Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update
Abstract
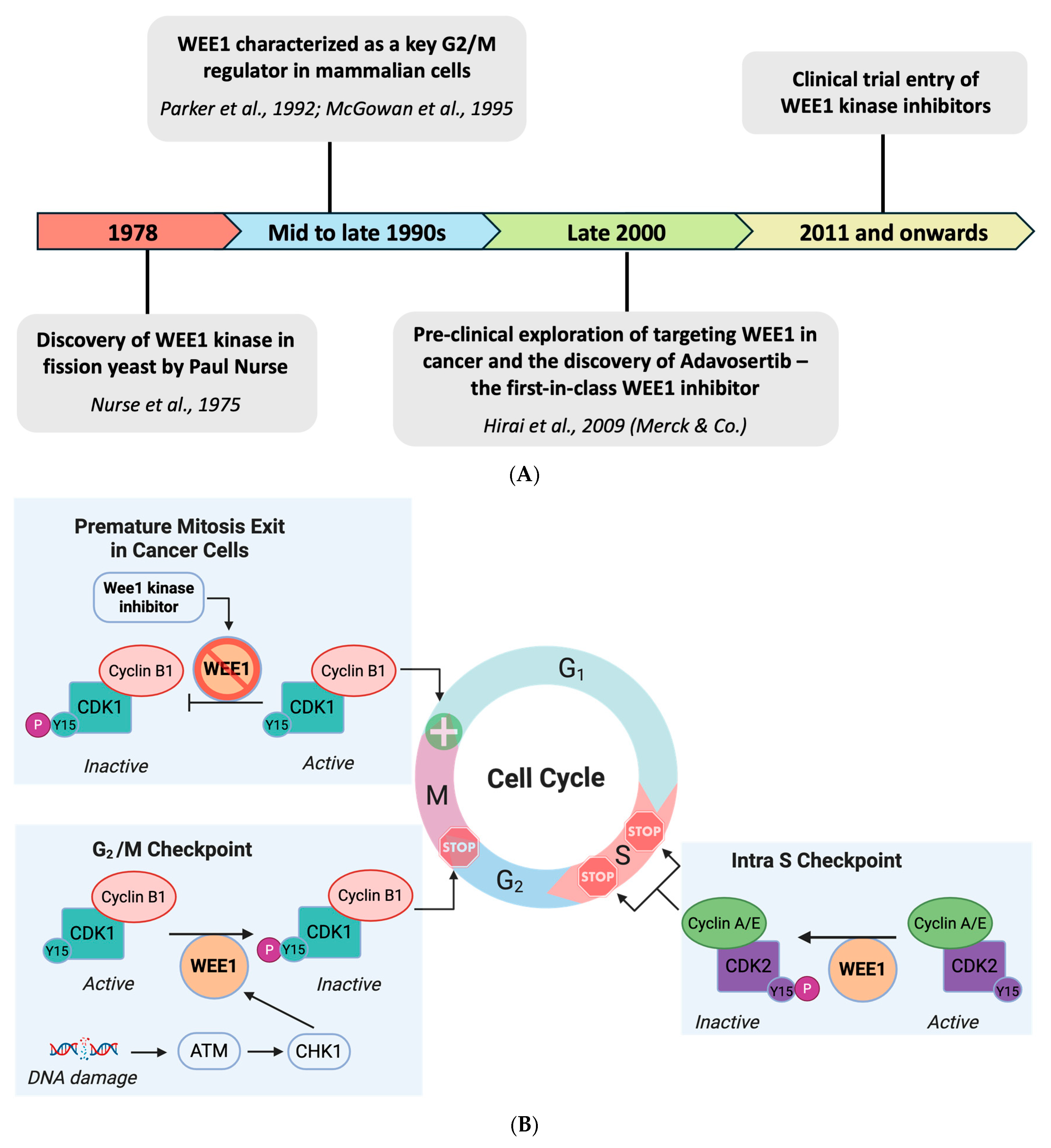
1. Introduction
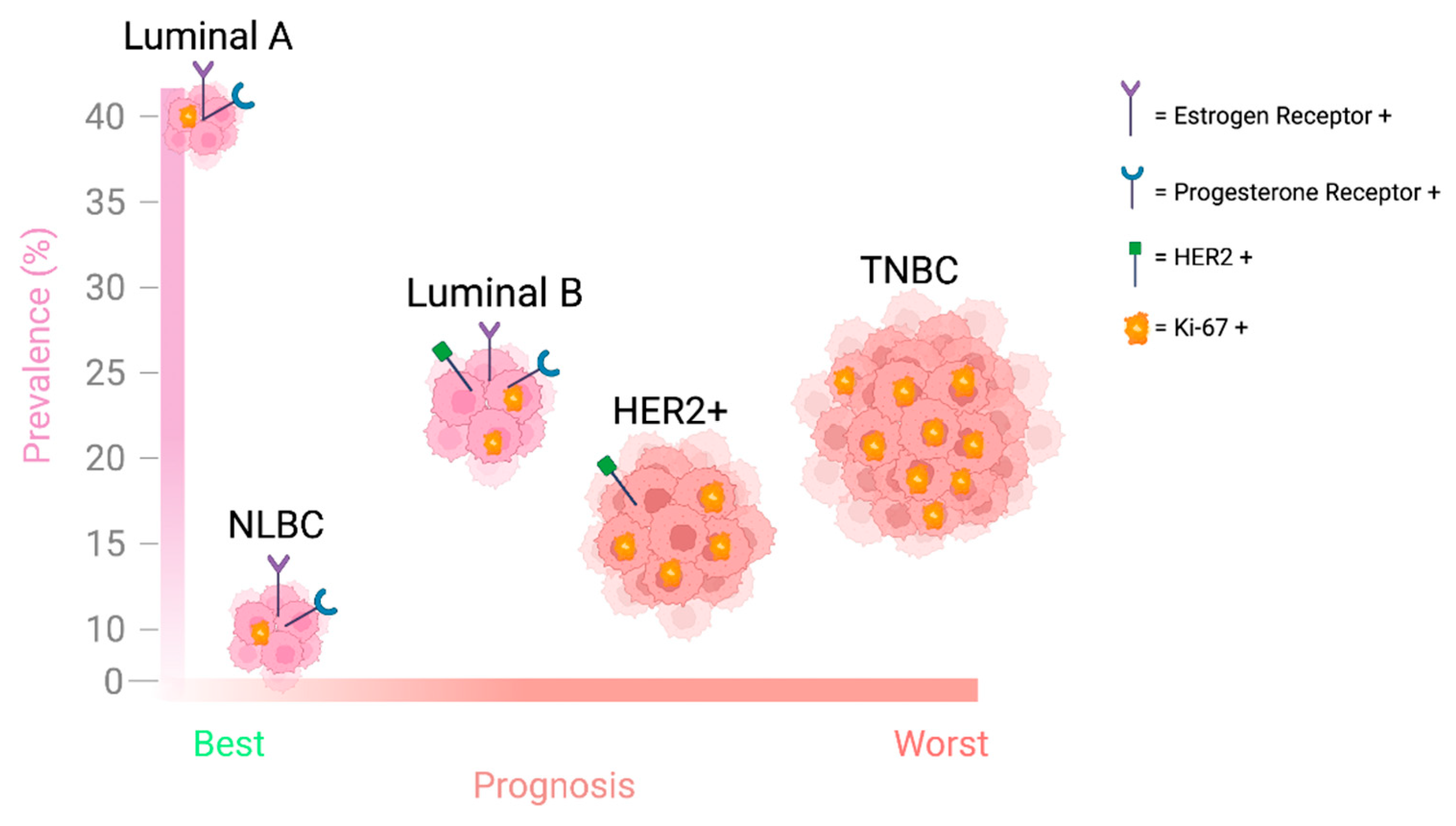
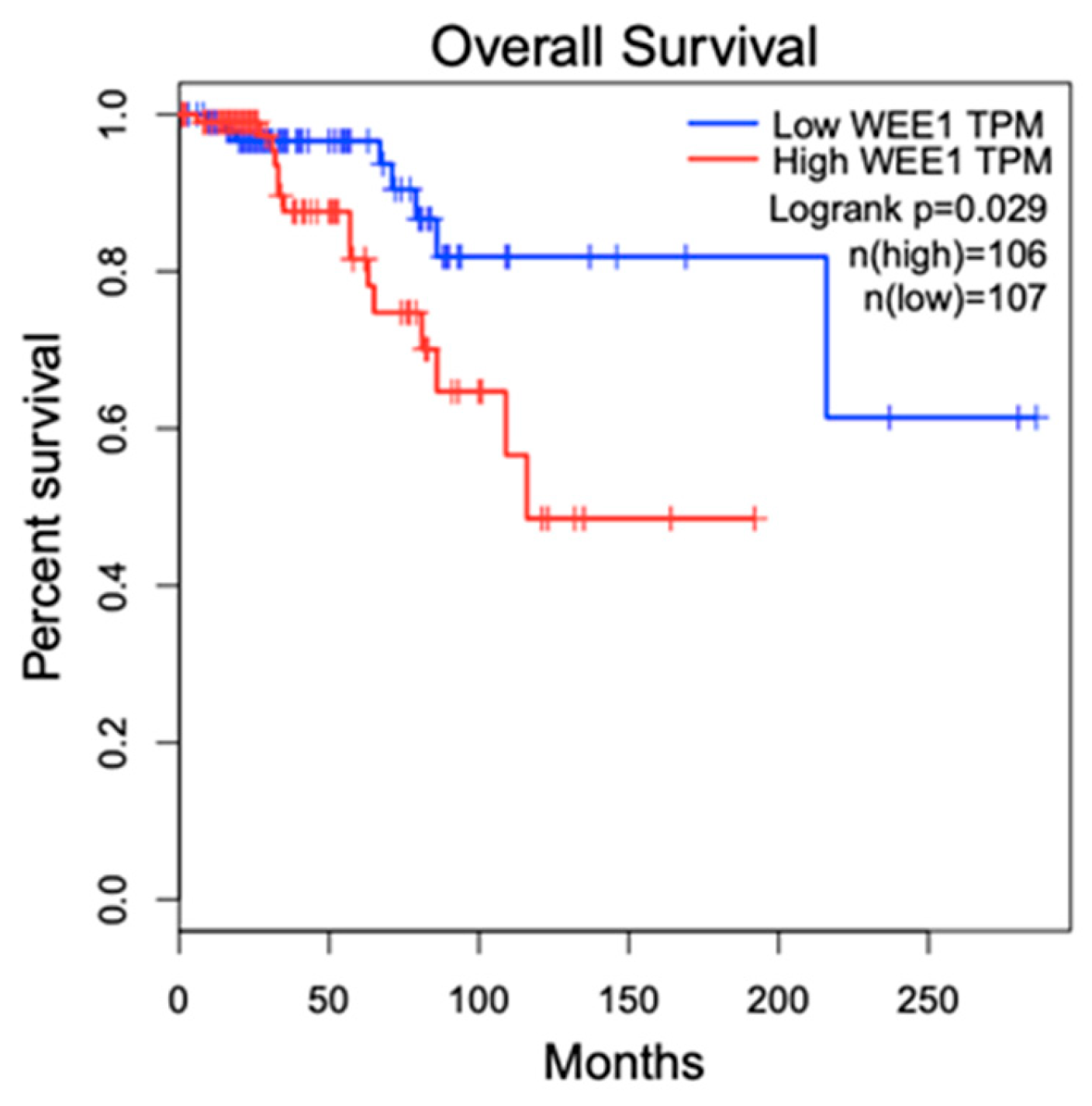
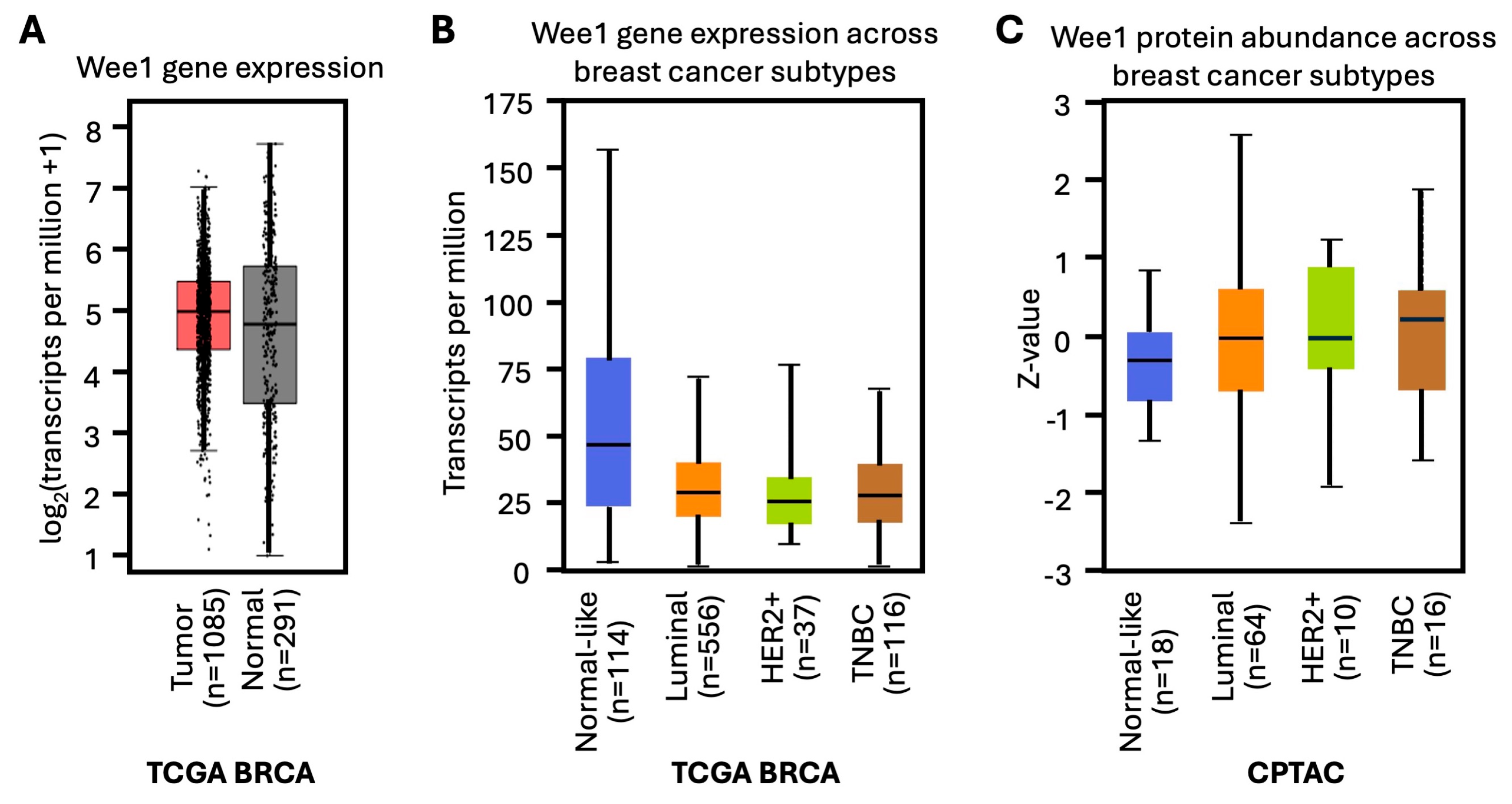
2. WEE1 Kinase in Breast Cancer
| Subtype | Receptor Status | Characteristics | Prevalence (%) | Treatment | Five-Year Relative Survival Rate |
|---|---|---|---|---|---|
| Luminal A (Lum A) |
|
| 60–70% | Hormonal therapy (Tamoxifen, Aromatase inhibitors) | ~94.4% |
| Luminal B (Lum B) |
|
| 60–70% | Hormonal therapy, targeted therapy | ~90.7% |
| HER2-overexpressing (HER2+) |
|
| 10–15% | Targeted therapy (HER2 inhibitors: Trastuzumab, Pertuzumab) | ~84.8% |
| Triple Negative Breast Cancer (TNBC) |
|
| 15–20% | Chemotherapy, surgery, radiation, PDL1 and PARP inhibitors | ~77.1% |
| Normal-like Breast Cancer (NLBC) | Variable |
| N/A | Chemotherapy, surgery, radiation | N/A |
3. WEE1 Kinase Inhibitors for Breast Cancer Treatment
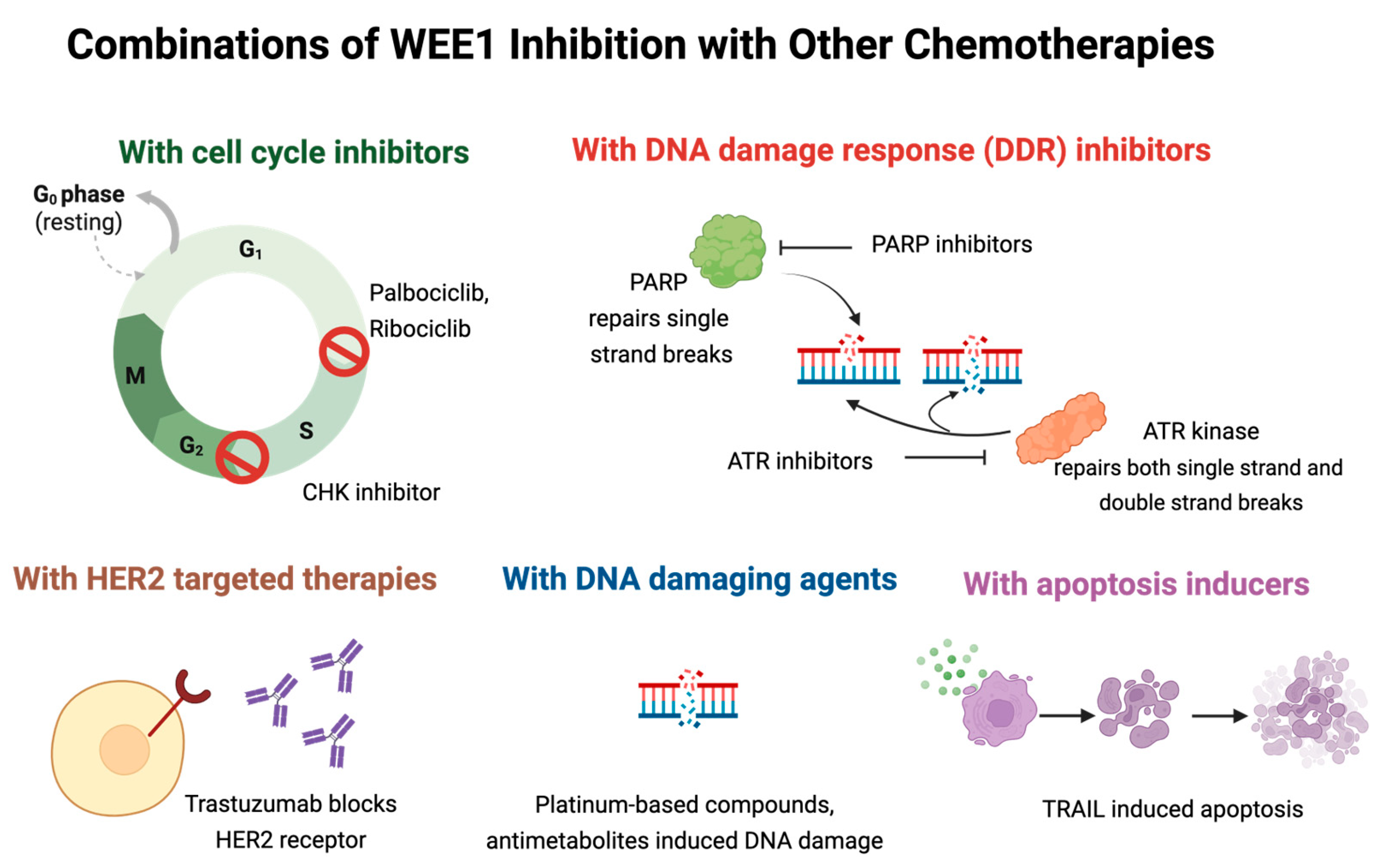
4. Combination Strategies to Enhance the Efficacy of WEE1 Kinase Inhibitors
4.1. Combination Therapy with CDK4/6 Inhibitors
4.2. Combination Therapy with ATR/CHK1 Inhibitors
4.3. Combination Therapy with PARP Inhibitors
4.4. Combination Therapy with Platinum Containing Compounds
4.5. Combination Therapy with HER2-Targeted Therapies
4.6. Combination Therapy with Apoptosis Inducers
4.7. Combination Therapy with Antimetabolites
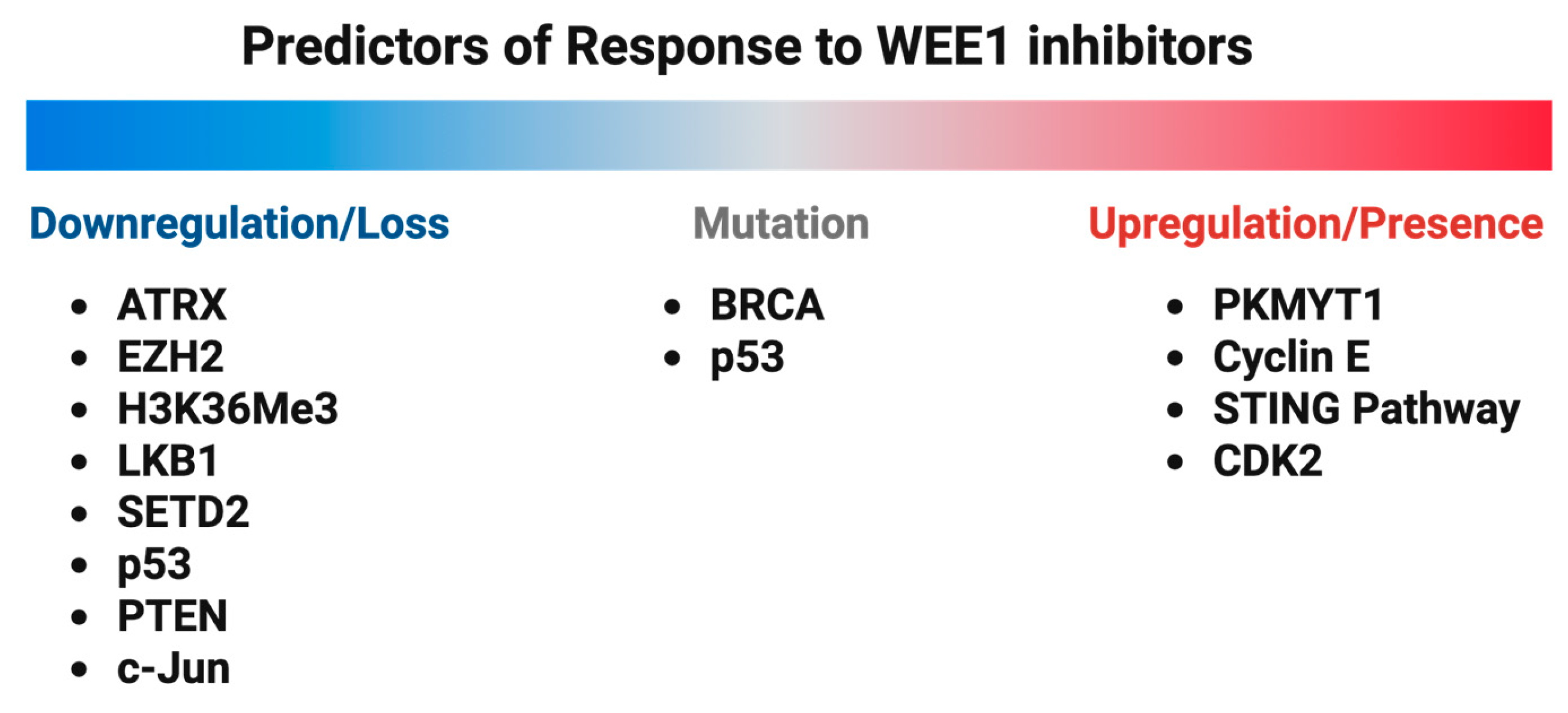
5. Resistance to WEE1 Kinase Inhibitors and Predictors of Response
5.1. PKMYT1 Upregulation
5.2. Cyclin E Overexpression
5.3. BRCA Mutations
5.4. ATRX Deficiency
5.5. EZH2 Deficiency and STING Pathway Activation
5.6. H3K36Me3 Deficiency
5.7. LKB1 Deficiency
5.8. SETD2 Deficiency
5.9. CDK2 Expression
5.10. p53 Deficiency/Mutations
5.11. PTEN Loss
5.12. c-Jun Loss
6. Clinical Trials Targeting WEE1 Kinase in Breast Cancer
| Clinicaltrials.gov | Clinical Study Title | Adavosertib Combination Regimen | Dose Information | Results/Adverse Effects |
|---|---|---|---|---|
| NCT03012477 | A Phase II Study of Cisplatin + Adavosertib in Metastatic Triple-negative Breast Cancer and Evaluation of pCDK1 as a Biomarker of Target Response |
|
|
|
| NCT02617277 | Open-label, multi-center, phase I study to assess safety and tolerability of Adavosertib plus durvalumab in patients with advanced solid tumors |
|
