Co-Culture Approaches in Cartilage and Bone Tissue Regeneration
Abstract
1. Introduction
2. Various Treatments Used for Cartilage and Bone Lesions
2.1. Autologous Chondrocyte Implantation
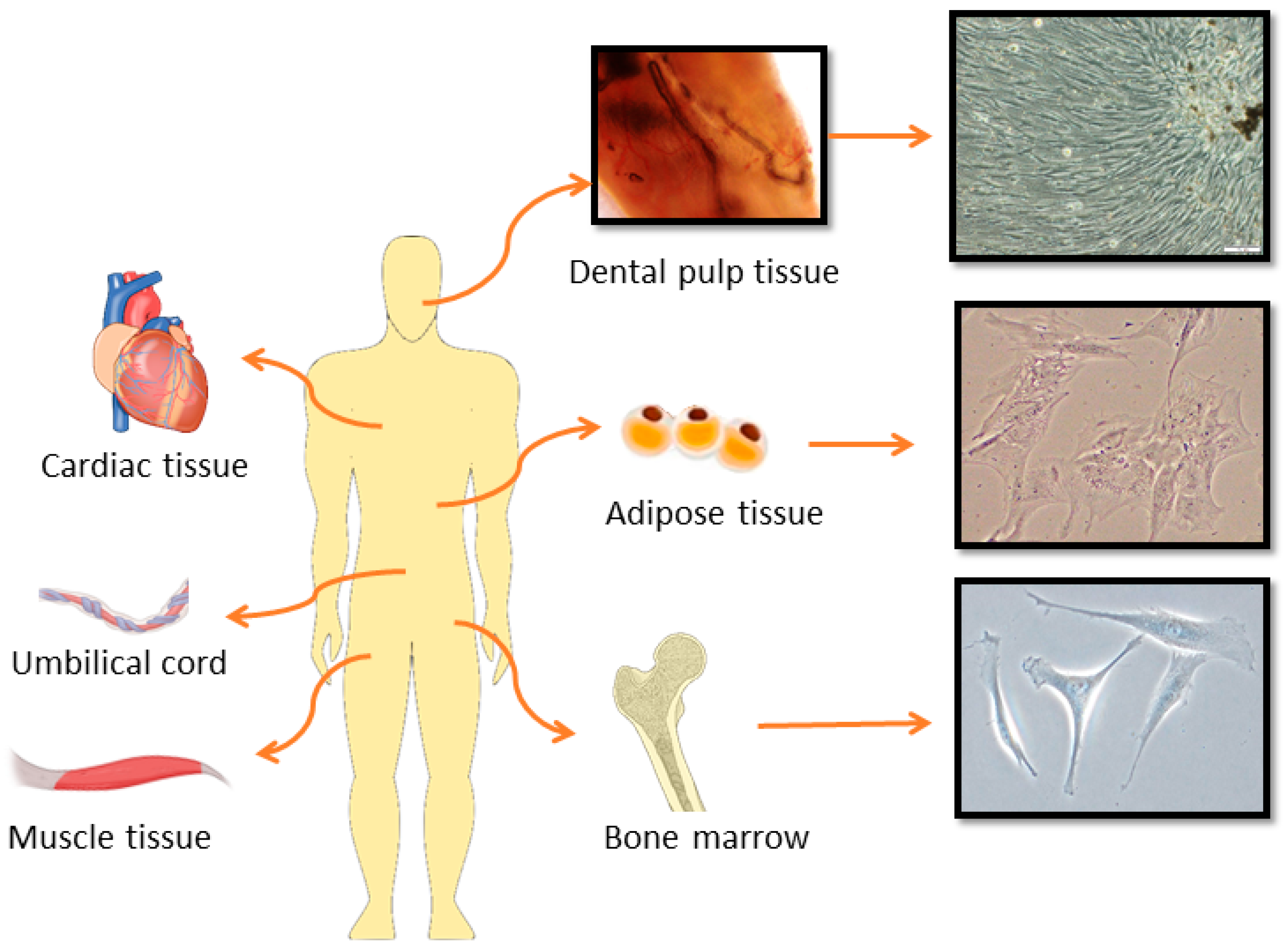
2.2. Stem Cell-Based Therapies in Cartilage and Bone Repair
2.2.1. Cartilage Tissue Repair
2.2.2. Bone Tissue Repair
3. Co-Culture Systems for Cartilage and Bone Regeneration
3.1. Co-Culture in Cartilage Regeneration
3.1.1. 2D Co-Culture Model for Cartilage Tissue Regeneration
3.1.2. Tissue Engineering Strategies Using 3D Co-Culture Models for Articular Cartilage Defects
3.2. Co-Culture in Bone Tissue Regeneration
3.2.1. 2D Co-Culture Models for Bone Regeneration
3.2.2. 3D Co-Culture Models for Bone Tissue Regeneration
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wuttisiriboon, K.; Tippayawat, P.; Daduang, J.; Limpaiboon, T. Three-Dimensional Silk Fibroin-Gelatin/Chondroitin Sulfate/Hyaluronic Acid–Aloe Vera Scaffold Supports In Vitro Chondrogenesis of Bone Marrow Mesenchymal Stem Cells and Reduces Inflammatory Effect. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Posniak, S.; Chung, J.H.Y.; Liu, X.; Mukherjee, P.; Gambhir, S.; Khansari, A.; Wallace, G.G. Bioprinting of Chondrocyte Stem Cell Co-Cultures for Auricular Cartilage Regeneration. ACS Omega 2022, 7, 5908–5920. [Google Scholar] [CrossRef] [PubMed]
- Brose, T.Z.; Kubosch, E.J.; Schmal, H.; Stoddart, M.J.; Armiento, A.R. Crosstalk Between Mesenchymal Stromal Cells and Chondrocytes: The Hidden Therapeutic Potential for Cartilage Regeneration. Stem Cell Rev. Rep. 2021, 17, 1647–1665. [Google Scholar] [CrossRef]
- Martín, A.R.; Patel, J.M.; Zlotnick, H.M.; Carey, J.L.; Mauck, R.L. Emerging Therapies for Cartilage Regeneration in Currently Excluded “Red Knee” Populations. NPJ Regen. Med. 2019, 4, 12. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Kim, K. Engineered Co-Culture Strategies Using Stem Cells for Facilitated Chondrogenic Differentiation and Cartilage Repair. Biotechnol. Bioprocess Eng. 2018, 23, 261–270. [Google Scholar] [CrossRef]
- Vinardell, T.; Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. A Comparison of the Functionality and In Vivo Phenotypic Stability of Cartilaginous Tissues Engineered from Different Stem Cell Sources. Tissue Eng. Part A 2012, 18, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous Chondrocyte Implantation: A Long-Term Follow-Up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Bi, M.; Yang, K.; Yu, T.; Wu, G.; Li, Q. Cell-Based Mechanisms and Strategies of Co-Culture System Both In Vivo and Vitro for Bone Tissue Engineering. Biomed. Pharmacother. 2023, 169, 115907. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–Culture Systems of Osteoblasts and Osteoclasts: Simulating In Vitro Bone Remodeling in Regenerative Approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The Master Cells in Bone Remodelling. Curr. Opin. Pharmacol. 2016, 28, 24–30. [Google Scholar] [CrossRef]
- Freitas, G.P.; Lopes, H.B.; Souza, A.T.P.; Oliveira, P.G.F.P.; Almeida, A.L.G.; Souza, L.E.B.; Coelho, P.G.; Beloti, M.M.; Rosa, A.L. Cell Therapy: Effect of Locally Injected Mesenchymal Stromal Cells Derived from Bone Marrow or Adipose Tissue on Bone Regeneration of Rat Calvarial Defects. Sci. Rep. 2019, 9, 13476. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Gagne, K.; Shaughnessy, M. Extracellular Signaling Molecules to Promote Fracture Healing and Bone Regeneration. Adv. Drug Deliv. Rev. 2015, 94, 3–12. [Google Scholar] [CrossRef]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; Tsuchida, A.I.; van Rijen, M.H.P.; Vonk, L.A.; Dhert, W.J.A.; Creemers, L.B.; Saris, D.B.F. Single-Stage Cell-Based Cartilage Regeneration Using a Combination of Chondrons and Mesenchymal Stromal Cells: Comparison with Microfracture. Am. J. Sports Med. 2013, 41, 2158–2166. [Google Scholar] [CrossRef]
