The Multi-Dimensional Role of Vitamin D in the Pathophysiology and Treatment of Diabetic Foot Ulcers: From Molecular Mechanisms to Clinical Translation
Abstract
1. Introduction
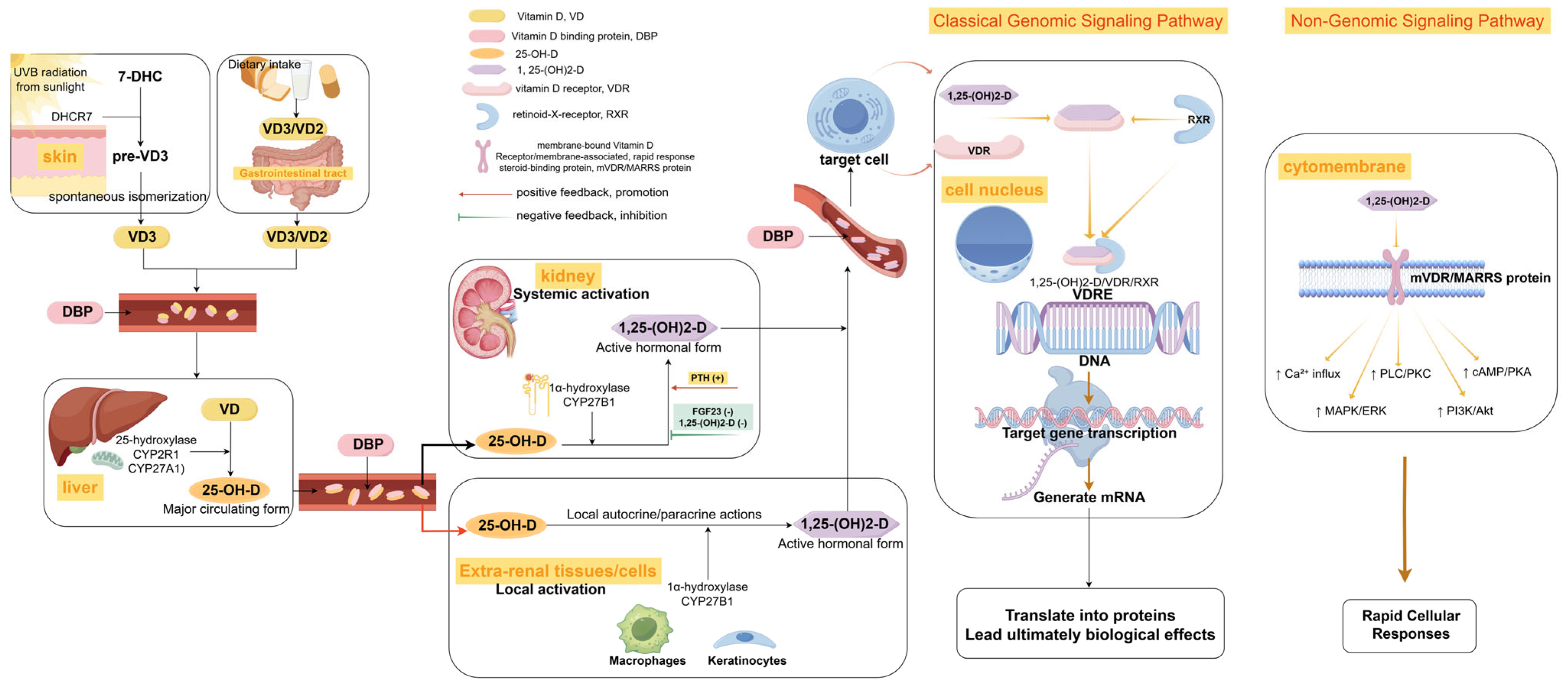
2. The Metabolic Profile of Vitamin D and DFUs
2.1. Different Forms of Vitamin D and Biological Significance
2.2. Characteristics of Vitamin D Metabolism in Patients with Diabetic Foot Ulcers
2.3. Abnormal Vitamin D Receptor (VDR) Signaling
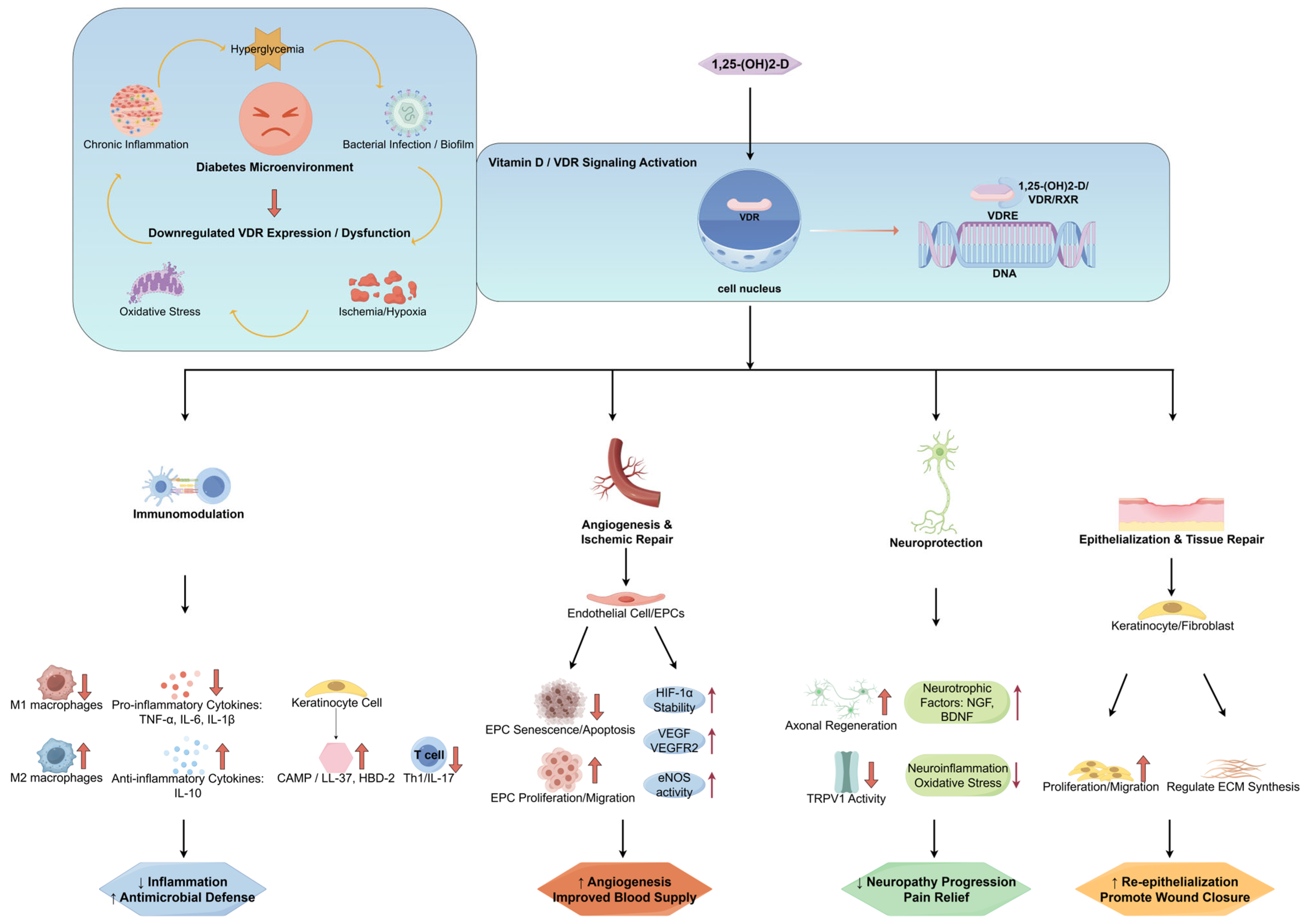
3. Molecular Mechanism of Interaction Between Vitamin D and DFUs
3.1. Regulation of the Immune Microenvironment
3.2. Angiogenesis and Ischemic Repair
3.3. Neuroprotective Effect
4. Application of Vitamin D in the Clinical Management of DFUs
4.1. Association Studies on Vitamin D Status with DFU Risk and Prognosis
4.2. Effect of Systemic Vitamin D Supplementation on DFUs
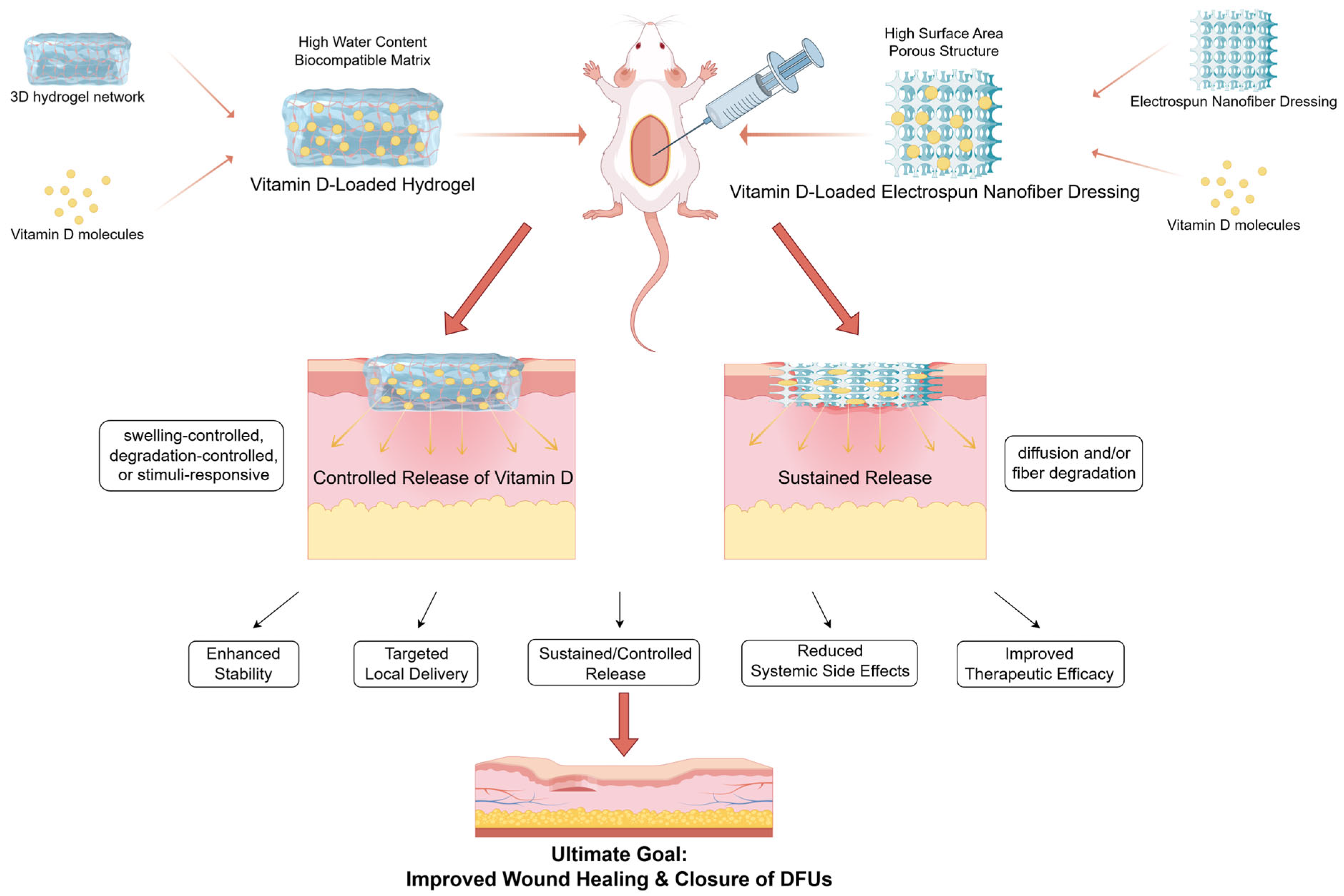
4.3. Innovative Exploration of Topical Application of Vitamin D or Its Analogues
5. Application of Vitamin D-Functionalized Materials in Wound Healing
6. Challenges and Future Research Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Chen, P.; Chuter, V.; Fitridge, R.; Game, F.; Hinchliffe, R.J.; Lazzarini, P.A.; Mills, J.; et al. Definitions and criteria for diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2016, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lazzarini, P.A.; McPhail, S.M.; van Netten, J.J.; Armstrong, D.G.; Pacella, R.E. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care 2020, 43, 964–974. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2022, 46, 209–221. [Google Scholar] [CrossRef]
- Almobarak, A.O.; Awadalla, H.; Osman, M.; Ahmed, M.H. Prevalence of diabetic foot ulceration and associated risk factors: An old and still major public health problem in Khartoum, Sudan? Ann. Transl. Med. 2017, 5, 340. [Google Scholar] [CrossRef]
- Fife, C.E.; Eckert, K.A.; Carter, M.J. Publicly Reported Wound Healing Rates: The Fantasy and the Reality. Adv. Wound Care 2018, 7, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Kamiński, A.; Bogacz, A.; Niezgoda-Nowak, J.T.; Podralska, M.; Górska, A.; Soczawa, M.; Czerny, B. The VDR rs1544410 and rs11568820 Variants and the Risk of Osteoporosis in the Polish Population. Int. J. Mol. Sci. 2025, 26, 481. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, Y.; Zhang, S.; Zhang, W.; Si, Z.; Bai, Y.; Wu, Y.; Fu, Y.; Zhang, Y.; Zhang, L.; et al. 1,25-Dihydroxyvitamin D regulates macrophage activation through FBP1/PKR and ameliorates arthritis in TNF-transgenic mice. J. Steroid Biochem. Mol. Biol. 2023, 228, 106251. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gong, Y.; Wu, Y.; Li, Y.; Fu, R.; Zhang, X.; Zhao, Q.; Zhi, X. 1,25(OH)2D3 improves diabetic wound healing by modulating inflammation and promoting angiogenesis. J. Steroid Biochem. Mol. Biol. 2024, 239, 106477. [Google Scholar] [CrossRef]
- Vaccaro, J.A.; Qasem, A.; Naser, S.A. Cathelicidin Mediates an Anti-Inflammatory Role of Active Vitamin D (Calcitriol) During M. paratuberculosis Infection. Front. Cell. Infect. Microbiol. 2022, 12, 875772. [Google Scholar] [CrossRef]
- Tang, W.; Chen, L.; Ma, W.; Chen, D.; Wang, C.; Gao, Y.; Ran, X. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2022, 13, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, D.; Chen, L.; Liu, G.; Sun, S.; Wang, C.; Gao, Y.; Ran, X. The correlation between serum vitamin D status and the occurrence of diabetic foot ulcers: A comprehensive systematic review and meta-analysis. Sci. Res. 2024, 14, 21932. [Google Scholar] [CrossRef]
- Atoum, M.F.; Al Shdaifat, A.; Al Hourani, H.; Al Hyari, M.; Zahran, R.; Abu Shaikh, H. Relationship of Serum Vitamin D Levels With Diabetic Foot in Patients With Type 2 Diabetes Mellitus: A Cross-Sectional Study. Int. J. Low. Extremity Wounds 2023, 15347346231205641. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Q.; Chen, T.C.; Holick, M.F. 1,25-dihydroxyvitamin D3: A novel agent for enhancing wound healing. J. Cell. Biochem. 1995, 59, 53–56. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, J.; Ye, X.; Peng, M.; Lan, Y.; Zhang, S.; Li, H. Effects of vitamin D supplementation on diabetic foot ulcer healing: A meta-analysis. Postgrad. Med. J. 2025, 101, 100–107. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef]
