, K.; Weijers, D.; Friml, J.; Østergaard, L.H. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 2016, 30, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [PubMed]
- Chapman, E.J.; Estelle, M. Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.-C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant Cell 2004, 16, S46–S60. [Google Scholar]
- Grossniklaus, U.; Schneitz, K. The molecular and genetic basis of ovule and megagametophyte development. Semin. Cell Dev. Biol. 1998, 9, 227–238. [Google Scholar]
- Chandler, J.W. The Hormonal Regulation of Flower Development. J. Plant Growth Regul. 2011, 30, 242–254. [Google Scholar]
- Cecchetti, V.; Altamura, M.M.; Falasca, G.; Costantino, P.; Cardarelli, M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 2008, 20, 1760–1774. [Google Scholar] [CrossRef]
- Han, H.; Adamowski, M.; Qi, L.; Alotaibi, S.S.; Friml, J. PIN-mediated polar auxin transport regulations in plant tropic responses. New Phytol. 2021, 232, 510–522. [Google Scholar] [CrossRef]
- Barbosa, I.C.R.; Hammes, U.Z.; Schwechheimer, C. Activation and Polarity Control of PIN-FORMED Auxin Transporters by Phosphorylation. Trends Plant Sci. 2018, 23, 523–538. [Google Scholar] [PubMed]
- Caumon, H.; Vernoux, T. A matter of time: Auxin signaling dynamics and the regulation of auxin responses during plant development. J. Exp. Bot. 2023, 74, 3887–3902. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tang, W.; Lin, W.; Li, W.; Zhou, X.; Li, Y.; Chen, R.; Zheng, R.; Qin, G.; Cao, W.; et al. ABLs and TMKs are co-receptors for extracellular auxin. Cell 2023, 186, 5457–5471.e17. [Google Scholar]
- Wu, M.F.; Yamaguchi, N.; Xiao, J.; Bargmann, B.; Estelle, M.; Sang, Y.; Wagner, D. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. Elife 2015, 4, e09269. [Google Scholar] [CrossRef]
- Yu, S.; Galvāo, V.C.; Zhang, Y.-c.; Horrer, D.; Zhang, T.-Q.; Hao, Y.-h.; Feng, Y.; Wang, S.; Schmid, M.; Wang, J.-W. Gibberellin Regulates the Arabidopsis Floral Transition through miR156-Targeted SQUAMOSA PROMOTER BINDING–LIKE Transcription Factors[W]. Plant Cell 2012, 24, 3320–3332. [Google Scholar] [CrossRef]
- Shi, L.; Li, C.; Lv, G.; Li, X.; Feng, W.; Bi, Y.; Wang, W.; Wang, Y.; Zhu, L.; Tang, W.; et al. The adaptor protein ECAP, the corepressor LEUNIG, and the transcription factor BEH3 interact and regulate microsporocyte generation in Arabidopsis. Plant Cell 2024, 36, 2531–2549. [Google Scholar]
- Xu, M.; Hu, T.; Zhao, J.; Park, M.Y.; Earley, K.W.; Wu, G.; Yang, L.; Poethig, R.S. Developmental Functions of miR156-Regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) Genes in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006263. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, J.; Pang, C.; Yu, H.; Guo, D.; Jiang, H.; Ding, M.; Chen, Z.; Tao, Q.; Gu, H.; et al. The molecular mechanism of sporocyteless/nozzle in controlling Arabidopsis ovule development. Cell Res. 2015, 25, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Yin, C.; Ma, M.; Zhou, Y.; Zheng, X.; Tu, X.; Fang, Y. Feedback regulation of auxin signaling through the transcription of H2A.Z and deposition of H2A.Z to SMALL AUXIN UP RNAs in Arabidopsis. New Phytol. 2022, 236, 1721–1733. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Parameswaran, S.; Ito, T.; Seubert, B.; Auer, M.; Rymaszewski, A.; Jia, G.; Owen, H.A.; Zhao, D. The SPOROCYTELESS/NOZZLE gene is involved in controlling stamen identity in Arabidopsis. Plant Physiol. 2009, 151, 1401–1411. [Google Scholar]
