Leptin-Upregulated Metastasis-Associated Protein 1 Promotes Vasculogenic Mimicry in Breast Cancer Cells
Abstract
1. Introduction
2. Results
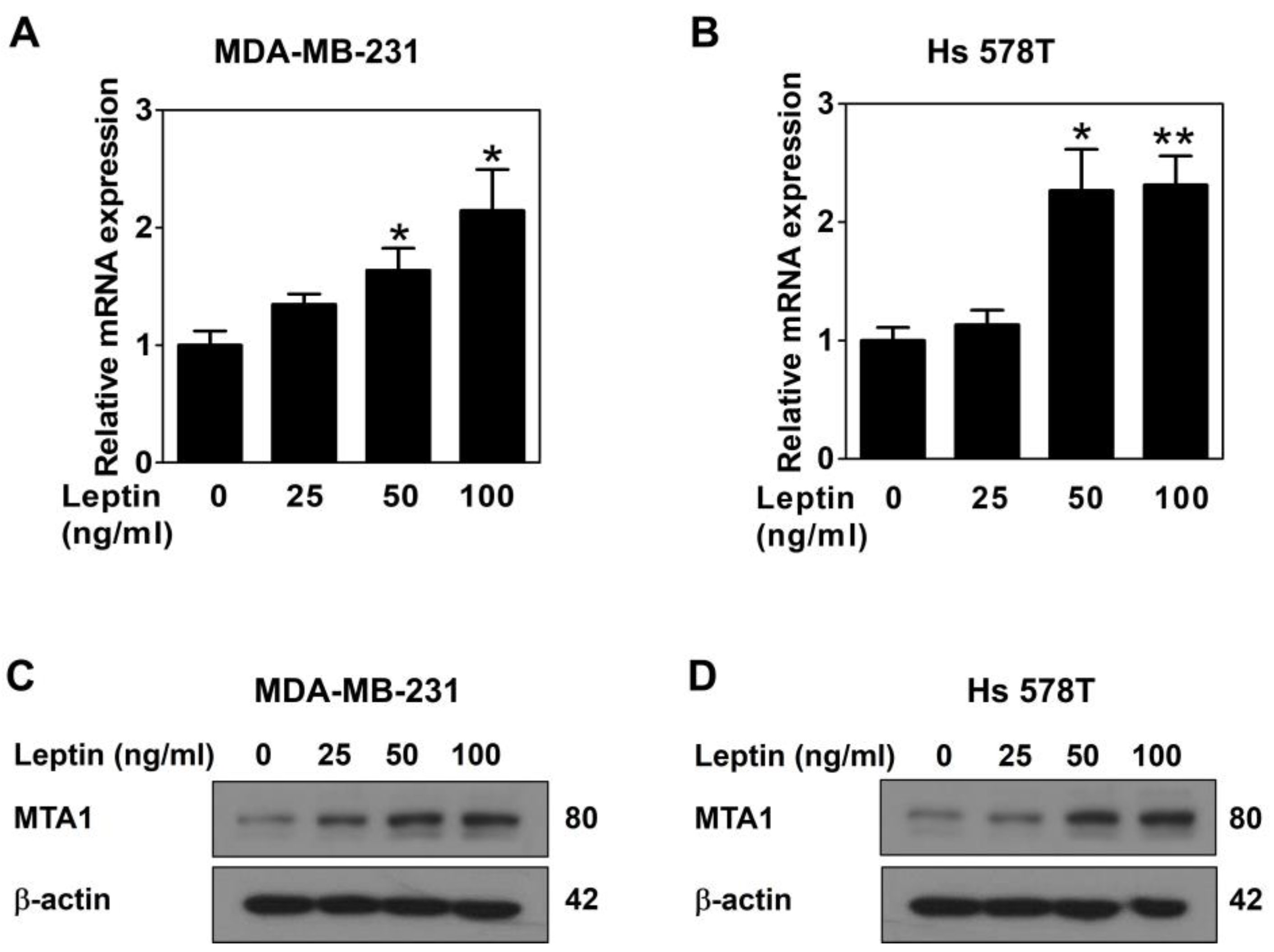
2.1. Leptin Upregulates MTA1 Expression via the Ob-R/STAT3 Pathway in Human Breast Cancer Cells
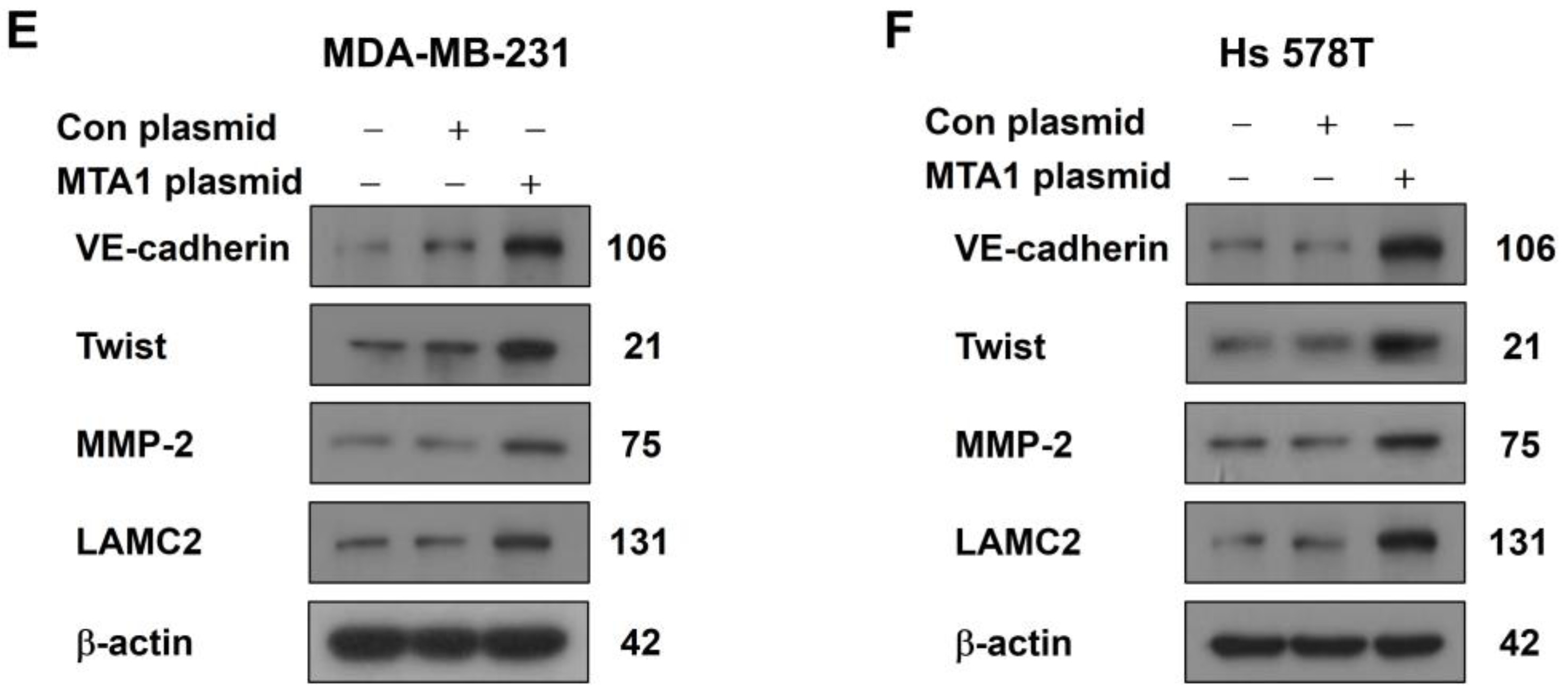
2.2. MTA1 Overexpression Promotes VM in Human Breast Cancer Cells
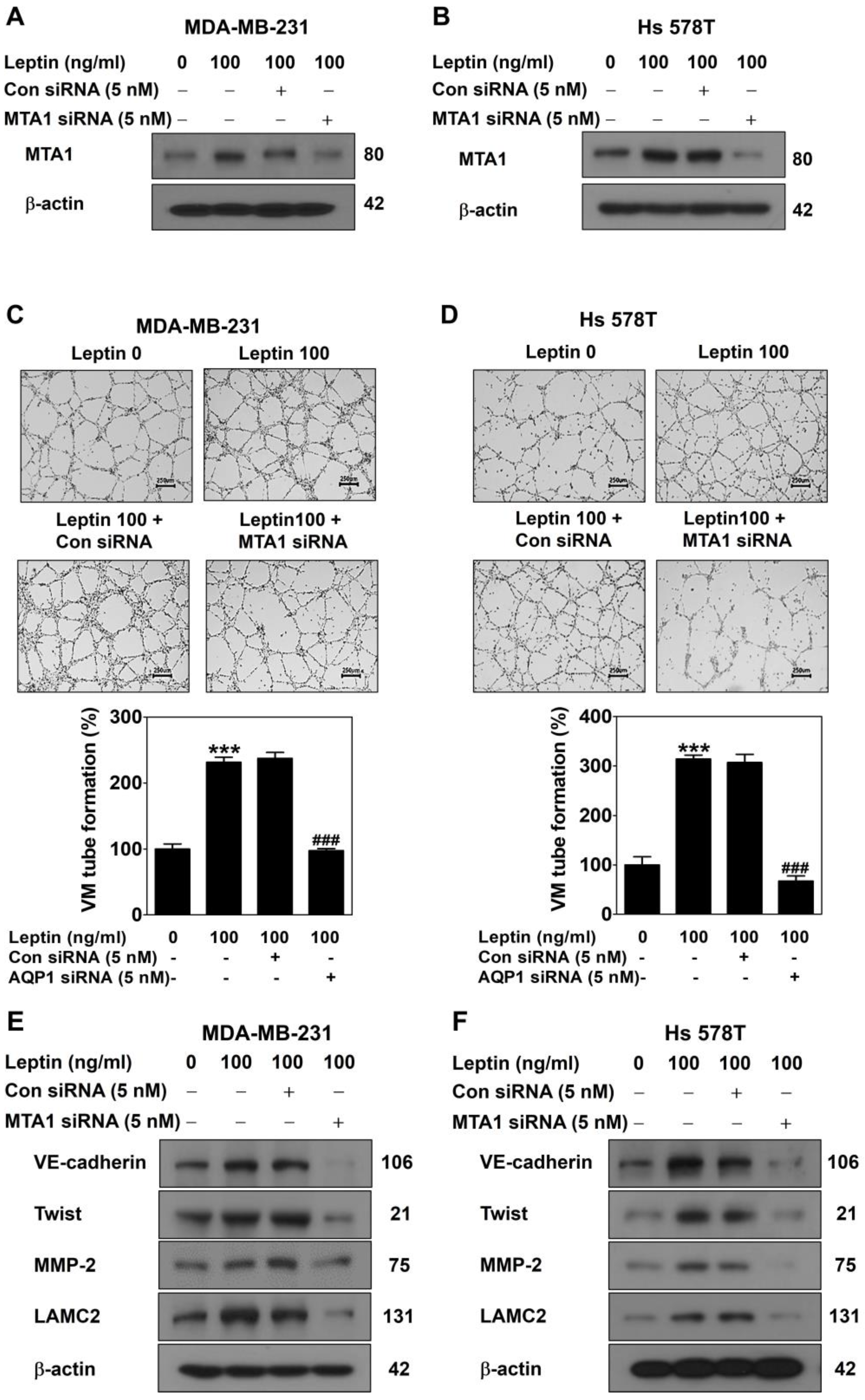
2.3. MTA1 Silencing Inhibits Leptin-Induced VM in Human Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Isolation and Quantitative Real-Time PCR
4.3. Western Blot Analysis
4.4. MTA1 Overexpression Using CRISPR Activation Plasmid
4.5. MTA1 Silencing Using Small Interfering RNA
4.6. Three-Dimensional (3D) Culture VM Tube Formation Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VM | Vasculogenic mimicry |
| MTA1 | Metastasis-associated protein 1 |
| STAT3 | Signal transducers and activators of transcription 3 |
| Ob-R | Leptin receptor |
| EMT | Epithelial-to-mesenchymal transition |
| VE-cadherin | Vascular endothelial cadherin |
| MMP-2 | Matrix metalloproteinase-2 |
| ECM | Extracellular matrix |
| LAMC2 | Laminin subunit 5 gamma-2 |
References
- Hujanen, R.; Almahmoudi, R.; Karinen, S.; Nwaru, B.I.; Salo, T.; Salem, A. Vasculogenic mimicry: A promising prognosticator in head and neck squamous cell carcinoma and esophageal cancer? A systematic review and meta-analysis. Cells 2020, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Provance, O.K.; Oria, V.O.; Tran, T.T.; Caulfield, J.I.; Zito, C.R.; Aguirre-Ducler, A.; Schalper, K.A.; Kluger, H.M.; Jilaveanu, L.B. Vascular mimicry as a facilitator of melanoma brain metastasis. Cell. Mol. Life Sci. 2024, 81, 188. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yao, N.; Cheng, S.; Li, L.; Liu, S.; Yang, Z.; Shang, G.; Zhang, D.; Yao, Z. Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer. Cancer Biol. Med. 2019, 16, 299. [Google Scholar]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer metastasis and treatment resistance: Mechanistic insights and therapeutic targeting of cancer stem cells and the tumor microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Deng, Q.; Yang, C.; Wang, D.; Jiang, G.; Yao, X.; He, X.; Ding, J.; Qiang, J. TEM8 marks neovasculogenic tumor-initiating cells in triple-negative breast cancer. Nat. Commun. 2021, 12, 4413. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Wang, S.; Jia, H.; Zhang, X.; Dong, Q.; Li, J. Wnt5a promotes VM formation by modulating the stemness and EMT progression of prostate cancer cell. Transl. Oncol. 2025, 51, 102155. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guadarrama, G.; García-Becerra, R.; Méndez-Pérez, E.A.; García-Quiroz, J.; Avila, E.; Díaz, L. Vasculogenic mimicry in breast cancer: Clinical relevance and drivers. Cells 2021, 10, 1758. [Google Scholar] [CrossRef]
- Dang, S.-M.; Yang, D.; Wang, Z.-Y.; Ding, X.-M.; Li, X.-L.; Li, D.-Y.; Li, D.-X. Vasculogenic mimicry: A pivotal mechanism contributing to drug resistance in antiangiogenic therapy. Oncol. Transl. Med. 2024, 10, 119–125. [Google Scholar]
- Fan, Y.-Z.; Sun, W. Molecular regulation of vasculogenic mimicry in tumors and potential tumor-target therapy. World J. Gastrointest. Surg. 2010, 2, 117. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hsiao, M. Leptin and cancer: Updated functional roles in carcinogenesis, therapeutic niches, and developments. Int. J. Mol. Sci. 2021, 22, 2870. [Google Scholar] [CrossRef]