- Herrmann, M.; Zelzer, S.; Cavalier, E.; Kleber, M.; Drexler-Helmberg, C.; Schlenke, P.; Curcic, P.; Keppel, M.H.; Enko, D.; Scharnagl, H.; et al. Functional Assessment of Vitamin D Status by a Novel Metabolic Approach: The Low Vitamin D Profile Concept. Clin. Chem. 2023, 69, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Bertholf, R.L.; Yi, X. Advances and challenges in the measurement of 1,25-dihydroxyvitamin D: A comprehensive review. Crit. Rev. Clin. Lab. Sci. 2023, 60, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Meyer, M.B.; Arevalo, C.; Tekguc, M.; Zhang, C.; Wang, J.S.; Castro Andrade, C.D.; Strauss, K.; Sato, T.; Benkusky, N.A.; et al. A parathyroid hormone/salt-inducible kinase signaling axis controls renal vitamin D activation and organismal calcium homeostasis. J. Clin. Investig. 2023, 133, e163627. [Google Scholar] [CrossRef]
- Fuchs, M.A.; Grabner, A.; Shi, M.; Murray, S.L.; Burke, E.J.; Latic, N.; Thiriveedi, V.; Roper, J.; Ide, S.; Abe, K.; et al. Intestinal Cyp24a1 regulates vitamin D locally independent of systemic regulation by renal Cyp24a1 in mice. J. Clin. Investig. 2024, 135, e179882. [Google Scholar] [CrossRef]
- Reichrath, J.; Zouboulis, C.C.; Vogt, T.; Holick, M.F. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev. Endocr. Metab. Disord. 2016, 17, 405–417. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brożyna, A.A.; Zmijewski, M.A.; Jóźwicki, W.; Jetten, A.M.; Mason, R.S.; Tuckey, R.C.; Elmets, C.A. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab. Investig. 2017, 97, 706–724. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Alexandridou, A.; Stokes, C.S.; Volmer, D.A. Measurement of Serum Free Vitamin D Concentrations: Importance, Challenges, and the Emerging Role of Mass Spectrometry. Clin. Chem. 2025, 71, 254–265. [Google Scholar] [CrossRef]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Karcioglu Batur, L.; Hekim, N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2021, 93, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, D.; Płomiński, J.; Augustyn, K.; Cieślińska, A. rs7041 and rs4588 Polymorphisms in Vitamin D Binding Protein Gene (VDBP) and the Risk of Diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hosen, M.B.; Faruk, M.O.; Hasan, M.M.; Kabir, Y.; Howlader, M.Z.H. Association of vitamin D and vitamin D binding protein (DBP) gene polymorphism with susceptibility of type 2 diabetes mellitus in Bangladesh. Gene 2017, 636, 42–47. [Google Scholar] [CrossRef]
- Setayesh, L.; Casazza, K.; Moradi, N.; Mehranfar, S.; Yarizadeh, H.; Amini, A.; Yekaninejad, M.S.; Mirzaei, K. Association of vitamin D-binding protein and vitamin D3 with insulin and homeostatic model assessment (HOMA-IR) in overweight and obese females. BMC Res. Notes 2021, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Briede, K.; Ugenskiene, R.; Valiukeviciene, S.; Gradauskiene, B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. Int. J. Mol. Sci. 2024, 25, 9021. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Nikparast, A.; Najd Hassan Bonab, L.; Mahdavi, M.; Daneshpour, M.S.; Hosseinpanah, F.; Mirmiran, P. Impact of daily vitamin D3 supplementation on the risk of vitamin D deficiency with the interaction of rs2282679 in vitamin D binding protein gene (GC) among overweight and obese children and adolescents: A one-year randomized controlled trial. Front. Nutr. 2022, 9, 1061496. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Y.; Luo, L.; Xu, M.; Deng, D.; Fang, Z.; Zhao, X.; Chen, M. Level of 25-hydroxyvitamin D and vitamin D receptor in diabetic foot ulcer and factor associated with diabetic foot ulcers. Diabetol. Metab. Syndr. 2023, 15, 30. [Google Scholar] [CrossRef]
- Song, Z.; Xiao, C.; Jia, X.; Luo, C.; Shi, L.; Xia, R.; Zhu, J.; Zhang, S. Vitamin D/VDR Protects Against Diabetic Kidney Disease by Restoring Podocytes Autophagy. Diabetes Metab. Syndr. Obes. 2021, 14, 1681–1693. [Google Scholar] [CrossRef]
