Harnessing Light Wavelengths to Enrich Health-Promoting Molecules in Tomato Fruits
Abstract
1. Introduction
2. Results
2.1. Influence of Light Quality Regimes on Biochemical Markers
2.2. Correlation Between Light Wavelengths and Biochemical Markers
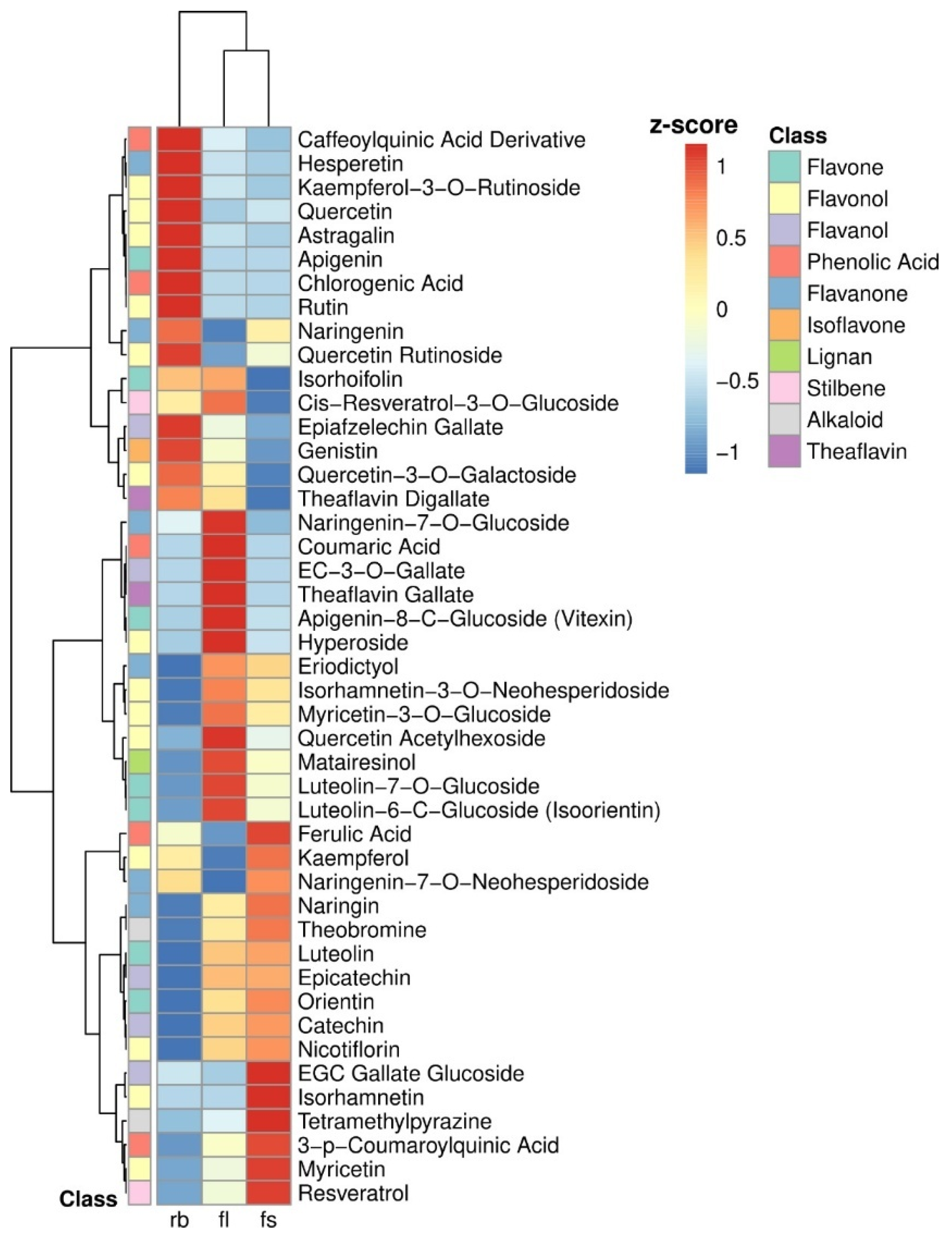
2.3. Cluster Analysis of the Polyphenolic Profile
3. Discussion
4. Materials and Methods
4.1. Plant Material, Experimental Set-Up
4.2. Biochemical Analyses and Polyphenolic Profile
4.2.1. Ferric Reducing Antioxidant Power (FRAP) Assay and DPPH Assay
4.2.2. Determination of Total Carotenoids, Lycopene, and Ascorbic Acid
4.2.3. Determination of Soluble Protein and Carbohydrate Content
4.2.4. Determination of Total Condensed Tannins, Anthocyanins, Flavonoids, and Polyphenols
4.2.5. Polyphenolic Profile
4.3. Statistical Analyses and Visualization of the Data
4.3.1. Assumption Testing
4.3.2. Biochemical Marker Analysis
4.3.3. Marker Effect Sizes
4.3.4. Spectral Analysis
4.3.5. HPLC Profiling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.R.; Vitale, E.; Guerretti, V.; Palumbo, G.; De Clemente, I.M.; Vitale, L.; Arena, C.; De Maio, A. Antioxidant Characterization of Six Tomato Cultivars and Derived Products Destined for Human Consumption. Antioxidants 2023, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nut. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T.; Arena, C. The Interplay between Light Quality and Biostimulant Application Affects the Antioxidant Capacity and Photosynthetic Traits of Soybean (Glycine max L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Alsina, I.; Erdberga, I.; Duma, M.; Alksnis, R.; Dubova, L. Changes in Greenhouse Grown Tomatoes Metabolite Content Depending on Supplemental Light Quality. Front. Nutr. 2022, 9, 830186. [Google Scholar] [CrossRef]
