Characterization and Expression Analysis of the PvTLP Gene Family in the Common Bean (Phaseolus vulgaris) in Response to Salt and Drought Stresses
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of the PvTLP Gene Family
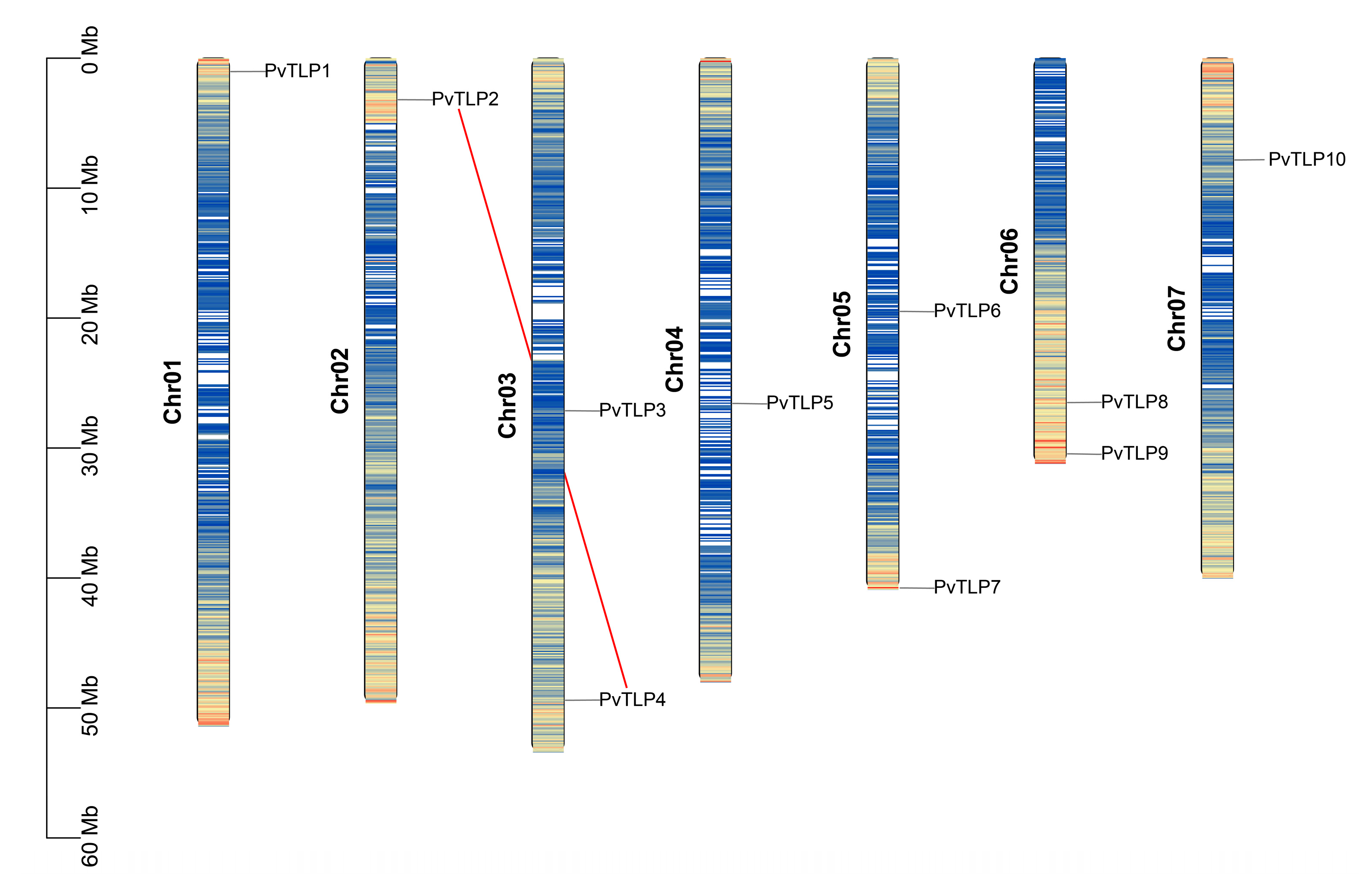
2.2. Chromosomal Localization and Gene Replication Events of the PvTLP Gene Family
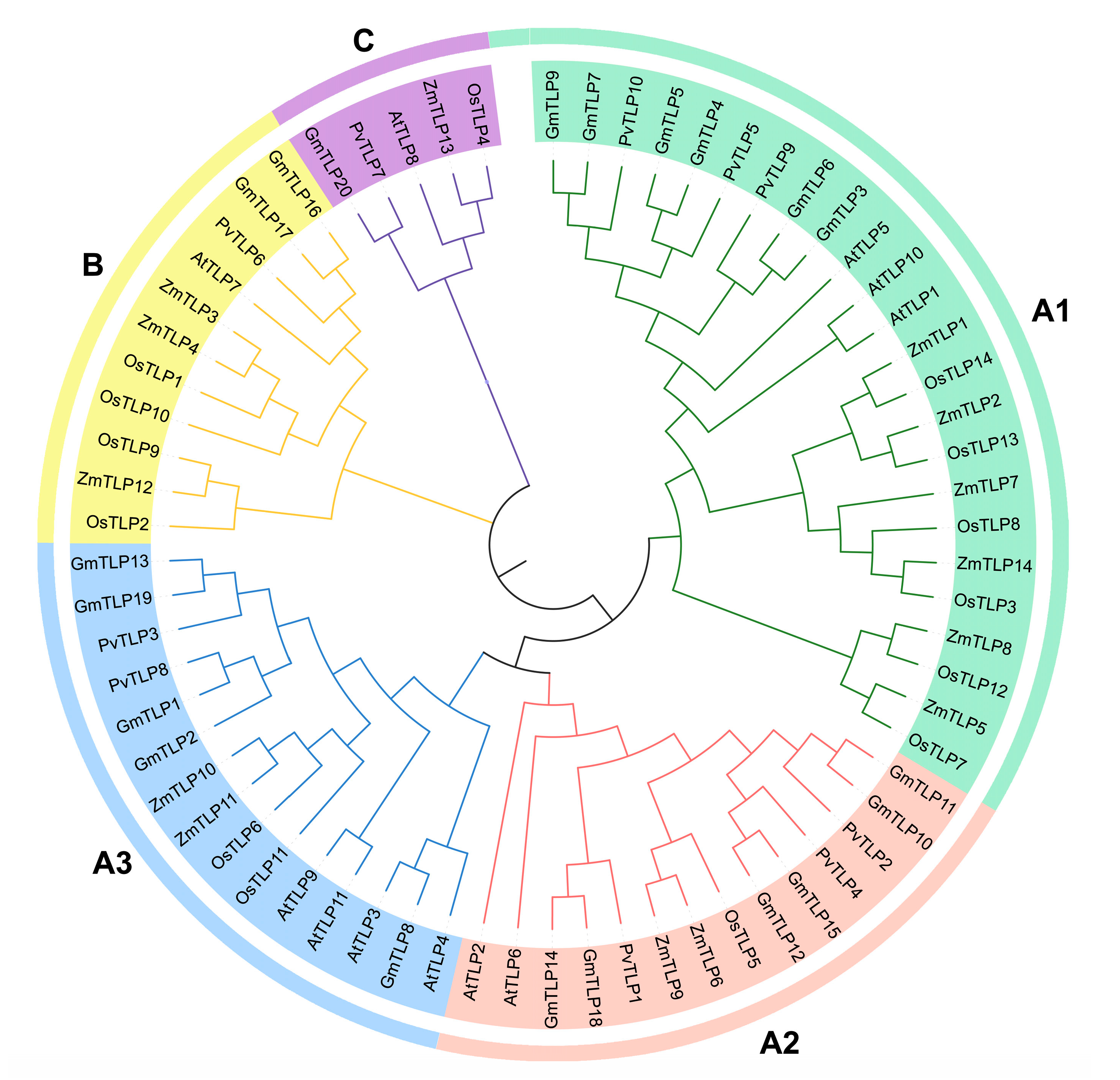
2.3. Phylogenetic Analysis of the PvTLP Gene Family
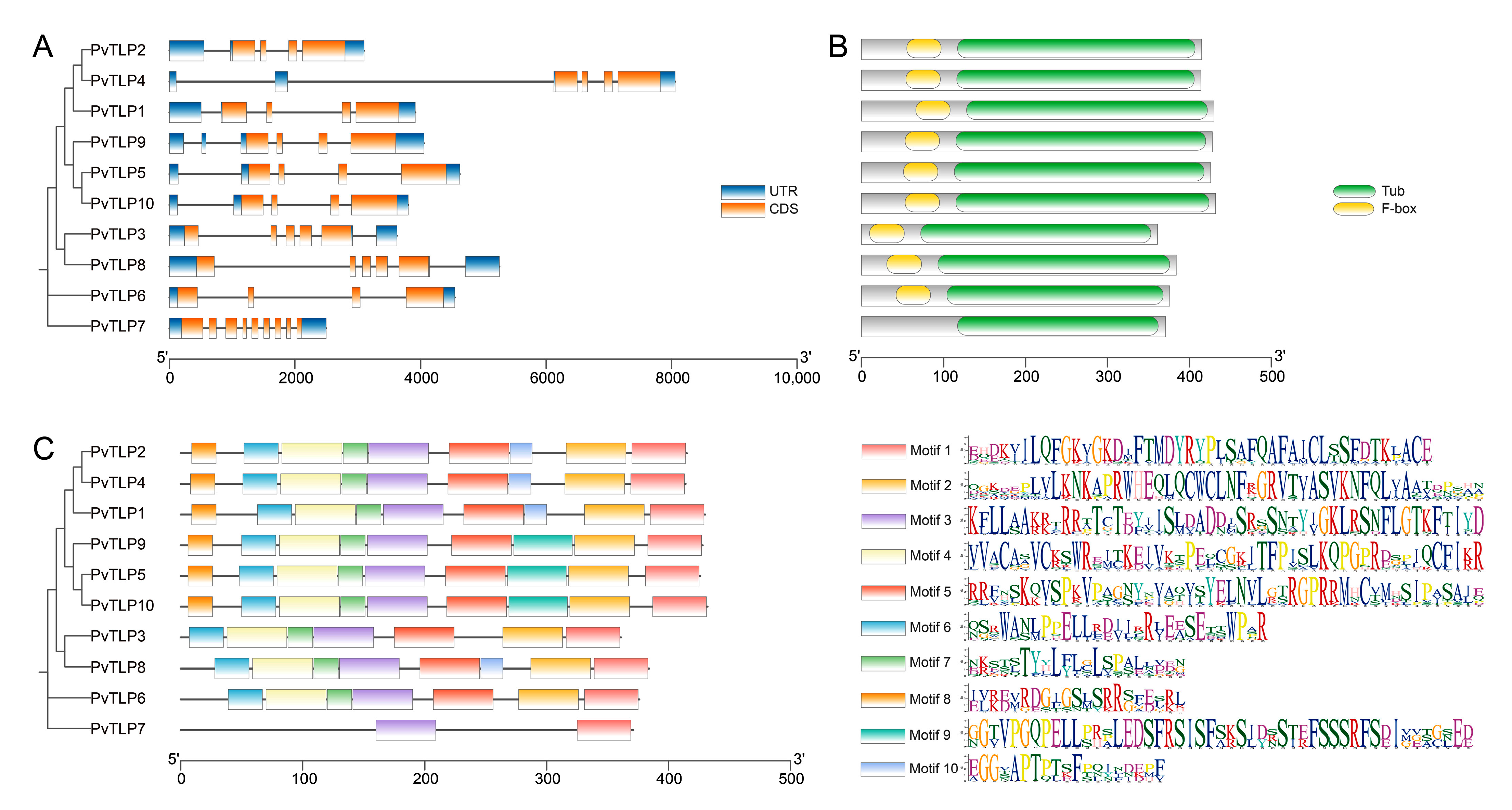
2.4. Gene Structure, Conserved Domain, and Motif Analysis of the PvTLP Gene Family
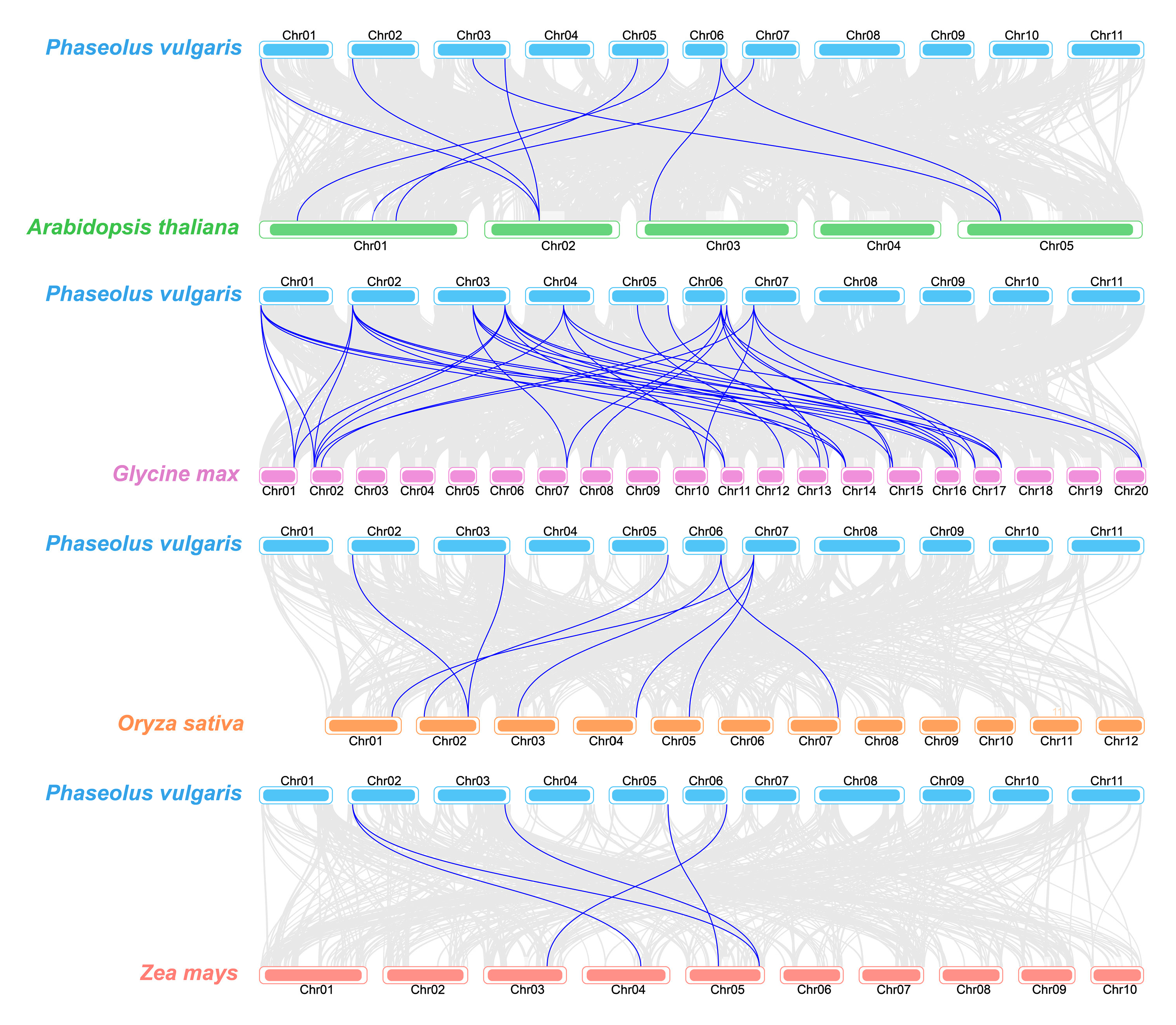
2.5. Synteny Analysis of TLP Genes
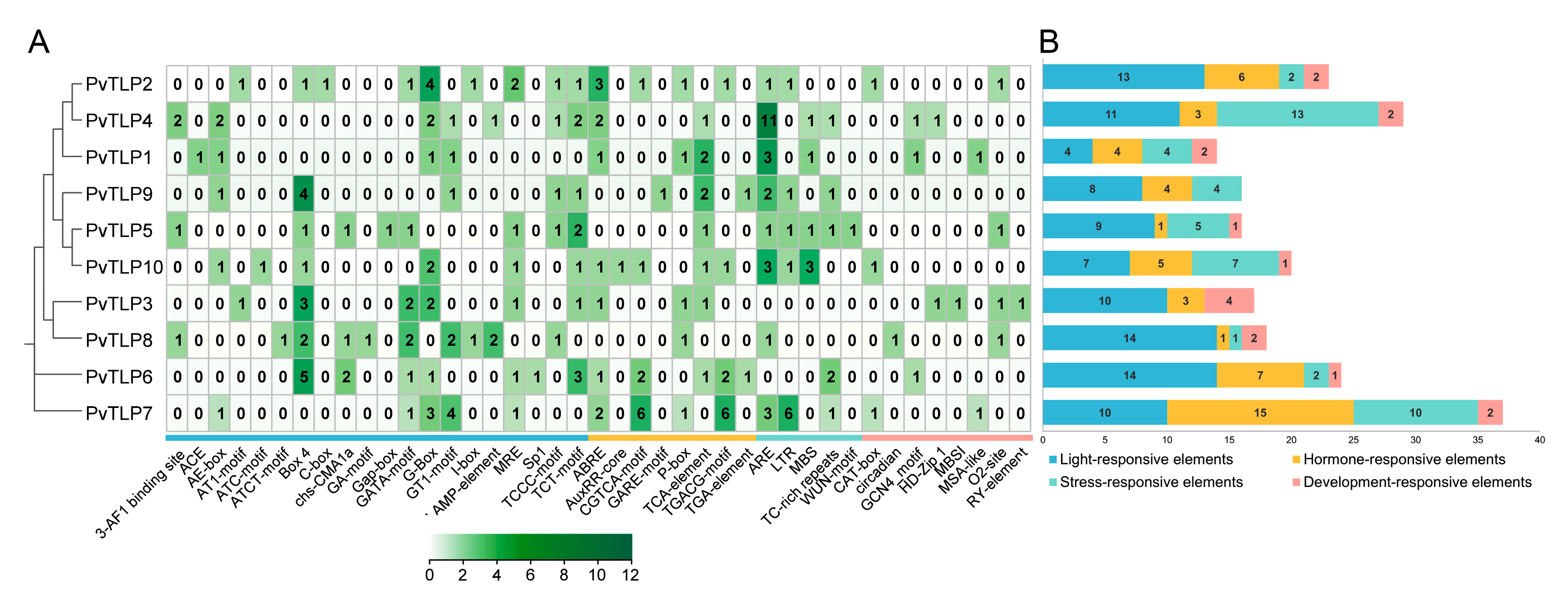
2.6. Analysis of Cis-Acting Elements in the PvTLP Promoter
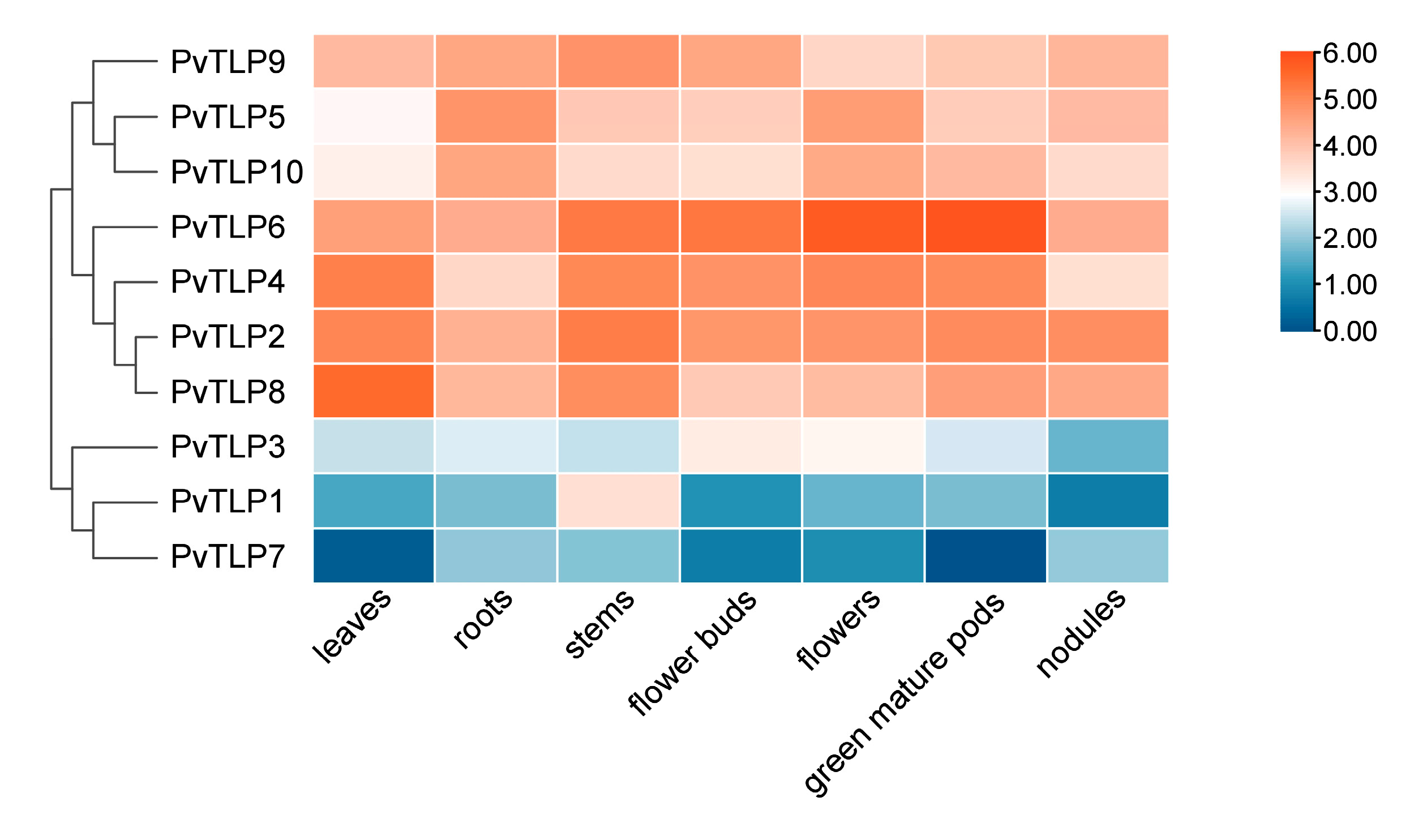
2.7. Expression Patterns of PvTLP Genes in Different Tissues
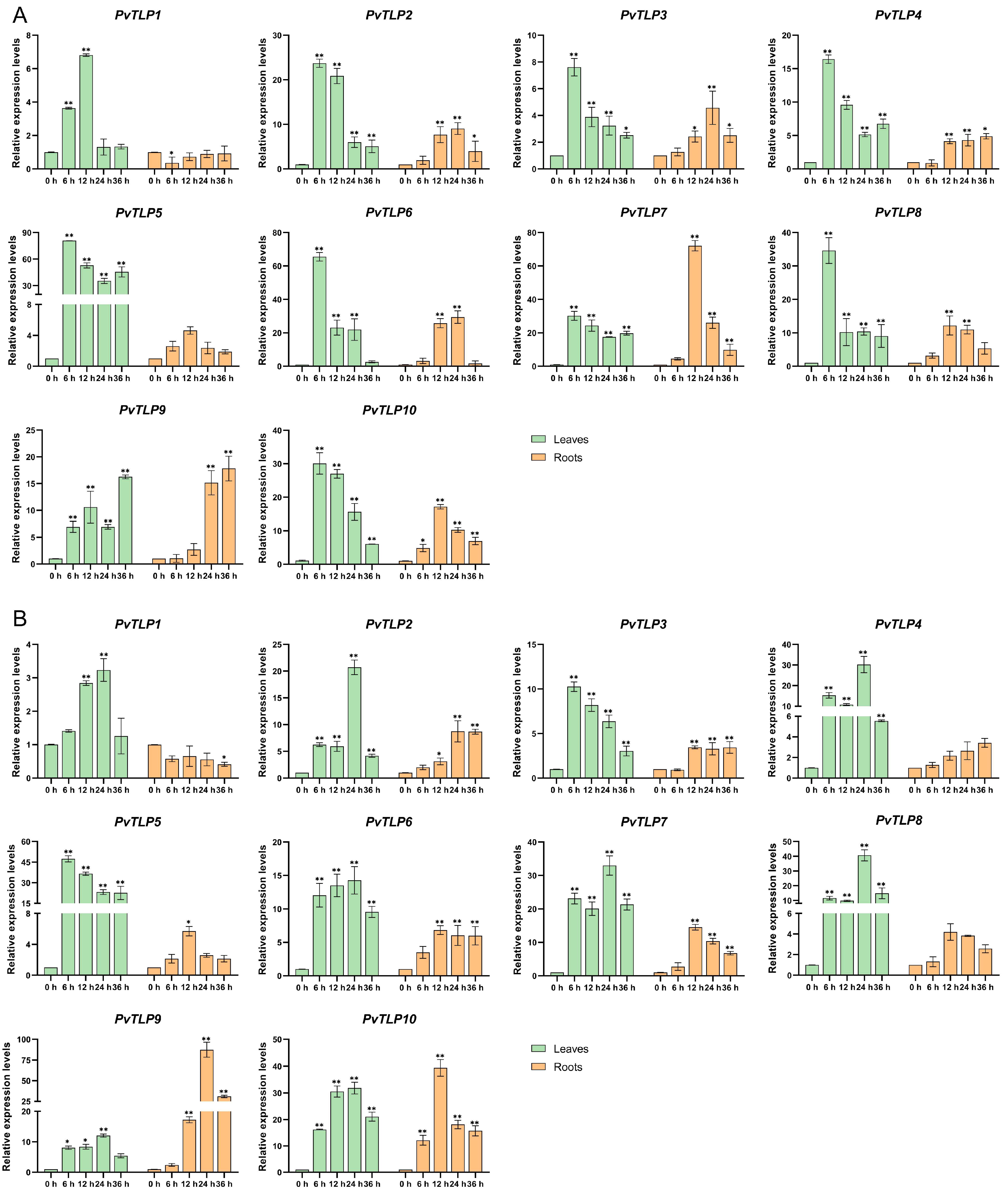
2.8. Expression Patterns of PvTLP Genes Under Salt and Drought Stresses
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of PvTLP Genes
4.2. Chromosomal Localization and Gene Replication Events
4.3. Multiple Sequence Alignment and Phylogenetic Analysis
4.4. Gene Structure, Conserved Domain, and Motif Analysis
4.5. Synteny Analysis
4.6. Promoter Cis-Acting Elements Analysis
4.7. Heatmap Analysis
4.8. Plant Material Source and Treatments
4.9. RNA Extraction and qRT-PCR Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bano, N.; Aalam, S.; Bag, S.K. Tubby-Like Proteins (TLPs) Transcription Factor in Different Regulatory Mechanism in Plants: A Review. Plant Mol. Biol. 2022, 110, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Kleyn, P.W.; Fan, W.; Kovats, S.G.; Lee, J.J.; Pulido, J.C.; Wu, Y.; Berkemeier, L.R.; Misumi, D.J.; Holmgren, L.; Charlat, O.; et al. Identification and Characterization of the Mouse Obesity Gene tubby: A Member of a Novel Gene Family. Cell 1996, 85, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, S.A.; North, M.A.; Nishina, P.L.; Berson, E.L.; Dryja, T.P. Recessive Mutations in the Gene Encoding the Tubby-Like Protein TULP1 in Patients with Retinitis Pigmentosa. Nat. Genet. 