- Ray, A.; Cleary, M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Bocian-Jastrzębska, A.; Malczewska-Herman, A.; Kos-Kudła, B. Role of leptin and adiponectin in carcinogenesis. Cancers 2023, 15, 4250. [Google Scholar] [CrossRef]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, leptin and breast cancer: Epidemiological evidence and proposed mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef]
- Han, D.S.; Lee, E.O. Leptin Promotes Vasculogenic Mimicry in Breast Cancer Cells by Regulating Aquaporin-1. Int. J. Mol. Sci. 2024, 25, 5215. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Gin, A.L.; Xu, Y.F.; Bruns, M.; Calloway, C.L.; Ding, W.Q. Metastasis-associated protein 1 (MTA1) is transferred by exosomes and contributes to the regulation of hypoxia and estrogen signaling in breast cancer cells. Cell Commun. Signal. 2019, 17, 13. [Google Scholar] [CrossRef]
- Sen, N.; Gui, B.; Kumar, R. Role of MTA1 in cancer progression and metastasis. Cancer Metastasis Rev. 2014, 33, 879–889. [Google Scholar] [CrossRef]
- Li, D.Q.; Kumar, R. Unravelling the Complexity and Functions of MTA Coregulators in Human Cancer. Adv. Cancer Res. 2015, 127, 1–47. [Google Scholar]
- Yao, Y.; Feng, S.; Xiao, M.; Li, Y.; Yang, L.; Gong, J. MTA1 promotes proliferation and invasion in human gastric cancer cells. Onco Targets Ther. 2015, 8, 1785–1794. [Google Scholar] [CrossRef]
- Wang, R.-A. MTA1—A stress response protein: A master regulator of gene expression and cancer cell behavior. Cancer Metastasis Rev. 2014, 33, 1001–1009. [Google Scholar] [CrossRef]