- Liu, C.; Lu, F.; Cui, X.; Cao, X. Histone methylation in higher plants. Annu. Rev. Plant Biol. 2010, 61, 395–420. [Google Scholar]
- Ito, T.; Wellmer, F.; Yu, H.; Das, P.; Ito, N.; Alves-Ferreira, M.; Riechmann, J.L.; Meyerowitz, E.M. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 2004, 430, 356–360. [Google Scholar] [PubMed]
- Chen, L.G.; Gao, Z.; Zhao, Z.; Liu, X.; Li, Y.; Zhang, Y.; Liu, X.; Sun, Y.; Tang, W. BZR1 Family Transcription Factors Function Redundantly and Indispensably in BR Signaling but Exhibit BRI1-Independent Function in Regulating Anther Development in Arabidopsis. Mol. Plant 2019, 12, 1408–1415. [Google Scholar] [PubMed]
- Shi, H.; Li, X.; Lv, M.; Li, J. BES1/BZR1 Family Transcription Factors Regulate Plant Development via Brassinosteroid-Dependent and Independent Pathways. Int. J. Mol. Sci. 2022, 23, 10149. [Google Scholar] [CrossRef]
- Li, X.; Lian, H.; Zhao, Q.; He, Y. MicroRNA166 Monitors SPOROCYTELESS/NOZZLE for Building of the Anther Internal Boundary. Plant Physiol. 2019, 181, 208–220. [Google Scholar] [PubMed]
- Cui, Y.; Hu, C.; Zhu, Y.; Cheng, K.; Li, X.; Wei, Z.; Xue, L.; Lin, F.; Shi, H.; Yi, J.; et al. CIK Receptor Kinases Determine Cell Fate Specification during Early Anther Development in Arabidopsis. Plant Cell 2018, 30, 2383–2401. [Google Scholar]
- Hord, C.L.H.; Sun, Y.-J.; Pillitteri, L.J.; Torii, K.U.; Wang, H.; Zhang, S.; Ma, H. Regulation of Arabidopsis Early Anther Development by the Mitogen-Activated Protein Kinases, MPK3 and MPK6, and the ERECTA and Related Receptor-Like Kinases. Mol. Plant 2008, 1, 645–658. [Google Scholar] [PubMed]
- Zhao, D.Z.; Wang, G.F.; Speal, B.; Ma, H. The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev. 2002, 16, 2021–2031. [Google Scholar]
- Huang, J.; Zhang, T.; Linstroth, L.; Tillman, Z.; Otegui, M.S.; Owen, H.A.; Zhao, D. Control of Anther Cell Differentiation by the Small Protein Ligand TPD1 and Its Receptor EMS1 in Arabidopsis. PLoS Genet. 2016, 12, e1006147. [Google Scholar]
- Yang, W.C.; Sundaresan, V. Genetics of gametophyte biogenesis in Arabidopsis. Curr. Opin. Plant Biol. 2000, 3, 53–57. [Google Scholar]
- Kaur, I.; Kathpalia, R.; Koul, M. Understanding megasporogenesis through model plants: Contemporary evidence and future insights. Int. J. Dev. Biol. 2024, 68, 9–17. [Google Scholar] [PubMed]
- Maheshwari, P. An Introduction to the Embryology of Angiosperms, 1st ed.; McGraw-Hill: New York, NY, USA, 1950. [Google Scholar]
- Gasser, C.S.; Broadhvest, J.; Hauser, B.A. Genetic analysis of ovule development. Annu. Rev. Plant Biol. 1998, 49, 1–24. [Google Scholar]
