Skin-Whitening, Antiwrinkle, and Moisturizing Effects of Astilboides tabularis (Hemsl.) Engl. Root Extracts in Cell-Based Assays and Three-Dimensional Artificial Skin Models
Abstract
1. Introduction
2. Results
2.1. TYR Inhibitory Activity
2.2. Elastase Inhibitory Activity
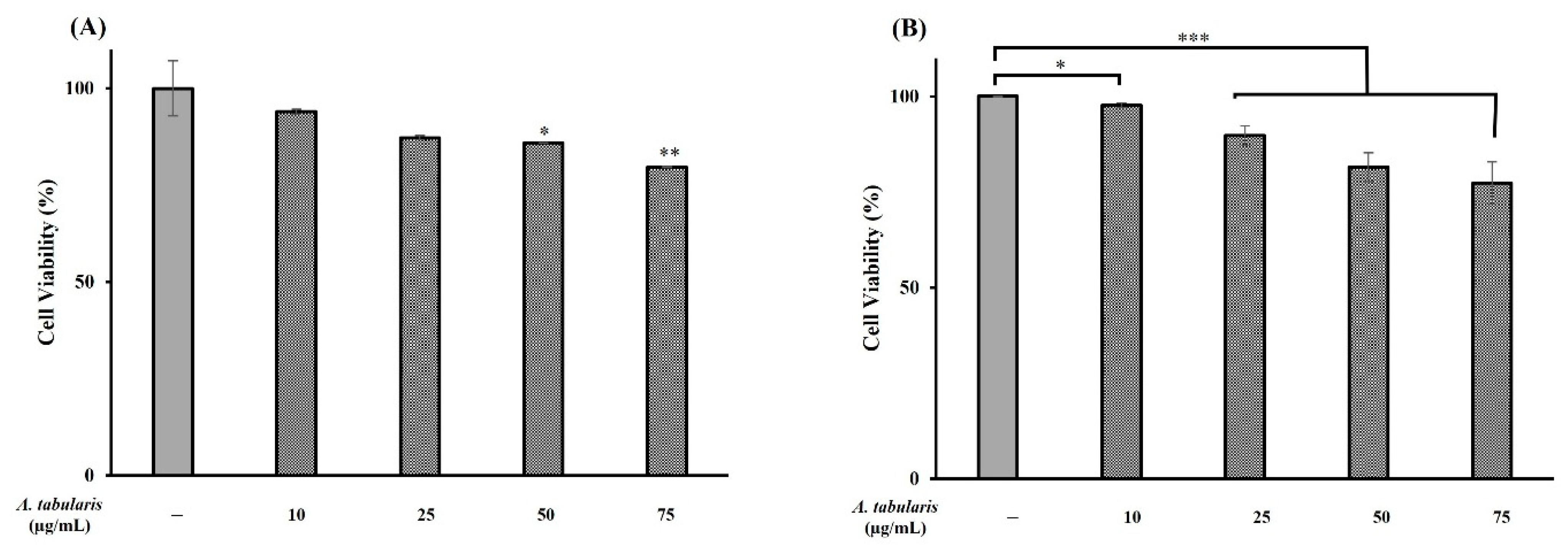
2.3. Cell Viability Assessment
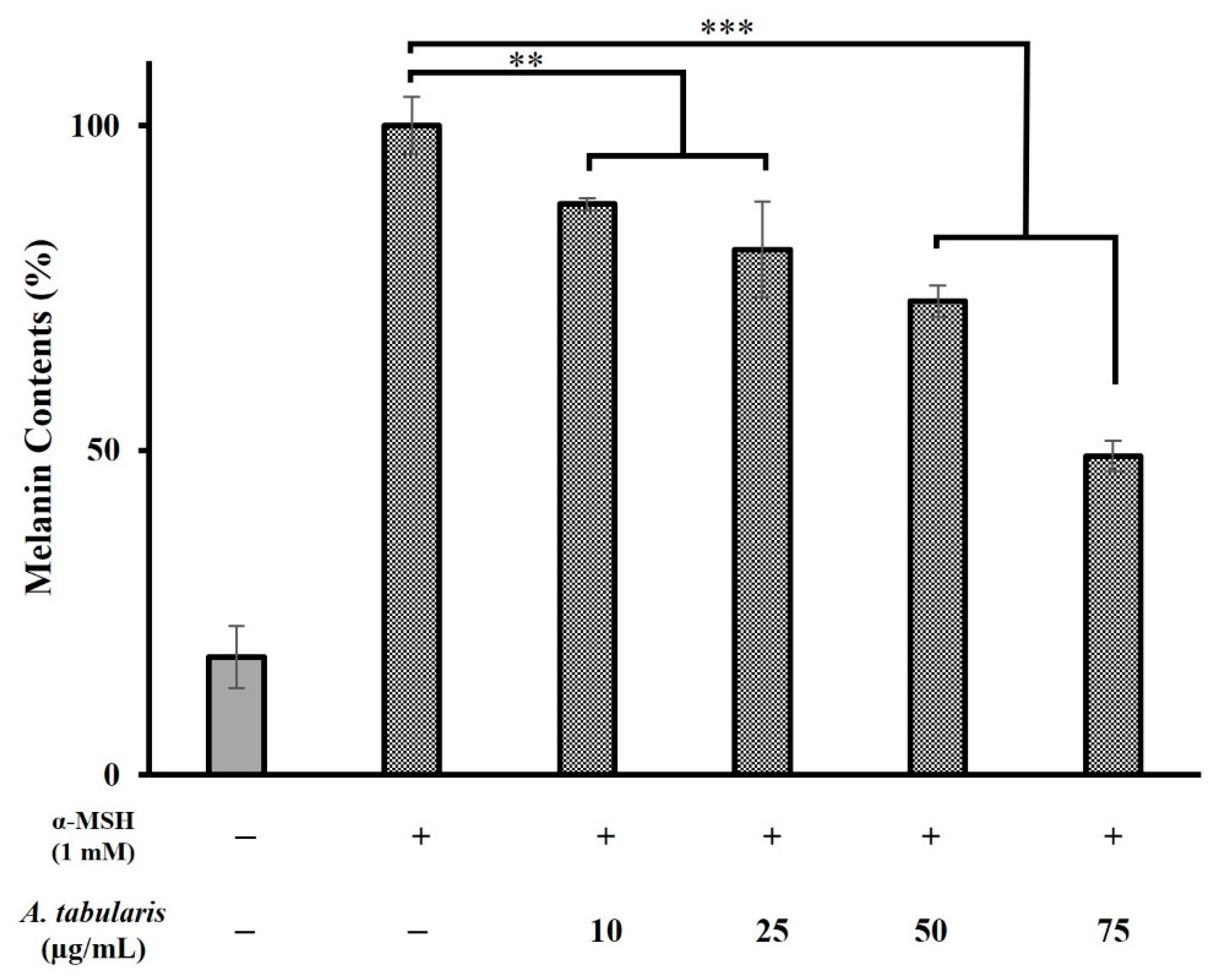
2.4. Melanin Production Inhibition in B16F10 Cells
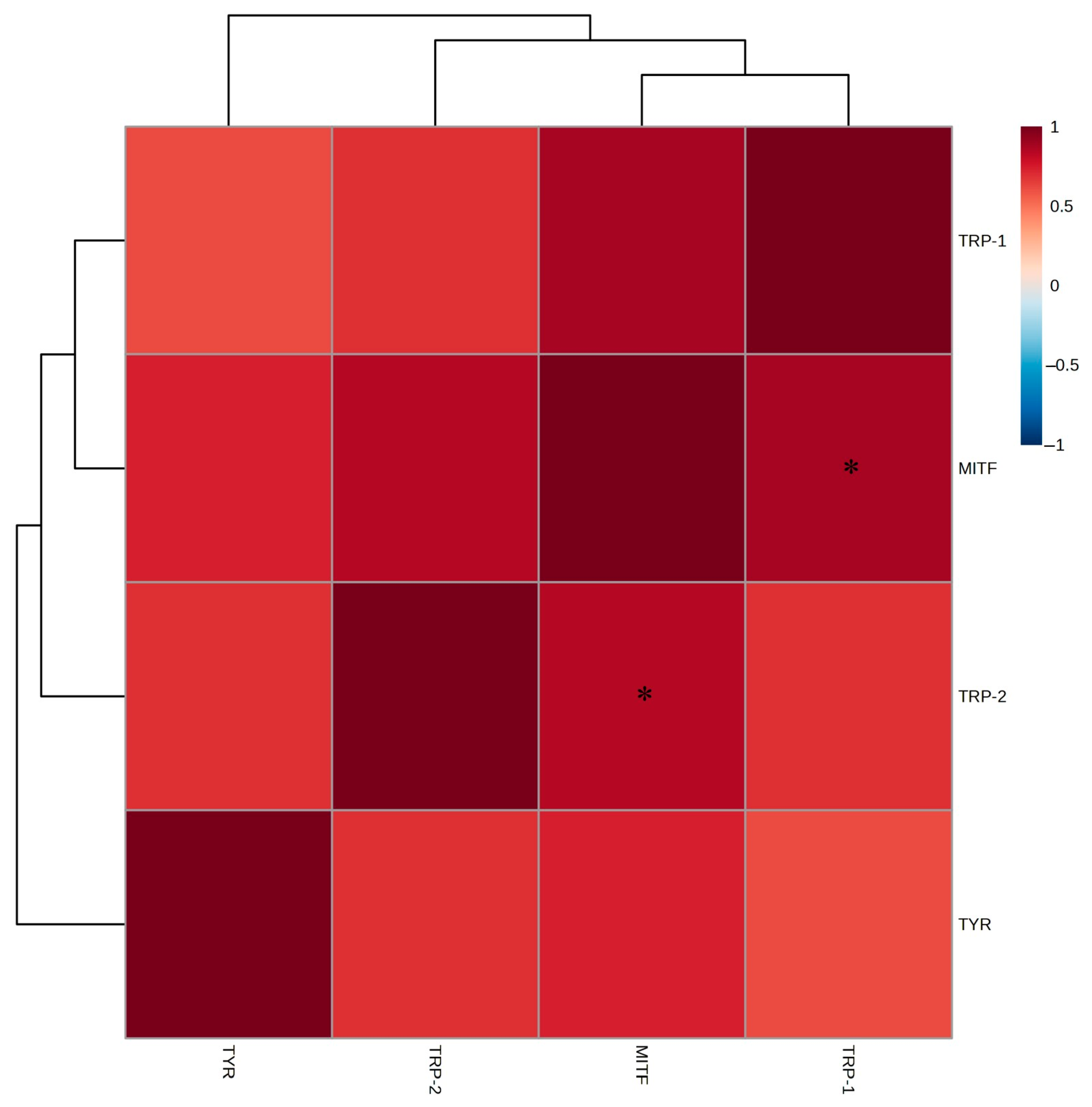
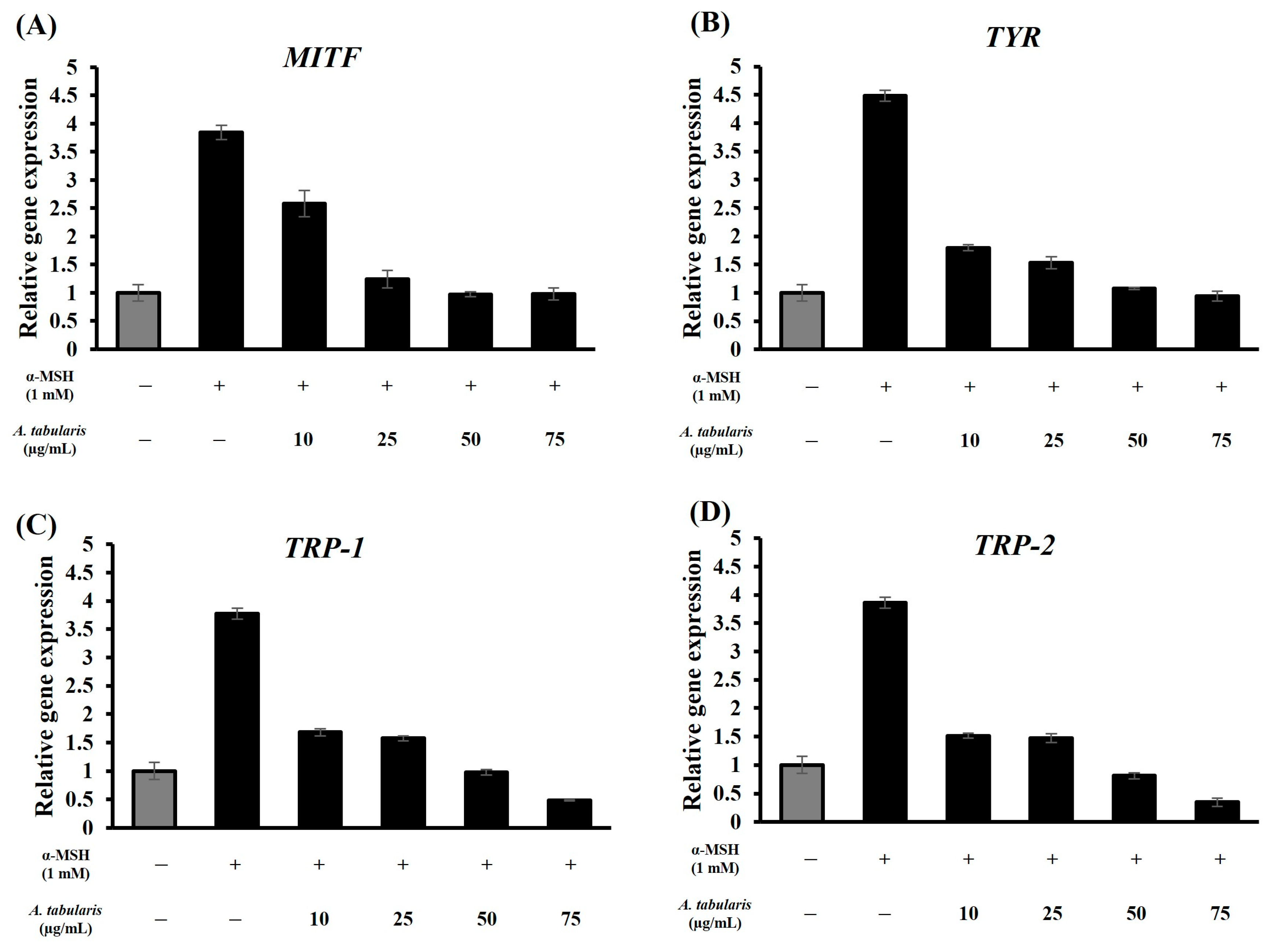
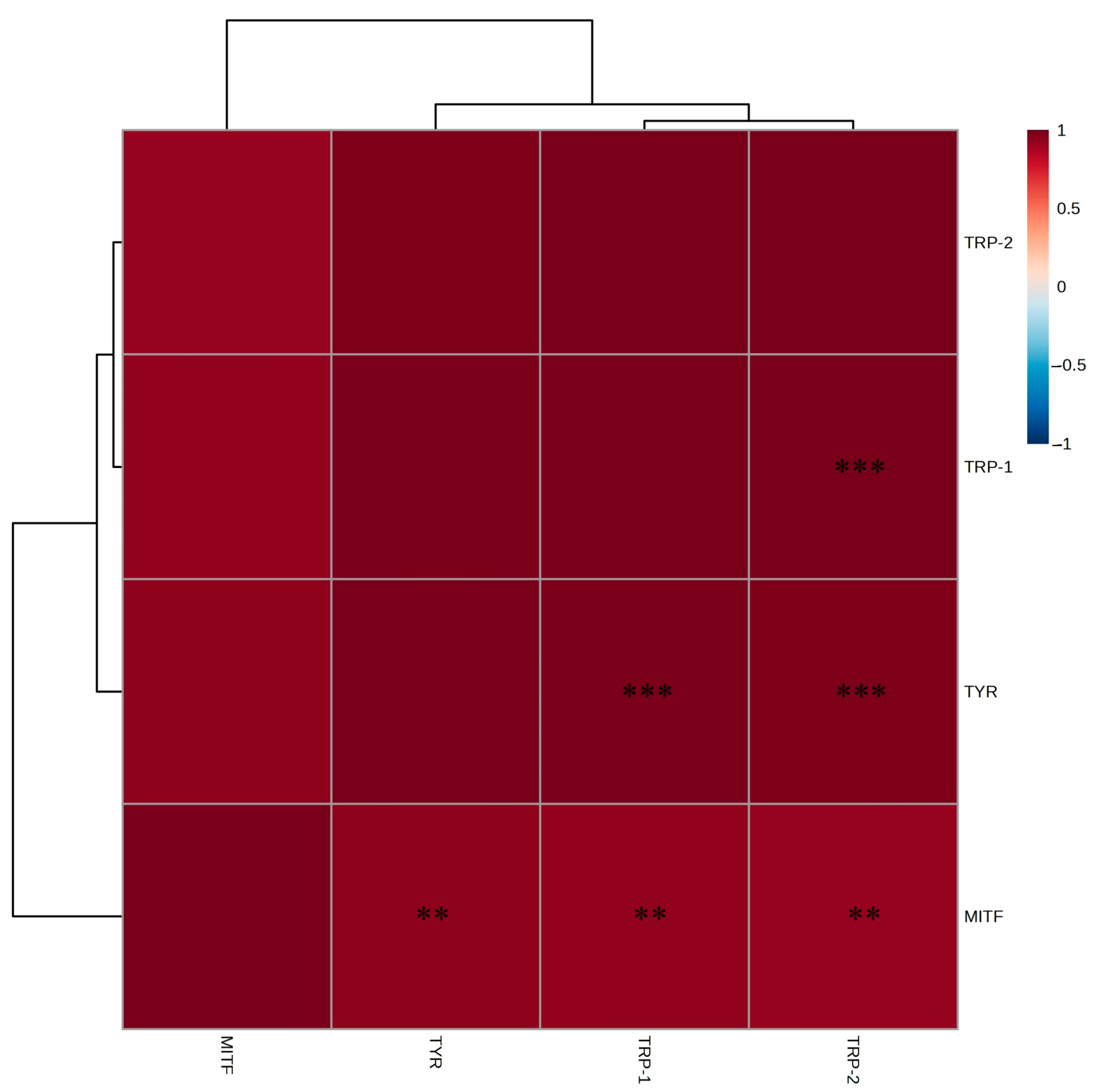
2.5. mRNA Expression Levels of Whitening-Related Factors in B16F10 Melanoma Cells
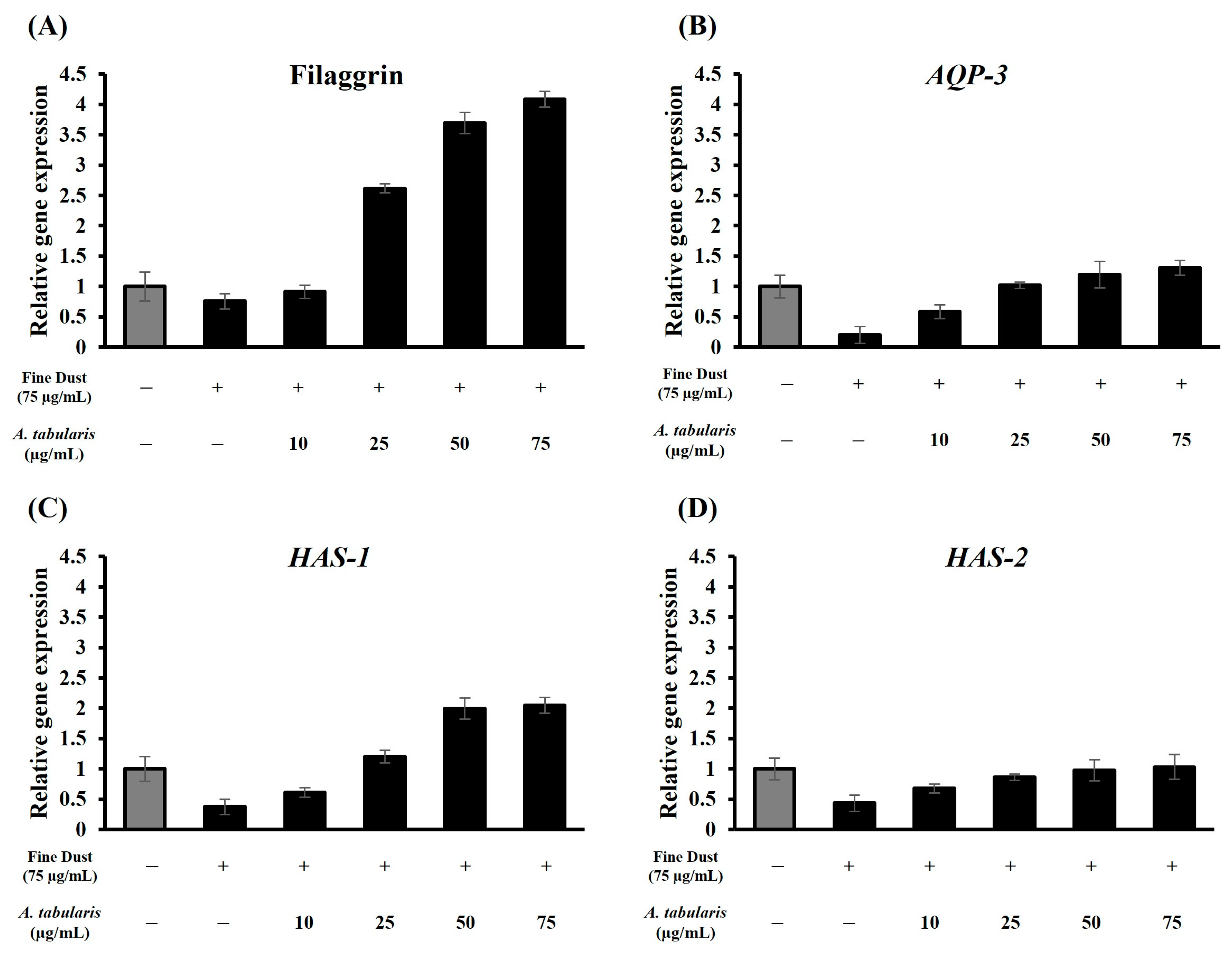
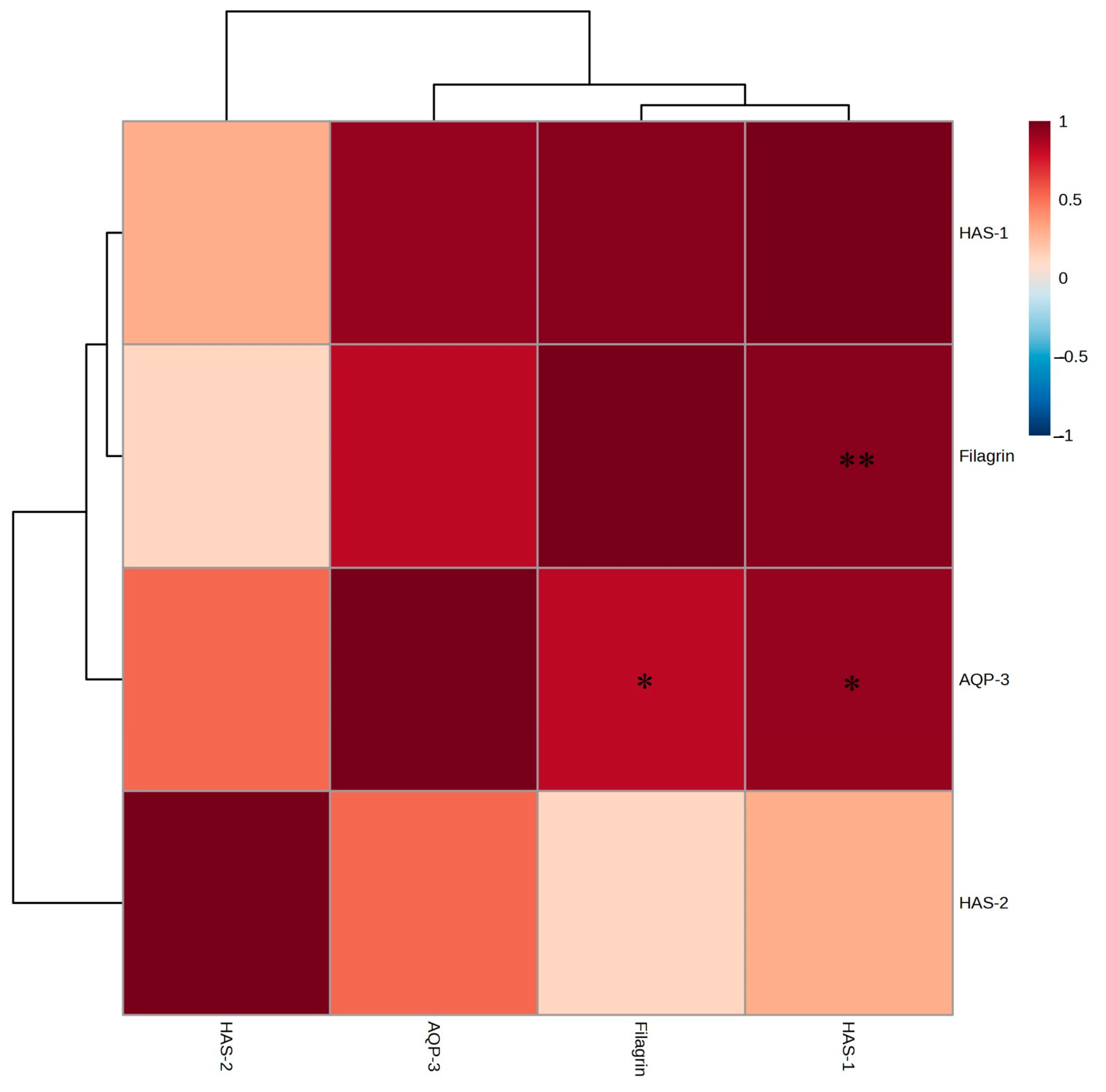
2.6. mRNA Expression Levels of Moisturizing Factors in HaCaT Cells
2.7. mRNA Expression Levels of Wrinkle-Related Factors in Detroit 551 Cells
2.8. Skin Irritation Test Using Artificial Skin Models
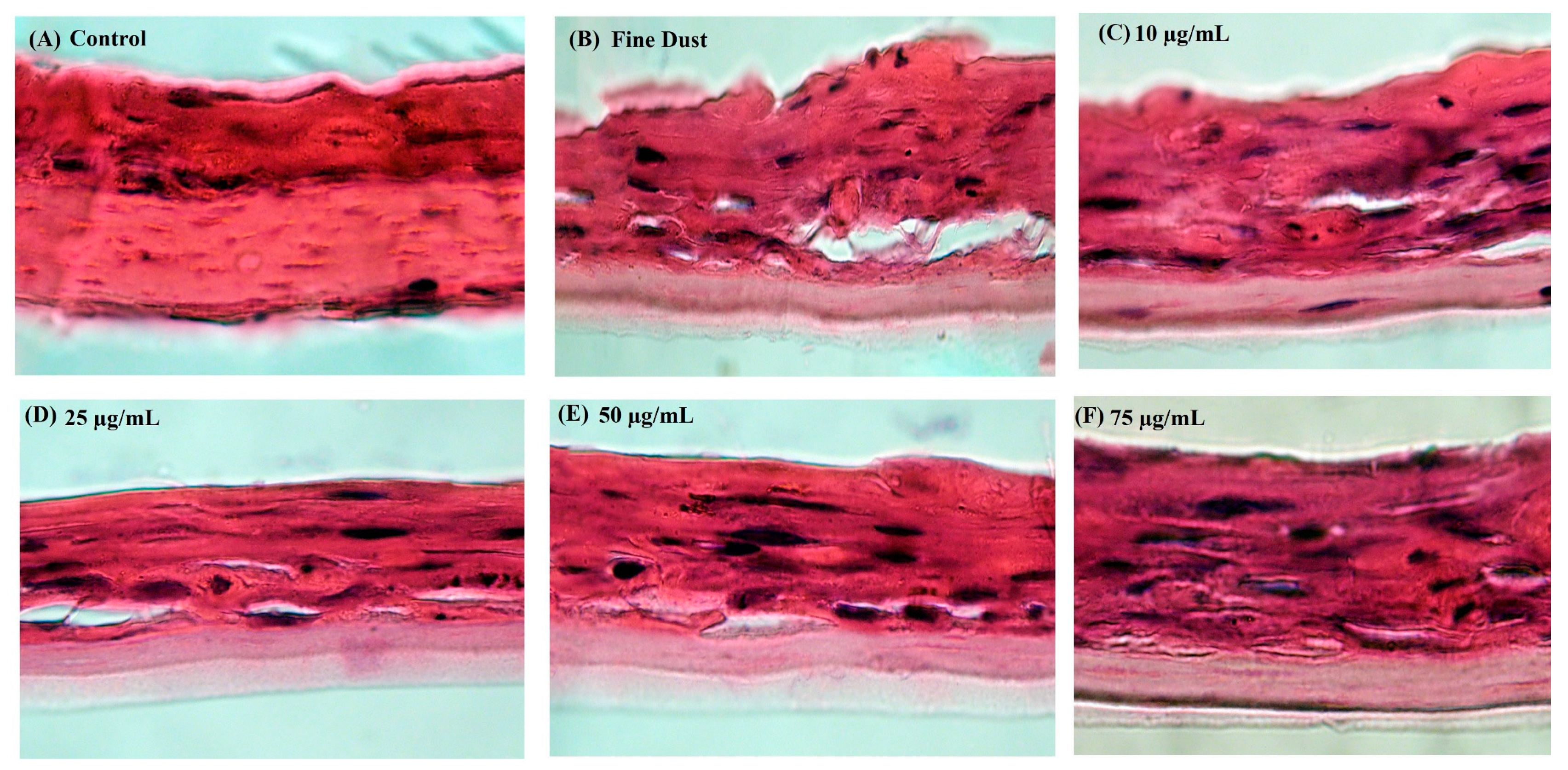
2.9. Fine Dust-Stimulated Skin Damage and Recovery Analysis in the Neoderm-ED Artificial Skin Model
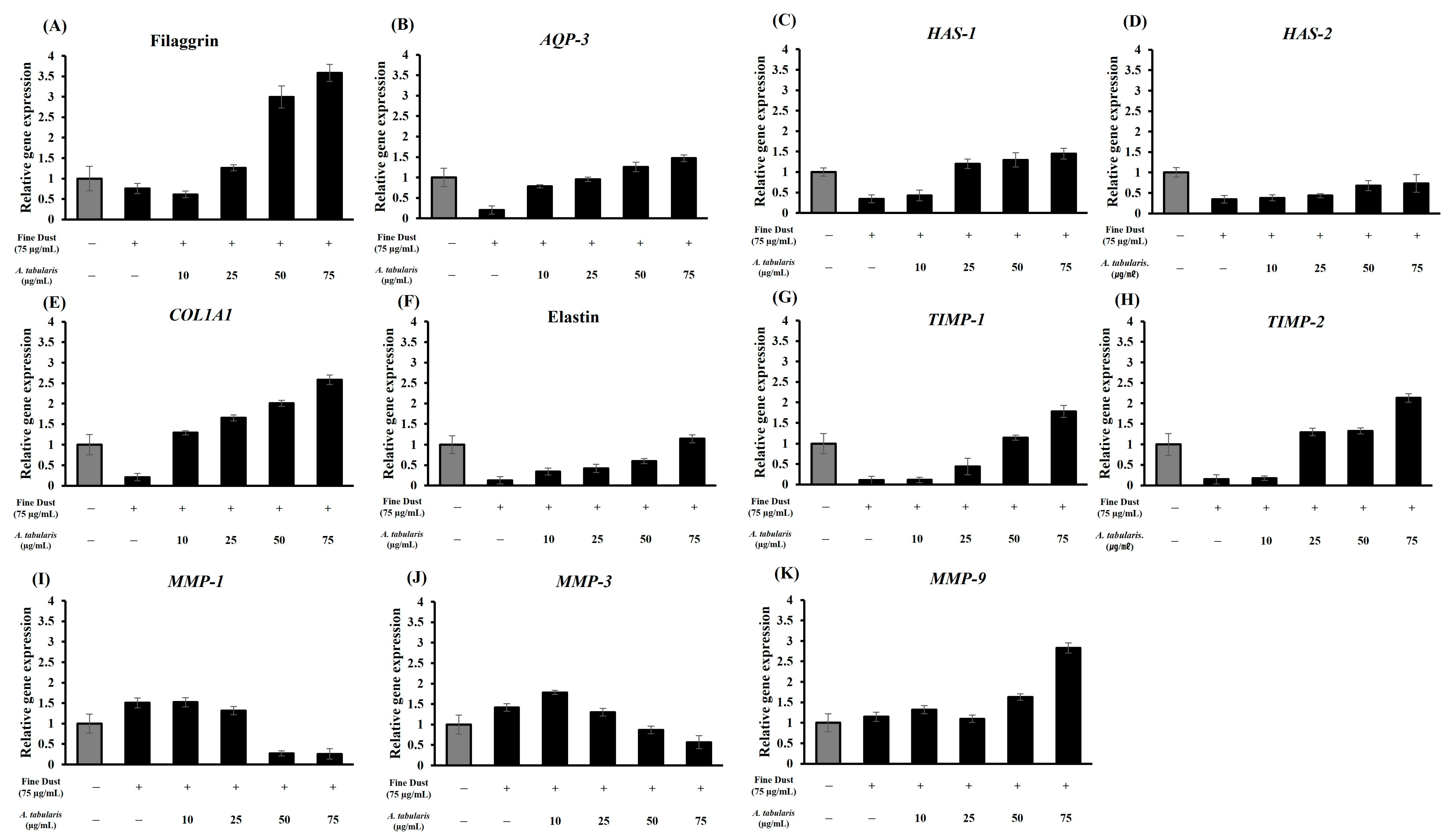
2.10. Analysis of the Skin Protection and Antiwrinkle Mechanisms in the Neoderm-ED Model
2.11. Analysis of Melanin Inhibitory Effects in the Neoderm-ME Artificial Skin Model
2.12. Analysis of Whitening-Related Gene Expression and Mechanisms in the Neoderm-ME Model
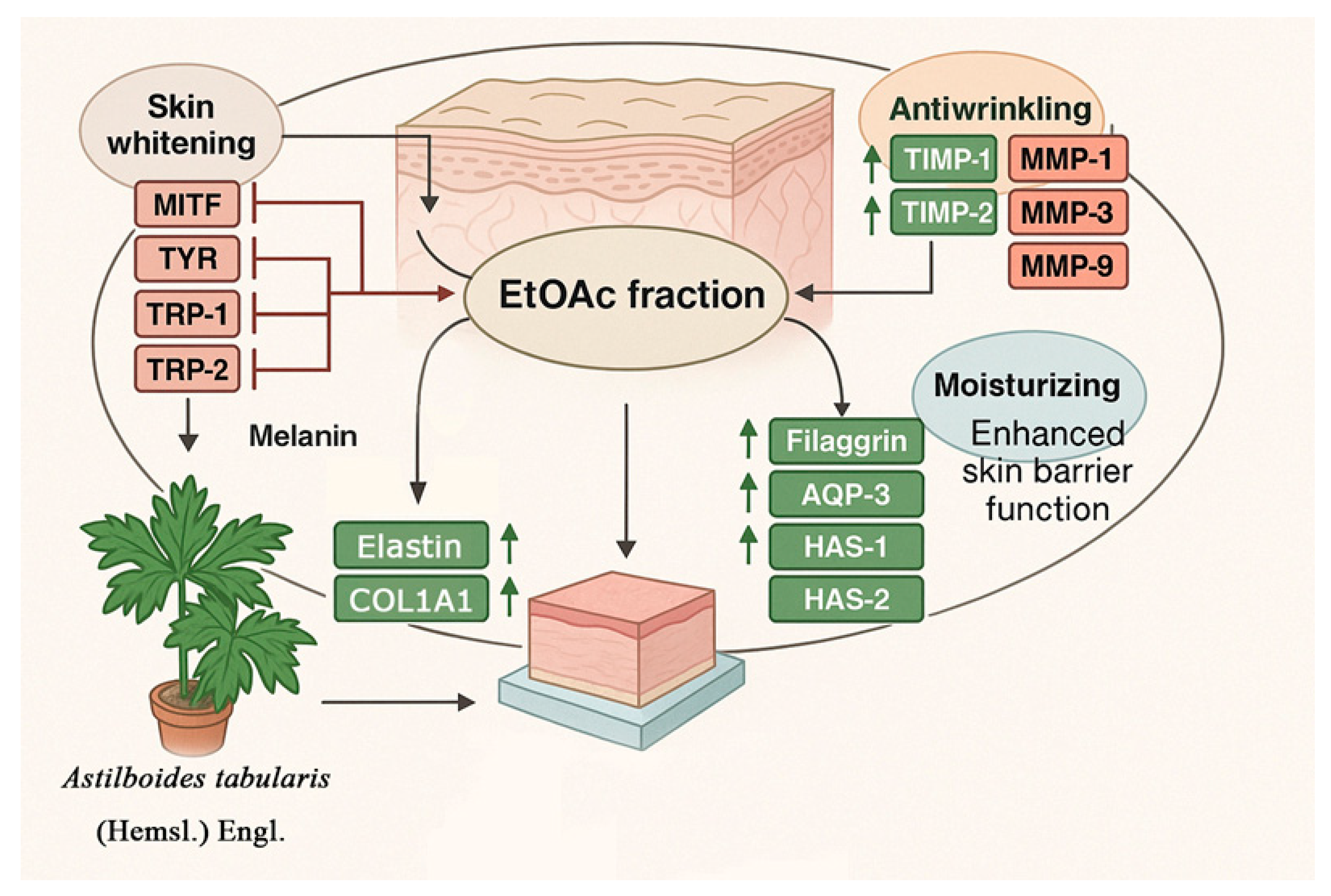
3. Discussion
4. Materials and Methods
4.1. Astilboides tabularis Root Extract and Fractions
Chemical Characterization of the EtOAc Fraction
4.2. Reagents and Instruments
4.3. TYR Inhibition Activity
4.4. Elastase Inhibition Activity
4.5. Cell Culture
4.6. MTT Assay
4.7. Measurement of Melanin Content