| Preliminary evidence of limited anti-tumor activity of Adavosertib + durvalumab. The most frequent (in >5% of patients) treatment-emergent grade ≥ 3 toxicities were
|
| NCT00648648 | Phase I study evaluating wee1 inhibitor Adavosertib as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors |
|
| Established tolerable doses of oral Adavosertib in +carboplatin/cisplatin/gemcitabine that exceed threshold pharmacokinetic levels for efficacy and preliminary pharmacodynamic. The most common treatment-related AEs were
|
| Drug | Sponsor | Mechanism and Key Properties | Clinical and Preclinical Highlights | References |
|---|---|---|---|---|
| Adavosertib | Astrazeneca, Cambridge, UK |
|
| [89,150] |
| PD0166285 | _ |
|
| [151,152,153] |
| PD0407824 | _ |
|
| [154] |
| Azenosertib | Zentalis Pharmaceuticals, New York, NY, USA |
|
| [113,155] |
| IMP7068 | IMPACT Therapeutics, Nanjing, China |
|
| [156,157] |
| SC-0191 | Shijiazhuang Zhikang Hongren New Drug Development Co., Ltd., Shijiazhuang, China |
|
| [158] |
| ATRN-1051 | Aprea Therapeutics, Doylestown, PA, USA |
|
| [159] |
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Russell, P.; Nurse, P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 1987, 49, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Rohe, A.; Platzer, C.; Najjar, A.; Erdmann, F.; Sippl, W. Regulation of G2/M Transition by Inhibition of WEE1 and PKMYT1 Kinases. Molecules 2017, 22, 2045. [Google Scholar] [CrossRef] [PubMed]
- Ghelli Luserna di Rora, A.; Cerchione, C.; Martinelli, G.; Simonetti, G. A WEE1 family business: Regulation of mitosis, cancer progression, and therapeutic target. J. Hematol. Oncol. 2020, 13, 126. [Google Scholar] [CrossRef]
- Solc, P.; Schultz, R.M.; Motlik, J. Prophase I arrest and progression to metaphase I in mouse oocytes: Comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol. Hum. Reprod. 2010, 16, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Ando, H.; Watanabe, N.; Kitamura, K.; Ito, K.; Okayama, H.; Miyamoto, T.; Agui, T.; Sasaki, M. Identification and characterization of human Wee1B, a new member of the Wee1 family of Cdk-inhibitory kinases. Genes. Cells 2000, 5, 839–847. [Google Scholar] [CrossRef]
- Geenen, J.J.J.; Schellens, J.H.M. Molecular Pathways: Targeting the Protein Kinase Wee1 in Cancer. Clin. Cancer Res. 2017, 23, 4540–4544. [Google Scholar] [CrossRef]
- Mitra, J.; Enders, G.H. Cyclin A/Cdk2 complexes regulate activation of Cdk1 and Cdc25 phosphatases in human cells. Oncogene 2004, 23, 3361–3367. [Google Scholar] [CrossRef]
