Breaking the Cycle: Can Vitamin D Bridge the Gap Between Gut Microbiota and Immune Dynamics in Multiple Sclerosis?
Abstract
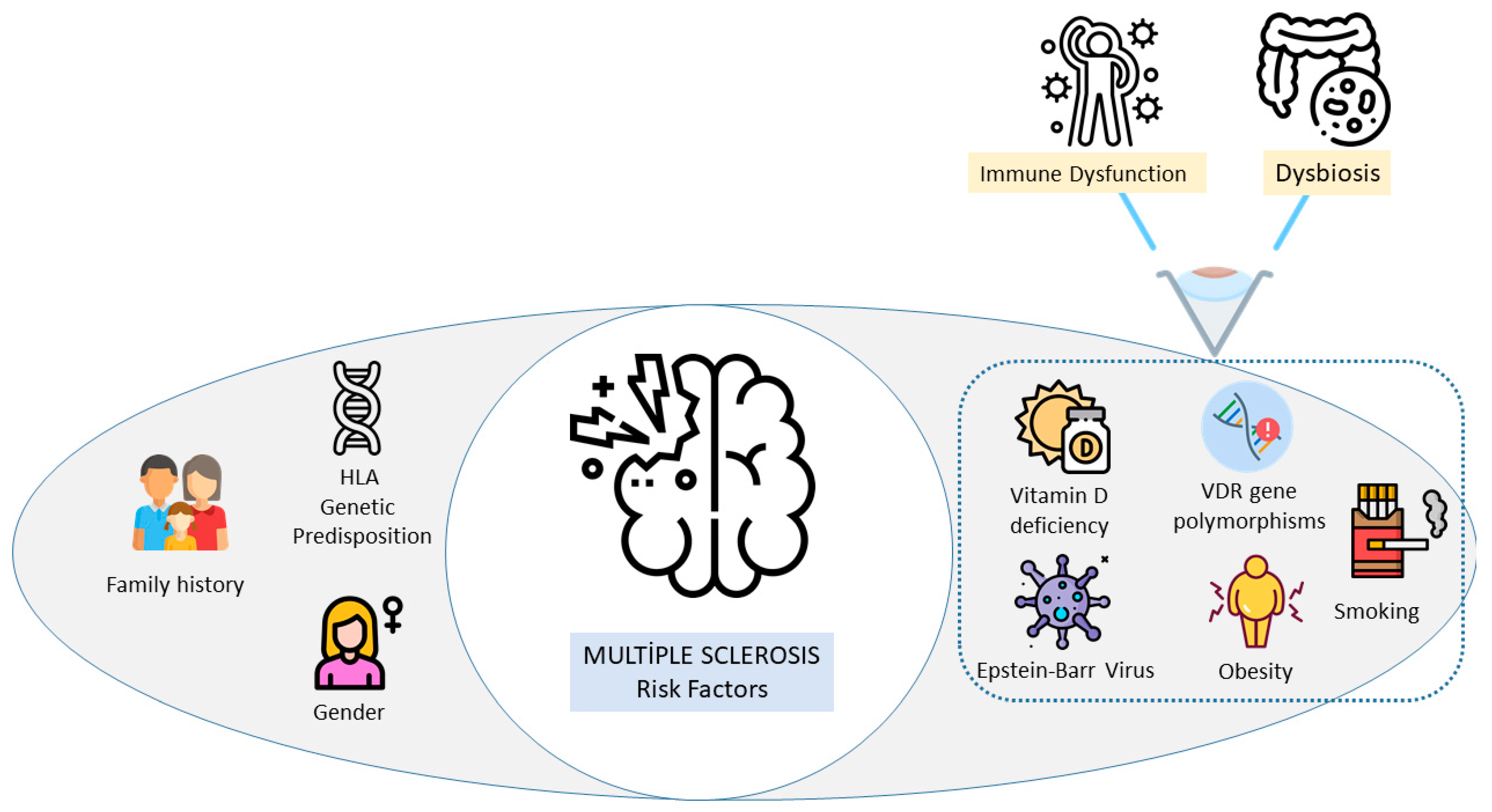
1. Introduction
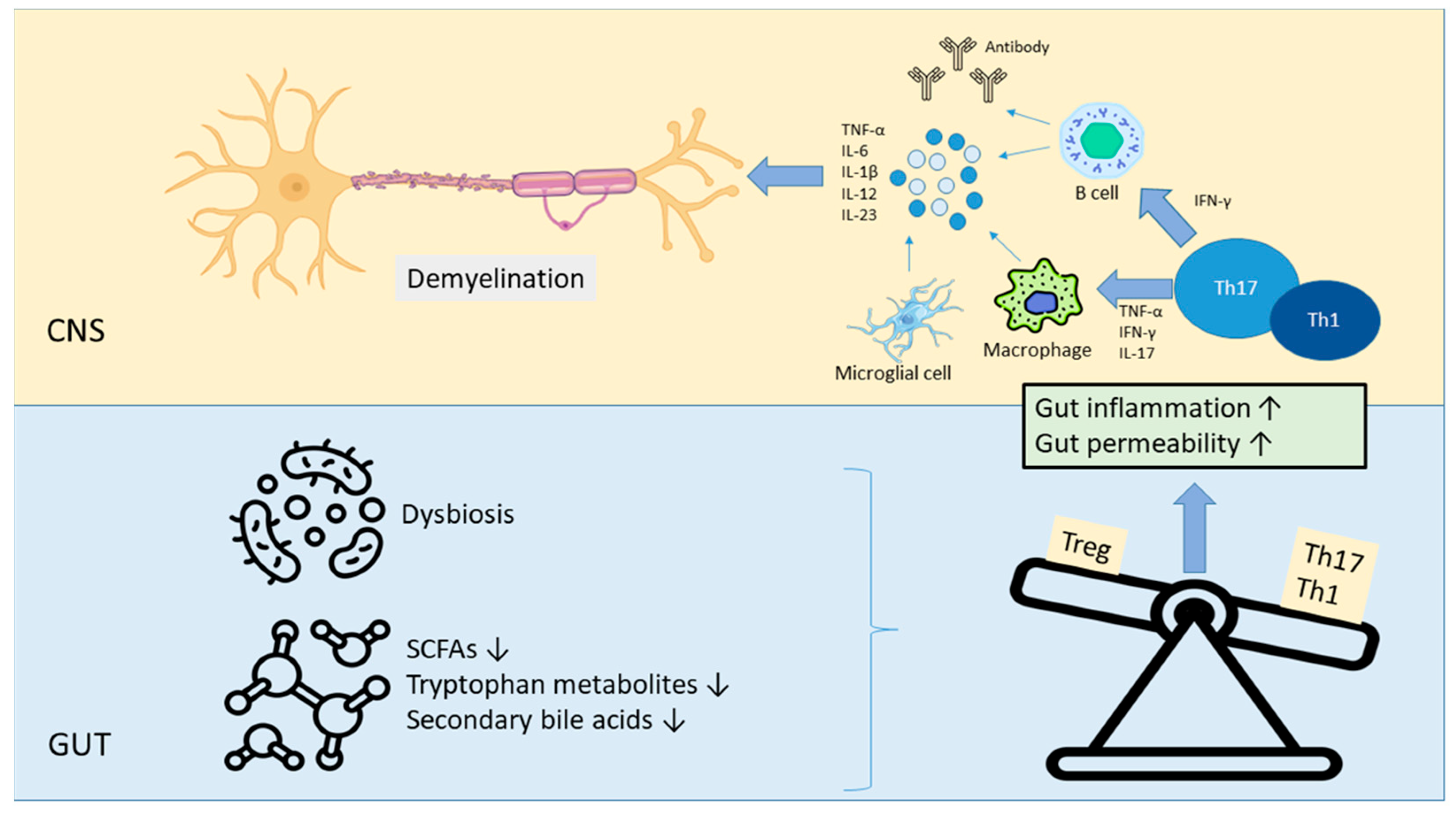
2. Immune Dysfunction and Microbiota Alterations in Multiple Sclerosis
2.1. Overview of the Innate and Adaptive Immune Systems
2.2. Immune Dysregulation in MS
2.3. Dysbiosis in MS: Key Microbial Alterations and Autoimmunity
2.4. Role of Microbiota-Derived Metabolites in Immune Homeostasis
3. Vitamin D, Gut Microbiota, and Immune Homeostasis: Mechanistic Insights and Clinical Evidence
3.1. The Effect of Vitamin D on Gut Microbiota Composition
3.2. The Impact of Gut Microbiota on Vitamin D Regulation
3.3. Vitamin D, Gut Microbiota, and Immune Homeostasis
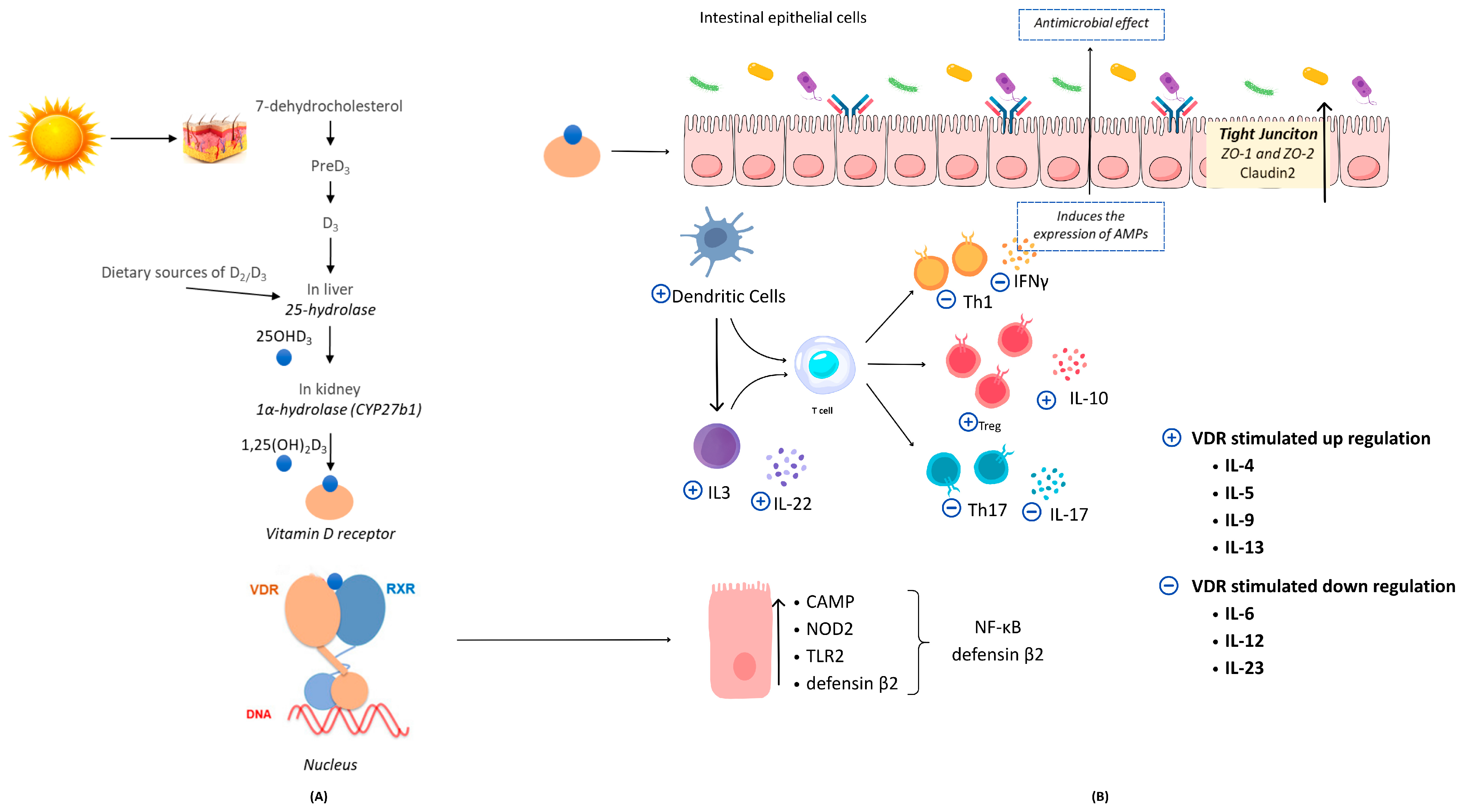
3.3.1. Vitamin D/VDR-RXR Complex Stimulates the Transcription of Antimicrobial Peptides
3.3.2. Vitamin D/VDR Signaling Pathway Enhances the Intestinal Mucosal Barrier Integrity
4. Therapeutic Implications and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| VDR | Vitamin D receptor |
| Th | T Helper Cells |
