Spindle Orientation Regulation Is Governed by Redundant Cortical Mechanosensing and Shape-Sensing Mechanisms
Abstract
1. Introduction
2. Results
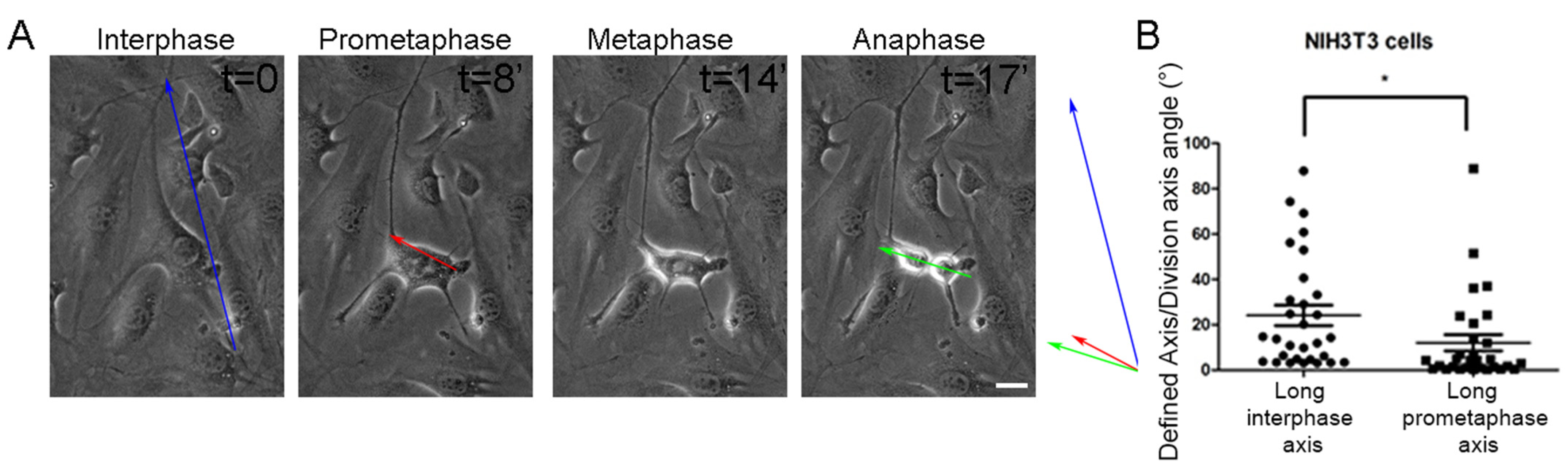
2.1. Geometric Anisotropy During Prometaphase Is a Better Predictor of SO than the Long Interphase Axis
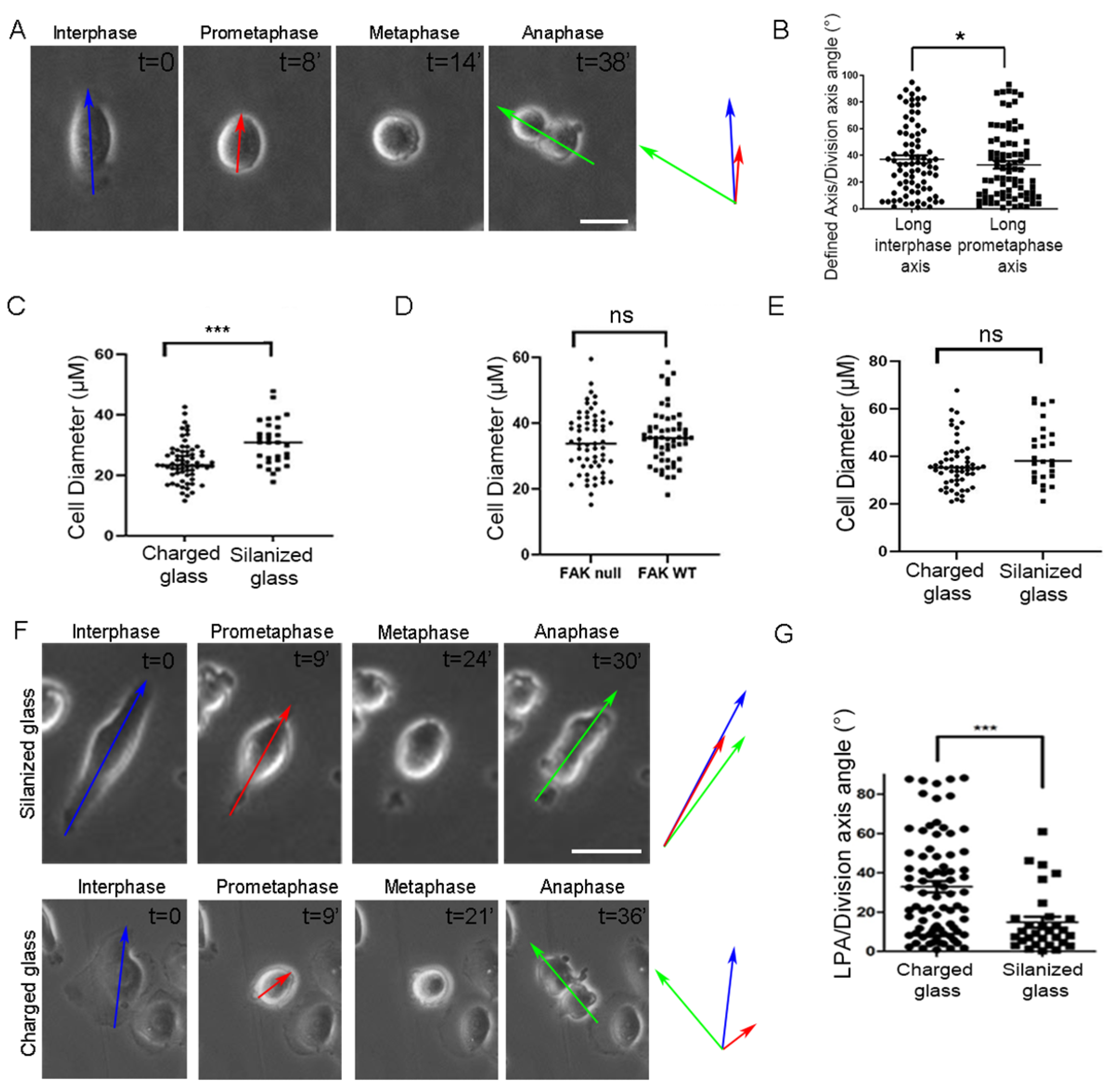
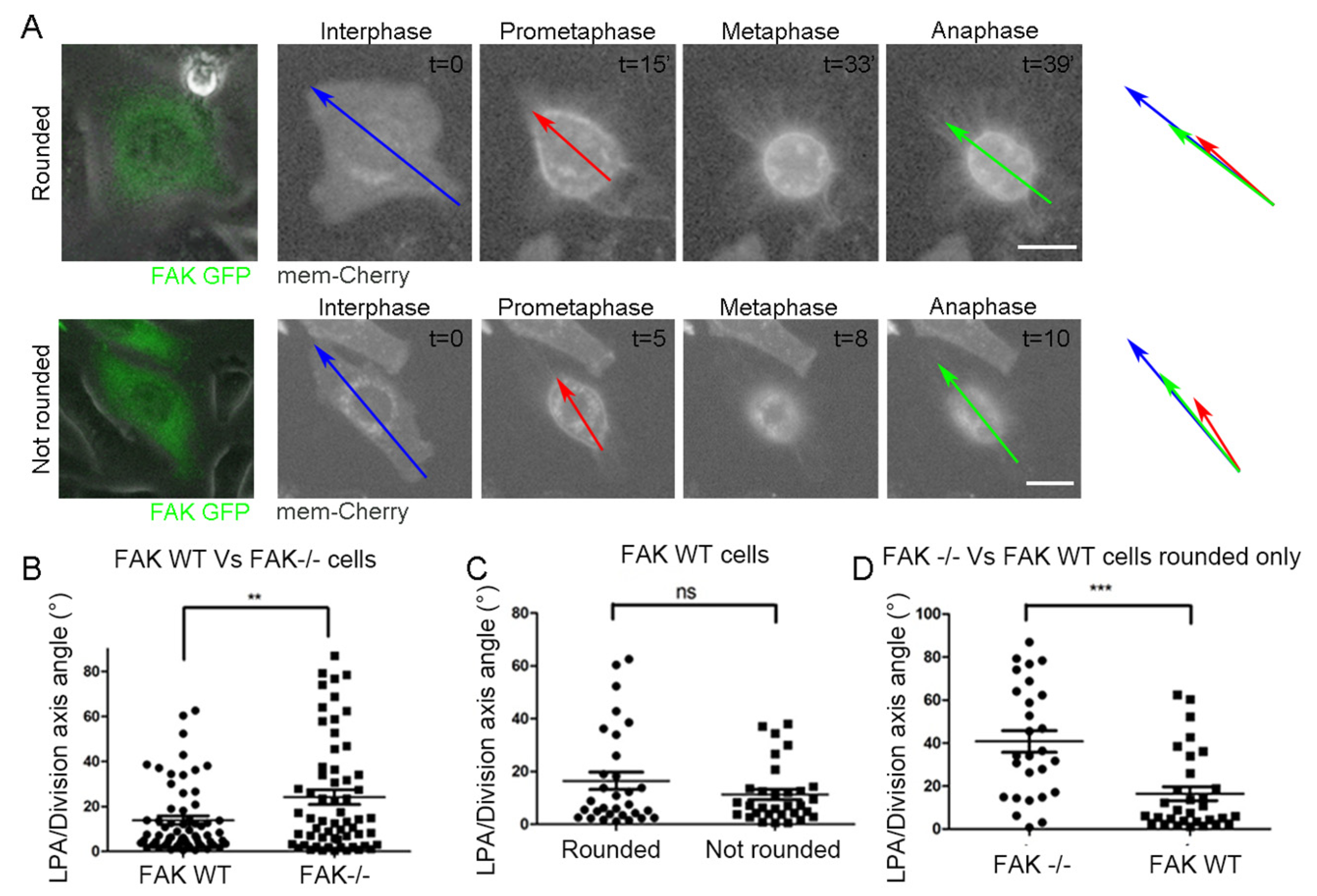
2.2. FAK Null Cells Fail to Orient Along the Long Interphase and Long Prometaphase Axis
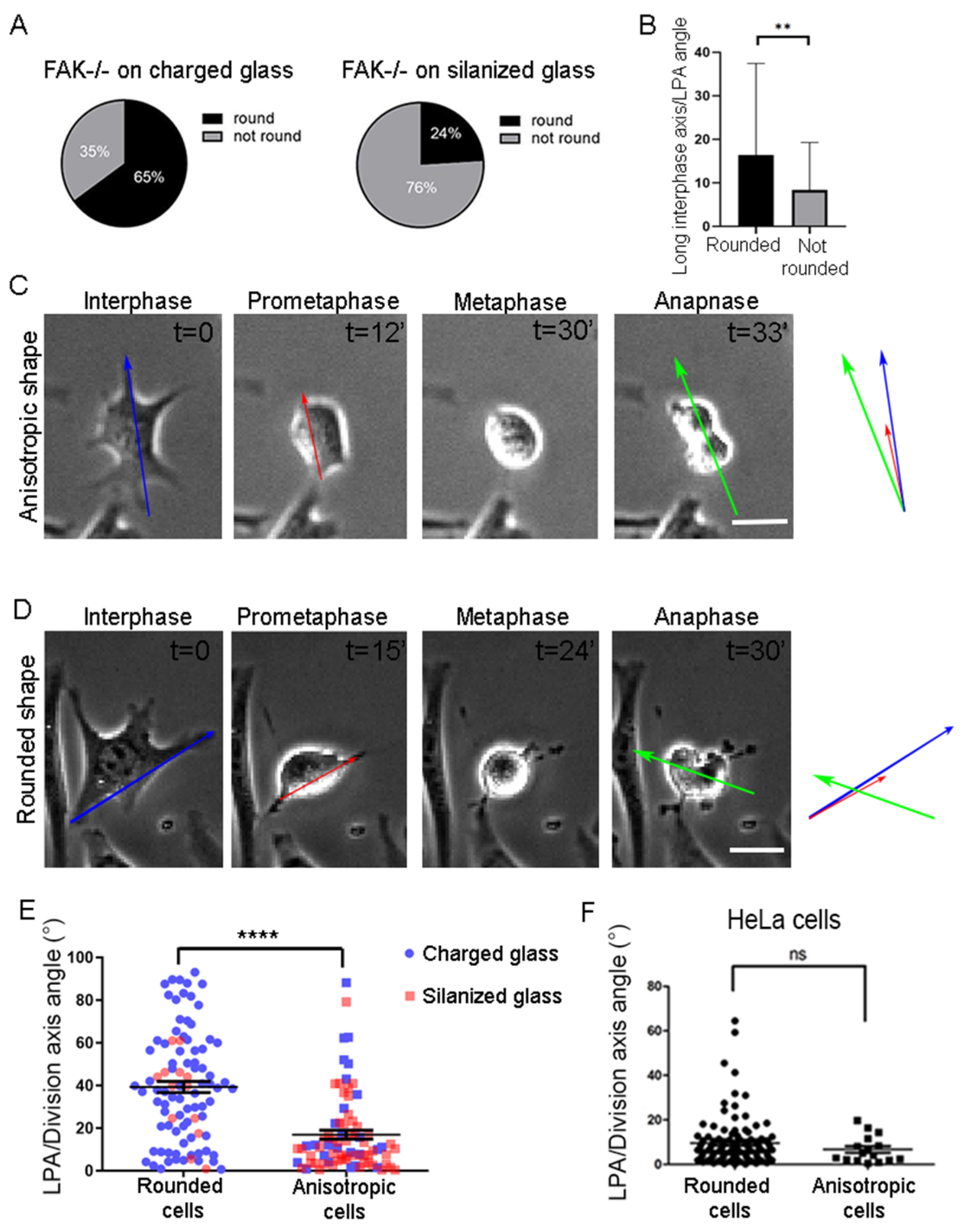
2.3. Increased Substrate Density Rescues FAK SO Defects
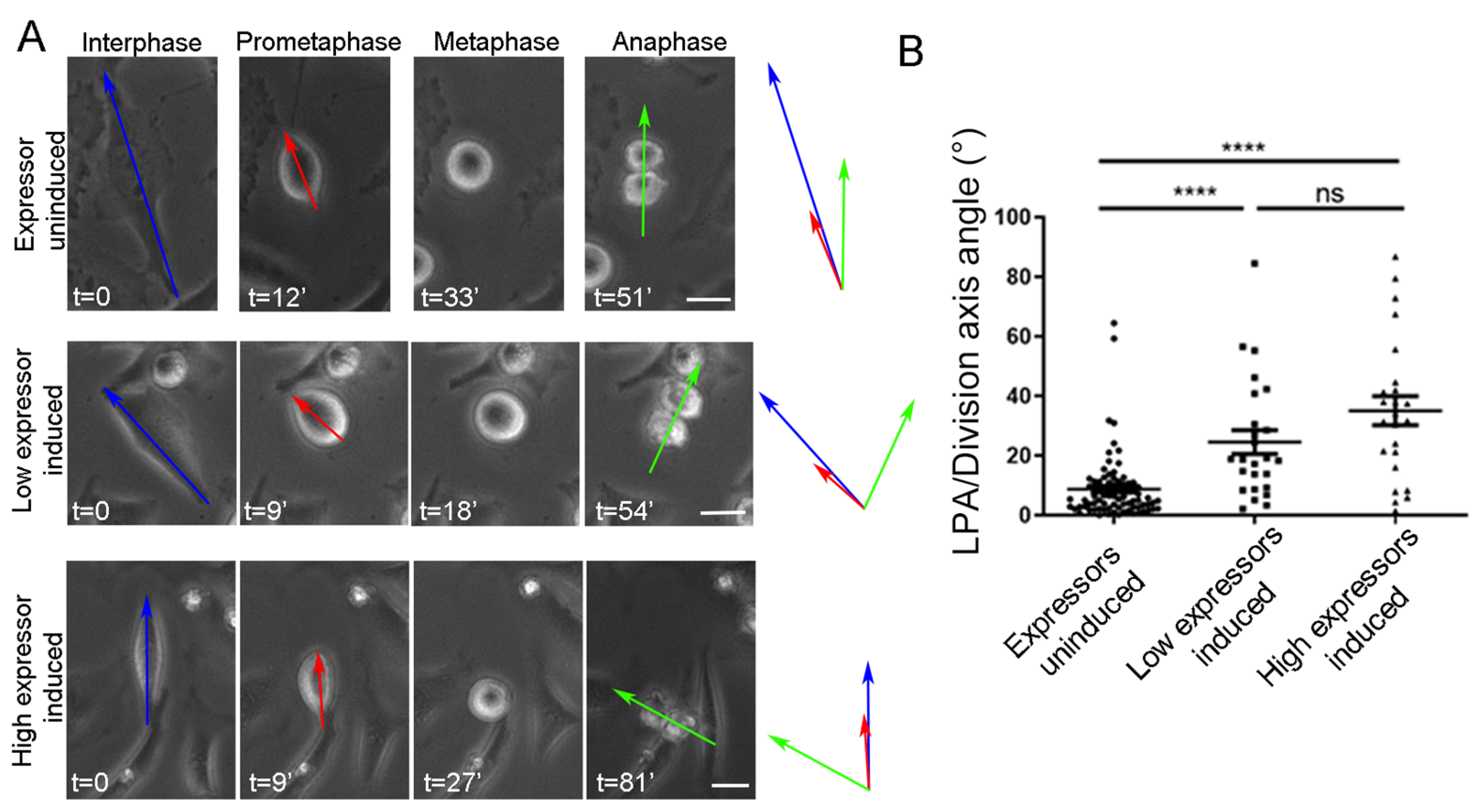
2.4. SO Is Governed by Parallel Acting Mechanisms
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Silanization of Coverslips
4.3. Substrate Coatings
4.4. Generation of Different Stiffness Polyacrylamide Gels
4.5. Imaging
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nestor-Bergmann, A.; Goddard, G.; Woolner, S. Force and the spindle: Mechanical cues in mitotic spindle orientation. Semin. Cell Dev. Biol. 2014, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Baena-López, L.A.; Baonza, A.; García-Bellido, A. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. CB 2005, 15, 1640–1644. [Google Scholar] [CrossRef]
- Quesada-Hernández, E.; Caneparo, L.; Schneider, S.; Winkler, S.; Liebling, M.; Fraser, S.E.; Heisenberg, C.-P. Stereotypical Cell Division Orientation Controls Neural Rod Midline Formation in Zebrafish. Curr. Biol. 2010, 20, 1966–1972. [Google Scholar] [CrossRef]
- Pease, J.C.; Tirnauer, J.S. Mitotic spindle misorientation in cancer--out of alignment and into the fire. J. Cell Sci. 2011, 124, 1007–1016. [Google Scholar] [CrossRef]