- Marchan, J.; Wittig, O.; Diaz-Solano, D.; Gomez, M.; Cardier, J.E. Enhanced Chondrogenesis from Chondrocytes Co-Cultured on Mesenchymal Stromal Cells: Implication for Cartilage Repair. Injury 2022, 53, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Eschen, C.; Kaps, C.; Widuchowski, W.; Fickert, S.; Zinser, W.; Niemeyer, P.; Roël, G. Clinical Outcome Is Significantly Better with Spheroid-Based Autologous Chondrocyte Implantation Manufactured with More Stringent Cell Culture Criteria. Osteoarthr. Cartil. Open 2020, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Gu, C.; Shen, Q.; Liu, J.; Zhou, Y.; Jin, Z.; Xiong, W.; Zhou, Y.; Tan, W. Hypoxia Alleviates Dexamethasone-Induced Inhibition of Angiogenesis in Cocultures of HUVECs and rBMSCs via HIF-1α. Stem Cell Res. Ther. 2020, 11, 343. [Google Scholar] [CrossRef]
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front. Cell Dev. Biol. 2021, 9, 674084. [Google Scholar] [CrossRef]
- Ogura, T.; Mosier, B.A.; Bryant, T.; Minas, T. A 20-Year Follow-Up After First-Generation Autologous Chondrocyte Implantation. Am. J. Sports Med. 2017, 45, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Hoffman, L.; Zak, L.; Tiefenboeck, T.; Aldrian, S.; Albrecht, C. Clinical Evaluation After Matrix-Associated Autologous Chondrocyte Transplantation. Bone Jt. Res. 2021, 10, 370–379. [Google Scholar] [CrossRef]
- Yue, L.; Lim, R.; Owens, B.D. Latest Advances in Chondrocyte-Based Cartilage Repair. Biomedicines 2024, 12, 1367. [Google Scholar] [CrossRef]
- Tierney, L.; Kuiper, J.H.; Roberts, S.; Snow, M.; Williams, M.; Harrington, M.B.; Harrison, P.; Gallacher, P.; Jermin, P.; Wright, K.T. Lower Cell Number, Lateral Defect Location and Milder Grade Are Associated with Improved Autologous Chondrocyte Implantation Outcome. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 1308–1320. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. [Google Scholar] [CrossRef] [PubMed]
- Baghaban Eslaminejad, M.; Taghiyar, L.; Falahi, F. Co-Culture of Mesenchymal Stem Cells with Mature Chondrocytes: Producing Cartilage Construct for Application in Cartilage Regeneration. Iran. J. Med. Sci. 2009, 34, 251–258. [Google Scholar]
- Pettit, R.J.; Everhart, J.S.; DiBartola, A.C.; Blackwell, R.E.; Flanigan, D.C. Time Matters: Knee Cartilage Defect Expansion and High-Grade Lesion Formation While Awaiting Autologous Chondrocyte Implantation. CARTILAGE 2021, 13, 1802S–1808S. [Google Scholar] [CrossRef] [PubMed]
- Zhidu, S.; Ying, T.; Rui, J.; Chao, Z. Translational Potential of Mesenchymal Stem Cells in Regenerative Therapies for Human Diseases: Challenges and Opportunities. Stem Cell Res. Ther. 2024, 15, 266. [Google Scholar] [CrossRef]
- Deszcz, I. Stem Cell-Based Therapy and Cell-Free Therapy as an Alternative Approach for Cardiac Regeneration. Stem Cells Int. 2023, 2023, 2729377. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem Versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. [Google Scholar] [CrossRef] [PubMed]
- Urlić, I.; Ivković, A. Cell Sources for Cartilage Repair—Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef]
- Sudarmono; Widyaputra, S.; Sitam, S.; Suherna, I.; Fitri, A.D.; Rachman, A. Comparison of Bone Regeneration in hADMSC Versus hUCBMSC with hBMMSC as a Reference: A Literature Review of Potential Bone Regeneration. Res. J. Pharm. Technol. 2021, 14, 1993–1998. [Google Scholar] [CrossRef]
- Jung, S.-H.; Nam, B.-J.; Choi, C.-H.; Kim, S.; Jung, M.; Chung, K.; Park, J.; Jung, Y.; Kim, S.-H. Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cell Implantation Versus Microdrilling Combined with High Tibial Osteotomy for Cartilage Regeneration. Sci. Rep. 2024, 14, 3333. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Yassin, M.A.; Rashad, A.; Espedal, H.; Idris, S.B.; Finne-Wistrand, A.; Mustafa, K.; Vindenes, H.; Fristad, I. Comparison of Bone Regenerative Capacity of Donor-Matched Human Adipose–Derived and Bone Marrow Mesenchymal Stem Cells. Cell Tissue Res. 2021, 383, 1061–1075. [Google Scholar] [CrossRef]