| CNS | Central Nervous System |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| PPMS | Primary Progressive Multiple Sclerosis |
| SPMS | Secondary Progressive Multiple Sclerosis |
| SCFAs | Short-Chain Fatty Acids |
| Tregs | T Regulatory Cells |
| IL | Interleukin |
| TNF-α | Tumor Necrosis Factor Alpha |
| AhR | Aryl Hydrocarbon Receptor |
| FGF-23 | Fibroblast Growth Factor-23 |
| RXR | Retinoid-X Receptor |
| AMPs | Antimicrobial Peptides |
| ZO | Zonula Occludens |
References
- Olejnik, P.; Roszkowska, Z.; Adamus, S.; Kasarełło, K. Multiple sclerosis: A narrative overview of current pharmacotherapies and emerging treatment prospects. Pharmacol. Rep. PR 2024, 76, 926–943. [Google Scholar] [CrossRef] [PubMed]
- Oliva Ramirez, A.; Keenan, A.; Kalau, O.; Worthington, E.; Cohen, L.; Singh, S. Prevalence and burden of multiple sclerosis-related fatigue: A systematic literature review. BMC Neurol. 2021, 21, 468. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- MS. MS International Federation. Atlas of MS. 2020–22 Worldwide Study. Available online: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms#about (accessed on 23 April 2025).