4.8. Fine Dust Preparation
4.9. Culture and Treatment of 3D Artificial Skin
4.10. Evaluation of Primary Skin Irritation Using Neoderm-ED and Neoderm-ME
4.11. Real-Time PCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| AQP-3 | aquaporin-3 |
| COL1A1 | collagen type I alpha 1 chain |
| EtOAc | ethyl acetate |
| H&E | hematoxylin and eosin |
| HAS | hyaluronan acid synthase |
| MITF | microphthalmia-associated transcription factor |
| MMP | matrix metalloproteinase |
| PM-10 | particulate matter ≤10 microns |
| RT-PCR | real-time polymerase chain reaction |
| TIMP | tissue inhibitor of metalloproteinases |
| TRP | tyrosinase-related protein |
| TYR | tyrosinase |
| α-MSH | alpha-melanocyte stimulating hormone |
References
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Liu, J.K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant properties of plant-derived phenolic compounds and their effect on skin fibroblast cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.K. Illustrated Book of Korean Medicinal Herbs; Kyo-Hak Publishing Co.: Seoul, Republic of Korea, 2003; p. 811. [Google Scholar]
- Yoon, Y.S.; Kim, K.A.; Yoo, K.O. Environmental characteristics of Astilboides tabularis (Hemsl.) Engl. habitats. Korean J. Plant Resour. 2015, 28, 64–78. [Google Scholar] [CrossRef]
- Ku, Y.B.; Oh, H.K.; Shim, K.Y.; Kim, M.S.; Lee, S.M. Growth Characteristics, Genetic Diversity and Conservation of Endangered Plants (I): The Case of Astilboides tabularis, Euchresta japonica, Echinosophora koreensis and Lilium cernuum; National Institute of Environmental Research of Korea: Incheon, Republic of Korea, 2006; pp. 1–60. [Google Scholar]