- Jin, Q.; Yuan, K.; Lin, W.; Niu, C.; Ma, R.; Huang, Z. Comparative Characterization of Mesenchymal Stem Cells from Human Dental Pulp and Adipose Tissue for Bone Regeneration Potential. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1577–1584. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, C.-H. Clinical Application of Mesenchymal Stem Cells for Cartilage Regeneration. Plast. Aesthetic Res. 2020, 7, 49. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, K.; Zhou, L.; Zheng, S. Adipose-Derived Stem Cells for Cartilage Tissue Engineering: A Bibliometric Analysis of Trends and Themes. JMDH 2025, 18, 3023–3037. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Ran, C.; Wang, W.; Piao, F.; Yang, J.; Tian, S.; Li, L.; Zhao, D. Immunoregulatory Paracrine Effect of Mesenchymal Stem Cells and Mechanism in the Treatment of Osteoarthritis. Front. Cell Dev. Biol. 2024, 12, 1411507. [Google Scholar] [CrossRef]
- Alsayegh, H.; Apostolidis, J.; Dhahi, T.; Mughir, A.; Alsafran, Z.; Adlan, T. Endovascular Management of Iatrogenic Arterial Injury Post Bone Marrow Biopsy: A Report of 3 Cases. Radiol. Case Rep. 2023, 18, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of Allogeneic vs Autologous Bone Marrow–Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients with Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Wright, K.T.; Sammons, R.L.; Jeys, L.; Snow, M.; Johnson, W.E.B. An In Vitro Comparison of the Incorporation, Growth, and Chondrogenic Potential of Human Bone Marrow Versus Adipose Tissue Mesenchymal Stem Cells in Clinically Relevant Cell Scaffolds Used for Cartilage Repair. Cartilage 2015, 6, 252–263. [Google Scholar] [CrossRef]
- Afizah, H.; Yang, Z.; Hui, J.H.P.; Ouyang, H.-W.; Lee, E.-H. A Comparison Between the Chondrogenic Potential of Human Bone Marrow Stem Cells (BMSCs) and Adipose-Derived Stem Cells (ADSCs) Taken from the Same Donors. Tissue Eng. 2007, 13, 659–666. [Google Scholar] [CrossRef]
- Romano, I.R.; D’Angeli, F.; Vicario, N.; Russo, C.; Genovese, C.; Lo Furno, D.; Mannino, G.; Tamburino, S.; Parenti, R.; Giuffrida, R. Adipose-Derived Mesenchymal Stromal Cells: A Tool for Bone and Cartilage Repair. Biomedicines 2023, 11, 1781. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-C.; Park, Y.-B.; Ha, C.-W.; Cole, B.J.; Lee, B.-K.; Jeong, H.-J.; Kim, M.-K.; Bin, S.-I.; Choi, C.-H.; Choi, C.H.; et al. Allogeneic Umbilical Cord Blood–Derived Mesenchymal Stem Cell Implantation Versus Microfracture for Large, Full-Thickness Cartilage Defects in Older Patients: A Multicenter Randomized Clinical Trial and Extended 5-Year Clinical Follow-Up. Orthop. J. Sports Med. 2021, 9, 2325967120973052. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells for Cartilage Regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Jiang, P.; Mao, L.; Qiao, L.; Lei, X.; Zheng, Q.; Li, D. Efficacy and Safety of Mesenchymal Stem Cell Injections for Patients with Osteoarthritis: A Meta-Analysis and Review of RCTs. Arch. Orthop. Trauma. Surg. 2021, 141, 1241–1251. [Google Scholar] [CrossRef]
- Shang, V.; Li, J.; Little, C.B.; Li, J.J. Understanding the Effects of Mesenchymal Stromal Cell Therapy for Treating Osteoarthritis Using an In Vitro Co-Culture Model. Eur. Cell Mater. 2023, 45, 143–157. [Google Scholar] [CrossRef]
- Padial-Molina, M.; O’Valle, F.; Lanis, A.; Mesa, F.; Dohan Ehrenfest, D.M.; Wang, H.-L.; Galindo-Moreno, P. Clinical Application of Mesenchymal Stem Cells and Novel Supportive Therapies for Oral Bone Regeneration. BioMed Res. Int. 2015, 2015, 341327. [Google Scholar] [CrossRef]
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352. [Google Scholar] [CrossRef]
- Majors, A.K.; Boehm, C.A.; Nitto, H.; Midura, R.J.; Muschler, G.F. Characterization of Human Bone Marrow Stromal Cells with Respect to Osteoblastic Differentiation. J. Orthop. Res. 2005, 15, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.; Behravan, J. Osteogenesis and Bone Remodeling: A Focus on Growth Factors and Bioactive Peptides. BioFactors 2020, 46, 326–340. [Google Scholar] [CrossRef]