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Khan, G.; Hashim, M.J. Epidemiology of Multiple Sclerosis: Global, Regional, National and Sub-National-Level Estimates and Future Projections. J. Epidemiol. Glob. Health 2025, 15, 21. [Google Scholar] [CrossRef]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Cagol, A.; Lorscheider, J.; Tsagkas, C.; Benkert, P.; Yaldizli, Ö.; Kuhle, J.; Derfuss, T.; Sormani, M.P.; Thompson, A.; et al. Harmonizing Definitions for Progression Independent of Relapse Activity in Multiple Sclerosis: A Systematic Review. JAMA Neurol. 2023, 80, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, E.; Bellinvia, A.; Fonderico, M.; Pastò, L.; Razzolini, L.; Totaro, R.; Spitaleri, D.; Lugaresi, A.; Cocco, E.; Onofrj, M.; et al. Progression is independent of relapse activity in early multiple sclerosis: A real-life cohort study. Brain A J. Neurol. 2022, 145, 2796–2805. [Google Scholar] [CrossRef]
- Haki, M.; Al-Biati, H.A.; Al-Tameemi, Z.S.; Ali, I.S.; Al-Hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, e37297. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Y.; Gong, Q.; Xu, X. Multiple Sclerosis: An Overview of Epidemiology, Risk Factors, and Serological Biomarkers. Acta Neurol. Scand. 2024, 2024, 7372789. [Google Scholar] [CrossRef]
- Goodin, D.S. The epidemiology, pathology and pathogenesis of MS: Therapeutic implications. Neurotherapeutics 2025, e00539. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- Penesová, A.; Dean, Z.; Kollár, B.; Havranová, A.; Imrich, R.; Vlček, M.; Rádiková, Ž. Nutritional intervention as an essential part of multiple sclerosis treatment? Physiol. Res. 2018, 67, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Barrie, W.; Yang, Y.; Irving-Pease, E.K.; Attfield, K.E.; Scorrano, G.; Jensen, L.T.; Armen, A.P.; Dimopoulos, E.A.; Stern, A.; Refoyo-Martinez, A.; et al. Elevated genetic risk for multiple sclerosis emerged in steppe pastoralist populations. Nature 2024, 625, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef]
- Wang, J.; Jelcic, I.; Mühlenbruch, L.; Haunerdinger, V.; Toussaint, N.C.; Zhao, Y.; Cruciani, C.; Faigle, W.; Naghavian, R.; Foege, M.; et al. HLA-DR15 Molecules Jointly Shape an Autoreactive T Cell Repertoire in Multiple Sclerosis. Cell 2020, 183, 1264–1281.e20. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Bjornevik, K.; Münz, C.; Cohen, J.I.; Ascherio, A. Epstein-Barr virus as a leading cause of multiple sclerosis: Mechanisms and implications. Nat. Rev. Neurol. 2023, 19, 160–171. [Google Scholar] [CrossRef]
- Heydarpour, P.; Manouchehrinia, A.; Beiki, O.; Mousavi, S.E.; Abdolalizadeh, A.; Lakeh, M.M.; Sahraian, M.A. Smoking and worsening disability in multiple sclerosis: A meta-analysis. Acta Neurol. Scand. 2018, 138, 62–69. [Google Scholar] [CrossRef]
- Manouchehrinia, A.; Huang, J.; Hillert, J.; Alfredsson, L.; Olsson, T.; Kockum, I.; Constantinescu, C.S. Smoking Attributable Risk in Multiple Sclerosis. Front. Immunol. 2022, 13, 840158. [Google Scholar] [CrossRef]
- Hedström, A.K.; Lima Bomfim, I.; Barcellos, L.; Gianfrancesco, M.; Schaefer, C.; Kockum, I.; Olsson, T.; Alfredsson, L. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 2014, 82, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine 2022, 76, 103798. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Álvarez, F.; Pérez-Matute, P.; Oteo, J.A.; Marzo-Sola, M.E. The influence of interferon β-1b on gut microbiota composition in patients with multiple sclerosis. Neurologia 2021, 36, 495–503. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Yan, X.; Shao, L.; Liu, X.; Zhou, D.; Zhang, L.; Yu, K.; Zhao, L. Alterations of the Fecal Microbiota in Chinese Patients With Multiple Sclerosis. Front. Immunol. 2020, 11, 590783. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in neuroinflammation and synaptic dysfunction: A focus on Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Corsini, S.; Kellingray, L.; Hegarty, C.; Le Gall, G.; Narbad, A.; Müller, M.; Tejera, N.; O’Toole, P.W.; Minihane, A.M.; et al. APOE genotype influences the gut microbiome structure and function in humans and mice: Relevance for Alzheimer’s disease pathophysiology. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 8221–8231. [Google Scholar]
- Chen, L.; Cao, H.; Wu, X.; Xu, X.; Ji, X.; Wang, B.; Zhang, P.; Li, H. Effects of oral health intervention strategies on cognition and microbiota alterations in patients with mild Alzheimer’s disease: A randomized controlled trial. Geriatr. Nurs. 2022, 48, 103–110. [Google Scholar] [CrossRef]
- Scheperjans, F.; Levo, R.; Bosch, B.; Lääperi, M.; Pereira, P.A.B.; Smolander, O.P.; Aho, V.T.E.; Vetkas, N.; Toivio, L.; Kainulainen, V.; et al. Fecal Microbiota Transplantation for Treatment of Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2024, 81, 925–938. [Google Scholar] [CrossRef]
- Yang, X.; He, X.; Xu, S.; Zhang, Y.; Mo, C.; Lai, Y.; Song, Y.; Yan, Z.; Ai, P.; Qian, Y.; et al. Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson’s disease. Food Funct. 2023, 14, 6828–6839. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Z.; Wang, J.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 2024, 15, 3003. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, D.; Laczmanski, L.; Budrewicz, S.; Pokryszko-Dragan, A.; Podbielska, M. Targeting gut microbiota: New therapeutic opportunities in multiple sclerosis. Gut Microbes 2023, 15, 2274126. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol. Investig. 2016, 45, 205–222. [Google Scholar] [CrossRef]
- Pröbstel, A.K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A.; et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol. 2020, 5, eabc7191. [Google Scholar] [CrossRef]
- Ordoñez-Rodriguez, A.; Roman, P.; Rueda-Ruzafa, L.; Campos-Rios, A.; Cardona, D. Changes in Gut Microbiota and Multiple Sclerosis: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4624. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ito, N.; Mindur, J.E.; Kumar, H.; Youssef, M.; Suresh, S.; Kulkarni, R.; Rosario, Y.; Balashov, K.E.; Dhib-Jalbut, S.; et al. Fecal Lcn-2 level is a sensitive biological indicator for gut dysbiosis and intestinal inflammation in multiple sclerosis. Front. Immunol. 2022, 13, 1015372. [Google Scholar] [CrossRef]
- Paraschiv, A.C.; Vacaras, V.; Nistor, C.; Vacaras, C.; Strilciuc, S.; Muresanu, D.F. The effect of multiple sclerosis therapy on gut microbiota dysbiosis: A longitudinal prospective study. Microb. Cell 2024, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ladakis, D.C.; Bhargava, P. The Role of Gut Dysbiosis and Potential Approaches to Target the Gut Microbiota in Multiple Sclerosis. CNS Drugs 2023, 37, 117–132. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Doyle, W.J.; Hill, K.; Ochoa-Repáraz, J. Gut microbiota in multiple sclerosis and animal models. FEBS J. 2025, 292, 1330–1356. [Google Scholar] [CrossRef]
- Buga, A.M.; Padureanu, V.; Riza, A.L.; Oancea, C.N.; Albu, C.V.; Nica, A.D. The Gut-Brain Axis as a Therapeutic Target in Multiple Sclerosis. Cells 2023, 12, 1872. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Marchesi, N.; Varesi, A.; Morozzi, M.; Mascione, L.; Ricevuti, G.; Esposito, C.; Galeotti, N.; Pascale, A. New therapeutic avenues in multiple sclerosis: Is there a place for gut microbiota-based treatments? Pharmacol. Res. 2024, 209, 107456. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Zierfuss, B.; Larochelle, C.; Prat, A. Blood-brain barrier dysfunction in multiple sclerosis: Causes, consequences, and potential effects of therapies. Lancet Neurol. 2024, 23, 95–109. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The Blood-Brain Barrier-A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10 (Suppl. 1), S17–S30. [Google Scholar] [CrossRef]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Zhang, H.-P.; Han, W.-J.; Zhao, D.-T.; Liao, H.-Y.; Ma, Y.-X.; Xu, B.; Li, L.-J.; Han, Y.; Liu, X.-H. Disease predisposition of human leukocyte antigen class II genes influences the gut microbiota composition in patients with primary biliary cholangitis. Front. Immunol. 2022, 13, 984697. [Google Scholar] [CrossRef] [PubMed]
- Andeweg, S.P.; Keşmir, C.; Dutilh, B.E. Quantifying the Impact of Human Leukocyte Antigen on the Human Gut Microbiota. Msphere 2021, 6, e0047621. [Google Scholar] [CrossRef]