- He, R.; Wei, J.; Zhang, J.; Tan, X.; Li, Y.; Gao, M.; Liu, H. Supplemental Blue Light Frequencies Improve Ripening and Nutritional Qualities of Tomato Fruits. Front. Plant Sci. 2022, 13, 888976. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Costanzo, G.; Paradiso, R.; Arena, C. Manipulation of light quality is an effective tool to regulate photosynthetic capacity and fruit antioxidant properties of Solanum lycopersicum L. cv. ‘Microtom’ in a controlled environment. PeerJ 2022, 10, e13677. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Spinelli, M.; Fontanarosa, C.; Paradiso, R.; Amoresano, A.; Arena, C. Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology 2023, 12, 1029. [Google Scholar] [CrossRef]
- Lima, I.H.A.; Rodrigues, A.A.; Resende, E.C.; da Silva, F.B.; Farnese, F.S.; Silva, L.J.; Rosa, M.; Reis, M.N.O.; Bessa, L.A.; de Oliveira, T.C.; et al. Light means power: Harnessing light spectrum and UV-B toenhance photosynthesis and rutin levels in microtomato plants. Front. Plant Sci. 2023, 14, 1261174. [Google Scholar] [CrossRef]
- Kim, E.Y.; Park, S.A.; Park, B.J.; Lee, Y.; Oh, M.M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; et al. Response of Tomato Fruit Quality Depends on Period of LED Supplementary Light. Front. Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef]
- Panjai, L.; Noga, G.; Fiebig, A.; Hunsche, M. Effects of continuous red light and short daily UV exposure during postharvest on carotenoid concentration and antioxidant capacity in stored tomatoes. Sci. Hortic. 2017, 226, 97–103. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.; Nicole, C.; Labrie, C.; Marcelis, L.F.M. Light Regulation of Vitamin C in Tomato Fruit Is Mediated through Photosynthesis. Environ. Exp. Bot. 2019, 158, 180–188. [Google Scholar] [CrossRef]
- Cozmuta, L.M.; Peter, A.; Nicula, C.; Vosgan, Z.; Giurgiulescu, L.; Vulpoi, A.; Baia, M. Effect of monochromatic Far-Red light on physical-nutritional-microbiological attributes of red tomatoes during storage. Sci. Hortic. 2016, 2011, 220–230. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct regulation of phyt oene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef]
- Castagna, A.; Chiavaro, E.; Dall’Asta, C.; Rinaldi, M.; Galaverna, G.; Ranieri, A. Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chem. 2013, 137, 151–158. [Google Scholar] [CrossRef]