- Shin, U.; Chandra, R.; Kang, H. In vitro and ex vitro propagations of Astilboides tabularis (Hemsl.) Engl. as a rare and endangered species. J. Hortic. 2019, 6, 261. [Google Scholar]
- Jin, Y.R.; Yu, Y.X.; Zhang, L.L.; Liu, X.Y.; Li, X.W.; Li, Z. Chemical constituents of Astilboides tabularis (Hemsl.) Engler. J. Jilin Univ. Sci. Ed. 2011, 49, 340–342. [Google Scholar]
- Yang, J.; Chanok, L.; Heekyu, K.; Kwon, Y.S.; Kim, M.J. Biological activities and phenolic compound content of Astilboides tabularis (Hemsl.) Engler extracts. Curr. Pharm. Biotechnol. 2020, 21, 1070–1078. [Google Scholar] [CrossRef]
- Lee, C.H. Studies on Chemical Compositions and Bioactivities of Astilboides tabularis (Hemsl.) Engl. Ph.D. Thesis, Kangwon National University, Chuncheon, Republic of Korea, 2016. [Google Scholar]
- Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W.; et al. Advances in biomedical functions of natural whitening substances in the treatment of skin pigmentation diseases. Pharmaceutics 2022, 14, 2308. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Vetrini, F.; Auricchio, A.; Du, J.; Angeletti, B.; Fisher, D.E.; Ballabio, A.; Marigo, V. The microphthalmia transcription factor (Mitf) controls expression of the ocular albinism type 1 gene: Link between melanin synthesis and melanosome biogenesis. Mol. Cell. Biol. 2004, 24, 6550–6559. [Google Scholar] [CrossRef]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, T.; Svirskis, D.; Merrilees, M.; Bolke, L.; Wu, Z. Effects of GHK-Cu on MMP and TIMP expression, collagen and elastin production, and facial wrinkle parameters. J. Aging Sci. 2016, 4, 166. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, D.Y.; Oh, H.; Kim, S.I.; Oh, S.Y.; Na, W.; Park, S.H.; Park, K.; Kim, J.I.; Kim, A.H.; et al. Dietary collagen hydrolysates ameliorate furrowed and parched skin caused by photoaging in hairless mice. Int. J. Mol. Sci. 2021, 22, 6137. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.W.; Escudero, D.O.; Lokeshwar, S.D.; Golshani, R.; Ekwenna, O.O.; Acosta, K.; Merseburger, A.S.; Soloway, M.; Lokeshwar, V.B. Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer 2011, 117, 1197–1209. [Google Scholar] [CrossRef]
- Bonté, F. Skin moisturization mechanisms: New data. Ann. Pharm. Fr. 2011, 69, 135–141. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A step toward more sustainable processes. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Balo, J.; Banga, I. The elastolytic activity of pancreatic extracts. Biochem. J. 1950, 46, 384–387. [Google Scholar] [CrossRef]
- Aroca, P.; Urabe, K.; Kobayashi, T.; Tsukamoto, K.; Hearing, V.J. Melanin biosynthesis patterns following hormonal stimulation. J. Biol. Chem. 1993, 268, 25650–25655. [Google Scholar] [CrossRef]
- Pavel, S. Dynamics of melanogenesis intermediates. J. Investig. Dermatol. 1993, 100, 162S–165S. [Google Scholar] [CrossRef]
- Fuller, B.B.; Meyskens, F.L., Jr. Endocrine responsiveness in human melanocyte and melanoma cell in culture. J. Natl. Cancer Inst. 1981, 66, 799–802. [Google Scholar] [PubMed]
- Kim, H.H.; Park, G.H.; Park, K.S.; Lee, J.Y.; Kim, T.H.; An, B.J. The effect of Aster glehni Fr. Schm. extracts on whitening and anti-wrinkle. J. Life Sci. 2010, 20, 1034–1040. [Google Scholar]