- Boncheva, I.; Poudrier, J.; Falcone, E.L. Role of the intestinal microbiota in host defense against respiratory viral infections. Curr. Opin. Virol. 2024, 66, 101410. [Google Scholar] [CrossRef]
- Mathew, S.; Smatti, M.K.; Al Ansari, K.; Nasrallah, G.K.; Al Thani, A.A.; Yassine, H.M. Mixed viral-bacterial infections and their effects on gut microbiota and clinical illnesses in children. Sci. Rep. 2019, 9, 865. [Google Scholar] [CrossRef]
- Sen, P.; Fan, Y.; Schlezinger, J.J.; Ehrlich, S.D.; Webster, T.F.; Hyötyläinen, T.; Pedersen, O.; Orešič, M. Exposure to environmental toxicants is associated with gut microbiome dysbiosis, insulin resistance and obesity. Environ. Int. 2024, 186, 108569. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Protano, C.; Antonucci, A.; Vitali, M.; Sessa, R. Impact of Air Pollution on the Composition and Diversity of Human Gut Microbiota in General and Vulnerable Populations: A Systematic Review. Toxics 2022, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yang, L.; Cheng, F.; Zhang, Z.; Li, J.; Liu, W.; Li, Y.; Chen, Y.; Bao, Y.; Chen, L. Gut microbiota are associated with psychological stress-induced defections in intestinal and blood–brain barriers. Front. Microbiol. 2019, 10, 3067. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; et al. Brazilian green propolis improves gut microbiota dysbiosis and protects against sarcopenic obesity. J. Cachexia Sarcopenia Muscle 2022, 13, 3028–3047. [Google Scholar] [CrossRef]
- Aragón-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Álvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.-G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Exploring the Role of Vitamin D and the Vitamin D Receptor in the Composition of the Gut Microbiota. Front. Biosci. 2023, 28, 116. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Fernandez, E. Vitamin D receptor polymorphisms and diseases. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 371, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Esmaeil Amini, M.; Shomali, N.; Bakhshi, A.; Rezaei, S.; Hemmatzadeh, M.; Hosseinzadeh, R.; Eslami, S.; Babaie, F.; Aslani, S.; Torkamandi, S.; et al. Gut microbiome and multiple sclerosis: New insights and perspective. Int. Immunopharmacol. 2020, 88, 107024. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Vivier, E.; Malissen, B. Innate and adaptive immunity: Specificities and signaling hierarchies revisited. Nat. Immunol. 2005, 6, 17–21. [Google Scholar] [CrossRef]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and genetic risk factors for MS: An integrated review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef]
- Jelcic, I.; Al Nimer, F.; Wang, J.; Lentsch, V.; Planas, R.; Jelcic, I.; Madjovski, A.; Ruhrmann, S.; Faigle, W.; Frauenknecht, K. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 2018, 175, 85–100.e23. [Google Scholar] [CrossRef]
- Jayaraman, S.; Prabhakar, B.S. Immune tolerance in autoimmune central nervous system disorders. In Neuroimmune Diseases: From Cells to the Living Brain; Springer: Cham, Switzerland, 2024; pp. 177–202. [Google Scholar] [CrossRef]
- Shimizu, F.; Nakamori, M. Blood–Brain Barrier Disruption in Neuroimmunological Disease. Int. J. Mol. Sci. 2024, 25, 10625. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Kanjevac, T.; Arsenijevic, D.; Radosavljevic, G.; Milovanovic, M.; Arsenijevic, N. Interleukin-17 in chronic inflammatory neurological diseases. Front. Immunol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Kaegi, C.; Wuest, B.; Crowley, C.; Boyman, O. Systematic review of safety and efficacy of second-and third-generation CD20-targeting biologics in treating immune-mediated disorders. Front. Immunol. 2022, 12, 788830. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Ni, S.; Liu, M.; Hu, K. The emerging role of microglia in the development and therapy of multiple sclerosis. Int. Immunopharmacol. 2024, 143, 113476. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Du, S.; Zhao, L.; Jain, S.; Sahay, K.; Rizvanov, A.; Lezhnyova, V.; Khaibullin, T.; Martynova, E.; Khaiboullina, S. Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front. Immunol. 2022, 13, 996469. [Google Scholar] [CrossRef]
- Alakhras, N.S.; Kaplan, M.H. Dendritic cells as a nexus for the development of multiple sclerosis and models of disease. Adv. Biol. 2023, 7, 2300073. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood–brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Suliman, B.A. Potential clinical implications of molecular mimicry-induced autoimmunity. Immun. Inflamm. Dis. 2024, 12, e1178. [Google Scholar] [CrossRef]
- Andreadou, M.; Ingelfinger, F.; De Feo, D.; Cramer, T.L.; Tuzlak, S.; Friebel, E.; Schreiner, B.; Eede, P.; Schneeberger, S.; Geesdorf, M. IL-12 sensing in neurons induces neuroprotective CNS tissue adaptation and attenuates neuroinflammation in mice. Nat. Neurosci. 2023, 26, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- von Essen, M.R.; Søndergaard, H.B.; Petersen, E.R.; Sellebjerg, F. IL-6, IL-12, and IL-23 STAT-pathway genetic risk and responsiveness of lymphocytes in patients with multiple sclerosis. Cells 2019, 8, 285. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Kerkering, J.; Kuehl, T.; Infante, A.G.; Paul, F.; Rosiewicz, K.S.; Siffrin, V.; Alisch, M. Inflammatory cytokines associated with multiple sclerosis directly induce alterations of neuronal cytoarchitecture in human neurons. J. Neuroimmune Pharmacol. 2023, 18, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Mehan, S.; Gupta, G.D.; Narula, A.S. Immune system dysregulation in the progression of multiple sclerosis: Molecular insights and therapeutic implications. Neuroscience 2024, 548, 9–26. [Google Scholar] [CrossRef]
- Wang, K.; Song, F.; Fernandez-Escobar, A.; Luo, G.; Wang, J.-H.; Sun, Y. The properties of cytokines in multiple sclerosis: Pros and cons. Am. J. Med. Sci. 2018, 356, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.O.; Ochoa-Repáraz, J. The gut microbiome in multiple sclerosis: A potential therapeutic avenue. Med. Sci. 2018, 6, 69. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ito, K.; Dhib-Jalbut, S. Interaction of the gut microbiome and immunity in multiple sclerosis: Impact of diet and immune therapy. Int. J. Mol. Sci. 2023, 24, 14756. [Google Scholar] [CrossRef]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Turner, T.A.; Lehman, P.; Ghimire, S.; Shahi, S.K.; Mangalam, A. Game of microbes: The battle within-gut microbiota and multiple sclerosis. Gut Microbes 2024, 16, 2387794. [Google Scholar] [CrossRef]
- Elsayed, N.S.; Aston, P.; Bayanagari, V.R.; Shukla, S.K. The gut microbiome molecular mimicry piece in the multiple sclerosis puzzle. Front. Immunol. 2022, 13, 972160. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Cerasoli, B.; Annibali, V.; Policano, C.; Lionetto, L.; Capi, M.; Mechelli, R.; Romano, S.; Fornasiero, A.; Mattei, G.; et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult. Scler. 2017, 23, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; Meuth, S.G.; Peixoto, C.A. Microbial Metabolites in Multiple Sclerosis: Implications for Pathogenesis and Treatment. Front. Neurosci. 2022, 16, 885031. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Wilck, N.; Haghikia, A.; Gold, R.; Mueller, D.N.; Linker, R.A. The role of the gut microbiota and microbial metabolites in neuroinflammation. Eur. J. Immunol. 2020, 50, 1863–1870. [Google Scholar] [CrossRef]
- Kopczyńska, J.; Kowalczyk, M. The potential of short-chain fatty acid epigenetic regulation in chronic low-grade inflammation and obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar]
- Zeng, Q.; Junli, G.; Liu, X.; Chen, C.; Sun, X.; Li, H.; Zhou, Y.; Cui, C.; Wang, Y.; Yang, Y.; et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem. Int. 2019, 129, 104468. [Google Scholar] [CrossRef]
- Olsson, A.; Gustavsen, S.; Nguyen, T.D.; Nyman, M.; Langkilde, A.R.; Hansen, T.H.; Sellebjerg, F.; Oturai, A.B.; Bach Søndergaard, H. Serum Short-Chain Fatty Acids and Associations With Inflammation in Newly Diagnosed Patients With Multiple Sclerosis and Healthy Controls. Front. Immunol. 2021, 12, 661493. [Google Scholar] [CrossRef]