- Zivanovic, B.; Vidovic, M.; Komic, S.M.; Jovanovic, L.; Kolarz, P.; Morina, F.; Jovanovic, S.V. Contents of phenolics and carotenoids in tomato grown under polytunnels with different UV-transmission rates. Turk. J. Agric. For. 2017, 41, 113–120. [Google Scholar] [CrossRef]
- Vitale, E.; Vitale, L.; Costanzo, G.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light Spectral Composition Influences Structural and Eco-Physiological Traits of Solanum lycopersicum L. cv. ‘Microtom’ in Response to High-LET Ionizing Radiation. Plants 2021, 10, 1752. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Xu, S.; Zhu, W. Ligh quality-controlled phytochemicals biosynthesis in vegetables and fruits. Agric. Sci. Technol. 2015, 16, 2029–2035. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, S.; Dai, S.; Wang, Q. Nitrate accumulation and expression patterns of genes involved in nitrate transport and assimilation in spinach. Molecules 2018, 23, 2231. [Google Scholar] [CrossRef]
- Knyazeva, I.V.; Panfilova, O.; Vershinina, O.; Smirnov, A.A.; Dorokhov, A.S.; Kahramanoğlu, I. The Effect of Nighttime LED Lighting on Tomato Growth, Yield, and Nutrient Content of Fruits. Horticulturae 2024, 10, 1259. [Google Scholar] [CrossRef]
- Zushi, K.; Suehara, C.; Shirai, M. Effect of light intensity and wavelengths on ascorbic acid content and the antioxidant system in tomato fruit grown in vitro. Sci. Hortic. 2020, 274, 109673. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, Q.C. Effects of intermittent light exposure with red and blue light emitting diodes on growth and carbohydrate accumulation of lettuce. Sci. Hortic. 2018, 234, 220–226. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effect of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef]
- Xie, B.; Wei, J.J.; Zhang, Y.T.; Song, S.W.; Sun, G.W.; Hao, Y.W.; Liu, H.C. Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef]