- Kang, B.-J.; Ryu, H.-H.; Park, S.S.; Koyama, Y.; Kikuchi, M.; Woo, H.-M.; Kim, W.H.; Kweon, O.-K. Comparing the Osteogenic Potential of Canine Mesenchymal Stem Cells Derived from Adipose Tissues, Bone Marrow, Umbilical Cord Blood, and Wharton’s Jelly for Treating Bone Defects. J. Vet. Sci. 2012, 13, 299–310. [Google Scholar] [CrossRef]
- Cui, C.; Lin, F.; Xia, L.; Zhang, X. Mesenchymal Stem Cells Therapy for the Treatment of Non-Union Fractures: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2025, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Buckwalter, J.A. The Role of Chondrocyte Senescence in the Pathogenesis of Osteoarthritis and in Limiting Cartilage Repair. J. Bone Jt. Surg. Am. 2003, 85-A (Suppl. S2), 106–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, W.; Wang, M.; Hao, C.; Lu, L.; Gao, S.; Zhang, X.; Li, X.; Chen, M.; Li, P.; et al. Co-Culture Systems-Based Strategies for Articular Cartilage Tissue Engineering. J. Cell. Physiol. 2018, 233, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Shariatzadeh, S.; Zafari, A.; Majd, A.; Niknejad, H. Recent Advances on Cell-Based Co-Culture Strategies for Prevascularization in Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 745314. [Google Scholar] [CrossRef]
- Paschos, N.K.; Brown, W.E.; Eswaramoorthy, R.; Hu, J.C.; Athanasiou, K.A. Advances in Tissue Engineering through Stem Cell-Based Co-Culture. J. Tissue Eng. Regen. Med. 2015, 9, 488–503. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Jeyaraman, N.; Nallakumarasamy, A.; Ramasubramanian, S.; Yadav, S. Critical Challenges and Frontiers in Cartilage Tissue Engineering. Cureus 2024, 16, e53095. [Google Scholar] [CrossRef]
- Mao, Y.; Block, T.; Singh-Varma, A.; Sheldrake, A.; Leeth, R.; Griffey, S.; Kohn, J. Extracellular Matrix Derived from Chondrocytes Promotes Rapid Expansion of Human Primary Chondrocytes In Vitro with Reduced Dedifferentiation. Acta Biomater. 2019, 85, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Kim, B.; Lee, S.; Lee, M. Co-Culture of Human Synovium-Derived Stem Cells and Chondrocytes Reduces Hypertrophy and Enhances In Vitro Chondrogenesis. Osteoarthr. Cartil. 2016, 24, S137–S138. [Google Scholar] [CrossRef]
- Deszcz, I.; Lis-Nawara, A.; Grelewski, P.; Dragan, S.; Bar, J. Utility of Direct 3D Co-Culture Model for Chondrogenic Differentiation of Mesenchymal Stem Cells on Hyaluronan Scaffold (Hyaff-11). Regen. Biomater. 2020, 7, 543–552. [Google Scholar] [CrossRef]
- Zheng, K.; Ma, Y.; Chiu, C.; Pang, Y.; Gao, J.; Zhang, C.; Du, D. Co-Culture Pellet of Human Wharton’s Jelly Mesenchymal Stem Cells and Rat Costal Chondrocytes as a Candidate for Articular Cartilage Regeneration: In Vitro and in Vivo Study. Stem Cell Res. Ther. 2022, 13, 386. [Google Scholar] [CrossRef]
- Li, X.; Liang, Y.; Xu, X.; Xiong, J.; Ouyang, K.; Duan, L.; Wang, D. Cell-to-Cell Culture Inhibits Dedifferentiation of Chondrocytes and Induces Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. BioMed Res. Int. 2019, 2019, 5871698. [Google Scholar] [CrossRef] [PubMed]
- Kubosch, E.J.; Heidt, E.; Bernstein, A.; Böttiger, K.; Schmal, H. The Trans-Well Coculture of Human Synovial Mesenchymal Stem Cells with Chondrocytes Leads to Self-Organization, Chondrogenic Differentiation, and Secretion of TGFβ. Stem Cell Res. Ther. 2016, 7, 64. [Google Scholar] [CrossRef]
- Liang, H.; Suo, H.; Wang, Z.; Feng, W. Progress in the Treatment of Osteoarthritis with Umbilical Cord Stem Cells. Hum. Cell 2020, 33, 470–475. [Google Scholar] [CrossRef]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees with Cartilage Defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, 8309256. [Google Scholar] [CrossRef]
- Jeyaraman, N.; Prajwal, G.S.; Jeyaraman, M.; Muthu, S.; Khanna, M. Chondrogenic Potential of Dental-Derived Mesenchymal Stromal Cells. Osteology 2021, 1, 149–174. [Google Scholar] [CrossRef]