- Pérez-Pérez, S.; Domínguez-Mozo, M.I.; Alonso-Gómez, A.; Medina, S.; Villarrubia, N.; Fernández-Velasco, J.I.; García-Martínez, M.; García-Calvo, E.; Estévez, H.; Costa-Frossard, L.; et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ 2020, 8, e10220. [Google Scholar] [CrossRef]
- Moles, L.; Delgado, S.; Gorostidi-Aicua, M.; Sepúlveda, L.; Alberro, A.; Iparraguirre, L.; Suárez, J.A.; Romarate, L.; Arruti, M.; Muñoz-Culla, M.; et al. Microbial dysbiosis and lack of SCFA production in a Spanish cohort of patients with multiple sclerosis. Front. Immunol. 2022, 13, 960761. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Paolicelli, G.; Fallarino, F.; Gargaro, M.; Vascelli, G.; De Zuani, M.; Fric, J.; Laznickova, P.; Kohoutkova, M.H.; Macchiarulo, A.; et al. A microbially produced AhR ligand promotes a Tph1-driven tolerogenic program in multiple sclerosis. Sci. Rep. 2024, 14, 6651. [Google Scholar] [CrossRef] [PubMed]
- Antonini Cencicchio, M.; Montini, F.; Palmieri, V.; Massimino, L.; Lo Conte, M.; Finardi, A.; Mandelli, A.; Asnicar, F.; Pavlovic, R.; Drago, D.; et al. Microbiota-produced immune regulatory bile acid metabolites control central nervous system autoimmunity. Cell Rep. Med. 2025, 6, 102028. [Google Scholar] [CrossRef]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Investig. 2020, 130, 3467–3482. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtfeger, L.A.; Montini, F.; Lanser, T.B.; Ekwudo, M.N.; Zurawski, J.; Tauhid, S.; Glanz, B.I.; Chu, R.; Bakshi, R.; Chitnis, T.; et al. Gut microbiota and metabolites are linked to disease progression in multiple sclerosis. Cell Rep. Med. 2025, 6, 102055. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Romano, S.; Mechelli, R.; Pizzolato Umeton, R.; Ferraldeschi, M.; Fornasiero, A.; Reniè, R.; Cerasoli, B.; Morena, E.; Romano, C.; et al. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics 2018, 15, 68–74. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Dopkins, N.; Nagarkatti, P.S.; Nagarkatti, M. The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology 2018, 154, 178–185. [Google Scholar] [CrossRef]
- Nitzan, Z.; Staun-Ram, E.; Volkowich, A.; Miller, A. Multiple sclerosis-associated gut microbiome in the israeli diverse populations: Associations with ethnicity, gender, disability status, Vitamin D levels, and mediterranean diet. Int. J. Mol. Sci. 2023, 24, 15024. [Google Scholar] [CrossRef]
- Cox, L.M.; Weiner, H.L. Microbiota signaling pathways that influence neurologic disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef]
- McCallum, G.; Tropini, C. The gut microbiota and its biogeography. Nat. Rev. Microbiol. 2024, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The Gut Microbiota in Multiple Sclerosis: An Overview of Clinical Trials. Cell Transpl. 2019, 28, 1507–1527. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, L.; Jiang, C.; Yang, X.; Huang, J. The Effect of Different Administration Time and Dosage of Vitamin D Supplementation in Patients with Multiple Sclerosis: A Meta-Analysis of Randomized Controlled Trials. Neuroimmunomodulation 2021, 28, 118–128. [Google Scholar] [CrossRef]
- López-Muñoz, P.; Torres-Costoso, A.I.; Fernández-Rodríguez, R.; Guzmán-Pavón, M.J.; De Arenas-Arroyo, S.N.; Basco-López, J.; Reina-Gutiérrez, S. Effect of Vitamin D Supplementation on Fatigue in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2861. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; He, L.; Liu, L.; Zhu, J.; Jin, T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2018, 23, 56–61. [Google Scholar] [CrossRef]
- Hanaei, S.; Sahraian, M.A.; Mohammadifar, M.; Ramagopalan, S.V.; Ghajarzadeh, M. Effect of vitamin D supplements on relapse rate and Expanded Disability Status Scale (EDSS) in multiple sclerosis (MS): A systematic review and meta-analysis. Int. J. Prev. Med. 2021, 12, 42. [Google Scholar]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef]
- Ali, A.; Wu, L.; Ali, S.S. Vitamin D and the microbiota connection: Understanding its potential to improve COPD outcomes. Egypt. J. Bronchol. 2024, 18, 20. [Google Scholar] [CrossRef]
- Fakhoury, H.M.; Kvietys, P.R.; AlKattan, W.; Al Anouti, F.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer. Res. 2020, 40, 551–556. [Google Scholar] [CrossRef]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of high doses of vitamin D 3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.-A.; Fazeli, M.; Meshkat, Z.; Khodashenas, E.; Esmaeili, H.; Mazloum, S.; Ferns, G.A.; Abdizadeh, M.F.; Ghayour-Mobarhan, M. The effects of high doses of vitamin D on the composition of the gut microbiome of adolescent girls. Clin. Nutr. ESPEN 2020, 35, 103–108. [Google Scholar] [CrossRef]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S. The association between vitamin D and gut microbiota: A systematic review of human studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Mirza, A.; Mao-Draayer, Y. The gut microbiome and microbial translocation in multiple sclerosis. Clin. Immunol. 2017, 183, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pröbstel, A.-K.; Baranzini, S.E. The role of the gut microbiome in multiple sclerosis risk and progression: Towards characterization of the “MS microbiome”. Neurotherapeutics 2018, 15, 126–134. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yan, J.; Zhi, C.; Zhou, Q.; Yuan, X. 1, 25 (OH) 2 D 3 deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Callejo, M.; Ramos, R.; Duarte, J.; Perez-Vizcaino, F. Impact of vitamin D deficit on the rat gut microbiome. Nutrients 2019, 11, 2564. [Google Scholar] [CrossRef]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. FGF23 and vitamin D metabolism. J. Bone Miner. Res. Plus 2021, 5, e10558. [Google Scholar] [CrossRef]
- Stacchiotti, V.; Rezzi, S.; Eggersdorfer, M.; Galli, F. Metabolic and functional interplay between gut microbiota and fat-soluble vitamins. Crit. Rev. Food Sci. Nutr. 2021, 61, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. The gut microbiota regulates endocrine vitamin D metabolism through fibroblast growth factor 23. Front. Immunol. 2018, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, M.; Akagi, D.; Makishima, M. Lithocholic acid is a vitamin D receptor ligand that acts preferentially in the ileum. Int. J. Mol. Sci. 2018, 19, 1975. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Deng, F. The role of vitamin D in immune system and inflammatory bowel disease. J. Inflamm. Res. 2022, 15, 3167–3185. [Google Scholar] [CrossRef]
- Sun, J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy 2016, 12, 1057–1058. [Google Scholar] [CrossRef]
- Szaleniec, M.; Wojtkiewicz, A.M.; Bernhardt, R.; Borowski, T.; Donova, M. Bacterial steroid hydroxylases: Enzyme classes, their functions and comparison of their catalytic mechanisms. Appl. Microbiol. Biotechnol. 2018, 102, 8153–8171. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-regulated gene expression profiles: Species-specificity and cell-specific effects on metabolism and immunity. Endocrinology 2021, 162, bqaa218. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Schedel, M.; Jia, Y.; Michel, S.; Takeda, K.; Domenico, J.; Joetham, A.; Ning, F.; Strand, M.; Han, J.; Wang, M. 1, 25D3 prevents CD8+ Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter. Nat. Commun. 2016, 7, 10213. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Mrad, M.F.; El Ayoubi, N.K.; Esmerian, M.O.; Kazan, J.M.; Khoury, S.J. Effect of vitamin D replacement on immunological biomarkers in patients with multiple sclerosis. Clin. Immunol. 2017, 181, 9–15. [Google Scholar] [CrossRef]
- Sotirchos, E.S.; Bhargava, P.; Eckstein, C.; Van Haren, K.; Baynes, M.; Ntranos, A.; Gocke, A.; Steinman, L.; Mowry, E.M.; Calabresi, P.A. Safety and immunologic effects of high-vs low-dose cholecalciferol in multiple sclerosis. Neurology 2016, 86, 382–390. [Google Scholar] [CrossRef]
- Toghianifar, N.; Ashtari, F.; Zarkesh-Esfahani, S.H.; Mansourian, M. Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. J. Neuroimmunol. 2015, 285, 125–128. [Google Scholar] [CrossRef]
- Al-Shammri, S.; Chattopadhyay, A.; Raghupathy, R. Vitamin D supplementation mediates a shift towards anti-inflammatory cytokine response in Multiple Sclerosis. Med. Princ. Pract. 2025, 12, 1–9. [Google Scholar] [CrossRef]
