Supercritical Carbon Dioxide-Processed Acellular Dermal Matrix Patch for Enhanced Wound Healing
Abstract
1. Introduction
2. Results
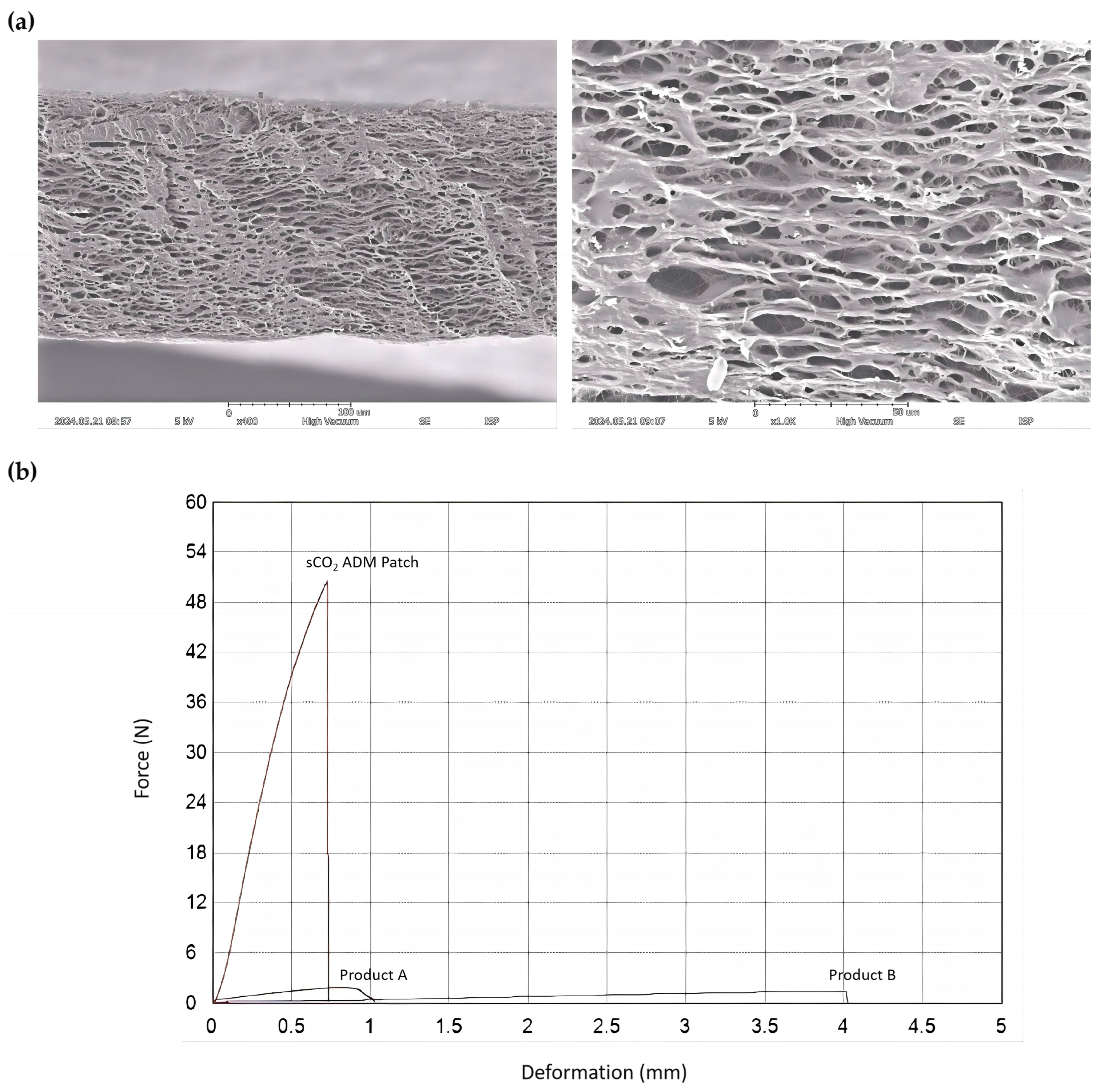
2.1. Characterization of sCO2 ADM Patch
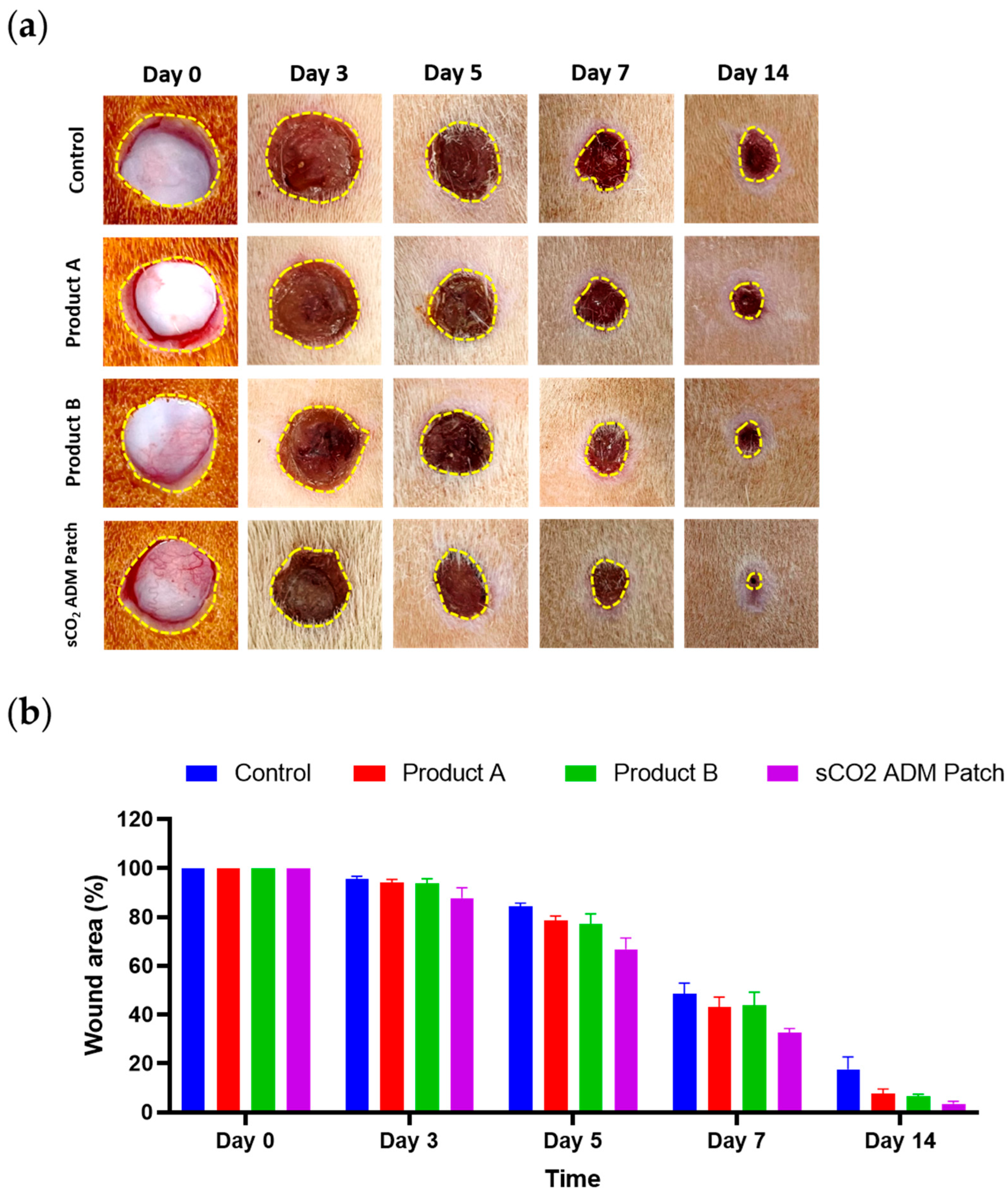
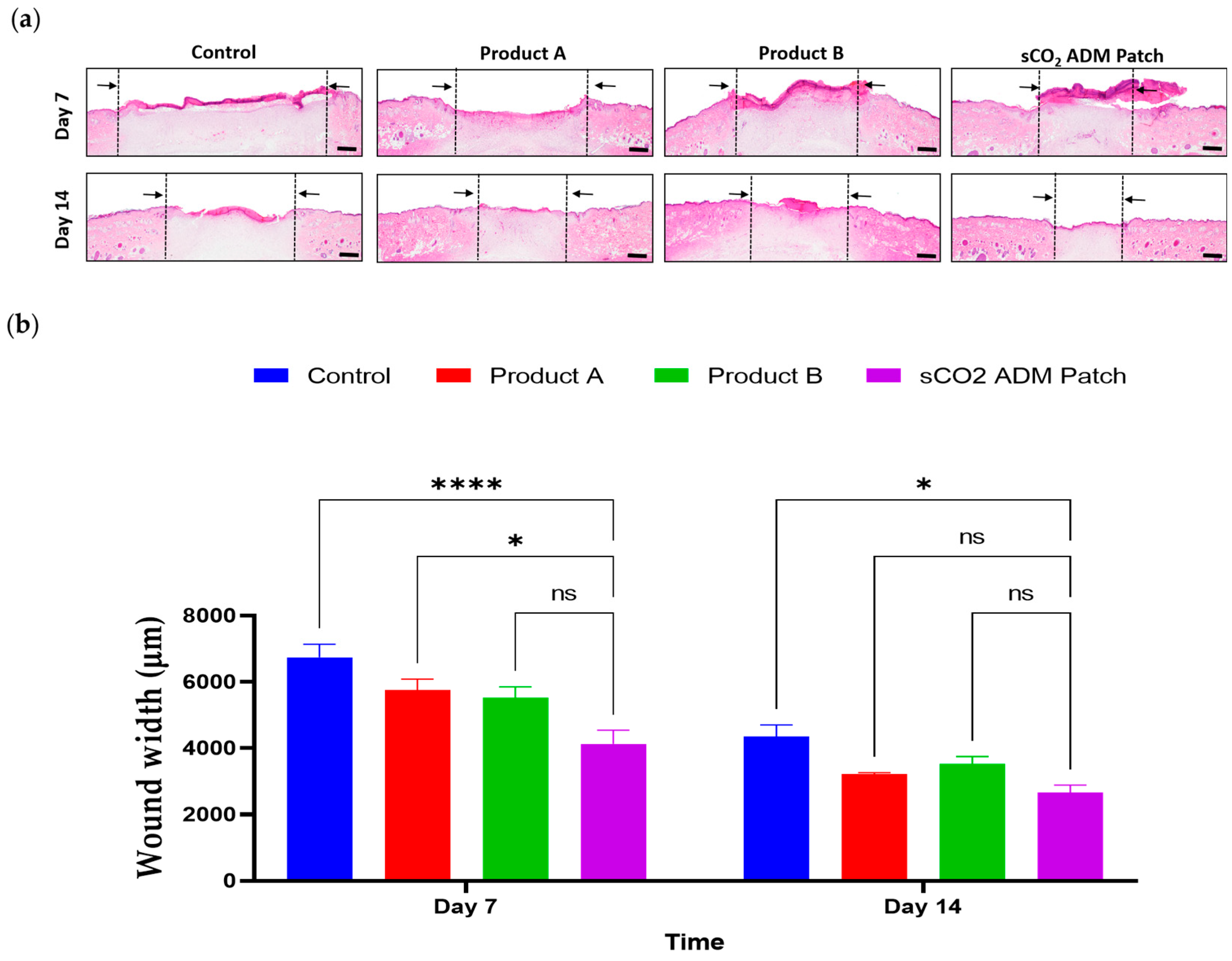
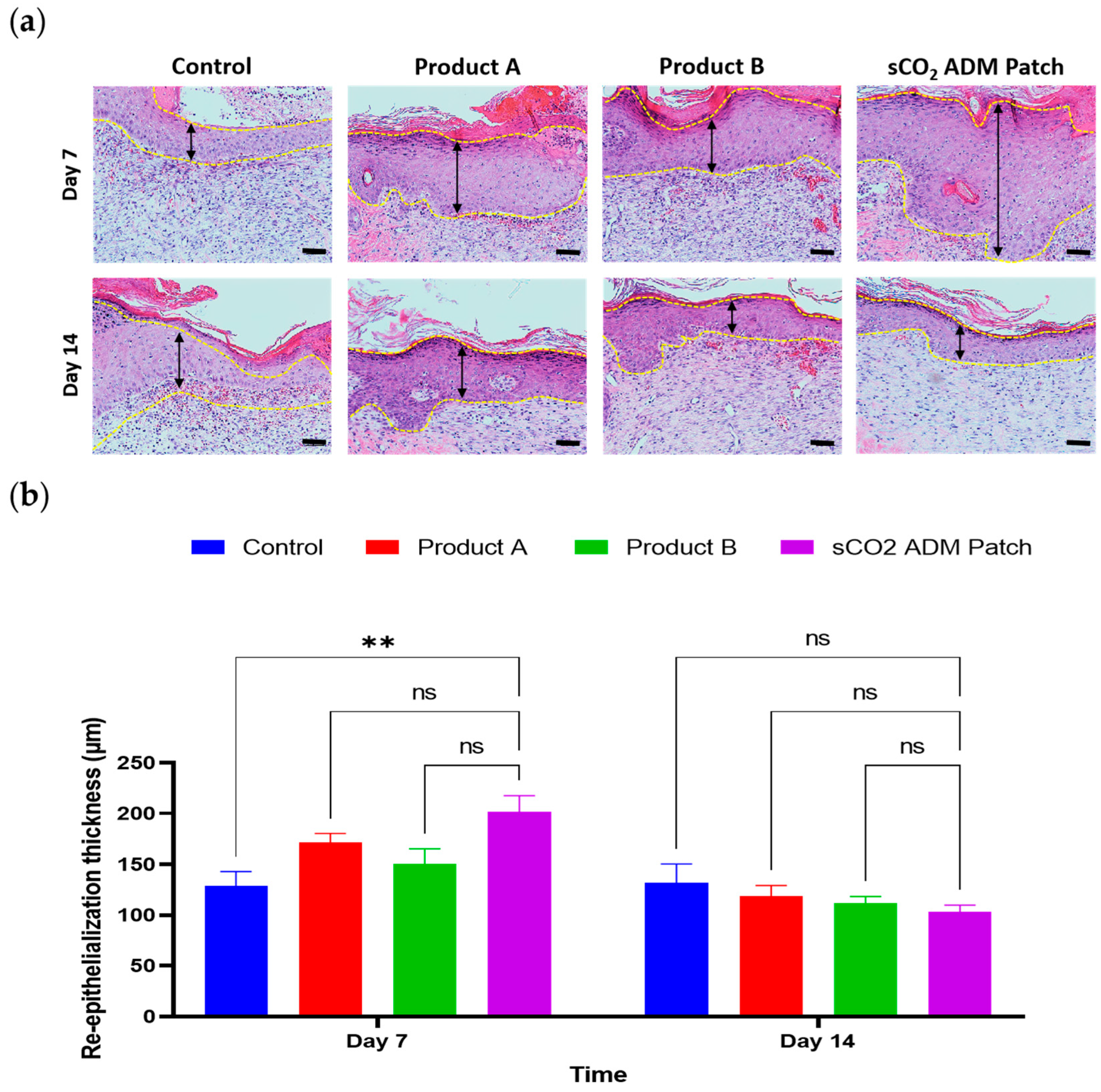
2.2. Macroscopic and Microscopic Observation of the Wound Healing Process
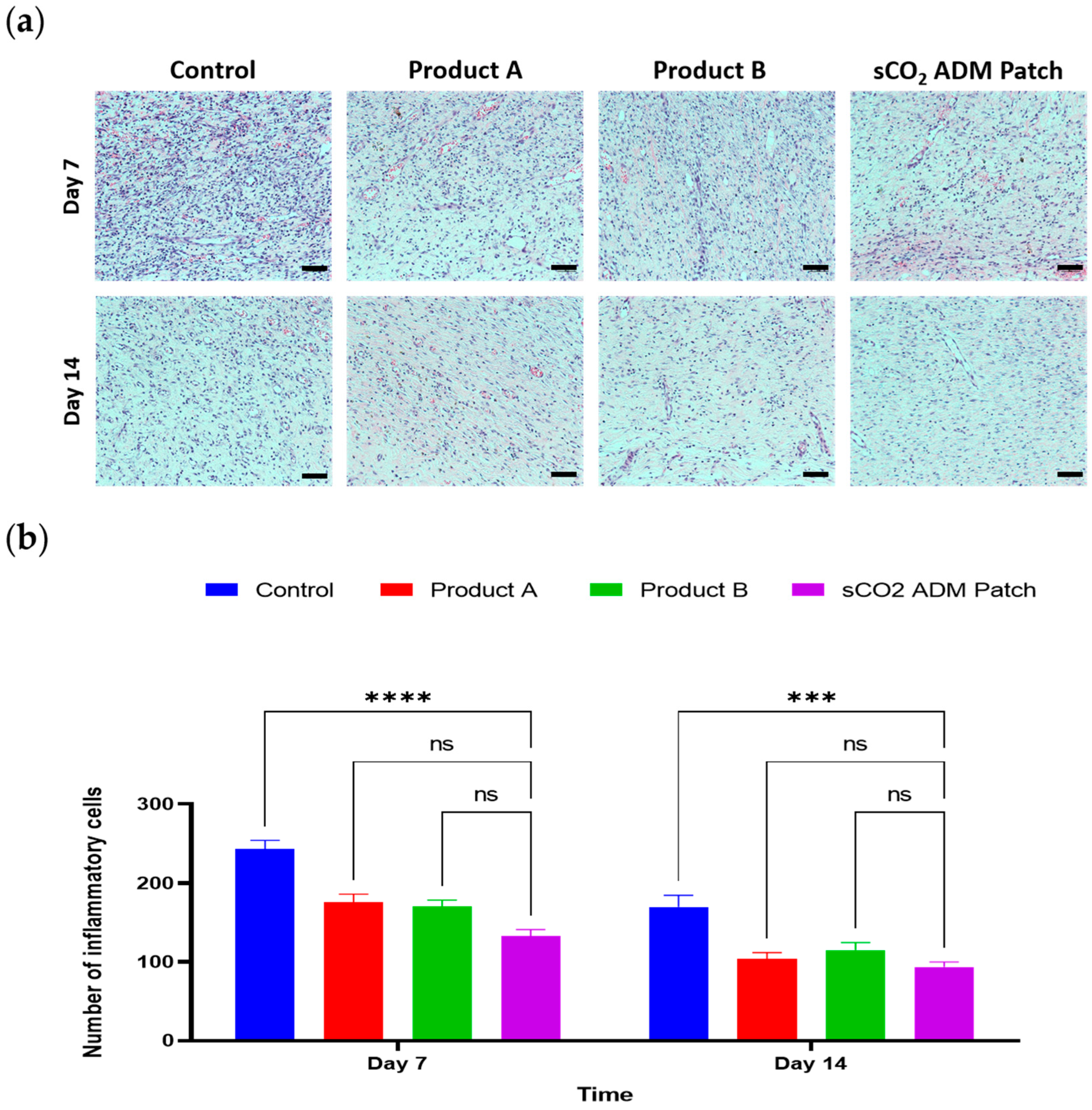
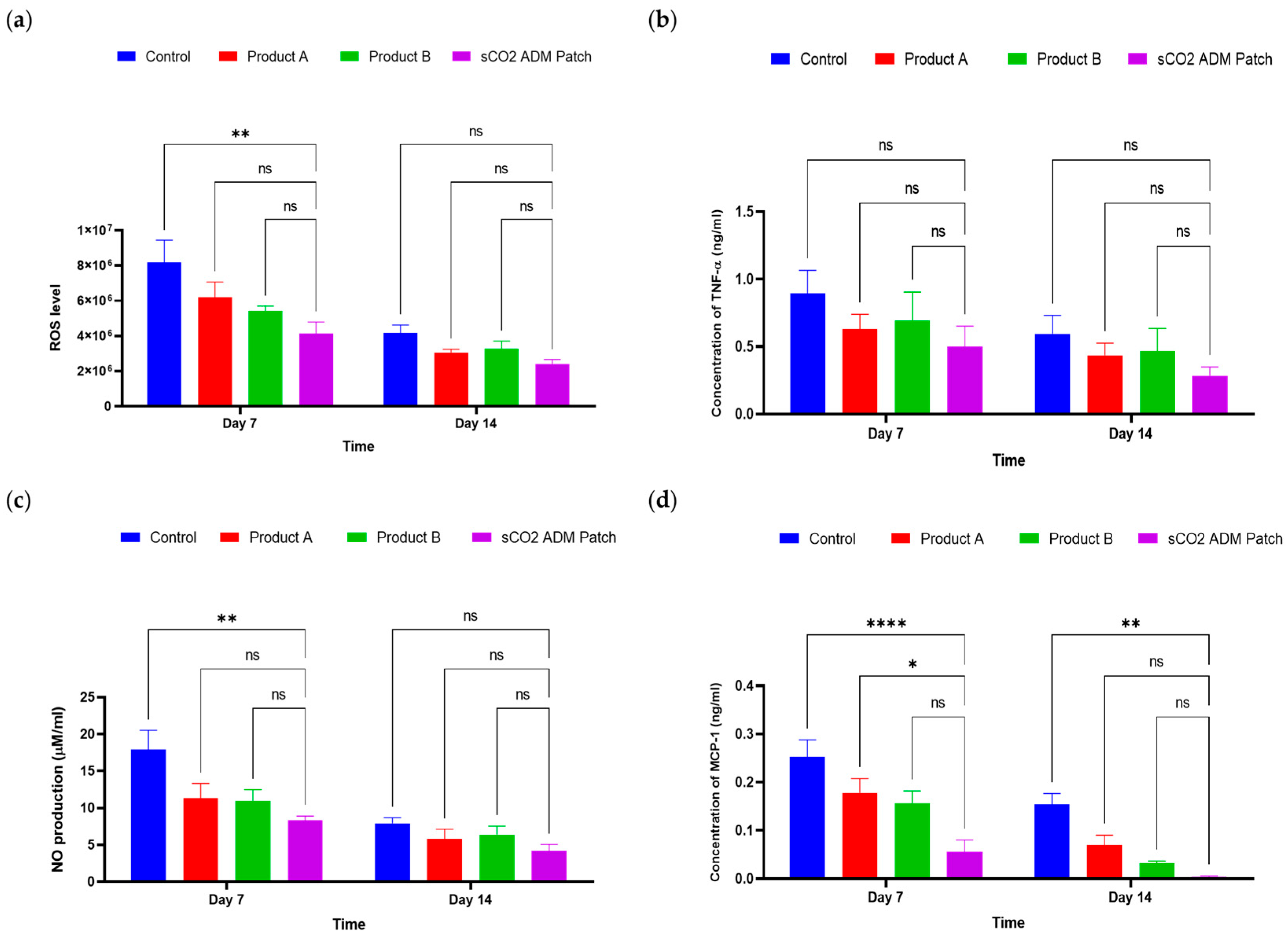
2.3. Effects of the Antiinflammation and Antioxidation by sCO2 ADM Patch During the Wound Healing Process
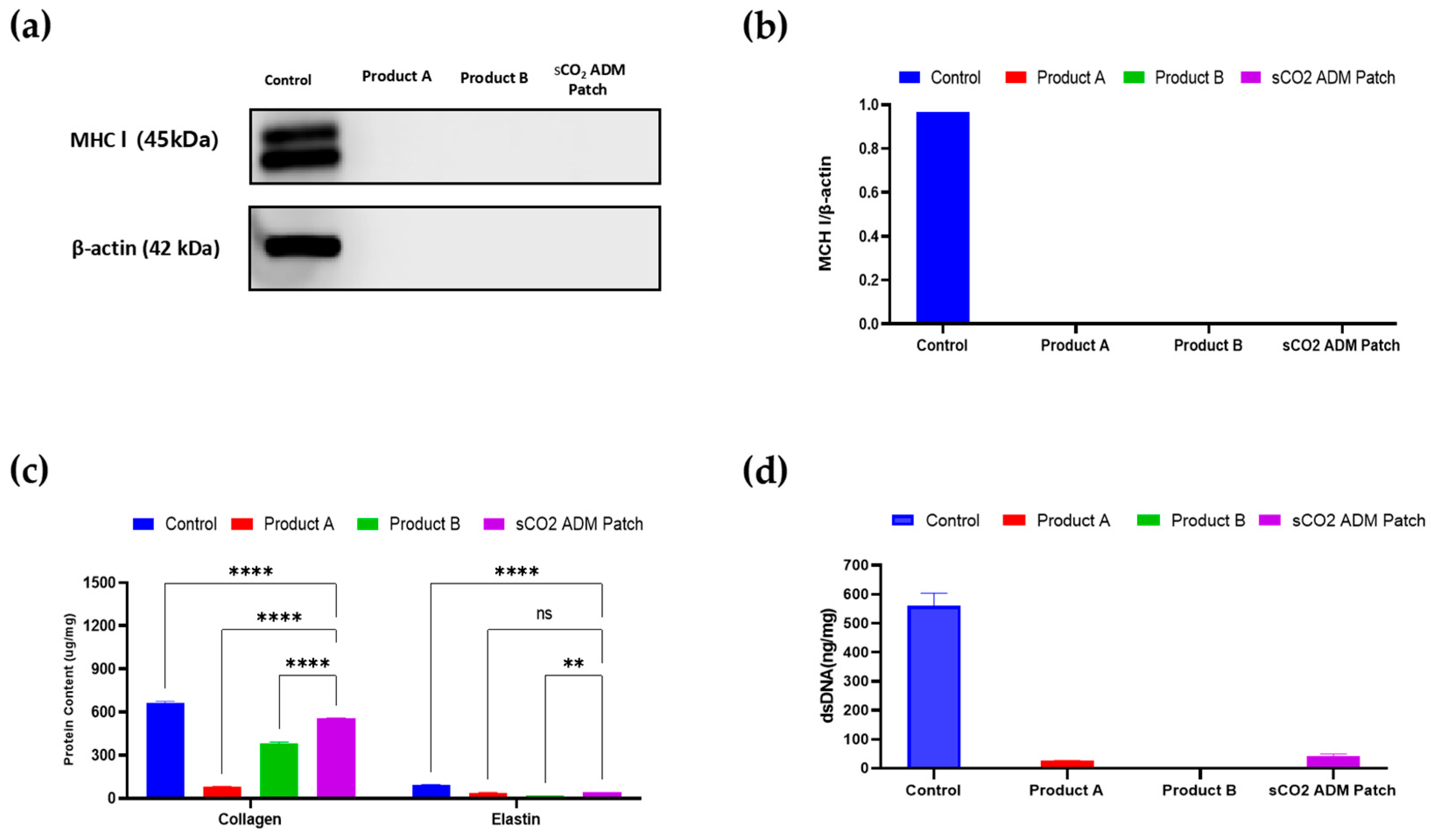
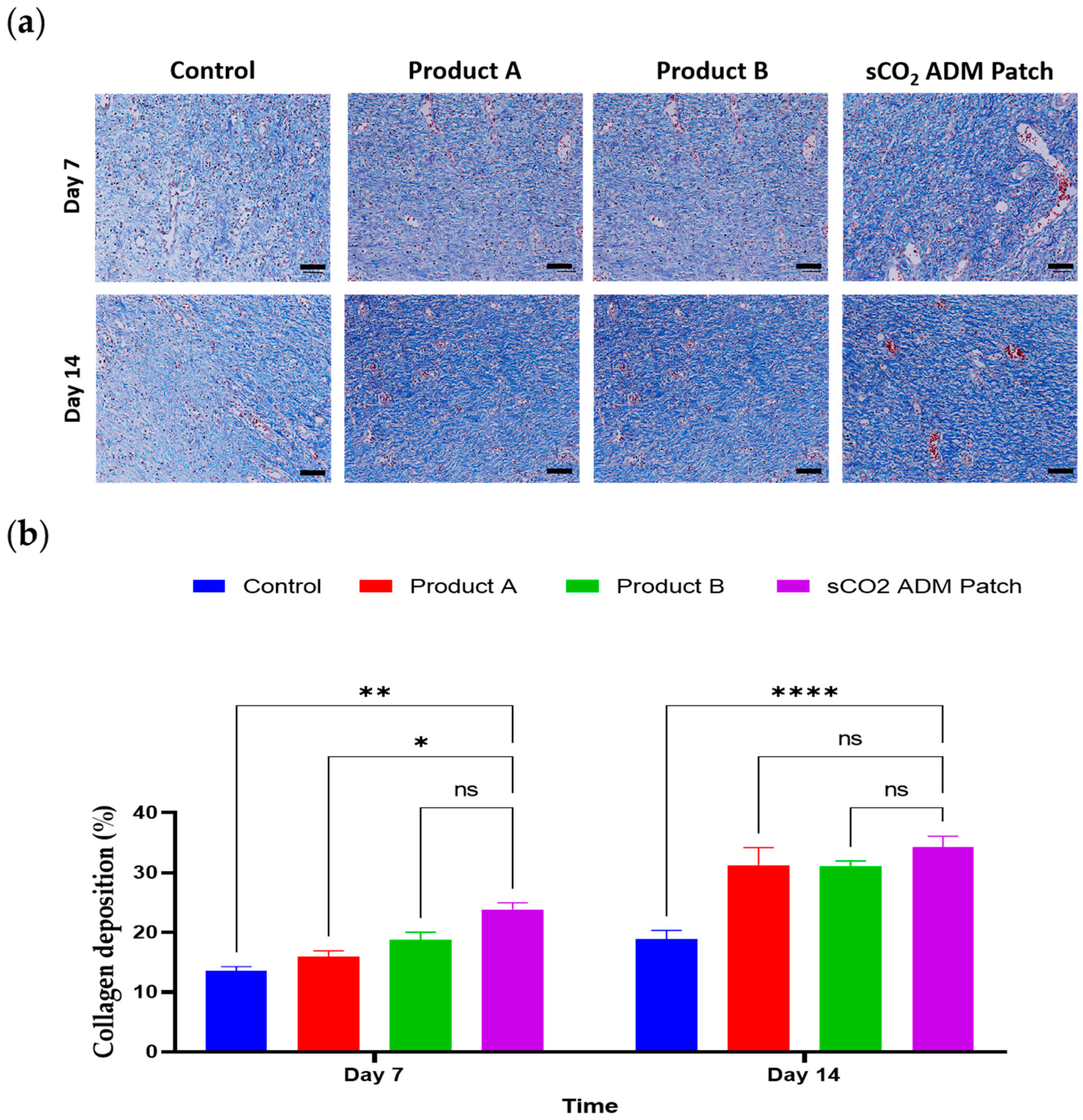
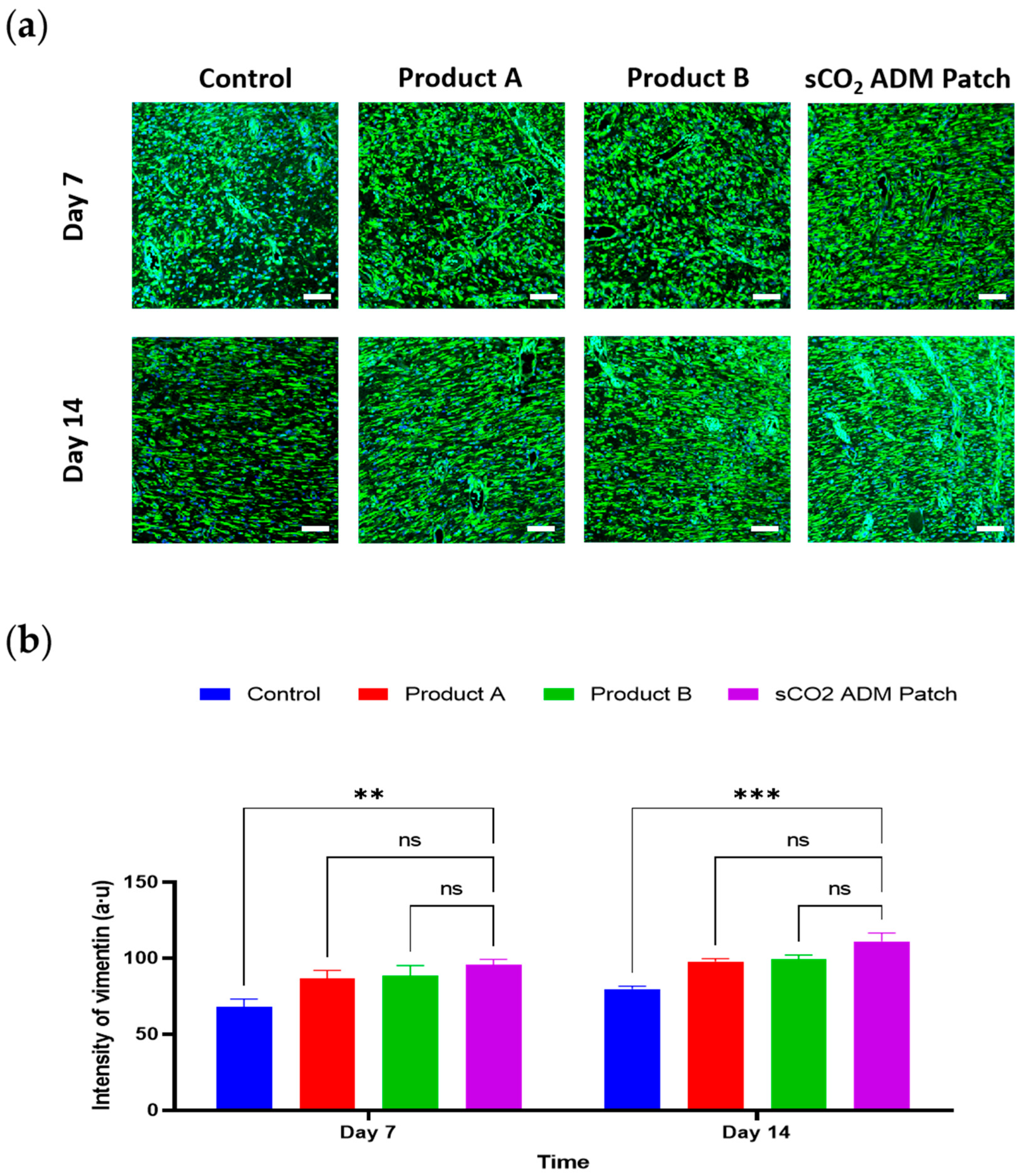
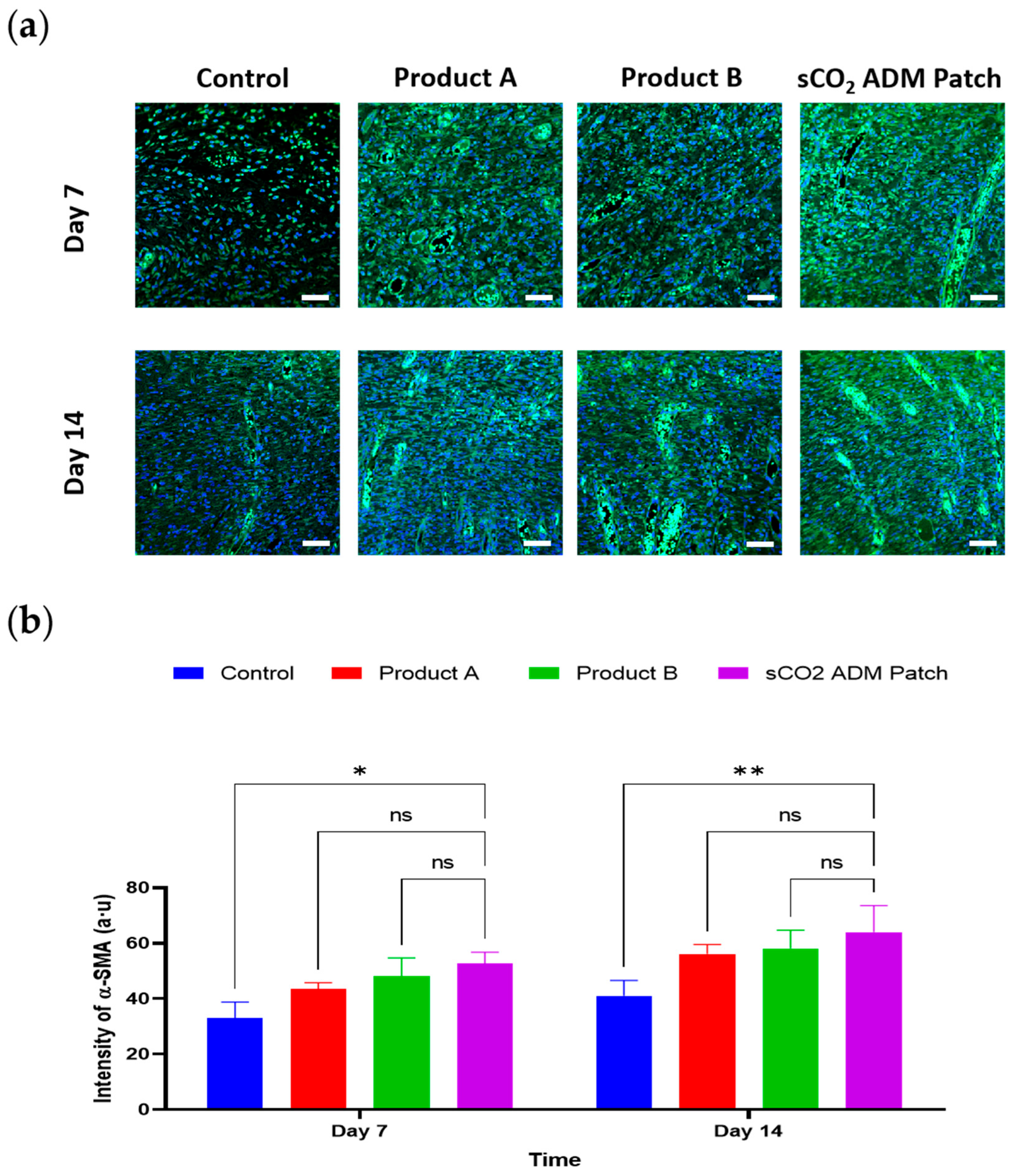
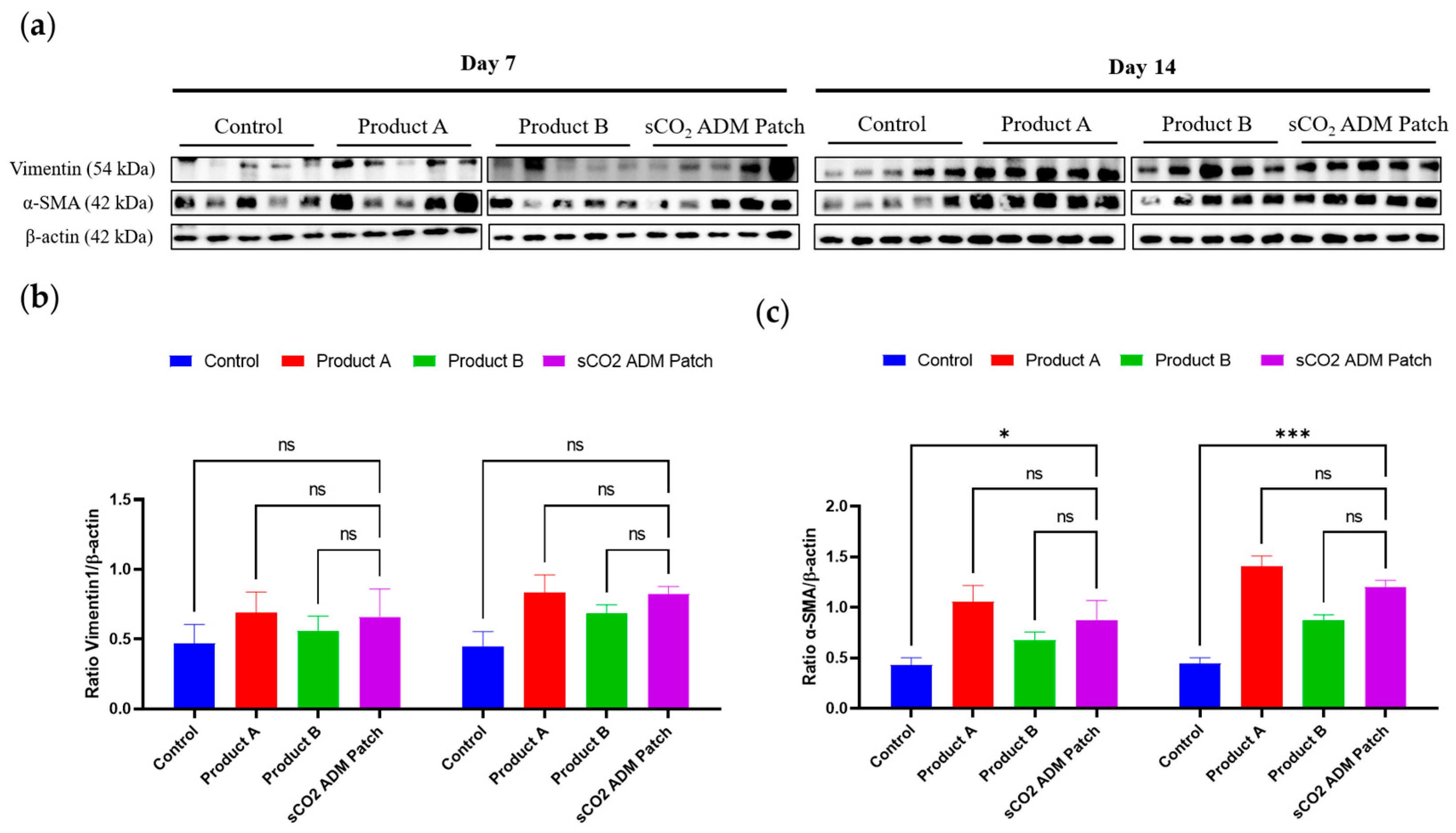
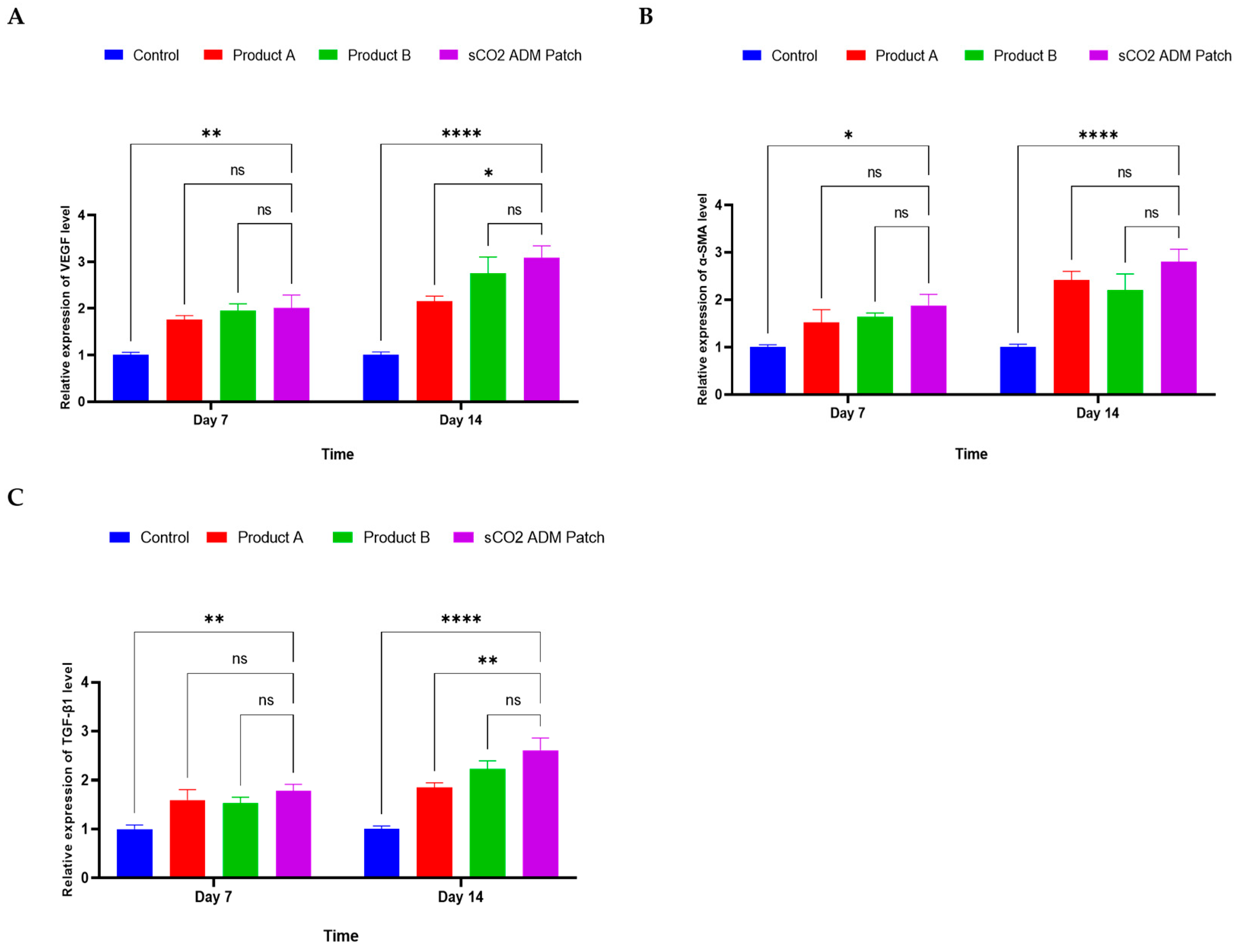
2.4. Acceleration of the Proliferation and Remodeling by sCO2 ADM During the Wound Healing Process
3. Discussion
4. Materials and Methods
4.1. Supercritical Carbon Dioxide Acellular Dermal Matrix Patch (sCO2 ADM Patch) Preparation
4.2. Scanning Electron Microscope (SEM) Imaging for sCO2 ADM Patch Morphology
4.3. Tensile Strength Testing
4.4. Immunogenicity Testing and Component Determination
4.5. Animal Experiment
4.6. Measurement of the Wound Healing Area
4.7. Histological Analysis
4.8. Hematoxylin and Eosin (H&E) Staining
4.9. Masson’s Trichrome (MT) Staining
4.10. Immunofluorescence (IF) Staining
4.11. Protein Preparation
4.12. Western Blot
4.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4.14. Nitric Oxide Quantification
4.15. 2′,7′-Dichlorofluorescein Diacetate (DCF-DA) Assay
4.16. RT-PCR