- Quyn, A.J.; Appleton, P.L.; Carey, F.A.; Steele, R.J.; Barker, N.; Clevers, H.; Ridgway, R.A.; Sansom, O.J.; Näthke, I.S. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 2010, 6, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Woolner, S.; Papalopulu, N. Spindle position in symmetric cell divisions during epiboly is controlled by opposing and dynamic apicobasal forces. Dev. Cell 2012, 22, 775–787. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.M.; Vincent, J.P. Oriented cell divisions in the extending germband of Drosophila. Development 2007, 134, 3049–3054. [Google Scholar] [CrossRef]
- Mao, Y.; Tournier, A.L.; Bates, P.A.; Gale, J.E.; Tapon, N.; Thompson, B.J. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 2011, 25, 131–136. [Google Scholar] [CrossRef]
- Tawk, M.; Araya, C.; Lyons, D.A.; Reugels, A.M.; Girdler, G.C.; Bayley, P.R.; Hyde, D.R.; Tada, M.; Clarke, J.D. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature 2007, 446, 797–800. [Google Scholar] [CrossRef]
- Buckley, S.A.; Appelbaum, F.R.; Walter, R.B. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013, 48, 630–641. [Google Scholar] [CrossRef]
- Petridou, N.I.; Skourides, P.A. FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat. Commun. 2014, 5, 5240. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, H.; Wan, Q.; Liu, J.; Xiao, Z.; Siderovski, D.P.; Du, Q. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 2010, 189, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wen, W.; Zheng, Z.; Shang, Y.; Wei, Z.; Xiao, Z.; Pan, Z.; Du, Q.; Wang, W.; Zhang, M. LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Gαi/LGN/NuMA pathways. Mol. Cell 2011, 43, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.C.; Tan, J.; Siemers, K.A.; Sim, J.Y.; Pruitt, B.L.; Nelson, W.J.; Gloerich, M. E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape. Proc. Natl. Acad. Sci. USA 2017, 114, E5845–E5853. [Google Scholar] [CrossRef]
- Peyre, E.; Jaouen, F.; Saadaoui, M.; Haren, L.; Merdes, A.; Durbec, P.; Morin, X. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J. Cell Biol. 2011, 193, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.E.; Huang, N.N.; Cho, H.; Miki, T.; Tall, G.G.; Kehrl, J.H. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol. Cell. Biol. 2010, 30, 3519–3530. [Google Scholar] [CrossRef]
- Minc, N.; Burgess, D.; Chang, F. Influence of cell geometry on division-plane positioning. Cell 2011, 144, 414–426. [Google Scholar] [CrossRef]
- O′Connell, C.B.; Wang, Y.L. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell 2000, 11, 1765–1774. [Google Scholar] [CrossRef]
- Strauss, B.; Adams, R.J.; Papalopulu, N. A default mechanism of spindle orientation based on cell shape is sufficient to generate cell fate diversity in polarised Xenopus blastomeres. Development 2006, 133, 3883–3893. [Google Scholar] [CrossRef]
- Théry, M.; Racine, V.; Pépin, A.; Piel, M.; Chen, Y.; Sibarita, J.-B.; Bornens, M. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 2005, 7, 947–953. [Google Scholar] [CrossRef]