- Smolders, J.; Peelen, E.; Thewissen, M.; Cohen Tervaert, J.W.; Menheere, P.; Hupperts, R.; Damoiseaux, J. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS ONE 2010, 5, e15235. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Role of vitamin D in the hygiene hypothesis: The interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef]
- Wampach, L.; Heintz-Buschart, A.; Hogan, A.; Muller, E.E.; Narayanasamy, S.; Laczny, C.C.; Hugerth, L.W.; Bindl, L.; Bottu, J.; Andersson, A.F. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front. Microbiol. 2017, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.A.; Pisani, L.P. The relationship among vitamin D, TLR4 pathway and preeclampsia. Mol. Biol. Rep. 2020, 47, 6259–6267. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M. Antimicrobial peptides—Mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Z.; Xia, B.; Zhang, Y.; Liu, X.; Yu, Y.; Tang, N.; Tong, X.; Wang, M.; Ye, X. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat. Biotechnol. 2022, 40, 921–931. [Google Scholar] [CrossRef]
- White, J.H. Emerging roles of vitamin D-induced antimicrobial peptides in antiviral innate immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef]
- Svensson, D.; Nebel, D.; Nilsson, B.-O. Vitamin D 3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production. Inflamm. Res. 2016, 65, 25–32. [Google Scholar] [CrossRef]
- Untersmayr, E.; Brandt, A.; Koidl, L.; Bergheim, I. The intestinal barrier dysfunction as driving factor of inflammaging. Nutrients 2022, 14, 949. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front. Immunol. 2020, 10, 499337. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, A.; Steenholdt, C.; Nielsen, O.H.; Jensen, K.B. Immune cell-derived signals governing epithelial phenotypes in homeostasis and inflammation. Trends Mol. Med. 2024, 30, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-g.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of vitamin D receptor leads to hyperfunction of claudin-2 in intestinal inflammatory responses. Inflamm. Bowel Dis. 2019, 25, 97–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Garrett, S.; Carroll, R.E.; Xia, Y.; Sun, J. Vitamin D receptor upregulates tight junction protein claudin-5 against colitis-associated tumorigenesis. Mucosal Immunol. 2022, 15, 683–697. [Google Scholar] [CrossRef]
- Lobo de Sá, F.D.; Backert, S.; Nattramilarasu, P.K.; Mousavi, S.; Sandle, G.I.; Bereswill, S.; Heimesaat, M.M.; Schulzke, J.-D.; Bücker, R. Vitamin D reverses disruption of gut epithelial barrier function caused by Campylobacter jejuni. Int. J. Mol. Sci. 2021, 22, 8872. [Google Scholar] [CrossRef]
- Xiang, L.; Du, T.; Zhang, J.; Zhang, Y.; Zhou, Y.; Zhao, Y.; Zhou, Y.; Ma, L. Vitamin D(3) supplementation shapes the composition of gut microbiota and improves some obesity parameters induced by high-fat diet in mice. Eur. J. Nutr. 2024, 63, 155–172. [Google Scholar] [CrossRef]
- Martens, P.J.; Centelles-Lodeiro, J.; Ellis, D.; Cook, D.P.; Sassi, G.; Verlinden, L.; Verstuyf, A.; Raes, J.; Mathieu, C.; Gysemans, C. High Serum Vitamin D Concentrations, Induced via Diet, Trigger Immune and Intestinal Microbiota Alterations Leading to Type 1 Diabetes Protection in NOD Mice. Front. Immunol. 2022, 13, 902678. [Google Scholar] [CrossRef] [PubMed]
- Hanson, T.; Constantine, E.; Nobles, Z.; Butler, E.; Renteria, K.M.; Teoh, C.M.; Koh, G.Y. Supplementation of Vitamin D(3) and Fructooligosaccharides Downregulates Intestinal Defensins and Reduces the Species Abundance of Romboutsia ilealis in C57BL/6J Mice. Nutrients 2024, 16, 2236. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Dong, Y. Vitamin D Decreases Plasma Trimethylamine-N-oxide Level in Mice by Regulating Gut Microbiota. BioMed Res. Int. 2020, 2020, 9896743. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; AlJaziri, Z.Y.; Almulhim, C.F.; Aldrees, A.S.; AlShakhs, Z.H.; AlAithan, R.I.; Alothman, F.A. Vitamin D Supplementation in Laboratory-Bred Mice: An In Vivo Assay on Gut Microbiome and Body Weight. Microbiol. Insights 2020, 13, 1178636120945294. [Google Scholar] [CrossRef]
- Wyatt, M.; Choudhury, A.; Von Dohlen, G.; Heileson, J.L.; Forsse, J.S.; Rajakaruna, S.; Zec, M.; Tfaily, M.M.; Greathouse, L. Randomized control trial of moderate dose vitamin D alters microbiota stability and metabolite networks in healthy adults. Microbiol. Spectr. 2024, 12, e0008324. [Google Scholar] [CrossRef] [PubMed]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef]
- Pham, H.; Waterhouse, M.; Rahman, S.; Baxter, C.; Duarte Romero, B.; McLeod, D.S.A.; Ebeling, P.R.; English, D.R.; Hartel, G.; O’Connell, R.L.; et al. The effect of vitamin D supplementation on the gut microbiome in older Australians-Results from analyses of the D-Health Trial. Gut Microbes 2023, 15, 2221429. [Google Scholar] [CrossRef] [PubMed]
- Drall, K.M.; Field, C.J.; Haqq, A.M.; de Souza, R.J.; Tun, H.M.; Morales-Lizcano, N.P.; Konya, T.B.; Guttman, D.S.; Azad, M.B.; Becker, A.B.; et al. Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: Implications for viral respiratory infections. Gut Microbes 2020, 12, 1799734. [Google Scholar] [CrossRef]
- Lei, W.T.; Huang, K.Y.; Jhong, J.H.; Chen, C.H.; Weng, S.L. Metagenomic analysis of the gut microbiome composition associated with vitamin D supplementation in Taiwanese infants. Sci. Rep. 2021, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, H.K.; Kang, C.D.; Choi, D.H.; Park, S.C.; Park, J.M.; Nam, S.J.; Chae, G.B.; Lee, K.Y.; Cho, H.; et al. High Dose Intramuscular Vitamin D3 Supplementation Impacts the Gut Microbiota of Patients With Clostridioides Difficile Infection. Front. Cell. Infect. Microbiol. 2022, 12, 904987. [Google Scholar] [CrossRef]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef]
- Küçük, A.; Bir, L.S.; Tekin, S.; Demir, S. Serum 25(OH) vitamin D level in Relapsing-Remitting Multiple Sclerosis and clinically isolated syndrome groups. Horm. Mol. Biol. Clin. Investig. 2022, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Darwish, H.; Farran, N.; Hannoun, S.; Tadros, N.; Yamout, B.; El Ayoubi, N.K.; Khoury, S.J. Serum vitamin D level is associated with speed of processing in multiple sclerosis patients. J. Steroid Biochem. Mol. Biol. 2020, 200, 105628. [Google Scholar] [CrossRef]
- Ayele, B.A.; Wuhib, M.Z.; Zenebe, B.G.; Metaferia, G.Z. Serum Vitamin D Level among Multiple Sclerosis Patients in the Tropics: Experience from a Private Clinic in Addis Ababa, Ethiopia. Ethiop. J. Health Sci. 2021, 31, 611–618. [Google Scholar]
- Kusumadewi, W.; Imran, D.; Witjaksono, F.; Pakasi, T.A.; Rusmana, A.I.; Pangeran, D.; Marwadhani, S.S.; Maharani, K.; Estiasari, R. Low vitamin D-25(OH) level in Indonesian multiple sclerosis and neuromyelitis optic patients. Mult. Scler. Relat. Disord. 2018, 25, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Lv, Z.; Fan, X.; Wang, L.; Han, F.; Wang, H.; Bi, S. Vitamin D status and the risk of multiple sclerosis: A systematic review and meta-analysis. Neurosci. Lett. 2014, 570, 108–113. [Google Scholar] [CrossRef]
- Grut, V.; Biström, M.; Salzer, J.; Stridh, P.; Lindam, A.; Alonso-Magdalena, L.; Andersen, O.; Jons, D.; Gunnarsson, M.; Vrethem, M.; et al. Free vitamin D(3) index and vitamin D-binding protein in multiple sclerosis: A presymptomatic case-control study. Eur. J. Neurol. 2022, 29, 2335–2342. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Munger, K.L.; Koch-Henriksen, N.; Hougaard, D.M.; Magyari, M.; Jørgensen, K.T.; Lundqvist, M.; Simonsen, J.; Jess, T.; Cohen, A.; et al. Neonatal vitamin D status and risk of multiple sclerosis: A population-based case-control study. Neurology 2017, 88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review. Nutrients 2021, 13, 4207. [Google Scholar] [CrossRef] [PubMed]
- Berezowska, M.; Coe, S.; Dawes, H. Effectiveness of Vitamin D Supplementation in the Management of Multiple Sclerosis: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 1301. [Google Scholar] [CrossRef]
- Langlois, J.; Denimal, D. Clinical and Imaging Outcomes after Vitamin D Supplementation in Patients with Multiple Sclerosis: A Systematic Review. Nutrients 2023, 15, 1945. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Multiple Sclerosis in Adults: Management. Available online: https://www.nice.org.uk/guidance/ng220/resources/multiple-sclerosis-in-adults-management-pdf-66143828948677 (accessed on 16 April 2025).