4.17. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADM | acellular dermal matrix |
| α-SMA | alpha smooth muscle actin |
| DCF-DA | 2′,7′-dichlorofluorescein diacetate |
| dECM | decellularized extracellular matrix |
| ECM | extracellular matrix |
| ELISA | enzyme-linked immunosorbent assay |
| H&E | hematoxylin and eosin |
| IF | immunofluorescence |
| MCP-1 | monocyte chemoattractant protein 1 |
| MHC | major histocompatibility complex |
| MT | Masson’s trichrome |
| NO | nitric oxide |
| PDGF | platelet-derived growth factor |
| PMSF | phenylmethylsulfonyl fluoride |
| ROS | reactive oxygen species |
| sCO2 | supercritical carbon dioxide |
| SEM | scanning electron microscope |
| SPF | Specific pathogen free |
| TGF-β1 | transforming growth factor-beta 1 |
| TNF-α | tumor necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
References
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martinez-Burnes, J.; Gomez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef] [PubMed]
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef]
- Crucianelli, L.; Ehrsson, H.H. The Role of the Skin in Interoception: A Neglected Organ? Perspect. Psychol. Sci. 2023, 18, 224–238. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Varga, J.F.A.; Bui-Marinos, M.P.; Katzenback, B.A. Frog Skin Innate Immune Defences: Sensing and Surviving Pathogens. Front. Immunol. 2018, 9, 3128. [Google Scholar] [CrossRef]