- Levin, J.; Friedlander, S.F.; Del Rosso, J.Q. Atopic dermatitis and the stratum corneum: Part 1: The role of filaggrin in the stratum corneum barrier and atopic skin. J. Clin. Aesthet. Dermatol. 2013, 6, 16. [Google Scholar] [PubMed]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Goldberga, I.; Li, R.; Duer, M.J. Collagen structure–function relationships from solid-state NMR spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. [Google Scholar] [CrossRef]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.M.; Watson, R.E.B. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef]
- Song, K.C.; Chang, T.S.; Lee, H.; Kim, J.; Park, J.H.; Hwang, G.S. Processed Panax ginseng (sun ginseng) increases type I collagen by regulating MMP-1 and TIMP-1 expression in human dermal fibroblasts. J. Ginseng Res. 2012, 36, 61. [Google Scholar] [CrossRef]
- Méchin, M.C.; Coudane, F.; Adoue, V.; Arnaud, J.; Duplan, H.; Charveron, M.; Schmitt, A.M.; Takahara, H.; Serre, G.; Simon, M. Deimination is regulated at multiple levels including auto-deimination of peptidylarginine deiminases. Cell. Mol. Life Sci. 2010, 67, 1491–1503. [Google Scholar] [CrossRef]
- Yoo, N.H.; Baek, Y.S.; Kim, H.K.; Lee, C.O.; Kim, M.J. Antioxidant and anti-inflammatory activities of Astilboides tabularis (Hemsl.) Engl. root extract. Molecules 2025, 30, 1892. [Google Scholar] [CrossRef]
- Koul, B.; Kumar, A.; Yadav, D.; Jin, J.O. Bergenia genus: Traditional uses, phytochemistry and pharmacology. Molecules 2020, 25, 5555. [Google Scholar] [CrossRef] [PubMed]
- Madaan, R.; Singla, R.K.; Kumar, S.; Dubey, A.K.; Kumar, D.; Sharma, P.; Bala, R.; Singla, S.; Shen, B. Bergenin–a biologically active scaffold: Nanotechnological perspectives. Curr. Top. Med. Chem. 2022, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Choi, E.M. Bergenin increases osteogenic differentiation and prevents methylglyoxal-induced cytotoxicity in MC3T3-E1 