- Den Haese, G.J.; Walworth, N.; Carr, A.M.; Gould, K.L. The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2. Mol. Biol. Cell 1995, 6, 371–385. [Google Scholar] [CrossRef]
- Solomon, M.J.; Harper, J.W.; Shuttleworth, J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993, 12, 3133–3142. [Google Scholar] [CrossRef]
- Lolli, G.; Johnson, L.N. CAK-Cyclin-dependent Activating Kinase: A key kinase in cell cycle control and a target for drugs? Cell Cycle 2005, 4, 572–577. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant p53 in cancer: From molecular mechanism to therapeutic modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Matheson, C.J.; Backos, D.S.; Reigan, P. Targeting WEE1 Kinase in Cancer. Trends Pharmacol. Sci. 2016, 37, 872–881. [Google Scholar] [CrossRef]
- Bucher, N.; Britten, C.D. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br. J. Cancer 2008, 98, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Stephens, P.A.; Middleton, F.K.; Curtin, N.J. Targeting the S and G2 checkpoint to treat cancer. Drug Discov. Today 2012, 17, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, S. Checkpoint Kinase and Wee1 Inhibitors as Anticancer Therapeutics. In DNA Repair in Cancer Therapy: Molecular Targets and Clinical Applications; Kelley, M., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 211–234. [Google Scholar]
- Do, K.; Doroshow, J.H.; Kummar, S. Wee1 kinase as a target for cancer therapy. Cell Cycle 2013, 12, 3159–3164. [Google Scholar] [CrossRef]
- Houghton, P.J.; Kurmasheva, R.T. Challenges and Opportunities for Childhood Cancer Drug Development. Pharmacol. Rev. 2019, 71, 671–697. [Google Scholar] [CrossRef]
- Aarts, M.; Sharpe, R.; Garcia-Murillas, I.; Gevensleben, H.; Hurd, M.S.; Shumway, S.D.; Toniatti, C.; Ashworth, A.; Turner, N.C. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2012, 2, 524–539. [Google Scholar] [CrossRef]
- Tominaga, Y.; Li, C.; Wang, R.H.; Deng, C.X. Murine Wee1 plays a critical role in cell cycle regulation and pre-implantation stages of embryonic development. Int. J. Biol. Sci. 2006, 2, 161–170. [Google Scholar] [CrossRef]
- Mir, S.E.; De Witt Hamer, P.C.; Krawczyk, P.M.; Balaj, L.; Claes, A.; Niers, J.M.; Van Tilborg, A.A.; Zwinderman, A.H.; Geerts, D.; Kaspers, G.J.; et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 2010, 18, 244–257. [Google Scholar] [CrossRef]