- Théry, M.; Jiménez-Dalmaroni, A.; Racine, V.; Bornens, M.; Jülicher, F. Experimental and theoretical study of mitotic spindle orientation. Nature 2007, 447, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Carpi, N.; Betz, T.; Bétard, A.; Chebah, M.; Azioune, A.; Bornens, M.; Sykes, C.; Fetler, L.; Cuvelier, D.; et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 2011, 13, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Petridou, N.I.; Skourides, P.A. A ligand-independent integrin β1 mechanosensory complex guides spindle orientation. Nat. Commun. 2016, 7, 10899. [Google Scholar] [CrossRef]
- Tarannum, N.; Hargreaves, D.; Ilieva, D.; Goddard, G.K.; Jensen, O.E.; Woolner, S. Mitotic spindle orientation and dynamics are fine-tuned by anisotropic tissue stretch via NuMA localisation. bioRxiv 2024. bioRxiv:2024.10.17.617436. [Google Scholar]
- Wang, H.B.; Dembo, M.; Hanks, S.K.; Wang, Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA 2001, 98, 11295–11300. [Google Scholar] [CrossRef]
- Ilić, D.; Furuta, Y.; Kanazawa, S.; Takeda, N.; Sobue, K.; Nakatsuji, N.; Nomura, S.; Fujimoto, J.; Okada, M.; Yamamoto, T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995, 377, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.D.; Ruest, P.J.; Fry, D.W.; Hanks, S.K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999, 19, 4806–4818. [Google Scholar] [CrossRef]
- Michael, K.E.; Dumbauld, D.W.; Burns, K.L.; Hanks, S.K.; García, A.J. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell 2009, 20, 2508–2519. [Google Scholar] [CrossRef]