- Song, C.; Wu, X.; Wei, Z.; Xu, Y.; Wang, Y.; Zhao, Y. Dental Pulp Stem Cells-Loaded Kartogenin-Modified Hydrogel Microspheres with Chondrocyte Differentiation Property for Cartilage Repair. Chem. Eng. J. 2024, 496, 153930. [Google Scholar] [CrossRef]
- Yuan, W.; Ferreira, L.d.A.Q.; Yu, B.; Ansari, S.; Moshaverinia, A. Dental-Derived Stem Cells in Tissue Engineering: The Role of Biomaterials and Host Response. Regen. Biomater. 2024, 11, rbad100. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Cortez de SantAnna, J.P.; Frisene, I.; Gazarini, J.P.; Gomes Pinheiro, C.C.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Franco Bueno, D. Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng. Part B Rev. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Longoni, A.; Utomo, L.; van Hooijdonk, I.E.; Bittermann, G.K.; Vetter, V.C.; Kruijt Spanjer, E.C.; Ross, J.; Rosenberg, A.J.; Gawlitta, D. The Chondrogenic Differentiation Potential of Dental Pulp Stem Cells. Eur. Cell Mater. 2020, 39, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Koyama, N.; Nakao, K.; Osawa, K.; Ikeno, M.; Yamanaka, S.; Okubo, Y.; Fujimura, K.; Bessho, K. Comparison of Human Mesenchymal Stem Cells Derived from Bone Marrow, Synovial Fluid, Adult Dental Pulp, and Exfoliated Deciduous Tooth Pulp. Int. J. Oral. Maxillofac. Surg. 2016, 45, 124–131. [Google Scholar] [CrossRef]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.M.; Nizak, R.; van Rijen, M.H.P.; Saris, D.B.F. Allogeneic MSCs and Recycled Autologous Chondrons Mixed in a One-Stage Cartilage Cell Transplantion: A First-in-Man Trial in 35 Patients. Stem Cells 2017, 35, 1984–1993. [Google Scholar] [CrossRef]
- Lee, C.S.; Burnsed, O.A.; Raghuram, V.; Kalisvaart, J.; Boyan, B.D.; Schwartz, Z. Adipose Stem Cells Can Secrete Angiogenic Factors That Inhibit Hyaline Cartilage Regeneration. Stem Cell Res. Ther. 2012, 3, 35. [Google Scholar] [CrossRef]
- Lee, J.-S.; Im, G.-I. Influence of Chondrocytes on the Chondrogenic Differentiation of Adipose Stem Cells. Tissue Eng. Part A 2010, 16, 3569–3577. [Google Scholar] [CrossRef]
- Pleumeekers, M.M.; Nimeskern, L.; Koevoet, J.L.M.; Karperien, M.; Stok, K.S.; van Osch, G.J.V.M. Trophic Effects of Adipose-Tissue-Derived and Bone-Marrow-Derived Mesenchymal Stem Cells Enhance Cartilage Generation by Chondrocytes in Co-Culture. PLoS ONE 2018, 13, e0190744. [Google Scholar] [CrossRef]
- Zuo, Q.; Cui, W.; Liu, F.; Wang, Q.; Chen, Z.; Fan, W. Co-Cultivated Mesenchymal Stem Cells Support Chondrocytic Differentiation of Articular Chondrocytes. Int. Orthop. (SICOT) 2013, 37, 747–752. [Google Scholar] [CrossRef]
- Muthu, S.; Jeyaraman, M.; Jain, R.; Gulati, A.; Jeyaraman, N.; Prajwal, G.S.; Mishra, P.C. Accentuating the Sources of Mesenchymal Stem Cells as Cellular Therapy for Osteoarthritis Knees—A Panoramic Review. Stem Cell Investig. 2021, 8, 13. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Lee, A.J.; Barabino, G.A. Coculture-Driven Mesenchymal Stem Cell-Differentiated Articular Chondrocyte-Like Cells Support Neocartilage Development. Stem Cells Transl. Med. 2012, 1, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature Induction of Hypertrophy during In Vitro Chondrogenesis of Human Mesenchymal Stem Cells Correlates with Calcification and Vascular Invasion After Ectopic Transplantation in SCID Mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Augello, A.; De Bari, C. The Regulation of Differentiation in Mesenchymal Stem Cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Wilkinson, K.J.; Iwamoto, M.; Otsuru, S.; Enomoto-Iwamoto, M. The Role of Hypertrophic Chondrocytes in Regulation of the Cartilage-to-Bone Transition in Fracture Healing. Bone Rep. 2022, 17, 101616. [Google Scholar] [CrossRef]
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing Chondrogenic Phenotype for Cartilage Tissue Engineering: Monoculture and Coculture of Articular Chondrocytes and Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2014, 20, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Owida, H.A.; De Las Heras Ruiz, T.; Dhillon, A.; Yang, Y.; Kuiper, N.J. Co-Culture of Chondrons and Mesenchymal Stromal Cells Reduces the Loss of Collagen VI and Improves Extracellular Matrix Production. Histochem. Cell Biol. 2017, 148, 625–638. [Google Scholar] [CrossRef]