- Takisawa, H.; Mimura, S.; Kubota, Y. Eukaryotic DNA replication: From pre-replication complex to initiation complex. Curr. Opin. Cell Biol. 2000, 12, 690–696. [Google Scholar] [CrossRef]
- Gu, Y.; Rosenblatt, J.; Morgan, D.O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992, 11, 3995–4005. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.R. Wee1-dependent mechanisms required for coordination of cell growth and cell division. J. Cell Sci. 2003, 116, 4883–4890. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Nahse, V.; Larsen, M.S.; Groth, P.; Clancy, T.; Lees, M.; Jorgensen, M.; Helleday, T.; Syljuasen, R.G.; Sorensen, C.S. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J. Cell Biol. 2010, 188, 629–638. [Google Scholar] [CrossRef]
- Salera-Vieira, J.; Maxwell, S. Creating the Joint Commission “super user”: An innovative plan to ensure survey readiness. Nurs. Womens Health 2012, 16, 159–162. [Google Scholar] [CrossRef]
- Dominguez-Kelly, R.; Martin, Y.; Koundrioukoff, S.; Tanenbaum, M.E.; Smits, V.A.; Medema, R.H.; Debatisse, M.; Freire, R. Wee1 controls genomic stability during replication by regulating the Mus81-Eme1 endonuclease. J. Cell Biol. 2011, 194, 567–579. [Google Scholar] [CrossRef]
- Li, C.; Shen, Q.; Zhang, P.; Wang, T.; Liu, W.; Li, R.; Ma, X.; Zeng, X.; Yin, Y.; Tao, K. Targeting MUS81 promotes the anticancer effect of WEE1 inhibitor and immune checkpoint blocking combination therapy via activating cGAS/STING signaling in gastric cancer cells. J. Exp. Clin. Cancer Res. 2021, 40, 315. [Google Scholar] [CrossRef]
- Martin, Y.; Dominguez-Kelly, R.; Freire, R. Novel insights into maintaining genomic integrity: Wee1 regulating Mus81/Eme1. Cell Div. 2011, 6, 21. [Google Scholar] [CrossRef]
- Nurse, P. Genetic control of cell size at cell division in yeast. Nature 1975, 256, 547–551. [Google Scholar] [CrossRef]
- Parker, L.L.; Piwnica-Worms, H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 1992, 257, 1955–1957. [Google Scholar] [CrossRef]
- McGowan, C.H.; Russell, P. Cell cycle regulation of human WEE1. EMBO J. 1995, 14, 2166–2175. [Google Scholar] [CrossRef]