- Hyunh, N.B.; Palma, C.S.D.; Rahikainen, R.; Mishra, A.; Azizi, L.; Verne, E.; Ferraris, S.; Hytönen, V.P.; Sanches Ribeiro, A.; Massera, J. Surface Modification of Bioresorbable Phosphate Glasses for Controlled Protein Adsorption. ACS Biomater. Sci. Eng. 2021, 7, 4483–4493. [Google Scholar] [CrossRef]
- Gerardo, H.; Lima, A.; Carvalho, J.; Ramos, J.R.D.; Couceiro, S.; Travasso, R.D.M.; das Neves, R.P.; Grãos, M. Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction. Sci. Rep. 2019, 9, 9086. [Google Scholar] [CrossRef]
- Vela-Alcántara, A.M.; Santiago-García, J.; Barragán-Palacios, M.; León-Chacón, A.; Domínguez-Pantoja, M.; Barceinas-Dávila, I.; Juárez-Aguilar, E.; Tamariz, E. Differential modulation of cell morphology, migration, and Neuropilin-1 expression in cancer and non-cancer cell lines by substrate stiffness. Front. Cell Dev. Biol. 2024, 12, 1352233. [Google Scholar] [CrossRef] [PubMed]
- Doss, B.L.; Pan, M.; Gupta, M.; Grenci, G.; Mège, R.-M.; Lim, C.T.; Sheetz, M.P.; Voituriez, R.; Ladoux, B. Cell response to substrate rigidity is regulated by active and passive cytoskeletal stress. Proc. Natl. Acad. Sci. USA 2020, 117, 12817–12825. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Karadaghy, A.; Zustiak, S. Simple polyacrylamide-based multiwell stiffness assay for the study of stiffness-dependent cell responses. J. Vis. Exp. 2015, 52643. [Google Scholar] [CrossRef]
- Li, J.; Cheng, L.; Jiang, H. Cell shape and intercellular adhesion regulate mitotic spindle orientation. Mol. Biol. Cell 2019, 30, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, I.; Kyriakou, M.; Charalambous, A.; Kalalidou, K.; Christodoulou, A.; Christoforou, M.; Skourides, P.A. FAK displacement from focal adhesions: A promising strategy to target processes implicated in cancer progression and metastasis. Cell Commun. Signal. CCS 2021, 19, 3. [Google Scholar] [CrossRef]