- Physicians Committee for Responsible Medicine. Nutrition Guide for Clinicians: Multiple Sclerosis. Available online: https://nutritionguide.pcrm.org/nutritionguide/view/Nutrition_Guide_for_Clinicians/1342098/all/Multiple_Sclerosis (accessed on 16 April 2025).
- Mavar, M.; Sorić, T.; Bagarić, E.; Sarić, A.; Matek Sarić, M. The Power of Vitamin D: Is the Future in Precision Nutrition through Personalized Supplementation Plans? Nutrients 2024, 16, 1176. [Google Scholar] [CrossRef]
- Dałek, P.; Drabik, D.; Wołczańska, H.; Foryś, A.; Jagas, M.; Jędruchniewicz, N.; Przybyło, M.; Witkiewicz, W.; Langner, M. Bioavailability by design-Vitamin D(3) liposomal delivery vehicles. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102552. [Google Scholar] [CrossRef]
- Cohen, Y.; Margier, M.; Lesmes, U.; Reboul, E.; Livney, Y.D. Mechanisms of absorption of vitamin D(3) delivered in protein nanoparticles in the absence and presence of fat. Food Funct. 2021, 12, 4935–4946. [Google Scholar] [CrossRef]
- Nah, H.; Lee, D.; Heo, M.; Lee, J.S.; Lee, S.J.; Heo, D.N.; Seong, J.; Lim, H.N.; Lee, Y.H.; Moon, H.J.; et al. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci. Technol. Adv. Mater. 2019, 20, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef]
- Srichomchey, P.; Sukprasert, S.; Khulasittijinda, N.; Voravud, N.; Sahakitrungruang, C.; Lumjiaktase, P. Vitamin D(3) Supplementation Promotes Regulatory T-Cells to Maintain Immune Homeostasis After Surgery for Early Stages of Colorectal Cancer. In Vivo 2023, 37, 286–293. [Google Scholar] [CrossRef]
- Eckard, A.R.; O’Riordan, M.A.; Rosebush, J.C.; Lee, S.T.; Habib, J.G.; Ruff, J.H.; Labbato, D.; Daniels, J.E.; Uribe-Leitz, M.; Tangpricha, V.; et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antivir. Ther. 2018, 23, 315–324. [Google Scholar] [CrossRef]
- Li, X.; Pan, C.; Ma, W.; Yang, T.; Wang, C.; Han, W.; Zhang, W.; Li, H.; Li, Z.; Zhao, T.; et al. Effects of dietary supplementation of fish oil plus vitamin D(3) on gut microbiota and fecal metabolites, and their correlation with nonalcoholic fatty liver disease risk factors: A randomized controlled trial. Food Funct. 2024, 15, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.; Gold, D.R.; Weiss, S.T.; Litonjua, A.A.; Lee-Sarwar, K.; Liu, Y.Y. Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation. Nutrients 2023, 15, 2059. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Coppi, F.; Severino, P.; Penna, C.; Pagliaro, P.; Dei Cas, A.; Bucciarelli, V.; Madonna, R.; Tarperi, C.; Schena, F.; et al. A Personalized Approach to Vitamin D Supplementation in Cardiovascular Health Beyond the Bone: An Expert Consensus by the Italian National Institute for Cardiovascular Research. Nutrients 2025, 17, 115. [Google Scholar] [CrossRef]
- Thouvenot, E.; Laplaud, D.; Lebrun-Frenay, C.; Derache, N.; Le Page, E.; Maillart, E.; Froment-Tilikete, C.; Castelnovo, G.; Casez, O.; Coustans, M.; et al. High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial. JAMA 2025, 333, 1413–1422. [Google Scholar] [CrossRef]
- Cassard, S.D.; Fitzgerald, K.C.; Qian, P.; Emrich, S.A.; Azevedo, C.J.; Goodman, A.D.; Sugar, E.A.; Pelletier, D.; Waubant, E.; Mowry, E.M. High-dose vitamin D(3) supplementation in relapsing-remitting multiple sclerosis: A randomised clinical trial. EClinicalMedicine 2023, 59, 101957. [Google Scholar] [CrossRef]
- Dörr, J.; Bäcker-Koduah, P.; Wernecke, K.D.; Becker, E.; Hoffmann, F.; Faiss, J.; Brockmeier, B.; Hoffmann, O.; Anvari, K.; Wuerfel, J.; et al. High-dose vitamin D supplementation in multiple sclerosis-results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320903474. [Google Scholar] [CrossRef] [PubMed]
- Muris, A.H.; Smolders, J.; Rolf, L.; Thewissen, M.; Hupperts, R.; Damoiseaux, J. Immune regulatory effects of high dose vitamin D(3) supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J. Neuroimmunol. 2016, 300, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.S.; Liu, Y.; Gray, O.M.; Baker, J.E.; Kolbe, S.C.; Ditchfield, M.R.; Egan, G.F.; Mitchell, P.J.; Harrison, L.C.; Butzkueven, H.; et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 2011, 77, 1611–1618. [Google Scholar] [CrossRef]
- De Vincentis, S.; Russo, A.; Milazzo, M.; Lonardo, A.; De Santis, M.C.; Rochira, V.; Simoni, M.; Madeo, B. How Much Vitamin D is Too Much? A Case Report and Review of the Literature. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1653–1659. [Google Scholar] [CrossRef]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Soric, T.; Saric, A.; Saric, M.M. Vitamin D, Public Health, and Personalized Nutrition. In Handbook of Public Health Nutrition; Springer: Cham, Switzerland, 2025. [Google Scholar]
- Salem, R.; Thomas, J.; Khattak, A.; Al Anouti, F. A digital heliometric device for monitoring sun exposure in cases of hypovitaminosis D: A qualitative analysis of user evaluations from the UAE. Inform. Med. Unlocked 2024, 50, 101568. [Google Scholar] [CrossRef]
- Ziemssen, T.; Haase, R. Digital Innovation in Multiple Sclerosis Management. Brain Sci. 2021, 12, 40. [Google Scholar] [CrossRef]
- Gashi, S.; Oldrati, P.; Moebus, M.; Hilty, M.; Barrios, L.; Ozdemir, F.; Kana, V.; Lutterotti, A.; Rätsch, G.; Holz, C. Modeling multiple sclerosis using mobile and wearable sensor data. NPJ Digit. Med. 2024, 7, 64. [Google Scholar] [CrossRef]
| Role of Gut Microbiota | Mechanism of Action | References |
|---|---|---|
| Gut microbiota alterations | Statistically significant differences in both alpha-diversity and beta-diversity Decrease in SCFA-producing bacteria such as Firmicutes, Roseburia, Coprococcus, Lachnospiraceae, Butyricicoccus, Faecalibacterium, Dorea, Lachnospira, and Prevotella Increase certain bacterial genera associated with pro-inflammatory responses, including Bacteroidetes, Akkermansia, and Ruminococcus species | [40] |