- Hirai, H.; Iwasawa, Y.; Okada, M.; Arai, T.; Nishibata, T.; Kobayashi, M.; Kimura, T.; Kaneko, N.; Ohtani, J.; Yamanaka, K.; et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol. Cancer Ther. 2009, 8, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; de la Garza Salazar, J.; Pritchard, K.; Amadori, D.; Haidinger, R.; Hudis, C.A.; Khaled, H.; Liu, M.C.; Martin, M.; Namer, M.; et al. The global breast cancer burden: Variations in epidemiology and survival. Clin. Breast Cancer 2005, 6, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, N.; Habibagahi, M.; Rosli, R.; Ghaderi, A.; Yusoff, K.; Hosseini, A.; Abdullah, S.; Jaberipour, M. Tumour suppressive effects of WEE1 gene silencing in breast cancer cells. Asian Pac. J. Cancer Prev. 2014, 14, 6605–6611. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Veronesi, U.; Boyle, P.; Goldhirsch, A.; Orecchia, R.; Viale, G. Breast cancer. Lancet 2005, 365, 1727–1741. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 2001, 358, 1389–1399. [Google Scholar] [CrossRef]
- Hulka, B.S. Epidemiology of susceptibility to breast cancer. Prog. Clin. Biol. Res. 1996, 395, 159–174. [Google Scholar]
- Colditz, G.A.; Kaphingst, K.A.; Hankinson, S.E.; Rosner, B. Family history and risk of breast cancer: Nurses’ health study. Breast Cancer Res. Treat. 2012, 133, 1097–1104. [Google Scholar] [CrossRef]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef]
- Allison, K.H. Molecular pathology of breast cancer: What a pathologist needs to know. Am. J. Clin. Pathol. 2012, 138, 770–780. [Google Scholar] [CrossRef]
- Shaath, H.; Elango, R.; Alajez, N.M. Molecular Classification of Breast Cancer Utilizing Long Non-Coding RNA (lncRNA) Transcriptomes Identifies Novel Diagnostic lncRNA Panel for Triple-Negative Breast Cancer. Cancers 2021, 13, 5350. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes. Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting Breast Cancer: An Overlook on Current Strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Afifi, N.; Barrero, C.A. Understanding Breast Cancer Aggressiveness and Its Implications in Diagnosis and Treatment. J. Clin. Med. 2023, 12, 1375. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kummel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Munoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Barchiesi, G.; Roberto, M.; Verrico, M.; Vici, P.; Tomao, S.; Tomao, F. Emerging Role of PARP Inhibitors in Metastatic Triple Negative Breast Cancer. Current Scenario and Future Perspectives. Front. Oncol. 2021, 11, 769280. [Google Scholar] [CrossRef]