- Gowing, L.R.; Tellam, R.L.; Banyard, M.R. Microfilament organization and total actin content are decreased in hybrids derived from the fusion of HeLa cells with human fibroblasts. J. Cell Sci. 1984, 69, 137–146. [Google Scholar] [CrossRef]
- Gruenstein, E.; Rich, A.; Weihing, R.R. Actin associated with membranes from 3T3 mouse fibroblast and HeLa cells. J. Cell Biol. 1975, 64, 223–234. [Google Scholar] [CrossRef]
- Théry, M.; Pépin, A.; Dressaire, E.; Chen, Y.; Bornens, M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil. Cytoskelet. 2006, 63, 341–355. [Google Scholar] [CrossRef]
- Nestor-Bergmann, A.; Stooke-Vaughan, G.A.; Goddard, G.K.; Starborg, T.; Jensen, O.E.; Woolner, S. Decoupling the Roles of Cell Shape and Mechanical Stress in Orienting and Cueing Epithelial Mitosis. Cell Rep. 2019, 26, 2088–2100.e2084. [Google Scholar] [CrossRef]
- Wyatt, T.P.J.; Harris, A.R.; Lam, M.; Cheng, Q.; Bellis, J.; Dimitracopoulos, A.; Kabla, A.J.; Charras, G.T.; Baum, B. Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc. Natl. Acad. Sci. USA 2015, 112, 5726–5731. [Google Scholar] [CrossRef]
- Matsumura, S.; Kojidani, T.; Kamioka, Y.; Uchida, S.; Haraguchi, T.; Kimura, A.; Toyoshima, F. Interphase adhesion geometry is transmitted to an internal regulator for spindle orientation via caveolin-1. Nat. Commun. 2016, 7, ncomms11858. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-P.; Aretz, J.; Fässler, R. CDK1–cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry. Nat. Cell Biol. 2022, 24, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.C.; Askari, J.A.; Humphries, J.D.; Humphries, M.J. Cell adhesion is regulated by CDK1 during the cell cycle. J. Cell Biol. 2018, 217, 3203–3218. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.P.; Helenius, J.; Stewart, M.P.; Cattin, C.J.; Hyman, A.A.; Muller, D.J. Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement. Nat. Cell Biol. 2015, 17, 148–159. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjisavva, R.; Skourides, P.A. Spindle Orientation Regulation Is Governed by Redundant Cortical Mechanosensing and Shape-Sensing Mechanisms. Int. J. Mol. Sci. 2025, 26, 5730. https://doi.org/10.3390/ijms26125730
Hadjisavva R, Skourides PA. Spindle Orientation Regulation Is Governed by Redundant Cortical Mechanosensing and Shape-Sensing Mechanisms. International Journal of Molecular Sciences. 2025; 26(12):5730. https://doi.org/10.3390/ijms26125730
Chicago/Turabian StyleHadjisavva, Rania, and Paris A. Skourides. 2025. "Spindle Orientation Regulation Is Governed by Redundant Cortical Mechanosensing and Shape-Sensing Mechanisms" International Journal of Molecular Sciences 26, no. 12: 5730. https://doi.org/10.3390/ijms26125730
APA StyleHadjisavva, R., & Skourides, P. A. (2025). Spindle Orientation Regulation Is Governed by Redundant Cortical Mechanosensing and Shape-Sensing Mechanisms. International Journal of Molecular Sciences, 26(12), 5730. https://doi.org/10.3390/ijms26125730