- Wang, B.; Qian, J.Y.; Tang, T.T.; Lin, L.L.; Yu, N.; Guo, H.L.; Ni, W.J.; Lv, L.L.; Wen, Y.; Li, Z.L.; et al. VDR/Atg3 Axis Regulates Slit Diaphragm to Tight Junction Transition via p62-Mediated Autophagy Pathway in Diabetic Nephropathy. Diabetes 2021, 70, 2639–2651. [Google Scholar] [CrossRef]
- Hou, X.; Xu, F.; Zhang, C.; Shuai, J.; Huang, Z.; Liang, Y.; Xu, X. Dexmedetomidine exerts neuroprotective effects during high glucose-induced neural injury by inhibiting miR-125b. Biosci. Rep. 2020, 40, BSR20200394. [Google Scholar] [CrossRef]
- Hu, Y.J.; Song, C.S.; Jiang, N. Single nucleotide variations in the development of diabetic foot ulcer: A narrative review. World J. Diabetes 2022, 13, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, L.X.; Wang, Y.T.; Li, Y.; Chen Md, H.L. Genetic Polymorphisms and the Risk of Diabetic Foot: A Systematic Review and Meta-Analyses. Int. J. Low Extrem. Wounds 2022, 21, 574–587. [Google Scholar] [CrossRef]
- Soroush, N.; Radfar, M.; Hamidi, A.K.; Abdollahi, M.; Qorbani, M.; Razi, F.; Esfahani, E.N.; Amoli, M.M. Vitamin D receptor gene FokI variant in diabetic foot ulcer and its relation with oxidative stress. Gene 2017, 599, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Klashami, Z.N.; Ahrabi, N.Z.; Ahrabi, Y.S.; Hasanzad, M.; Asadi, M.; Amoli, M.M. The vitamin D receptor gene variants, ApaI, TaqI, BsmI, and FokI in diabetic foot ulcer and their association with oxidative stress. Mol. Biol. Rep. 2022, 49, 8627–8639. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.J.; Baral, T.; Benson, R.; Munisamy, M.; Saravu, K.; Rodrigues, G.S.; Sunil Krishna, M.; Shetty, S.; Kumar, A.; Miraj, S.S. Association of vitamin D status and vitamin D receptor polymorphism in diabetic foot ulcer patients: A prospective observational study in a South-Indian tertiary healthcare facility. Int. Wound J. 2024, 21, e70027. [Google Scholar] [CrossRef]
- Gonzalez-Curiel, I.; Trujillo, V.; Montoya-Rosales, A.; Rincon, K.; Rivas-Calderon, B.; deHaro-Acosta, J.; Marin-Luevano, P.; Lozano-Lopez, D.; Enciso-Moreno, J.A.; Rivas-Santiago, B. 1,25-dihydroxyvitamin D3 induces LL-37 and HBD-2 production in keratinocytes from diabetic foot ulcers promoting wound healing: An in vitro model. PLoS ONE 2014, 9, e111355. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Indra, A.K.; Ganguli-Indra, G.; Le, M.N.; Wang, H.; Hollins, R.R.; Reilly, D.A.; Carlson, M.A.; Gallo, R.L.; et al. 1α,25-dihydroxyvitamin D3-eluting nanofibrous dressings induce endogenous antimicrobial peptide expression. Nanomedicine 2018, 13, 1417–1432. [Google Scholar] [CrossRef]
- Wu, X.; He, W.; Mu, X.; Liu, Y.; Deng, J.; Liu, Y.; Nie, X. Macrophage polarization in diabetic wound healing. Burns Trauma 2022, 10, tkac051. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhu, X.; Guo, Y.; Yang, Y.; Jiang, Y.; Liu, B. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J. Cell. Physiol. 2019, 234, 6917–6926. [Google Scholar] [CrossRef]
- Luo, W.J.; Dong, X.W.; Ye, H.; Zhao, Q.S.; Zhang, Q.B.; Guo, W.Y.; Liu, H.W.; Xu, F. Vitamin D 1,25-Dihydroxyvitamin D3 reduces lipid accumulation in hepatocytes by inhibiting M1 macrophage polarization. World J. Gastrointest. Oncol. 2024, 16, 4685–4699. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, L.; Zhang, Z.; Li, J.; Qu, M.; Zhou, Q. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp. Eye Res. 2021, 209, 108668. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tan, J.; Yang, S.; Li, A.; Liu, J.; Zhang, W.; Zhang, H.; Liu, Y. Paricalcitol ameliorates diabetic nephropathy by promoting EETs and M2 macrophage polarization and inhibiting inflammation by regulating VDR/CYP2J2 axis. Faseb J. 2024, 38, e70108. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; You, H.; Kwon, D.H.; Son, Y.; Lee, G.Y.; Han, S.N. Transcriptome analysis of T cells from Ldlr(-/-) mice and effects of in vitro vitamin D treatment. J. Nutr. Biochem. 2023, 109510. [Google Scholar] [CrossRef]