1998, 18, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Nishina, P.M.; Naggert, J.K. The Tubby-Like Proteins, a Family with Roles in Neuronal Development and Function. J. Cell Sci. 2002, 115, 9–14. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Jackson, P.K. The Tubby Family Proteins. Genome Biol. 2011, 12, 225. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Kong, Y. The Tubby-Like Proteins Kingdom in Animals and Plants. Gene 2018, 642, 16–25. [Google Scholar] [CrossRef]
- Boggon, T.J.; Shan, W.S.; Santagata, S.; Myers, S.C.; Shapiro, L. Implication of Tubby Proteins as Transcription Factors by Structure-Based Functional Analysis. Science 1999, 286, 2119–2125. [Google Scholar] [CrossRef]
- Thallmair, V.; Schultz, L.; Zhao, W.; Marrink, S.J.; Oliver, D.; Thallmair, S. Two Cooperative Binding Sites Sensitize PI(4,5)P2 Recognition by the Tubby Domain. Sci. Adv. 2022, 8, eabp9471. [Google Scholar] [CrossRef]
- Reitz, M.U.; Bissue, J.K.; Zocher, K.; Attard, A.; Hückelhoven, R.; Becker, K.; Imani, J.; Eichmann, R.; Schäfer, P. The Subcellular Localization of Tubby-Like Proteins and Participation in Stress Signaling and Root Colonization by the Mutualist Piriformospora indica. Plant Physiol. 2012, 160, 349–364. [Google Scholar] [CrossRef]
- Santagata, S.; Boggon, T.J.; Baird, C.L.; Gomez, C.A.; Zhao, J.; Shan, W.S.; Myszka, D.G.; Shapiro, L. G-Protein Signaling Through Tubby Proteins. Science 2001, 292, 2041–2050. [Google Scholar] [CrossRef]
- Thulasi Devendrakumar, K.; Copeland, C.; Adamchek, C.; Zhong, X.; Huang, X.; Gendron, J.M.; Li, X. Arabidopsis Tubby Domain-Containing F-Box Proteins Positively Regulate Immunity by Modulating PI4Kβ Protein Levels. New Phytol. 2023, 240, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, N.; Yao, W.; Li, X.; Zhou, Y.; Li, H. The Biological Function and Roles in Phytohormone Signaling of the F-Box Protein in Plants. Agronomy 2021, 11, 2360. [Google Scholar] [CrossRef]