- de Windt, T.S.; Saris, D.B.F.; Slaper-Cortenbach, I.C.M.; van Rijen, M.H.P.; Gawlitta, D.; Creemers, L.B.; de Weger, R.A.; Dhert, W.J.A.; Vonk, L.A. Direct Cell–Cell Contact with Chondrocytes Is a Key Mechanism in Multipotent Mesenchymal Stromal Cell-Mediated Chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef]
- Muthu, S.; Mir, A.A.; Kumar, R.; Yadav, V.; Jeyaraman, M.; Khanna, M. What Is the Clinically Significant Ideal Mesenchymal Stromal Cell Count in the Management of Osteoarthritis of the Knee?—Meta-Analysis of Randomized Controlled Trials. J. Clin. Orthop. Trauma. 2022, 25, 101744. [Google Scholar] [CrossRef]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced Chondrocyte Proliferation and Mesenchymal Stromal Cells Chondrogenesis in Coculture Pellets Mediate Improved Cartilage Formation. J. Cell. Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef]
- Scalzone, A.; Ferreira, A.M.; Tonda-Turo, C.; Ciardelli, G.; Dalgarno, K.; Gentile, P. The Interplay Between Chondrocyte Spheroids and Mesenchymal Stem Cells Boosts Cartilage Regeneration Within a 3D Natural-Based Hydrogel. Sci. Rep. 2019, 9, 14630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic Differentiation of Human Mesenchymal Stem Cells: A Comparison Between Micromass and Pellet Culture Systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef]
- Rogan, H.; Yang, F. Optimizing 3D Co-Culture Models to Enhance Synergy Between Adipose-Derived Stem Cells and Chondrocytes for Cartilage Tissue Regeneration. Regen. Eng. Transl. Med. 2019, 5, 270–279. [Google Scholar] [CrossRef]
- Nazempour, A.; Van Wie, B.J. Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine. Ann. Biomed. Eng. 2016, 44, 1325–1354. [Google Scholar] [CrossRef]
- Prasadam, I.; Akuien, A.; Friis, T.E.; Fang, W.; Mao, X.; Crawford, R.W.; Xiao, Y. Mixed Cell Therapy of Bone Marrow-Derived Mesenchymal Stem Cells and Articular Cartilage Chondrocytes Ameliorates Osteoarthritis Development. Lab. Invest. 2018, 98, 106–116. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, X.; Guan, J.; Wu, M.; Zhou, J. Co-Implantation of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Increase the Viability of Chondrocytes in Rat Osteo-Chondral Defects. Oncol. Lett. 2018, 15, 7021–7027. [Google Scholar] [CrossRef]
- Babur, B.K.; Ghanavi, P.; Levett, P.; Lott, W.B.; Klein, T.; Cooper-White, J.J.; Crawford, R.; Doran, M.R. The Interplay Between Chondrocyte Redifferentiation Pellet Size and Oxygen Concentration. PLoS ONE 2013, 8, e58865. [Google Scholar] [CrossRef]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The Therapeutic Potential of Three-Dimensional Multipotent Mesenchymal Stromal Cell Spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef]
- Hazrati, A.; Malekpour, K.; Soudi, S.; Hashemi, S.M. Mesenchymal Stromal/Stem Cells Spheroid Culture Effect on the Therapeutic Efficacy of These Cells and Their Exosomes: A New Strategy to Overcome Cell Therapy Limitations. Biomed. Pharmacother. 2022, 152, 113211. [Google Scholar] [CrossRef]
- Lam, A.T.L.; Reuveny, S.; Oh, S.K.-W. Human Mesenchymal Stem Cell Therapy for Cartilage Repair: Review on Isolation, Expansion, and Constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chien, A.J.; Guo, J.L.; Smith, B.T.; Watson, E.; Pearce, H.A.; Koons, G.L.; Navara, A.M.; Lam, J.; Scott, D.W.; et al. Chondrogenesis of Cocultures of Mesenchymal Stem Cells and Articular Chondrocytes in Poly(l-Lysine)-Loaded Hydrogels. J. Control. Release 2020, 328, 710–721. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Singh, Y.P.; Mandal, B.B. Silk Fibroin Scaffold-Based 3D Co-Culture Model for Modulation of Chondrogenesis without Hypertrophy via Reciprocal Cross-Talk and Paracrine Signaling. ACS Biomater. Sci. Eng. 2019, 5, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Yamada, S.; Takazawa, S.; Hattori, S.; Okada, T.; Ohuchi, H. Comparative Study on Clinical Outcomes in Autologous Chondrocyte Implantation Using Three-Dimensional Cultured JACC® with Collagen Versus Periosteum Coverings. Sci. Rep. 2024, 14, 9834. [Google Scholar] [CrossRef]