- Pakala, S.B.; Rayala, S.K.; Wang, R.A.; Ohshiro, K.; Mudvari, P.; Reddy, S.D.; Zheng, Y.; Pires, R.; Casimiro, S.; Pillai, M.R.; et al. MTA1 promotes STAT3 transcription and pulmonary metastasis in breast cancer. Cancer Res. 2013, 73, 3761–3770. [Google Scholar] [CrossRef]
- Lee, M.-H.; Koh, D.; Na, H.; Ka, N.-L.; Kim, S.; Kim, H.-J.; Hong, S.; Shin, Y.K.; Seong, J.K.; Lee, M.-O. MTA1 is a novel regulator of autophagy that induces tamoxifen resistance in breast cancer cells. Autophagy 2018, 14, 812–824. [Google Scholar] [CrossRef]
- Vattem, C.; Pakala, S.B. Metastasis-associated protein 1: A potential driver and regulator of the hallmarks of cancer. J. Biosci. 2022, 47, 23. [Google Scholar] [CrossRef]
- Fouladzadeh, A.; Dorraki, M.; Min, K.K.M.; Cockshell, M.P.; Thompson, E.J.; Verjans, J.W.; Allison, A.; Bonder, C.S.; Abbott, D. The development of tumour vascular networks. Commun. Biol. 2021, 4, 1111. [Google Scholar] [CrossRef]
- Andonegui-Elguera, M.A.; Alfaro-Mora, Y.; Caceres-Gutierrez, R.; Caro-Sanchez, C.H.S.; Herrera, L.A.; Diaz-Chavez, J. An Overview of Vasculogenic Mimicry in Breast Cancer. Front. Oncol. 2020, 10, 220. [Google Scholar] [CrossRef]
- Ando, S.; Catalano, S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat. Rev. Endocrinol. 2011, 8, 263–275. [Google Scholar] [CrossRef]
- Yan, D.; Avtanski, D.; Saxena, N.K.; Sharma, D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3-and MTA1/Wnt1 protein-dependent pathways. J. Biol. Chem. 2012, 287, 8598–8612. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, B.; Qi, L.; Li, H.; Gao, J.; Leng, X. Zinc finger E-box binding homeobox 1 promotes vasculogenic mimicry in colorectal cancer through induction of epithelial-to-mesenchymal transition. Cancer Sci. 2012, 103, 813–820. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zheng, M.; Tang, Y.L.; Liang, X.H. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncol. Lett. 2013, 6, 1174–1180. [Google Scholar] [CrossRef]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef]
- Buettner, C.; Pocai, A.; Muse, E.D.; Etgen, A.M.; Myers, M.G., Jr.; Rossetti, L. Critical role of STAT3 in leptin’s metabolic actions. Cell. Metab. 2006, 4, 49–60. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, F.; Perez-Perez, A.; de la Cruz-Merino, L.; Sanchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Saxena, N.K.; Vertino, P.M.; Anania, F.A.; Sharma, D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J. Biol. Chem. 2007, 282, 13316–13325. [Google Scholar] [CrossRef]
- Kumar, R.; Wang, R.-A. Structure, expression and functions of MTA genes. Gene 2016, 582, 112–121. [Google Scholar] [CrossRef]
- Gururaj, A.E.; Singh, R.R.; Rayala, S.K.; Holm, C.; Den Hollander, P.; Zhang, H.; Balasenthil, S.; Talukder, A.H.; Landberg, G.; Kumar, R. MTA1, a transcriptional activator of breast cancer amplified sequence 3. Proc. Natl. Acad. Sci. USA 2006, 103, 6670–6675. [Google Scholar] [CrossRef]
- Han, D.S.; Lee, E.O. Sp1 plays a key role in vasculogenic mimicry of human prostate cancer cells. Int. J. Mol. Sci. 2022, 23, 1321. [Google Scholar] [CrossRef]
- Yeo, C.; Lee, H.J.; Lee, E.O. Serum promotes vasculogenic mimicry through the EphA2/VE-cadherin/AKT pathway in PC-3 human prostate cancer cells. Life Sci. 2019, 221, 267–273. [Google Scholar] [CrossRef]


| mRNA | Primer Sequences |
|---|---|