- Quaresma, J.A.S. Organization of the Skin Immune System and Compartmentalized Immune Responses in Infectious Diseases. Clin. Microbiol. Rev. 2019, 32, e00034-18. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.X.; Ngoc Chien, P.; Thi Nga, P.; Zhang, X.R.; Ngan Giang, N.; Thi Thuy Le, L.; Trinh, T.T.; Zhou, S.Y.; Nam, S.Y.; Heo, C.Y. Enhancing wound healing through innovative technologies: Microneedle patches and iontophoresis. Front. Bioeng. Biotechnol. 2024, 12, 1468423. [Google Scholar] [CrossRef]
- Serra, M.B.; Barroso, W.A.; da Silva, N.N.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflam. 2017, 2017, 3406215. [Google Scholar] [CrossRef]
- Canedo-Dorantes, L.; Canedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in Healing Chronic Wounds: Opportunities and Challenges. Mol. Pharm. 2021, 18, 550–575. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef]
- Valentin, J.E.; Stewart-Akers, A.M.; Gilbert, T.W.; Badylak, S.F. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng. Part. A 2009, 15, 1687–1694. [Google Scholar] [CrossRef]
- Loots, M.A.; Lamme, E.N.; Zeegelaar, J.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J. Invest. Dermatol. 1998, 111, 850–857. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S. What role does the extracellular matrix serve in skin grafting and wound healing? Burns 1994, 20 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef]
- Raghow, R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994, 8, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elcin, A.E.; Elcin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109942. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Lorenzini, G.; Casella, D.; Liso, F.G.; De Pascalis, A.; Cinotti, E.; Rubegni, P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Dermatol. Ther. 2021, 34, e14987. [Google Scholar] [CrossRef]