- Mesallati, T.; Buckley, C.T.; Kelly, D.J. Engineering Cartilaginous Grafts Using Chondrocyte-Laden Hydrogels Supported by a Superficial Layer of Stem Cells. J. Tissue Eng. Regen. Med. 2017, 11, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Amann, E.; Wolff, P.; Breel, E.; van Griensven, M.; Balmayor, E.R. Hyaluronic Acid Facilitates Chondrogenesis and Matrix Deposition of Human Adipose Derived Mesenchymal Stem Cells and Human Chondrocytes Co-Cultures. Acta Biomater. 2017, 52, 130–144. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. Articular Chondrocyte Redifferentiation in 3D Co-Cultures with Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2014, 20, 514–523. [Google Scholar] [CrossRef]
- Chu, Y.-Y.; Hikita, A.; Asawa, Y.; Hoshi, K. Advancements in Chondrocyte 3-Dimensional Embedded Culture: Implications for Tissue Engineering and Regenerative Medicine. Biomed. J. 2024, 48, 100786. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Wang, F.-K.; Zhao, D.-P.; Dai, X.-M.; Han, X.-S. Coculture of Vascular Endothelial Cells and Adipose-Derived Stem Cells as a Source for Bone Engineering. Ann. Plast. Surg. 2012, 69, 91. [Google Scholar] [CrossRef]
- Schmid, F.V.; Kleinhans, C.; Schmid, F.F.; Kluger, P.J. Osteoclast Formation Within a Human Co-Culture System on Bone Material as an In Vitro Model for Bone Remodeling Processes. J. Funct. Morphol. Kinesiol. 2018, 3, 17. [Google Scholar] [CrossRef]
- Maria, S.; Swanson, M.H.; Enderby, L.T.; D’Amico, F.; Enderby, B.; Samsonraj, R.M.; Dudakovic, A.; van Wijnen, A.J.; Witt-Enderby, P.A. Melatonin-Micronutrients Osteopenia Treatment Study (MOTS): A Translational Study Assessing Melatonin, Strontium (Citrate), Vitamin D3 and Vitamin K2 (MK7) on Bone Density, Bone Marker Turnover and Health Related Quality of Life in Postmenopausal Osteopenic Women Following a One-Year Double-Blind RCT and on Osteoblast-Osteoclast Co-Cultures. Aging 2017, 9, 256–285. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; van den Beucken, J.J.J.P.; Yang, F.; Both, S.K.; Cui, F.-Z.; Pan, J.; Jansen, J.A. Coculture of Osteoblasts and Endothelial Cells: Optimization of Culture Medium and Cell Ratio. Tissue Eng. Part C Methods 2011, 17, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; He, Y.; Li, Y.; He, X. Endothelial Progenitor Cells Promote Osteogenic Differentiation in Co-Cultured with Mesenchymal Stem Cells via the MAPK-Dependent Pathway. Stem Cell Res. Ther. 2020, 11, 537. [Google Scholar] [CrossRef]
- Jia, L.; Gu, W.; Zhang, Y.; Ji, Y.; Liang, J.; Wen, Y.; Xu, X. The Crosstalk Between HDPSCs and HUCMSCs on Proliferation and Osteogenic Genes Expression in Coculture System. Int. J. Med. Sci. 2017, 14, 1118–1129. [Google Scholar] [CrossRef]
- Herath, T.D.K.; Larbi, A.; Teoh, S.H.; Kirkpatrick, C.J.; Goh, B.T. Neutrophil-Mediated Enhancement of Angiogenesis and Osteogenesis in a Novel Triple Cell Co-Culture Model with Endothelial Cells and Osteoblasts. J. Tissue Eng. Regen. Med. 2018, 12, e1221–e1236. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Li, S.; Zhou, Y.; Geng, Y.; Liu, S.; Wu, W.; Forouzanfar, T.; Wu, G.; Zhang, Z.; Zhou, M. A Novel Method to Improve the Osteogenesis Capacity of hUCMSCs with Dual-Directional Pre-Induction Under Screened Co-Culture Conditions. Cell Prolif. 2020, 53, e12740. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hong, N.; Liu, H.; Wang, J.; Li, Y.; Wu, S. Differentiated Adipose-Derived Stem Cell Cocultures for Bone Regeneration in RADA16-I In Vitro. J. Cell Physiol. 2018, 233, 9458–9472. [Google Scholar] [CrossRef]