- Liu, C.C.; Ahammed, G.J.; Wang, G.T.; Xu, C.J.; Chen, K.S.; Zhou, Y.H.; Yu, J.Q. CRY1a plays a critical role in the regulation of phytohormone homeostasis plant development and carotenoid metabolism in fruits. Plant Cell Environ. 2019, 41, 2. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, W.; Ma, Y.; Liang, H.; Zhao, X. Effects of light-emitting diode illumination on the quality of fresh-cut cherry tomatoes during refrigerated storage. Int. J. Food Sci. Technol. 2021, 56, 2041–2052. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Noelia Castillejo, L.; Artés–Hernández, F. Effect of postharvest visible spectrum LED lighting on quality and bioactive compounds of tomatoes during shelf life. LWT 2023, 174, 114420. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H.; Zhao, Y.; Cheng, X.; Wang, L.; Guo, X. Effect of light quality on polyphenol biosynthesis in three varieties of mung bean sprouts with different color seed coats. Plant Cell Rep. 2023, 42, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beypnd vegetables: Effects of indoor LED light on specialized matabolites biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food. Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, M.; Crown, O.; Akinmoladun, A.; Akindahunsi, A. Rutin and quercetin show greater efficacy than nifedipin in ameliorating hemodynamic, redox, and metabolite imbalances in sodium chloride-induced hypertensive rats. Hum. Exp. Toxicol. 2013, 33, 602–608. [Google Scholar] [CrossRef]
- da Rocha, E.V.; Falchetti, F.; Pernomian, L.; Blascke de Mello, M.M.; Parente, J.M.; Nogueira, R.C.; Gomes, B.Q.; Bertozi, G.; Sanches-Lopes, J.M.; Tanus-Santos, J.E.; et al. Quercetin decreases cardiac hypertrophic mediators and maladaptive coronary arterial remodeling in renovascular hypertensive rats without improving cardiac function. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 939–949. [Google Scholar] [CrossRef]
- Sharma, S.; Asgar, A.; Javed, A.; Jasjeet, K.S.; Baboota, S. Rutin: Therapeutic Potential and Recent Advances in Drug Delivery. Expert Opin. Investig. Drug 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, H.O.; Kim, J.Y.; Kwon, K.H.; Cha, H.S.; Kim, J.H. An effect of light emitting diode (LED) irradiation treatment on the amplification of functional components of immature strawberry. Hortic. Environ. Biotechnol. 2011, 52, 35–39. [Google Scholar] [CrossRef]