- Rodríguez-Carlos, A.; Trujillo, V.; Gonzalez-Curiel, I.; Marin-Luevano, S.; Torres-Juarez, F.; Santos-Mena, A.; Rivas-Santiago, C.; Enciso-Moreno, J.A.; Zaga-Clavellina, V.; Rivas-Santiago, B. Host Defense Peptide RNase 7 Is Down-regulated in the Skin of Diabetic Patients with or without Chronic Ulcers, and its Expression is Altered with Metformin. Arch. Med. Res. 2020, 51, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, C.; Henrich, M.; Liu, P.T.; Yacoubian, V.; Wang, J.; Chun, R.; Adams, J.S. High-Throughput CAMP Assay (HiTCA): A Novel Tool for Evaluating the Vitamin D-Dependent Antimicrobial Response. Nutrients 2023, 15, 1380. [Google Scholar] [CrossRef]
- Benson, R.; Unnikrishnan, M.K.; Kurian, S.J.; Velladath, S.U.; Rodrigues, G.S.; Chandrashekar Hariharapura, R.; Muraleedharan, A.; Bangalore Venkateshiah, D.; Banerjee, B.; Mukhopadhyay, C.; et al. Vitamin D attenuates biofilm-associated infections via immunomodulation and cathelicidin expression: A narrative review. Expert. Rev. Anti. Infect. Ther. 2023, 21, 15–27. [Google Scholar] [CrossRef]
- Lowry, M.B.; Guo, C.; Zhang, Y.; Fantacone, M.L.; Logan, I.E.; Campbell, Y.; Zhang, W.; Le, M.; Indra, A.K.; Ganguli-Indra, G.; et al. A mouse model for vitamin D-induced human cathelicidin antimicrobial peptide gene expression. J. Steroid Biochem. Mol. Biol. 2020, 198, 105552. [Google Scholar] [CrossRef]
- Tiwari, S.; Pratyush, D.D.; Gupta, B.; Dwivedi, A.; Chaudhary, S.; Rayicherla, R.K.; Gupta, S.K.; Singh, S.K. Prevalence and severity of vitamin D deficiency in patients with diabetic foot infection. Br. J. Nutr. 2013, 109, 99–102. [Google Scholar] [CrossRef]
- Tiwari, S.; Pratyush, D.D.; Gupta, S.K.; Singh, S.K. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br. J. Nutr. 2014, 112, 1938–1943. [Google Scholar] [CrossRef]
- Danny Darlington, C.J.; Kumar, S.S.; Jagdish, S.; Sridhar, M.G. Evaluation of Serum Vitamin D Levels in Diabetic Foot Infections: A Cross-Sectional Study in a Tertiary Care Center in South India. Iran. J. Med. Sci. 2019, 44, 474–482. [Google Scholar]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019, 187, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, L.; Zhang, J.; Zou, L.; Jia, Z.; Han, X.; Zhao, L.; Song, M.; Zhang, Z.; Zong, J.; et al. Identification of angiogenesis-related genes in diabetic foot ulcer using machine learning algorithms. Heliyon 2023, 9, e23003. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Abdelhady, R.; Elhemely, M.A.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Negm, S.; et al. Nanoscale Systems for Local Activation of Hypoxia-Inducible Factor-1 Alpha: A New Approach in Diabetic Wound Management. Int. J. Nanomed. 2024, 19, 13735–13762. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L. Role of vitamin D-vitamin D receptor signaling on hyperoxia-induced bronchopulmonary dysplasia in neonatal rats. Pediatr. Pulmonol. 2021, 56, 2335–2344. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Z.; Guo, L.; Liu, R.; Li, R.; Chu, X.; Yang, J.; Luo, J.; Chen, F.; Deng, M. Hypoxia Mediates Runt-Related Transcription Factor 2 Expression via Induction of Vascular Endothelial Growth Factor in Periodontal Ligament Stem Cells. Mol. Cells 2019, 42, 763–772. [Google Scholar]
- Arfian, N.; Kusuma, M.H.; Anggorowati, N.; Nugroho, D.B.; Jeffilano, A.; Suzuki, Y.; Ikeda, K.; Emoto, N. Vitamin D upregulates endothelin-1, ETBR, eNOS mRNA expression and attenuates vascular remodelling and ischemia in kidney fibrosis model in mice. Physiol. Res. 2018, 67 (Suppl. S1), S137–S147. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Dawoud, H.; Malinski, T. Nanomedical studies of the restoration of nitric oxide/peroxynitrite balance in dysfunctional endothelium by 1,25-dihydroxy vitamin D3—Clinical implications for cardiovascular diseases. Int. J. Nanomed. 2018, 13, 455–466. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Xu, K.; Lin, L. Protective Effects of 1,25 Dihydroxyvitamin D3 against High-Glucose-Induced Damage in Human Umbilical Vein Endothelial Cells Involve Activation of Nrf2 Antioxidant Signaling. J. Vasc. Res. 2021, 58, 267–276. [Google Scholar] [CrossRef]