- Caputo, R.; Piezzo, M.; Martinelli, C.; von Arx, C.; Pantano, F.; Guarino, A.; Botticelli, A.; Rizzo, A.; Orlando, L.; Sanò, M.V.; et al. Sacituzumab govitecan for the treatment of metastatic triple-negative breast cancer patients: A multicenter realworld updated analysis. ESMO Open 2025, 10, 104925. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, M.; Zhang, Z.; Li, Z.; Ni, D.; Ashton, A.W.; Tang, H.Y.; Speicher, D.W.; Pestell, R.G. Leronlimab, a humanized monoclonal antibody to CCR5, blocks breast cancer cellular metastasis and enhances cell death induced by DNA damaging chemotherapy. Breast Cancer Res. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Pestell, R.G.; Cristofanilli, M.; Adams, D.; Dolezal, M.; Rui, H.; Arman, C.; Joseph, M.; Cunningham, B.; Lalezari, J.; Rugo, H.S. Observed survival following treatment with Leronlimab in patients with metastatic triple-negative breast cancer (mTNBC). ESMO Open 2025, 10, 104940. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res. Treat. 2018, 170, 213–219. [Google Scholar] [CrossRef]
- McDermott, A.M.; Miller, N.; Wall, D.; Martyn, L.M.; Ball, G.; Sweeney, K.J.; Kerin, M.J. Identification and validation of oncologic miRNA biomarkers for luminal A-like breast cancer. PLoS ONE 2014, 9, e87032. [Google Scholar] [CrossRef]
- Karami, F.; Mehdipour, P. A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed. Res. Int. 2013, 2013, 928562. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer; HN, M., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Vassilopoulos, A.; Tominaga, Y.; Kim, H.S.; Lahusen, T.; Li, B.; Yu, H.; Gius, D.; Deng, C.X. WEE1 murine deficiency induces hyper-activation of APC/C and results in genomic instability and carcinogenesis. Oncogene 2015, 34, 3023–3035. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Goncalves, A.; Birnbaum, D. The therapeutic response of ER+/HER2- breast cancers differs according to the molecular Basal or Luminal subtype. NPJ Breast Cancer 2020, 6, 8. [Google Scholar] [CrossRef]
- De Nonneville, A.; Finetti, P.; Birnbaum, D.; Mamessier, E.; Bertucci, F. WEE1 Dependency and Pejorative Prognostic Value in Triple-Negative Breast Cancer. Adv. Sci. 2021, 8, e2101030. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, Y.; Kang, H.; Yim, Y.S.; Kim, S.J.; Song, J.; Chun, K.H. Targeting the WEE1 kinase as a molecular targeted therapy for gastric cancer. Oncotarget 2016, 7, 49902–49916. [Google Scholar] [CrossRef]