- Williamson, G. Protection against developing type 2 diabetes by coffee consumption: Assessment of the role of chlorogenic acid and metabolites on glycaemic responses. Food Funct. 2020, 11, 4826–4833. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.B.; Li, X.; Choi, S.R.; Park, S.; Park, J.S.; Lim, Y.P.; Park, S.U. Accumulation of Phenylpropanoids by White, Blue, and Red Light Irradiation and Their Organ-Specific Distribution in Chinese Cabbage (Brassica rapa ssp. pekinensis). J. Agric. Food Chem. 2015, 63, 6772–6778. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.R.; Liu, J.; Fu, L.; Shu, T.; Yang, L.; Zhang, X.; Jiang, Z.H.; Bai, L. Anti-Entry Activity of Natural Flavonoids against SARS-CoV-2 by Targeting Spike RBD. Viruses 2023, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersicum esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative studies on different citrus cultivars: A revaluation of waste mandarin components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays et al. Determination of Antioxidant Capacity. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Composit. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Davis, A.R.; Fish, W.W.; Perkins-Veazie, P. A rapid spectrophotometric method for analyzing lycopene content in tomsto and tomato products. Postharvest Biol. Technol. 2003, 28, 425–430. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef]
- Im, J.; Ko, J.; Kim, H.Y.; Ha, B.K. Biochemical Responses of Soybean (Glycine max L. Merr) to Proton Beam Irradiation. Plant Breed. Biotechnol. 2017, 5, 97–105. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate chemistry. In Methods in Carbohydrate Chemistry; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962; Volume 17. [Google Scholar]
- Sun, B.; Richardo-da-Silva, J.M.; Sprenger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Yang, C.P.; Lindquist, P.; Anderson, O.R.; Rabino, I. Photocontrol of Anthocyanin Synthesis: III. The Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chen, S.J.; Hsu, C.K.; Chang, C.T.; Chou, S.T. Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther. Food Chem. 2005, 91, 419–424. [Google Scholar] [CrossRef]