- Miao, D.; Goltzman, D. Mechanisms of action of vitamin D in delaying aging and preventing disease by inhibiting oxidative stress. Vitam. Horm. 2023, 121, 293–318. [Google Scholar]
- Li, Y.; Zhou, Q.; Pei, C.; Liu, B.; Li, M.; Fang, L.; Sun, Y.; Li, Y.; Meng, S. Hyperglycemia and Advanced Glycation End Products Regulate miR-126 Expression in Endothelial Progenitor Cells. J. Vasc. Res. 2016, 53, 94–104. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, B.; Tian, Y.; Hu, M.; Zhang, G.; Di, Z.; Wang, X.; Liu, Z.; Gu, N.; Liu, Y. AGEs Decreased SIRT3 Expression and SIRT3 Activation Protected AGEs-Induced EPCs’ Dysfunction and Strengthened Anti-oxidant Capacity. Inflammation 2017, 40, 473–485. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, F.; Liu, Y.; Yi, Z.; Wang, X.; Wu, Y.; Gong, P. 1α,25-Dihydroxyvitamin D3 promotes angiogenesis by alleviating AGEs-induced autophagy. Arch. Biochem. Biophys. 2021, 712, 109041. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Weng, Y.; Lin, S.; Lin, J.; Huang, Z.; Huang, W.; Cai, X. 1,25(OH)2D3 Strengthens the Vasculogenesis of Multipotent Mesenchymal Stromal Cells from Rat Bone Marrow by Regulating the PI3K/AKT Pathway. Drug Des. Devel. Ther. 2020, 14, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, H.; Chen, Y.; He, J.; Zhu, L.; Yang, B.; Zhao, W. High glucose-induced injury in human umbilical vein endothelial cells is alleviated by vitamin D supplementation through downregulation of TIPE1. Diabetol. Metab. Syndr. 2024, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, M.; Esmaeili-Bandboni, A.; Emami, M.R.; Naeini, F.; Zarezadeh, M.; Javanbakht, M.H. The effects of all trans retinoic acid, vitamin D3 and their combination on plasma levels of miRNA-125a-5p, miRNA-34a, and miRNA-126 in an experimental model of diabetes. Avicenna J. Phytomed 2022, 12, 67–76. [Google Scholar]
- Veves, A.; Backonja, M.; Malik, R.A. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain. Med. 2008, 9, 660–674. [Google Scholar] [CrossRef]
- Eid, S.A.; Rumora, A.E.; Beirowski, B.; Bennett, D.L.; Hur, J.; Savelieff, M.G.; Feldman, E.L. New perspectives in diabetic neuropathy. Neuron 2023, 111, 2623–2641. [Google Scholar] [CrossRef]
- Riaz, S.; Malcangio, M.; Miller, M.; Tomlinson, D.R. A vitamin D3 derivative (CB1093) induces nerve growth factor and prevents neurotrophic deficits in streptozotocin-diabetic rats. Diabetologia 1999, 42, 1308–1313. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Lu, J.; Watsky, M.A. Effects of 1,25-Vitamin D3 and 24,25-Vitamin D3 on Corneal Nerve Regeneration in Diabetic Mice. Biomolecules 2023, 13, 1754. [Google Scholar] [CrossRef]
- Dimova, R.; Tankova, T.; Chakarova, N. Vitamin D in the Spectrum of Prediabetes and Cardiovascular Autonomic Dysfunction. J. Nutr. 2017, 147, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Fatehi, M.; Soni, S.; Panigrahi, R.; Philippaert, K.; Yu, Y.; Kelly, R.; Boonen, B.; Barr, A.; Golec, D. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 2020, 598, 4321–4338. [Google Scholar] [CrossRef] [PubMed]
- Gündüz Başçıl, S.; Gölgeli, A. Investigation of antinociceptive effects of vitamin D and EB1089 in rats. Agri 2022, 34, 229–234. [Google Scholar] [CrossRef]
- Robilotto, G.L.; Mohapatra, D.P.; Shepherd, A.J.; Mickle, A.D. Role of Src kinase in regulating protein kinase C mediated phosphorylation of TRPV1. Eur. J. Pain 2022, 26, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Britti, E.; Delaspre, F.; Sanz-Alcázar, A.; Medina-Carbonero, M.; Llovera, M.; Purroy, R.; Mincheva-Tasheva, S.; Tamarit, J.; Ros, J. Calcitriol increases frataxin levels and restores mitochondrial function in cell models of Friedreich Ataxia. Biochem. J. 2021, 478, 1–20. [Google Scholar] [CrossRef]
- Qiao, J.; Ma, H.; Chen, M.; Bai, J. Vitamin D alleviates neuronal injury in cerebral ischemia-reperfusion via enhancing the Nrf2/HO-1 antioxidant pathway to counteract NLRP3-mediated pyroptosis. J. Neuropathol. Exp. Neurol. 2023, 82, 722–733. [Google Scholar] [CrossRef]