- Drews, G.N.; Yadegari, R. Development and Function of the Angiosperm Female Gametophyte. Annu. Rev. Genet. 2002, 36, 99–124. [Google Scholar] [PubMed]
- Hater, F.; Nakel, T.; Groß-Hardt, R. Reproductive Multitasking: The Female Gametophyte. Annu. Rev. Plant Biol. 2020, 71, 517–546. [Google Scholar]
- Cai, H.; Liu, L.; Ma, S.; Aslam, M.; Qin, Y. Insights into the role of phytohormones in plant female germline cell specification. Curr. Opin. Plant Biol. 2023, 75, 102439. [Google Scholar]
- Bencivenga, S.; Simonini, S.; Benkova, E.; Colombo, L. The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis. Plant Cell 2012, 24, 2886–2897. [Google Scholar]
- Ceccato, L.; Masiero, S.; Sinha Roy, D.; Bencivenga, S.; Roig-Villanova, I.; Ditengou, F.A.; Palme, K.; Simon, R.; Colombo, L. Maternal control of PIN1 is required for female gametophyte development in Arabidopsis. PLoS ONE 2013, 8, e66148. [Google Scholar]
- Shen, X.; He, J.; Ping, Y.; Guo, J.; Hou, N.; Cao, F.; Li, X.; Geng, D.; Wang, S.; Chen, P.; et al. The positive feedback regulatory loop of miR160-Auxin Response Factor 17-HYPONASTIC LEAVES 1 mediates drought tolerance in apple trees. Plant Physiol. 2022, 188, 1686–1708. [Google Scholar]
- Pessino, S.; Cucinotta, M.; Colono, C.; Costantini, E.; Perrone, D.; Di Marzo, M.; Callizaya Terceros, G.; Petrella, R.; Mizzotti, C.; Azzaro, C.; et al. Auxin response factor 10 insensitive to miR160regulation induces apospory-like phenotypes in Arabidopsis. iScience 2024, 27, 111115. [Google Scholar]
- Mizzotti, C.; Ezquer, I.; Paolo, D.; Rueda-Romero, P.; Guerra, R.F.; Battaglia, R.; Rogachev, I.; Aharoni, A.; Kater, M.M.; Caporali, E.; et al. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genet. 2014, 10, e1004856. [Google Scholar]
- Takei, T.; Tsukada, M.; Tamura, K.; Hara-Nishimura, I.; Fukao, Y.; Kurihara, Y.; Matsui, M.; Saze, H.; Tsuzuki, M.; Watanabe, Y.; et al. ARGONAUTE1-binding Tudor domain proteins function in small interfering RNA production for RNA-directed DNA methylation. Plant Physiol. 2024, 195, 1333–1346. [Google Scholar] [PubMed]
- Herud-Sikimic, O.; Stiel, A.C.; Kolb, M.; Shanmugaratnam, S.; Berendzen, K.W.; Feldhaus, C.; Hocker, B.; Jurgens, G. A biosensor for the direct visualization of auxin. Nature 2021, 592, 768–772. [Google Scholar]
- Mendes, M.A.; Petrella, R.; Cucinotta, M.; Vignati, E.; Gatti, S.; Pinto, S.C.; Bird, D.C.; Gregis, V.; Dickinson, H.; Tucker, M.R.; et al. The RNA-dependent DNA methylation pathway is required to restrict SPOROCYTELESS/NOZZLE expression to specify a single female germ cell precursor in Arabidopsis. Development 2020, 147, dev194274. [Google Scholar] [PubMed]
- Zhao, X.; Bramsiepe, J.; Van Durme, M.; Komaki, S.; Prusicki, M.A.; Maruyama, D.; Forner, J.; Medzihradszky, A.; Wijnker, E.; Harashima, H.; et al. RETINOBLASTOMA RELATED1 mediates germline entry in Arabidopsis. Science 2017, 356, eaaf6532. [Google Scholar]