| Gut-associated immune system | Increased Th1 and Th17 responses due to dysbiosis (microbial antigens, microbial metabolites or dendritic cell mediated effect) Decreased Treg cells due to dysbiosis Autoimmunity due to increased Th1 and Th17 responses Increased levels of pro-inflammatory cytokines Microbial metabolites such as SCFAs alter the expression of immune genes by acting as histone deacetylase inhibitors | [75,79,85,86,89,96] |
| Gut–brain axis | Decreased production of SCFAs and impaired intestinal barrier integrity due to dysbiosis (leaky gut) CNS effects and neuro-inflammation due to the passage of exogenous molecules such as bacterial products and metabolites into the bloodstream due to increased intestinal permeability Impaired synthesis of neurotransmitters such as gamma-aminobutyric acid and dopamine due to dysbiosis | [75,93,94,108,109] |
| Sample | Mechanism of Action | References |
|---|---|---|
| Effect of vitamin D supplementation on gut microbiota and intestinal barrier integrity | ||
| Male C57BL/6 J mice | Prevention of endotoxemia and maintenance of intestinal barrier function Serum LPS concentration ↓ Caecum ZO-1 and Occludin mRNA relative expression ↑ Improved α-diversity and β-diversity of the gut microbiota Relative abundance of Firmicutes ↓ Relative abundance of Bacteroidetes and Proteobacteria ↑ Gut dysbiosis ↓ | [178] |
| Non-obese diabetic mice | Abundance of Lachnospiraceae_FCS020 and Ruminiclostridium_9 ↑ Abundance of Marvinbryantia ↓ | [179] |
| C57BL/6J Mice | Colonic ZO-1 and Occludin mRNA relative expression ↑ Relative abundance of Dubosiella newyorkensis ↑ | [180] |
| Female C57BL/6J mice | Plasma TMA and TMAO ↓ Increased gut microbial α-diversity indices Relative abundance of Firmicutes ↓ Relative abundance of Bacteroidetes ↑ Bacteroidetes/Firmicutes ratio ↑ Relative abundance of Akkermansia and Ruminiclostridium ↑ | [181] |
| Male BALB/C mice | Percentages of Pseudomonas aeruginosa and Salmonella/Shigella spp. ↓ | [182] |
| Healthy adults | Bifidobacteriaceae family ↑ | [183] |
| Increased gut microbial diversity Relative abundance of Firmicutes ↓ Relative abundance of Bacteroidetes ↑ Bacteroidetes/Firmicutes ratio ↑ Relative abundance of Actinobacteria and Verrucomicrobia ↑ Relative abundance of Bifidobacterium and Akkermansia ↑ Relative abundance of Roseburia, Ruminococcus, and Fecalibacterium ↓ | [128] | |
| Relative abundance of Faecalibacterium spp., Clostridia, and Ruminococcaceae ↓ Firmicutes/Bacteroidetes ratio ↓ | [130] | |
| No difference was observed in terms of α-diversity. Abundance of genus Lachnospira ↑ Abundance of genus Blautia ↓ Abundance of genus Coprococcus ↑ (>75 nmol/L) Abundance of genus Ruminococcus ↓ (<50 nmol/L) | [184] | |
| MS | Majority of operational taxonomic units ↓ Akkermansia, Faecalibacterium, and Coprococcus in untreated MS ↑ | [132] |
| Older adults (60–84 y) | No significant difference was observed in gut microbiota composition | [185] |
| Infants | Abundance of genus Bilophila, Megamonas and Peptostreptococcus ↓ | [186] |
| Relative abundance of Bifidobacterium, Streptococcus, and Lactobacillus ↑ Proportion of Bifidobacterium correlated vitamin D circulating level | [187] | |
| Clostridioides Difficile Infected Patients | Abundance of genus Proteobacteria, Enterobacteriaceae and Escherichia ↓ Abundance of Lachnospiraceae, Ruminococcaceae, Christensenellaceae, Bifidobacteriaceae, and Sutterellaceae ↑ | [188] |
| Effect of vitamin D supplementation on immune regulation | ||
| Male C57BL/6 J mice | TNF-α, IL-1β ↓ | [178] |
| Non-obese diabetic mice | Splenic FoxP3+ Treg Cells ↑ IL-10 secretion CD4+ T Cells ↑ Type 1 Diabetes Mellitus incidence ↓ | [179] |
| Weaned C57BL/6 mice | Inhibit pro-inflammatory cytokines including IL-1, IL-8, IL-17, and TNF-α | [189] |
| 25-hydroxyvitamin D-deficient RRMS patients | ↓ IFN-γ secretion by CD4+ T cells received 10,000 IU/week cholecalciferol for 3 months | [156] |
| RRMS patients | High-dose group (received 10,000 IU/week cholecalciferol for 6 months) ↓ IL-17 production by CD4+ T cells | [157] |
| RRMS patients | Control group (received 50,000 IU cholecalciferol every 5 days for 12 weeks) ↓ IL-17 levels | [158] |
| MS patients | Received 50,000 IU cholecalciferol once weekly ↓ TNF-α:IL-5 ratio, ↓ TNF-α:IL-6 ratio, ↓ TNF-α:IL-10 ratio, and ↓ IFN-γ:IL-10 ratio at the 12-month follow-up | [159] |
| RRMS patients | Received 20,000 IU/d cholecalciferol for 12 weeks ↑ %IL-17 production by CD4+ T cells | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gençer Bingöl, F.; Kocyigit, E.; Çelik, E.; Ağagündüz, D.; Budán, F. Breaking the Cycle: Can Vitamin D Bridge the Gap Between Gut Microbiota and Immune Dynamics in Multiple Sclerosis? Int. J. Mol. Sci. 2025, 26, 5464. https://doi.org/10.3390/ijms26125464
Gençer Bingöl F, Kocyigit E, Çelik E, Ağagündüz D, Budán F. Breaking the Cycle: Can Vitamin D Bridge the Gap Between Gut Microbiota and Immune Dynamics in Multiple Sclerosis? International Journal of Molecular Sciences. 2025; 26(12):5464. https://doi.org/10.3390/ijms26125464
Chicago/Turabian StyleGençer Bingöl, Feray, Emine Kocyigit, Elif Çelik, Duygu Ağagündüz, and Ferenc Budán. 2025. "Breaking the Cycle: Can Vitamin D Bridge the Gap Between Gut Microbiota and Immune Dynamics in Multiple Sclerosis?" International Journal of Molecular Sciences 26, no. 12: 5464. https://doi.org/10.3390/ijms26125464
APA StyleGençer Bingöl, F., Kocyigit, E., Çelik, E., Ağagündüz, D., & Budán, F. (2025). Breaking the Cycle: Can Vitamin D Bridge the Gap Between Gut Microbiota and Immune Dynamics in Multiple Sclerosis? International Journal of Molecular Sciences, 26(12), 5464. https://doi.org/10.3390/ijms26125464