- Xiao, W.; Jang, J. F-Box Proteins in Arabidopsis. Trends Plant Sci. 2000, 5, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Lee, C.L.; Chen, P.H.; Wu, S.H.; Yang, C.C.; Shaw, J.F. Molecular Analyses of the Arabidopsis TUBBY-Like Protein Gene Gamily. Plant Physiol. 2004, 134, 1586–1597. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Wang, X.; Gu, S.; Yu, J.; Liang, G.; Yan, C.; Xu, C. Genomewide Comparative Phylogenetic and Molecular Evolutionary Analysis of Tubby-Like Protein Family in Arabidopsis, Rice, and Poplar. Genomics 2008, 92, 246–253. [Google Scholar] [CrossRef]
- Bano, N.; Fakhrah, S.; Mohanty, C.S.; Bag, S.K. Genome-Wide Identification and Evolutionary Analysis of Gossypium Tubby-Like Protein (TLP) Gene Family and Expression Analyses during Salt and Drought Stress. Front. Plant Sci. 2021, 12, 667929. [Google Scholar] [CrossRef]
- Zeng, Y.; Wen, J.; Fu, J.; Geng, H.; Dan, Z.; Zhao, W.; Xu, W.; Huang, W. Genome-Wide Identification and Comprehensive Analysis of Tubby-Like Protein Gene Family in Multiple Crops. Front. Plant Sci. 2022, 13, 1093944. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Dong, X.; Wang, F.; Cao, L.; Li, S.; Liu, Z.; Zhang, X.; Guo, Y.D.; Zhao, B.; et al. Expression Analysis and Functional Characterization of Tomato Tubby-Like Protein Family. Plant Sci. 2022, 324, 111454. [Google Scholar] [CrossRef]
- Xu, J.N.; Xing, S.S.; Zhang, Z.R.; Chen, X.S.; Wang, X.Y. Genome-Wide Identification and Expression Analysis of the Tubby-Like Protein Family in the Malus Domestica Genome. Front. Plant Sci. 2016, 7, 1693. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Chang, L.; Sun, R.; Wu, R.; Zhong, C.; Gao, Y.; Zhang, H.; Wei, L.; Wei, Y.; et al. Genome-Wide Identification, Characterization, and Expression Analysis of Tubby-Like Protein (TLP) Gene Family Members in Woodland Strawberry (Fragaria vesca). Int. J. Mol. Sci. 2022, 23, 11961. [Google Scholar] [CrossRef]
- Dong, M.Y.; Fan, X.W.; Pang, X.Y.; Li, Y.Z. Decrypting Tubby-Like Protein Gene Family of Multiple Functions in Starch Root Crop Cassava. AoB Plants 2019, 11, plz075. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Khurana, P.; Khurana, J.P. AtTLP2, a Tubby-Like Protein, Plays Intricate Roles in Abiotic Stress Signaling. Plant Cell Rep. 2023, 42, 235–252. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Ahmed, R.I.; Wang, Y.; Hu, R.; Zhou, G.; Kong, Y. Tubby-Like Protein 2 Regulates Homogalacturonan Biosynthesis in Arabidopsis Seed Coat Mucilage. Plant Mol. Biol. 2019, 99, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Song, W.M.; Jin, Y.L.; Jiang, C.M.; Yang, Y.; Li, B.; Huang, W.J.; Liu, H.; Zhang, H.X. Characterization of Arabidopsis Tubby-Like Proteins and Redundant Function of AtTLP3 and AtTLP9 in Plant Response to ABA and Osmotic Stress. Plant Mol. Biol. 2014, 86, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Wardhan, V.; Jahan, K.; Gupta, S.; Chennareddy, S.; Datta, A.; Chakraborty, S.; Chakraborty, N. Overexpression of CaTLP1, A Putative Transcription Factor in Chickpea (Cicer arietinum L.), Promotes Stress Tolerance. Plant Mol. Biol. 2012, 79, 479–493. [Google Scholar] [CrossRef]
- Wardhan, V.; Pandey, A.; Chakraborty, S.; Chakraborty, N. Chickpea Transcription Factor CaTLP1 Interacts with Protein Kinases, Modulates ROS Accumulation and Promotes ABA-Mediated Stomatal Closure. Sci. Rep. 2016, 6, 38121. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Wang, F.; Lv, H.; Cao, M.; Zhang, N.; Li, F.; Wang, H.; Li, X.; Yuan, X.; et al. A Tubby-Like Protein CsTLP8 Acts in the ABA Signaling Pathway and Negatively Regulates Osmotic Stresses Tolerance during Seed Germination. BMC Plant Biol. 2021, 21, 340. [Google Scholar] [CrossRef]
- Lisciani, S.; Marconi, S.; Le Donne, C.; Camilli, E.; Aguzzi, A.; Gabrielli, P.; Gambelli, L.; Kunert, K.; Marais, D.; Vorster, B.J.; et al. Legumes and Common Beans in Sustainable Diets: Nutritional Quality, Environmental Benefits, Spread and Use in Food Preparations. Front. Nutr. 2024, 11, 1385232. [Google Scholar] [CrossRef]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef]
- Losa, A.; Vorster, J.; Cominelli, E.; Sparvoli, F.; Paolo, D.; Sala, T.; Ferrari, M.; Carbonaro, M.; Marconi, S.; Camilli, E.; et al. Drought and Heat Affect Common Bean Minerals and Human Diet—What We Know and Where to Go. Food Energy Secur. 2022, 11, e351. [Google Scholar] [CrossRef]