- Cao, L.; Wang, S.; Venglat, P.; Zhao, L.; Cheng, Y.; Ye, S.; Qin, Y.; Datla, R.; Zhou, Y.; Wang, H. Arabidopsis ICK/KRP cyclin-dependent kinase inhibitors function to ensure the formation of one megaspore mother cell and one functional megaspore per ovule. PLoS Genet. 2018, 14, e1007230. [Google Scholar]
- Gentric, N.; Masoud, K.; Journot, R.P.; Cognat, V.; Chabouté, M.E.; Noir, S.; Genschik, P. The F-Box-Like Protein FBL17 Is a Regulator of DNA-Damage Response and Colocalizes with RETINOBLASTOMA RELATED1 at DNA Lesion Sites. Plant Physiol. 2020, 183, 1295–1305. [Google Scholar]
- Zhao, L.; Cai, H.; Su, Z.; Wang, L.; Huang, X.; Zhang, M.; Chen, P.; Dai, X.; Zhao, H.; Palanivelu, R.; et al. KLU suppresses megasporocyte cell fate through SWR1-mediated activation of WRKY28 expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E526–E535. [Google Scholar]
- Nardeli, S.M.; Arge, L.W.P.; Artico, S.; de Moura, S.M.; Tschoeke, D.A.; de Freitas Guedes, F.A.; Grossi-de-Sa, M.F.; Martinelli, A.P.; Alves-Ferreira, M. Global gene expression profile and functional analysis reveal the conservation of reproduction-associated gene networks in Gossypium hirsutum. Plant Reprod. 2024, 37, 215–227. [Google Scholar]
- Schmidt, A.; Wuest, S.E.; Vijverberg, K.; Baroux, C.; Kleen, D.; Grossniklaus, U. Transcriptome analysis of the Arabidopsis megaspore mother cell uncovers the importance of RNA helicases for plant germline development. PLoS Biol. 2011, 9, e1001155. [Google Scholar] [CrossRef]
- Liu, H.; Luo, Q.; Tan, C.; Song, J.; Zhang, T.; Men, S. Biosynthesis- and transport-mediated dynamic auxin distribution during seed development controls seed size in Arabidopsis. Plant J. 2023, 113, 1259–1277. [Google Scholar] [PubMed]
- Yang, Q.; Wang, J.; Zhang, S.; Zhan, Y.; Shen, J.; Chang, F. ARF3-Mediated Regulation of SPL in Early Anther Morphogenesis: Maintaining Precise Spatial Distribution and Expression Level. Int. J. Mol. Sci. 2023, 24, 11740. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Liu, M.; Ai, G.; Zhang, X.; Wang, J.; Tian, S.; Yuan, L. A sexually and vegetatively reproducible diploid seedless watermelon inducer via ClHAP2 mutation. Nat. Plants 2024, 10, 1446–1452. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.-Y.; Wang, P.; Lv, Y.; Wang, B.; Zhao, M.-R.; Dong, X.-W. Auxin Orchestrates Germ Cell Specification in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 3257. https://doi.org/10.3390/ijms26073257
Yu T-Y, Wang P, Lv Y, Wang B, Zhao M-R, Dong X-W. Auxin Orchestrates Germ Cell Specification in Arabidopsis. International Journal of Molecular Sciences. 2025; 26(7):3257. https://doi.org/10.3390/ijms26073257
Chicago/Turabian StyleYu, Tian-Ying, Ping Wang, Yue Lv, Bo Wang, Ming-Ri Zhao, and Xin-Wei Dong. 2025. "Auxin Orchestrates Germ Cell Specification in Arabidopsis" International Journal of Molecular Sciences 26, no. 7: 3257. https://doi.org/10.3390/ijms26073257
APA StyleYu, T.-Y., Wang, P., Lv, Y., Wang, B., Zhao, M.-R., & Dong, X.-W. (2025). Auxin Orchestrates Germ Cell Specification in Arabidopsis. International Journal of Molecular Sciences, 26(7), 3257. https://doi.org/10.3390/ijms26073257