- Ramey-Ward, A.N.; Walthall, H.P.; Smith, S.; Barrows, T.H. Human keratin matrices promote wound healing by modulating skin cell expression of cytokines and growth factors. Wound Repair. Regen. 2024, 32, 257–267. [Google Scholar] [CrossRef]
- Chen, H.; Yin, B.; Hu, B.; Zhang, B.; Liu, J.; Jing, Y.; Fan, Z.; Tian, Y.; Wei, X.; Zhang, W. Acellular fish skin enhances wound healing by promoting angiogenesis and collagen deposition. Biomed. Mater. 2021, 16, 045011. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, A.; Singh, L.; Roy, P.; Mishra, N.C. Silk fibroin protein modified acellular dermal matrix for tissue repairing and regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 313–324. [Google Scholar] [CrossRef]
- Guo, X.; Mu, D.; Gao, F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: A systematic review and meta-analysis. Int. J. Surg. 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef]
- Lin, W.; Qi, X.; Guo, W.; Liang, D.; Chen, H.; Lin, B.; Deng, X. A barrier against reactive oxygen species: Chitosan/acellular dermal matrix scaffold enhances stem cell retention and improves cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 383. [Google Scholar] [CrossRef]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 2022, 128, 137–144. [Google Scholar] [CrossRef]
- du Plessis, M.I.; Cottler, P.S.; Campbell, C.A. Acellular Dermal Matrix Favorably Modulates the Healing Response after Surgery. Plast. Reconstr. Surg. 2022, 150, 290e–299e. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef]