- Dutta, T.; Neelapu, N.R.R.; Wani, S.H.; Challa, S. Response of Pulses to Drought and Salinity Stress Response: A Physiological Perspective. In Pulse Improvement; Wani, S.H., Jain, M., Eds.; Springer: Cham, Switzerland, 2018; Volume 4, pp. 77–98. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry Beans (Phaseolus vulgaris L.) as a Vital Component of Sustainable Agriculture and Food Security–A Review. Legume Sci. 2022, 5, e155. [Google Scholar] [CrossRef]
- Altaf, A.; Zada, A.; Hussain, S.; Gull, S.; Ding, Y.; Tao, R.; Zhu, M.; Zhu, X. Genome-Wide Identification, Characterization, and Expression Analysis of TUBBY Gene Family in Wheat (Triticum aestivum L.) under Biotic and Abiotic Stresses. Agronomy 2022, 12, 1121. [Google Scholar] [CrossRef]
- Wang, T.; Hu, J.; Ma, X.; Li, C.; Yang, Q.; Feng, S.; Li, M.; Li, N.; Song, X. Identification, Evolution and Expression Analyses of Whole Genome-Wide TLP Gene Family in Brassica napus. BMC Genom. 2020, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cheng, Y.; Yi, L.; He, H.; Zhan, S.; Yang, P. Genome-Wide Identification of the Tubby-Like Protein (TLPs) Family in Medicinal Model Plant Salvia miltiorrhiza. PeerJ 2021, 9, e11403. [Google Scholar] [CrossRef] [PubMed]
- Mejia, S.; Santos, J.L.B.; Noutsos, C. Comprehensive Genome-Wide Natural Variation and Expression Analysis of Tubby-Like Proteins Gene Family in Brachypodium distachyon. Plants 2024, 13, 987. [Google Scholar] [CrossRef]
- Ullah, I.; Uzair, M.; Rehman, O.U.; Attacha, S.; Attia, K.A.; Ahmad, S.; Salman, M.; Ali, H.; Rashid, M.A.R.; Abushady, A.M.; et al. Characterization of the Tubby-Like Proteins (TLPs) Gene Family in Cucumis sativus L.: Insights into the Evolution, Structure, and Expression Patterns under Salt Stress. Genet. Resour. Crop Evol. 2023, 71, 2019–2036. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of Duplicate Genes in Exon–Intron Structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Wolf, Y.I.; Sorokin, A.V.; Mirkin, B.G.; Koonin, E.V. Remarkable Interkingdom Conservation of Intron Positions and Massive, Lineage-Specific Intron Loss and Gain in Eukaryotic Evolution. Curr. Biol. 2003, 13, 1512–1517. [Google Scholar] [CrossRef]