- Fei, S.; Fan, J.; Cao, J.; Chen, H.; Wang, X.; Pan, Q. Vitamin D deficiency increases the risk of diabetic peripheral neuropathy in elderly type 2 diabetes mellitus patients by predominantly increasing large-fiber lesions. Diabetes Res. Clin. Pract. 2024, 209, 111585. [Google Scholar] [CrossRef]
- Pang, C.; Yu, H.; Cai, Y.; Song, M.; Feng, F.; Gao, L.; Li, K.; Chen, Y.; Xie, J.; Cheng, Y.; et al. Vitamin D and diabetic peripheral neuropathy: A multi-centre nerve conduction study among Chinese patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2023, 39, e3679. [Google Scholar] [CrossRef]
- Alam, U.; Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Asghar, O.; Jeziorska, M.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Vitamin D deficiency is associated with painful diabetic neuropathy. Diabetes Metab. Res. Rev. 2021, 37, e3361. [Google Scholar] [CrossRef]
- Putz, Z.; Tordai, D.; Hajdú, N.; Vági, O.E.; Kempler, M.; Békeffy, M.; Körei, A.E.; Istenes, I.; Horváth, V.; Stoian, A.P.; et al. Vitamin D in the Prevention and Treatment of Diabetic Neuropathy. Clin. Ther. 2022, 44, 813–823. [Google Scholar] [CrossRef]
- Smart, H.; AlGhareeb, A.M.; Smart, S.A. 25-Hydroxyvitamin D Deficiency: Impacting Deep-Wound Infection and Poor Healing Outcomes in Patients With Diabetes. Adv. Skin. Wound Care 2019, 32, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, L.; Ma, W.; Liu, G.; Chen, D.; Wang, C.; Gao, Y.; Ran, X. Association of vitamin D status with all-cause mortality and outcomes among Chinese individuals with diabetic foot ulcers. J. Diabetes Investig. 2023, 14, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kinesya, E.; Santoso, D.; Gde Arya, N.; Putri Cintya, E.; Seriari Ambarini, P.; Kinesya, B.; Stephanie Kartjito, M.; Mannagalli, Y. Vitamin D as adjuvant therapy for diabetic foot ulcers: Systematic review and meta-analysis approach. Clin. Nutr. ESPEN 2023, 54, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, R.; Pourbagheri, H.; Momen-Heravi, M.; Bahmani, F.; Shadi, J.; Soleimani, Z.; Asemi, Z. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complications 2017, 31, 766–772. [Google Scholar] [CrossRef]
- Kamble, A.; Ambad, R.; Padamwar, M.; Kakade, A.; Yeola, M. To study the effect of oral vitamin D supplements on wound healing in patient with diabetic foot ulcer and its effect on lipid metabolism. Int. J. Res. Pharm. Sci. 2020, 11, 2701–2706. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Haratian-Arab, M.; MoeinTavakkoli, H.; Nadjarzadeh, A. Comparative effect of two different doses of vitamin D on diabetic foot ulcer and inflammatory indices among the type 2 diabetic patients a randomized clinical trial. Iran. J. Diabetes Obes. 2017, 8, 164–171. [Google Scholar]
- Halschou-Jensen, P.M.; Sauer, J.; Bouchelouche, P.; Fabrin, J.; Brorson, S.; Ohrt-Nissen, S. Improved Healing of Diabetic Foot Ulcers After High-dose Vitamin D: A Randomized Double-blinded Clinical Trial. Int. J. Low Extrem. Wounds 2021, 15347346211020268. [Google Scholar] [CrossRef]
- Perna, S. The enigma of vitamin D supplementation in aging with obesity. Minerva Gastroenterol. 2022, 68, 459–462. [Google Scholar] [CrossRef]
- Shroff, R.; Wan, M.; Nagler, E.V.; Bakkaloglu, S.; Cozzolino, M.; Bacchetta, J.; Edefonti, A.; Stefanidis, C.J.; Vande Walle, J.; Ariceta, G.; et al. Clinical practice recommendations for treatment with active vitamin D analogues in children with chronic kidney disease Stages 2-5 and on dialysis. Nephrol. Dial. Transplant. 2017, 32, 1114–1127. [Google Scholar] [CrossRef]
- Cardoso, M.M.A.; Machado-Rugolo, J.; Lima, S.A.M.; Andrade, L.G.M.; Curado, D.S.P.; Ponce, D. Cost-effectiveness analysis of intravenous paricalcitol vs. oral calcitriol in the treatment of hyperparathyroidism secondary to chronic kidney disease. J. Bras. Nefrol. 2023, 45, 95–101. [Google Scholar] [CrossRef]
- Reichrath, J.; Saternus, R.; Vogt, T. Challenge and perspective: The relevance of ultraviolet (UV) radiation and the vitamin D endocrine system (VDES) for psoriasis and other inflammatory skin diseases. Photochem. Photobiol. Sci. 2017, 16, 433–444. [Google Scholar] [CrossRef]