- Badylak, S.F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann. Biomed. Eng. 2014, 42, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, X.; Liu, X.; Wen, G.; Yu, Y. Recent advances in decellularized biomaterials for wound healing. Mater. Today Bio 2023, 19, 100589. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N.; Schulze-Makuch, D. Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef]
- de Wit, R.J.J.; van Dis, D.J.; Bertrand, M.E.; Tiemessen, D.; Siddiqi, S.; Oosterwijk, E.; Verhagen, A. Scaffold-based tissue engineering: Supercritical carbon dioxide as an alternative method for decellularization and sterilization of dense materials. Acta Biomater. 2023, 155, 323–332. [Google Scholar] [CrossRef]
- Porta, G.D.; Reverchon, E.; Maffulli, N. Biomaterials and Supercritical Fluid Technologies: Which Perspectives to Fabricate Artificial Extracellular Matrix? Curr. Pharm. Des. 2017, 23, 3759–3771. [Google Scholar] [CrossRef]
- Duarte, M.M.; Silva, I.V.; Eisenhut, A.R.; Bionda, N.; Duarte, A.R.C.; Oliveira, A.L. Contributions of supercritical fluid technology for advancing decellularization and postprocessing of viable biological materials. Mater. Horiz. 2022, 9, 864–891. [Google Scholar] [CrossRef]
- White, A.; Burns, D.; Christensen, T.W. Effective terminal sterilization using supercritical carbon dioxide. J. Biotechnol. 2006, 123, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A.; Oliveira, A.L. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosales, V.; Magarinos, B.; Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A. Combined sterilization and fabrication of drug-loaded scaffolds using supercritical CO2 technology. Int. J. Pharm. 2022, 612, 121362. [Google Scholar] [CrossRef]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; Gonzalez, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 520–540. [Google Scholar] [CrossRef]