- Wagner, A. Genetic Redundancy Caused by Gene Duplications and Its Evolution in Networks of Transcriptional Regulators. Biol. Cybern. 1996, 74, 557–567. [Google Scholar] [CrossRef]
- Chapman, B.A.; Bowers, J.E.; Feltus, F.A.; Paterson, A.H. Buffering of Crucial Functions by Paleologous Duplicated Genes May Contribute Cyclicality to Angiosperm Genome Duplication. Proc. Natl. Acad. Sci. USA 2006, 103, 2730–2735. [Google Scholar] [CrossRef]
- Li, Z.; Woo, H.R.; Guo, H. Genetic Redundancy of Senescence-Associated Transcription Factors in Arabidopsis. J. Exp. Bot. 2018, 69, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 Co-Operate as Negative Regulators of Leaf Senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, T.; Jiang, J.; Deng, L.; Liu, Y.; Zhong, Z.; Fu, H.; Yang, B.; Zhang, L. The Chloroplast GATA-Motif of Mahonia bealei Participates in Alkaloid-Mediated Photosystem Inhibition during Dark to Light Transition. J. Plant Physiol. 2023, 280, 153894. [Google Scholar] [CrossRef]
- Mallappa, C.; Yadav, V.; Negi, P.; Chattopadhyay, S. A Basic Leucine Zipper Transcription Factor, G-Box-Binding Factor 1, Regulates Blue Light-Mediated Photomorphogenic Growth in Arabidopsis. J. Biol. Chem. 2006, 281, 22190–22199. [Google Scholar] [CrossRef] [PubMed]
- Harter, K.; Kircher, S.; Frohnmeyer, H.; Krenz, M.; Nagy, F.; Schäfer, E. Light-Regulated Modification and Nuclear Translocation of Cytosolic G-Box Binding Factors in Parsley. Plant Cell 1994, 6, 545–559. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Su, D.; Feng, Y.; Zhao, H.; Deng, H.; Liu, M. Comprehensive Profiling of Tubby-Like Protein Expression Uncovers Ripening-Related TLP Genes in Tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2020, 21, 1000. [Google Scholar] [CrossRef] [PubMed]