- Gisondi, P.; Gracia-Cazaña, T.; Kurzen, H.; Galván, J. Calcipotriol/Betamethasone Dipropionate for the Treatment of Psoriasis: Mechanism of Action and Evidence of Efficacy and Safety versus Topical Corticosteroids. J. Clin. Med. 2024, 13, 4484. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Lu, J.; Watsky, M. Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules 2023, 13, 1065. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.I.A.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Sahrapeyma, H.; Ghorbani, S. A promising wound dressing based on alginate hydrogels containing vitamin D3 cross-linked by calcium carbonate/d-glucono-δ-lactone. Biomed. Eng. Lett. 2020, 10, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, J.H.; Baek, S.W.; Lee, J.K.; Park, S.Y.; Choi, B.; Kim, T.H.; Min, K.; Han, D.K. Controlled vitamin D delivery with injectable hyaluronic acid-based hydrogel for restoration of tendinopathy. J. Tissue Eng. 2022, 13, 20417314221122089. [Google Scholar] [CrossRef]
- Kim, C.; Park, S.; Rho, S.J.; Kim, Y.-R. Rice flour-based filled hydrogel: An effective vitamin D encapsulation system as influenced by rice flour variety. Food Sci. Biotechnol. 2025, 34, 1617–1629. [Google Scholar] [CrossRef]
- Brahmbhatt, A.; NievesTorres, E.; Yang, B.; Edwards, W.D.; Roy Chaudhury, P.; Lee, M.K.; Kong, H.; Mukhopadhyay, D.; Kumar, R.; Misra, S. The role of Iex-1 in the pathogenesis of venous neointimal hyperplasia associated with hemodialysis arteriovenous fistula. PLoS ONE 2014, 9, e102542. [Google Scholar] [CrossRef]
- Cai, C.; Kilari, S.; Zhao, C.; Singh, A.K.; Simeon, M.L.; Misra, A.; Li, Y.; Takahashi, E.; Kumar, R.; Misra, S. Adventitial delivery of nanoparticles encapsulated with 1α, 25-dihydroxyvitamin D3 attenuates restenosis in a murine angioplasty model. Sci. Rep. 2021, 11, 4772. [Google Scholar] [CrossRef]
- Macido, A. Diabetic Foot Ulcers and Vitamin D Status: A Literature Review. SAGE Open Nurs. 2018, 4, 2377960818789027. [Google Scholar] [CrossRef]
- Greenhagen, R.M.; Frykberg, R.G.; Wukich, D.K. Serum vitamin D and diabetic foot complications. Diabet. Foot Ankle 2019, 10, 1579631. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.J.; Miraj, S.S.; Benson, R.; Munisamy, M.; Saravu, K.; Rodrigues, G.S.; Rao, M. Vitamin D Supplementation in Diabetic Foot Ulcers: A Current Perspective. Curr. Diabetes Rev. 2021, 17, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, F.; Jamali, M.; Guimarães, N.S.; Radkhah, N.; Jamilian, P.; Wang, Q. The role of vitamin D in diabetic foot ulcer; an umbrella review of meta-analyses. Front. Nutr. 2024, 11, 1454779. [Google Scholar] [CrossRef] [PubMed]
| Comparison Aspect | Tang et al., 2025 (Current Review) | Macido, 2018 [110] | Greenhagen et al., 2019 [111] | Kurian et al., 2021 [112] | Putz et al., 2022 [90] | Liu et al., 2024 [113] |
|---|---|---|---|---|---|---|
| Title | The Multi-Dimensional Role of Vitamin D in the Pathophysiology and Treatment of Diabetic Foot Ulcers: from Molecular Mechanisms to Clinical Translation | Diabetic Foot Ulcers and Vitamin D Status: A Literature Review | Serum vitamin D and diabetic foot complications | Vitamin D Supplementation in Diabetic Foot Ulcers: A Current Perspective | Vitamin D in the Prevention and Treatment of Diabetic Neuropathy | The role of vitamin D in diabetic foot ulcer; an umbrella review of meta-analyses |
| Article Type | Narrative Review | Literature Review | Original Research | Narrative Review | Narrative Review | Umbrella Review of Meta-Analyses |
| Primary Theme | The multidimensional, integrated role and translation of vitamin D in DFUs: from molecular mechanisms to biomaterial applications | A early review on vitamin D status and DFUs/DFI links, highlighting insufficient evidence | Clinical association between vitamin D levels with diabetic foot complications in a specific cohort | The effects of vitamin D on DFUs risk factors and healing, as well as supplementation considerations | The specific link between vitamin D deficiency and diabetic neuropathy, as well as supplementation perspective | Summary and systematic quality assessment of the existing meta-analytic evidence on the association of vitamin D and DFUs |