- Hennessy, R.S.; Jana, S.; Tefft, B.J.; Helder, M.R.; Young, M.D.; Hennessy, R.R.; Stoyles, N.J.; Lerman, A. Supercritical carbon dioxide-based sterilization of decellularized heart valves. JACC Basic. Transl. Sci. 2017, 2, 71–84. [Google Scholar] [CrossRef]
- Scognamiglio, F.; Blanchy, M.; Borgogna, M.; Travan, A.; Donati, I.; Bosmans, J.; Foulc, M.P.; Bouvy, N.D.; Paoletti, S.; Marsich, E. Effects of supercritical carbon dioxide sterilization on polysaccharidic membranes for surgical applications. Carbohydr. Polym. 2017, 173, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mei, J.; Xie, J. The regulation of carbon dioxide on food microorganisms: A review. Food Res. Int. 2023, 172, 113170. [Google Scholar] [CrossRef]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Gadre, M.; Kasturi, M.; Agarwal, P.; Vasanthan, K.S. Decellularization and Their Significance for Tissue Regeneration in the Era of 3D Bioprinting. ACS Omega 2024, 9, 7375–7392. [Google Scholar] [CrossRef] [PubMed]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chan, V.; Corridon, P.R. Decellularized blood vessel development: Current state-of-the-art and future directions. Front. Bioeng. Biotechnol. 2022, 10, 951644. [Google Scholar] [CrossRef] [PubMed]
- Allu, I.; Sahi, A.K.; Koppadi, M.; Gundu, S.; Sionkowska, A. Decellularization Techniques for Tissue Engineering: Towards Replicating Native Extracellular Matrix Architecture in Liver Regeneration. J. Funct. Biomater. 2023, 14, 518. [Google Scholar] [CrossRef]