- Reňák, D.; Dupl’áková, N.; Honys, D. Wide-Scale Screening of T-DNA Lines for Transcription Factor Genes Affecting Male Gametophyte Development in Arabidopsis. Sex. Plant Repord. 2012, 25, 39–60. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, X.; Ruan, L.; Xu, Y.; Li, K. Molecular Analyses of the Tubby-Like Protein Gene Family and Their Response to Salt and High Temperature in the Foxtail Millet (Setaria italica). Funct. Integr. Genom. 2024, 24, 170. [Google Scholar] [CrossRef]
- Du, F.; Xu, J.N.; Zhan, C.Y.; Yu, Z.B.; Wang, X.Y. An Obesity-Like Gene MdTLP7 from Apple (Malus × Domestica) Enhances Abiotic Stress Tolerance. Biochem. Bioph. Res. Co. 2014, 445, 394–397. [Google Scholar] [CrossRef]
- Du, F.; Xu, J.N.; Li, D.; Wang, X.Y. The Identification of Novel and Differentially Expressed Apple-Tree Genes under Low-Temperature Stress Using High-Throughput Illumina Sequencing. Mol. Biol. Rep. 2015, 42, 569–580. [Google Scholar] [CrossRef]
- Xu, J.; Xing, S.; Sun, Q.; Zhan, C.; Liu, X.; Zhang, S.; Wang, X. The Expression of a Tubby-Like Protein from Malus domestica (MdTLP7) Enhances Abiotic Stress Tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The Conserved Domain Database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB Bioinformatics Resource Portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, D.; Sun, B.; Zhao, Y.; Ma, Q. Genome-Wide Identification and Comparative Analysis of the TUBBY-Like Protein Gene Family in Maize. Genes Genom. 2015, 38, 25–36. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) V6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Mu, H.; Chen, J.; Huang, W.; Huang, G.; Deng, M.; Hong, S.; Ai, P.; Gao, C.; Zhou, H. OmicShare Tools: A Zero-Code Interactive Online Platform for Biological Data Analysis and Visualization. iMeta 2024, 3, e228. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene Name | Gene ID | CDS (bp) | Protein (aa 1) | Intron | MW 2 (Da) | pI 3 | Instability Index | Aliphatic Index | GRAVY 4 | Subcellular Localization 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| PvTLP1 | Phvul.001G013500 | 1290 | 429 | 3 | 47,795.75 | 9.34 | 60.34 | 80.93 | −0.341 | Nucl |
| PvTLP2 | Phvul.002G031500 | 1245 | 414 | 3 | 46,047.76 | 9.46 | 56.58 | 80.05 | −0.346 | Nucl |