- Magnussen, G.I.; Holm, R.; Emilsen, E.; Rosnes, A.K.; Slipicevic, A.; Florenes, V.A. High expression of Wee1 is associated with poor disease-free survival in malignant melanoma: Potential for targeted therapy. PLoS ONE 2012, 7, e38254. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Hashizume, R.; Yang, X.; Kolkowitz, I.; Olow, A.K.; Phillips, J.; Smirnov, I.; Tom, M.W.; Prados, M.D.; James, C.D.; et al. Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro Oncol. 2014, 16, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Murrow, L.M.; Garimella, S.V.; Jones, T.L.; Caplen, N.J.; Lipkowitz, S. Identification of WEE1 as a potential molecular target in cancer cells by RNAi screening of the human tyrosine kinome. Breast Cancer Res. Treat. 2010, 122, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Scheithauer, H.; Pietschmann, S.; Hoffmann, M.; Rossler, J.; Graf, N.; Baumert, B.G.; Christiansen, H.; Kortmann, R.D.; Kramm, C.M.; et al. Reirradiation as part of a salvage treatment approach for progressive non-pontine pediatric high-grade gliomas: Preliminary experiences from the German HIT-HGG study group. Radiat. Oncol. 2014, 9, 177. [Google Scholar] [CrossRef]
- Caretti, V.; Hiddingh, L.; Lagerweij, T.; Schellen, P.; Koken, P.W.; Hulleman, E.; van Vuurden, D.G.; Vandertop, W.P.; Kaspers, G.J.; Noske, D.P.; et al. WEE1 kinase inhibition enhances the radiation response of diffuse intrinsic pontine gliomas. Mol. Cancer Ther. 2013, 12, 141–150. [Google Scholar] [CrossRef]
- Chaudhuri, L.; Vincelette, N.D.; Koh, B.D.; Naylor, R.M.; Flatten, K.S.; Peterson, K.L.; McNally, A.; Gojo, I.; Karp, J.E.; Mesa, R.A.; et al. CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo. Haematologica 2014, 99, 688–696. [Google Scholar] [CrossRef]
- Kreahling, J.M.; Foroutan, P.; Reed, D.; Martinez, G.; Razabdouski, T.; Bui, M.M.; Raghavan, M.; Letson, D.; Gillies, R.J.; Altiok, S. Wee1 inhibition by MK-1775 leads to tumor inhibition and enhances efficacy of gemcitabine in human sarcomas. PLoS ONE 2013, 8, e57523. [Google Scholar] [CrossRef]
- Tamura, K. Development of cell-cycle checkpoint therapy for solid tumors. Jpn. J. Clin. Oncol. 2015, 45, 1097–1102. [Google Scholar] [CrossRef]
- Di Sante, G.; Page, J.; Jiao, X.; Nawab, O.; Cristofanilli, M.; Skordalakes, E.; Pestell, R.G. Recent advances with cyclin-dependent kinase inhibitors: Therapeutic agents for breast cancer and their role in immuno-oncology. Expert Rev. Anticancer Ther. 2019, 19, 569–587. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Lu, H.; Wang, H.; Feng, H.; Xu, J.; Zhang, B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life 2020, 72, 1012–1022. [Google Scholar] [CrossRef]