osteoblasts. Cytotechnology 2018, 70, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Monteiro e Silva, S.A.; Calixto, G.M.F.; Cajado, J.; De Carvalho, P.C.A.; Rodero, C.F.; Chorilli, M.; Leonardi, G.R. Gallic acid-loaded gel formulation combats skin oxidative stress: Development, characterization and ex vivo biological assays. Polymers 2017, 9, 391. [Google Scholar] [CrossRef]
- Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef]
- Kumar, K.S.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar] [CrossRef]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef]
- Chaikul, P.; Khat-Udomkiri, N.; Iangthanarat, K.; Manosroi, J.; Manosroi, A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur. J. Pharm. Sci. 2019, 131, 39–49. [Google Scholar] [CrossRef]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Najar, M.H.; Zargar, M.I. Evaluation of antioxidant and antimicrobial activities of bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur. J. Med. Chem. 2011, 46, 2415–2420. [Google Scholar] [CrossRef]
- Hashim, P.; Sidek, H.; Helan, M.H.M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene composition and bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Jain, G.K.; Shankar, R.; Kulshrestha, D.K.; Dhawan, B.N. Asiaticoside-induced elevation of antioxidant levels in healing wounds. Phytother Res. 1999, 13, 50–54. [Google Scholar] [CrossRef]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Cannell, R.J.; Kellam, S.J.; Owsianka, A.M.; Walker, J.M. Results of a large scale screen of microalgae for the production of protease inhibitors. Planta Med. 1988, 54, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, S.H.; Jung, H.S.; Ryu, Y.H.; Jung, K.M.; Lim, K.M.; Park, C.B. Interlaboratory validation of KeraSkin™ for replacement of skin irritation test using animal model. J. Altern. Anim. Exp. 2010, 4, 5–13. [Google Scholar]