| PvTLP3 | Phvul.003G100700 | 1083 | 360 | 4 | 40,469.27 | 9.09 | 57.71 | 79.67 | −0.302 | Nucl |
| PvTLP4 | Phvul.003G254900 | 1242 | 413 | 3 | 46,210.86 | 9.21 | 61.47 | 77.92 | −0.334 | Chlo |
| PvTLP5 | Phvul.004G095000 | 1278 | 425 | 3 | 47,566.43 | 9.56 | 67.49 | 76.40 | −0.371 | Nucl |
| PvTLP6 | Phvul.005G082200 | 1128 | 375 | 3 | 41,640.27 | 9.33 | 53.17 | 67.09 | −0.476 | Mito |
| PvTLP7 | Phvul.005G183800 | 1113 | 370 | 8 | 42,252.31 | 9.60 | 37.45 | 65.62 | −0.607 | Nucl |
| PvTLP8 | Phvul.006G161100 | 1152 | 383 | 4 | 43,206.85 | 9.46 | 60.29 | 76.37 | −0.327 | Mito |
| PvTLP9 | Phvul.006G210500 | 1284 | 427 | 3 | 47,950.04 | 9.47 | 63.82 | 76.28 | −0.363 | Nucl |
| PvTLP10 | Phvul.007G079900 | 1296 | 431 | 3 | 48,120.22 | 9.71 | 60.90 | 75.31 | −0.349 | Nucl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhao, M.; Li, J.; Qiu, F.; Wang, Y.; Zhao, J.; Chang, J.; Hao, X. Characterization and Expression Analysis of the PvTLP Gene Family in the Common Bean (Phaseolus vulgaris) in Response to Salt and Drought Stresses. Int. J. Mol. Sci. 2025, 26, 5702. https://doi.org/10.3390/ijms26125702
Dong X, Zhao M, Li J, Qiu F, Wang Y, Zhao J, Chang J, Hao X. Characterization and Expression Analysis of the PvTLP Gene Family in the Common Bean (Phaseolus vulgaris) in Response to Salt and Drought Stresses. International Journal of Molecular Sciences. 2025; 26(12):5702. https://doi.org/10.3390/ijms26125702
Chicago/Turabian StyleDong, Xue, Min Zhao, Jia Li, Fuyi Qiu, Yan Wang, Jiandong Zhao, Jianwu Chang, and Xiaopeng Hao. 2025. "Characterization and Expression Analysis of the PvTLP Gene Family in the Common Bean (Phaseolus vulgaris) in Response to Salt and Drought Stresses" International Journal of Molecular Sciences 26, no. 12: 5702. https://doi.org/10.3390/ijms26125702
APA StyleDong, X., Zhao, M., Li, J., Qiu, F., Wang, Y., Zhao, J., Chang, J., & Hao, X. (2025). Characterization and Expression Analysis of the PvTLP Gene Family in the Common Bean (Phaseolus vulgaris) in Response to Salt and Drought Stresses. International Journal of Molecular Sciences, 26(12), 5702. https://doi.org/10.3390/ijms26125702