- Rozila, I.; Azari, P.; Munirah, S.; Safwani, W.K.Z.W.; Pingguan-Murphy, B.; Chua, K.H. Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System. Polymers 2021, 13, 597. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, G.; Wang, Y.; Xu, W.; Hu, M. Indirect Co-Culture of Osteoblasts and Endothelial Cells In Vitro Based on a Biomimetic 3D Composite Hydrogel Scaffold to Promote the Proliferation and Differentiation of Osteoblasts. PLoS ONE 2024, 19, e0298689. [Google Scholar] [CrossRef]
- Tong, X.; Chen, J.; Wang, R.; Hou, D.; Wu, G.; Liu, C.; Pathak, J.L. The Paracrine Effect of Hyaluronic Acid-Treated Endothelial Cells Promotes BMP-2-Mediated Osteogenesis. Bioengineering 2023, 10, 1227. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Qiao, Q.; Weir, M.D.; Schneider, A.; Masri, R.; Lynch, C.D.; Zhang, N.; Zhang, K.; Bai, Y.; et al. Calvaria Defect Regeneration via Human Periodontal Ligament Stem Cells and Prevascularized Scaffolds in Athymic Rats. J. Dent. 2023, 138, 104690. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, T.; Wang, F.; Liu, X.; Wang, Z. Endothelial Progenitor Cells with Stem Cells Enhance Osteogenic Efficacy. Am. J. Transl. Res. 2020, 12, 2409–2424. [Google Scholar] [PubMed]
- Ma, Y.; Yang, S.; He, Q.; Zhang, D.; Chang, J. The Role of Immune Cells in Post-Stroke Angiogenesis and Neuronal Remodeling: The Known and the Unknown. Front. Immunol. 2021, 12, 784098. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Zuo, X.; Shen, T.; Yu, S.; Deng, L.; Gao, C. Migration of Endothelial Cells and Mesenchymal Stem Cells into Hyaluronic Acid Hydrogels with Different Moduli Under Induction of Pro-Inflammatory Macrophages. J. Mater. Chem. B 2019, 7, 5478–5489. [Google Scholar] [CrossRef]
- Tang, H.; Husch, J.F.A.; Zhang, Y.; Jansen, J.A.; Yang, F.; van den Beucken, J.J.J.P. Coculture with Monocytes/Macrophages Modulates Osteogenic Differentiation of Adipose-Derived Mesenchymal Stromal Cells on Poly(Lactic-Co-Glycolic) Acid/Polycaprolactone Scaffolds. J. Tissue Eng. Regen. Med. 2019, 13, 785–798. [Google Scholar] [CrossRef]
- Zhao, D.; Xue, C.; Lin, S.; Shi, S.; Li, Q.; Liu, M.; Cai, X.; Lin, Y. Notch Signaling Pathway Regulates Angiogenesis via Endothelial Cell in 3D Co-Culture Model. J. Cell. Physiol. 2017, 232, 1548–1558. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Kim, H.-W. Co-Culture of Human Dental Pulp Stem Cells and Endothelial Cells Using Porous Biopolymer Microcarriers: A Feasibility Study for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-H.; Guo, Y.; Zhu, J.-Y.; Tang, C.-Y.; Zhao, Y.-Q.; Zhou, H.-D. Spheroid Co-Culture of BMSCs with Osteocytes Yields Ring-Shaped Bone-like Tissue That Enhances Alveolar Bone Regeneration. Sci. Rep. 2022, 12, 14636. [Google Scholar] [CrossRef]
- Suh, J.-D.; Lim, K.T.; Jin, H.; Kim, J.; Choung, P.-H.; Chung, J.H. Effects of Co-Culture of Dental Pulp Stem Cells and Periodontal Ligament Stem Cells on Assembled Dual Disc Scaffolds. Tissue Eng. Regen. Med. 2014, 11, 47–58. [Google Scholar] [CrossRef]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]


| Stem Cell Type | Advantages | Disadvantages |
|---|---|---|
| Bone Marrow-Derived MSCs (BMSCs) |
|
|
| Adipose-Derived MSCs (ADMSCs) |
|
|
| Human Dental Pulp Stem Cells (hDPSCs) |
|
|
| Human Umbilical Cord MSCs (hUC-MSCs) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deszcz, I.; Bar, J. Co-Culture Approaches in Cartilage and Bone Tissue Regeneration. Int. J. Mol. Sci. 2025, 26, 5711. https://doi.org/10.3390/ijms26125711
Deszcz I, Bar J. Co-Culture Approaches in Cartilage and Bone Tissue Regeneration. International Journal of Molecular Sciences. 2025; 26(12):5711. https://doi.org/10.3390/ijms26125711
Chicago/Turabian StyleDeszcz, Iwona, and Julia Bar. 2025. "Co-Culture Approaches in Cartilage and Bone Tissue Regeneration" International Journal of Molecular Sciences 26, no. 12: 5711. https://doi.org/10.3390/ijms26125711
APA StyleDeszcz, I., & Bar, J. (2025). Co-Culture Approaches in Cartilage and Bone Tissue Regeneration. International Journal of Molecular Sciences, 26(12), 5711. https://doi.org/10.3390/ijms26125711