- Moulehi, I.; Soumaya, B.; Ines, O.; Moufida, S.T. Variety and Ripening Impact on Phenolic Composition and Antioxidant Activity of Mandarin (Citrus reticulate Blanco) and Bitter Orange (Citrus aurantium L.) Seeds Extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef]
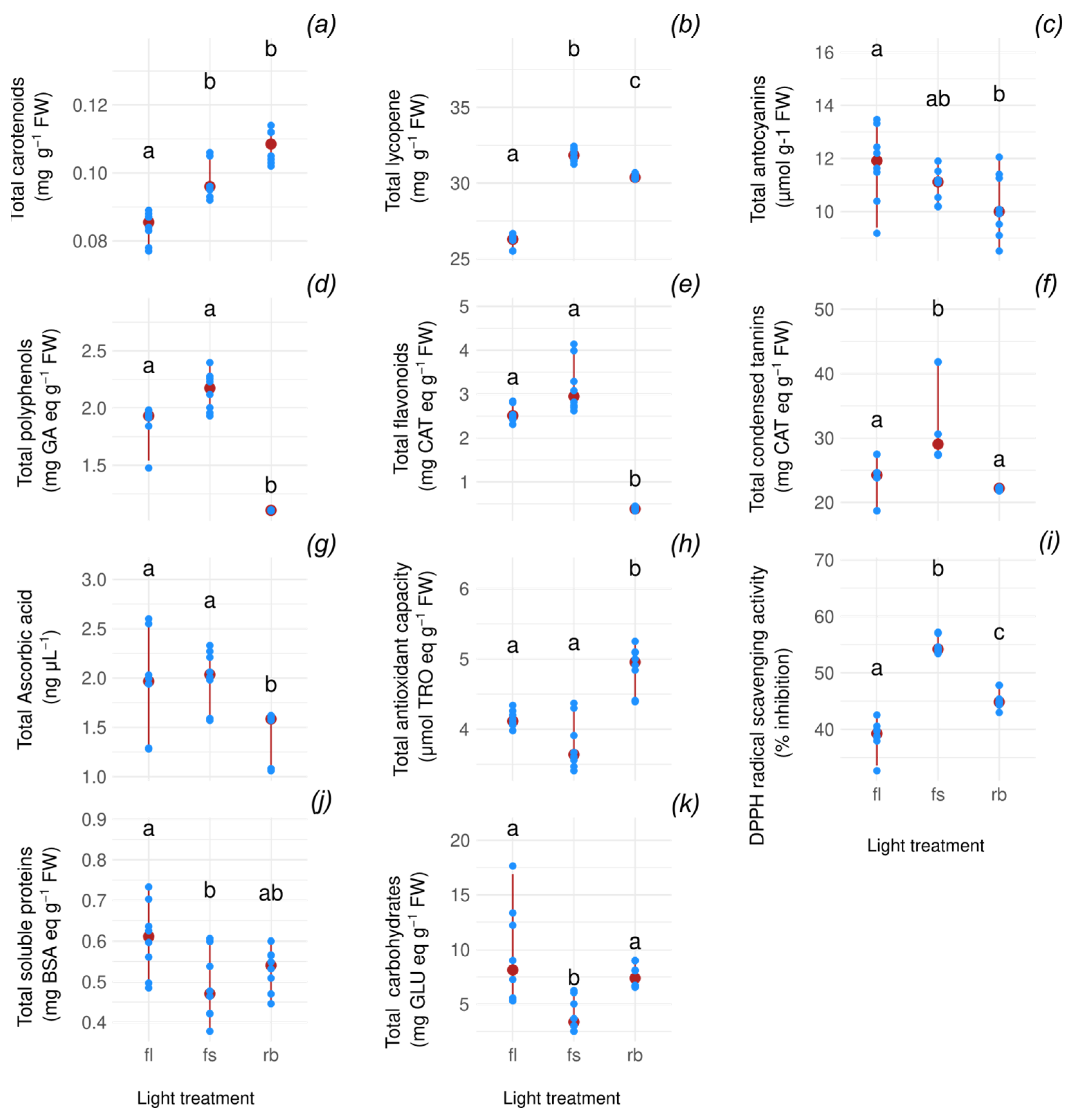
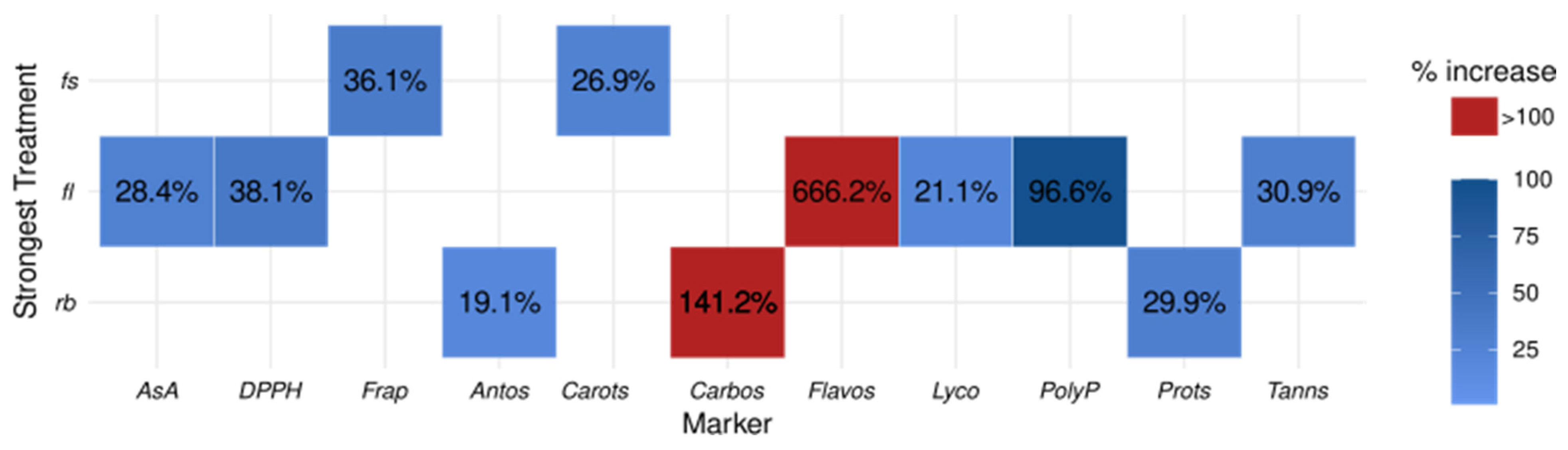

| Marker | Strongest | Weakest | FC |
|---|---|---|---|
| Carotenoids | RB | FL | 1.27 |
| Lycopene | FS | FL | 1.21 |
| Anthocyanins | FL | RB | 1.19 |
| Polyphenols | FS | RB | 1.97 |
| Flavonoids | FS | RB | 7.66 |
| Tannins | FS | RB | 1.31 |
| Ascorbic Acid | FS | RB | 1.28 |
| Antioxidant capacity | RB | FS | 1.36 |
| Antioxidant activity | FS | FL | 1.38 |
| Soluble proteins | FL | FS | 1.30 |
| Carbohydrates | FL | FS | 2.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hay Mele, B.; Vitale, E.; Velikova, V.; Tsonev, T.; Fontanarosa, C.; Spinelli, M.; Amoresano, A.; Arena, C. Harnessing Light Wavelengths to Enrich Health-Promoting Molecules in Tomato Fruits. Int. J. Mol. Sci. 2025, 26, 5712. https://doi.org/10.3390/ijms26125712
Hay Mele B, Vitale E, Velikova V, Tsonev T, Fontanarosa C, Spinelli M, Amoresano A, Arena C. Harnessing Light Wavelengths to Enrich Health-Promoting Molecules in Tomato Fruits. International Journal of Molecular Sciences. 2025; 26(12):5712. https://doi.org/10.3390/ijms26125712
Chicago/Turabian StyleHay Mele, Bruno, Ermenegilda Vitale, Violeta Velikova, Tsonko Tsonev, Carolina Fontanarosa, Michele Spinelli, Angela Amoresano, and Carmen Arena. 2025. "Harnessing Light Wavelengths to Enrich Health-Promoting Molecules in Tomato Fruits" International Journal of Molecular Sciences 26, no. 12: 5712. https://doi.org/10.3390/ijms26125712
APA StyleHay Mele, B., Vitale, E., Velikova, V., Tsonev, T., Fontanarosa, C., Spinelli, M., Amoresano, A., & Arena, C. (2025). Harnessing Light Wavelengths to Enrich Health-Promoting Molecules in Tomato Fruits. International Journal of Molecular Sciences, 26(12), 5712. https://doi.org/10.3390/ijms26125712