| Molecular Mechanisms | Discusses in detail the specific roles of immune, vascular, neuro, AMPs in DFUs microenvironment | Summarizes the metabolic pathways of vitamin D and its effects on skin cells (keratinocytes and fibroblasts) | Limited | General overview of immune, vascular, neurological mechanisms | Focuses on the investigation of neuroprotective mechanisms | Mentions immunomodulation as background in discussion |
| Vitamin D Forms and VDR | Analyzes the different forms of vitamin D, and the dysregulation, polymorphisms and dysfunction of VDR | Mentions the physiological metabolism of vitamin D and VDR | Mentions only serum 25(OH)D | Mentions vitamin D metabolism and VDR, but no further discussion is made | Focuses on the VDR of nervous system rather than the systemic one | Mentions only serum 25(OH)D |
| Biomaterials and Drug Delivery | Detailed discussion of intelligent delivery systems of vitamin D, including nanofibers and hydrogels | Not covered | Not covered | Not covered | Not covered | Not covered |
| Translational Medicine | Emphasizes basic-clinical-materials translational pathway and MDT integration | Literature review and initial inference | Clinical observation research | Links physiological mechanisms with clinical supplementation | Links neuropathology with clinical supplementation | Systematic assessment of clinical evidence, guiding future translational research directions |
| Consideration of Special Populations | Explicitly discusses the individualized strategies for obese and renal insufficiency populations | Not specifically discussed | Not specifically discussed | Briefly mentions it in the conclusion | Mentions the lack of consideration of the differences among the population in the limitations of discussion | Not directly involved |
| Main Findings and Conclusions | Explores the multi-mechanistic impact of vitamin D on DFUs, notes supplementation potential despite lacking strong evidence, highlights material delivery as innovation, and calls for high-quality RCTs | Vitamin D deficiency may be associated with DFUs and foot infections, but evidence is minimal; more research is needed | Lower vitamin D levels are associated with PAD, DFI, and DFUs in diabetic patients; no significant difference for patients with CN | Vitamin D has protective roles in immune systems, vascular systems and wound healing; it could be a preferred adjuvant in the management of DFUs | Vitamin D deficiency may play roles in DPN, DFUs and CAN; supplementation is effective for neuropathic pain, may slow neural damage | Pooled meta-analyses suggest that low vitamin D levels are linked to DFU risk, and supplementation may be beneficial; the results need to be interpreted with caution due to the potential bias |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Chen, S.; Zhang, S.; Ran, X. The Multi-Dimensional Role of Vitamin D in the Pathophysiology and Treatment of Diabetic Foot Ulcers: From Molecular Mechanisms to Clinical Translation. Int. J. Mol. Sci. 2025, 26, 5719. https://doi.org/10.3390/ijms26125719
Tang W, Chen S, Zhang S, Ran X. The Multi-Dimensional Role of Vitamin D in the Pathophysiology and Treatment of Diabetic Foot Ulcers: From Molecular Mechanisms to Clinical Translation. International Journal of Molecular Sciences. 2025; 26(12):5719. https://doi.org/10.3390/ijms26125719
Chicago/Turabian StyleTang, Weiwei, Shengqiu Chen, Shuxia Zhang, and Xingwu Ran. 2025. "The Multi-Dimensional Role of Vitamin D in the Pathophysiology and Treatment of Diabetic Foot Ulcers: From Molecular Mechanisms to Clinical Translation" International Journal of Molecular Sciences 26, no. 12: 5719. https://doi.org/10.3390/ijms26125719
APA StyleTang, W., Chen, S., Zhang, S., & Ran, X. (2025). The Multi-Dimensional Role of Vitamin D in the Pathophysiology and Treatment of Diabetic Foot Ulcers: From Molecular Mechanisms to Clinical Translation. International Journal of Molecular Sciences, 26(12), 5719. https://doi.org/10.3390/ijms26125719