- Kim, M.J.; Kim, B.H. Skin irritation test of Samhwang-Sasimtang using newly developed reconstructed human epidermis, KeraSkin™. J. Altern. Anim. Exp. 2013, 7, 5–9. [Google Scholar]








| Primer Name | Sequences | |
|---|---|---|
| GADPH | Forward | 5′-GGTTGTCTCCTGCGACTTCA-3′ |
| Reverse | 5′-TGGTCCAGGGTTTCTTACTCC-3′ | |
| MITF | Forward | 5′-CATCATCAGCCTGGAATCAA-3′ |
| Reverse | 5′-TCAAGTTTCCAGAGACGGGT-3′ | |
| TYR | Forward | 5′-TCTTCACCATGCTTTTGTGG-3′ |
| Reverse | 5′-ATAGGTGCATTGGCTTCTGG-3′ | |
| TRP-1 | Forward | 5′-TGGTCTGTGAATCCTTGGAA-3′ |
| Reverse | 5′-CATTTCCAGCTGGGTTTCTC-3′ | |
| TRP-2 | Forward | 5′-CGTGCTGAACAAGGAATGC-3′ |
| Reverse | 5′-CGAAGGATATAAGGGCCACTC-3′ | |
| Primer Name | Sequences | |
|---|---|---|
| GADPH | Forward | 5′-CAATGAATACGGCTACAGCAAC-3′ |
| Reverse | 5′-AGGGAGATGCTCAGTGTTGG-3′ | |
| MMP-1 | Forward | 5′-ACCAAGGAGCGAAGATAG-3′ |
| Reverse | 5′-CAGGCGGAGTATGAGATAA-3′ | |
| MMP-3 | Forward | 5′-ACAAAGGATACAACAGGGACCAA-3′ |
| Reverse | 5′-CCAGGGAGTGGCCAATTTC-3′ | |
| MMP-9 | Forward | 5′-CCCACTGCTGGCCCTTCTA-3′ |
| Reverse | 5′-TCACGTTGCAGGCATCGT-3′ | |
| COL1A1 | Forward | 5′-AGGGCCAAGACGAAGACATC-3′ |
| Reverse | 5′-AGATCACGTCATCGCACAACA-3′ | |
| HAS1 | Forward | 5′-GTGCGGGTACTGGACGA-3′ |
| Reverse | 5′-GACCGCTGATGCAGGATACA-3′ | |
| HAS2 | Forward | 5′-GCAGTGTAAGATATTGGATGGC-3′ |
| Reverse | 5′-CCCATAAATTCTTGATTGTACCAATCTTC-3′ | |
| AQP3 | Forward | 5′-GGGACCAGTCGGAAGGGAT-3′ |
| Reverse | 5′-CACAGATGGACAGGCTGCCT-3′ | |
| Elastin | Forward | 5′-CTTCAGAGCAGTTCCCATTC-3′ |
| Reverse | 5′-AATCCCCAAATATCCAGGACAA-3′ | |