- Ngan Giang, N.; Le, L.T.T.; Ngoc Chien, P.; Trinh, T.T.; Thi Nga, P.; Zhang, X.R.; Jin, Y.X.; Zhou, S.Y.; Han, J.; Nam, S.Y.; et al. Assessment of inflammatory suppression and fibroblast infiltration in tissue remodelling by supercritical CO2 acellular dermal matrix (scADM) utilizing Sprague Dawley models. Front. Bioeng. Biotechnol. 2024, 12, 1407797. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Basok, Y.B.; Grigoriev, A.M.; Nemets, E.A.; Kirillova, A.D.; Kirsanova, L.A.; Lazhko, A.E.; Subbot, A.; Kravchik, M.V.; Khesuani, Y.D.; et al. Decellularization of cartilage microparticles: Effects of temperature, supercritical carbon dioxide and ultrasound on biochemical, mechanical, and biological properties. J. Biomed. Mater. Res. A 2023, 111, 543–555. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjug Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Gangwar, A.K.; Kumar, N.; Khangembam, S.D.; Kumar, V.; Singh, R. Primary chicken embryo fibroblasts seeded acellular dermal matrix (3-D ADM) improve regeneration of full thickness skin wounds in rats. Tissue Cell 2015, 47, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Zhou, G.; Liu, Y.; Cao, Y. Biological Evaluation of Acellular Cartilaginous and Dermal Matrixes as Tissue Engineering Scaffolds for Cartilage Regeneration. Front. Cell Dev. Biol. 2020, 8, 624337. [Google Scholar] [CrossRef]
- Wang, T.; Xue, Y.; Zhang, W.; Zheng, Z.; Peng, X.; Zhou, Y. Collagen sponge scaffolds loaded with Trichostatin A pretreated BMSCs-derived exosomes regulate macrophage polarization to promote skin wound healing. Int. J. Biol. Macromol. 2024, 269, 131948. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, C.; Gan, B.; Liu, W.; Liang, J.; Fan, Z.; Wen, Y.; Yang, Y.; Peng, X.; Zhou, Y. Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110611. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Qian, L.W.; Fourcaudot, A.B.; Yamane, K.; You, T.; Chan, R.K.; Leung, K.P. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair. Regen. 2016, 24, 26–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Le, L.T.T.; Jin, Y.; Jin, C.; Giang, N.N.; Trinh, T.-T.T.; Lee, Y.H.; Shin, Y.W.; Bae, J.W.; Chien, P.N.; et al. Supercritical Carbon Dioxide-Processed Acellular Dermal Matrix Patch for Enhanced Wound Healing. Int. J. Mol. Sci. 2025, 26, 5715. https://doi.org/10.3390/ijms26125715
Zhang X, Le LTT, Jin Y, Jin C, Giang NN, Trinh T-TT, Lee YH, Shin YW, Bae JW, Chien PN, et al. Supercritical Carbon Dioxide-Processed Acellular Dermal Matrix Patch for Enhanced Wound Healing. International Journal of Molecular Sciences. 2025; 26(12):5715. https://doi.org/10.3390/ijms26125715
Chicago/Turabian StyleZhang, Xinrui, Linh Thi Thuy Le, Yongxun Jin, Caijun Jin, Nguyen Ngan Giang, Thuy-Tien Thi Trinh, Yong Hyun Lee, Yong Woo Shin, Jin Woo Bae, Pham Ngoc Chien, and et al. 2025. "Supercritical Carbon Dioxide-Processed Acellular Dermal Matrix Patch for Enhanced Wound Healing" International Journal of Molecular Sciences 26, no. 12: 5715. https://doi.org/10.3390/ijms26125715
APA StyleZhang, X., Le, L. T. T., Jin, Y., Jin, C., Giang, N. N., Trinh, T.-T. T., Lee, Y. H., Shin, Y. W., Bae, J. W., Chien, P. N., & Heo, C. Y. (2025). Supercritical Carbon Dioxide-Processed Acellular Dermal Matrix Patch for Enhanced Wound Healing. International Journal of Molecular Sciences, 26(12), 5715. https://doi.org/10.3390/ijms26125715