| β-actin | S: 5′-AAGAGAGGCATCCTCACCCT-3′ AS: 5′-ATCTCTTGCTCGAAGTCCAG-3′ |
| MTA1 | S: 5′-CCAGGACCAAACCGCAGTAACA-3′ AS: 5′-GTCAGCTTCGTCGTGTGCAGAT-3′ |
| Antibody | Company | Dilution | Product No. |
|---|---|---|---|
| MTA1 | Santa Cruz | 1:500 | SC-17773 |
| β-actin | Sigma-Aldrich | 1:20,000 | A5316 |
| p-STAT3 | CST | 1:1000 | 9145 |
| STAT3 | CST | 1:5000 | 12640 |
| MMP-2 | Abcam | 1:1000 | ab86607 |
| LAMC2 | Abcam | 1:1000 | ab96327 |
| VE-cadherin | Abgent | 1:1000 | AP2724a |
| Twist | Abcam | 1:1000 | ab50887 |
| Goat anti-rabbit IgG-HRP | CST | 1:5000 | 7074P2 |
| Goat anti-mouse IgG-HRP | Bio-Rad | 1:5000 | STAR120P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.-S.; Wang, S.-I.; Lee, S.-H.; Lee, E.-O. Leptin-Upregulated Metastasis-Associated Protein 1 Promotes Vasculogenic Mimicry in Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 5726. https://doi.org/10.3390/ijms26125726
Han D-S, Wang S-I, Lee S-H, Lee E-O. Leptin-Upregulated Metastasis-Associated Protein 1 Promotes Vasculogenic Mimicry in Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(12):5726. https://doi.org/10.3390/ijms26125726
Chicago/Turabian StyleHan, Deok-Soo, Seung-Il Wang, Seung-Hyeon Lee, and Eun-Ok Lee. 2025. "Leptin-Upregulated Metastasis-Associated Protein 1 Promotes Vasculogenic Mimicry in Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 12: 5726. https://doi.org/10.3390/ijms26125726
APA StyleHan, D.-S., Wang, S.-I., Lee, S.-H., & Lee, E.-O. (2025). Leptin-Upregulated Metastasis-Associated Protein 1 Promotes Vasculogenic Mimicry in Breast Cancer Cells. International Journal of Molecular Sciences, 26(12), 5726. https://doi.org/10.3390/ijms26125726