| TIMP-1 | Forward | 5′-TGACATCCGGTTCGTCTACA-3′ |
| Reverse | 5′-TGCAGTTTTCCAGCAATGAG-3′ | |
| TIMP-2 | Forward | 5′-GCGGTCAGTGAGAAGGAAGTGGA-3′ |
| Reverse | 5′-GAGGAGGGGGCCGTGTAGATAAAC-3′ | |
| Filaggrin | Forward | 5′-AAGCTTCATGGTGATGCGAC-3′ |
| Reverse | 5′-TCAAGCAGAAGAGGAAGGCA-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, N.H.; Lee, H.S.; Park, S.M.; Baek, Y.S.; Kim, M.J. Skin-Whitening, Antiwrinkle, and Moisturizing Effects of Astilboides tabularis (Hemsl.) Engl. Root Extracts in Cell-Based Assays and Three-Dimensional Artificial Skin Models. Int. J. Mol. Sci. 2025, 26, 5725. https://doi.org/10.3390/ijms26125725
Yoo NH, Lee HS, Park SM, Baek YS, Kim MJ. Skin-Whitening, Antiwrinkle, and Moisturizing Effects of Astilboides tabularis (Hemsl.) Engl. Root Extracts in Cell-Based Assays and Three-Dimensional Artificial Skin Models. International Journal of Molecular Sciences. 2025; 26(12):5725. https://doi.org/10.3390/ijms26125725
Chicago/Turabian StyleYoo, Nam Ho, Hyun Sook Lee, Sung Min Park, Young Sun Baek, and Myong Jo Kim. 2025. "Skin-Whitening, Antiwrinkle, and Moisturizing Effects of Astilboides tabularis (Hemsl.) Engl. Root Extracts in Cell-Based Assays and Three-Dimensional Artificial Skin Models" International Journal of Molecular Sciences 26, no. 12: 5725. https://doi.org/10.3390/ijms26125725
APA StyleYoo, N. H., Lee, H. S., Park, S. M., Baek, Y. S., & Kim, M. J. (2025). Skin-Whitening, Antiwrinkle, and Moisturizing Effects of Astilboides tabularis (Hemsl.) Engl. Root Extracts in Cell-Based Assays and Three-Dimensional Artificial Skin Models. International Journal of Molecular Sciences, 26(12), 5725. https://doi.org/10.3390/ijms26125725