- Jandial, D.D.; Krill, L.S.; Chen, L.; Wu, C.; Ke, Y.; Xie, J.; Hoang, B.H.; Zi, X. Induction of G2M Arrest by Flavokawain A, a Kava Chalcone, Increases the Responsiveness of HER2-Overexpressing Breast Cancer Cells to Herceptin. Molecules 2017, 22, 462. [Google Scholar] [CrossRef] [PubMed]
- Sand, A.; Piacsek, M.; Donohoe, D.L.; Duffin, A.T.; Riddell, G.T.; Sun, C.; Tang, M.; Rovin, R.A.; Tjoe, J.A.; Yin, J. WEE1 inhibitor, AZD1775, overcomes trastuzumab resistance by targeting cancer stem-like properties in HER2-positive breast cancer. Cancer Lett. 2020, 472, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, J.; Yu, Q.; Wu, X.; Pan, A.; Li, L. Evaluation of CCND1 amplification and CyclinD1 expression: Diffuse and strong staining of CyclinD1 could have same predictive roles as CCND1 amplification in ER positive breast cancers. Am. J. Transl. Res. 2016, 8, 142–153. [Google Scholar] [PubMed]
- Li, Z.; Chen, K.; Jiao, X.; Wang, C.; Willmarth, N.E.; Casimiro, M.C.; Li, W.; Ju, X.; Kim, S.H.; Lisanti, M.P.; et al. Cyclin D1 integrates estrogen-mediated DNA damage repair signaling. Cancer Res. 2014, 74, 3959–3970. [Google Scholar] [CrossRef]
- Lee, R.J.; Albanese, C.; Fu, M.; D’Amico, M.; Lin, B.; Watanabe, G.; Haines, G.K., 3rd; Siegel, P.M.; Hung, M.C.; Yarden, Y.; et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol. Cell Biol. 2000, 20, 672–683. [Google Scholar] [CrossRef]
- Abdelmalak, M.; Singh, R.; Anwer, M.; Ivanchenko, P.; Randhawa, A.; Ahmed, M.; Ashton, A.W.; Du, Y.; Jiao, X.; Pestell, R. The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance. Cancers 2022, 14, 5388. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef]
- Choi, Y.J.; Li, X.; Hydbring, P.; Sanda, T.; Stefano, J.; Christie, A.L.; Signoretti, S.; Look, A.T.; Kung, A.L.; von Boehmer, H.; et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell 2012, 22, 438–451. [Google Scholar] [CrossRef]
- Walker, A.J.; Wedam, S.; Amiri-Kordestani, L.; Bloomquist, E.; Tang, S.; Sridhara, R.; Chen, W.; Palmby, T.R.; Fourie Zirkelbach, J.; Fu, W.; et al. FDA Approval of Palbociclib in Combination with Fulvestrant for the Treatment of Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2016, 22, 4968–4972. [Google Scholar] [CrossRef]
- Pancholi, S.; Ribas, R.; Simigdala, N.; Schuster, E.; Nikitorowicz-Buniak, J.; Ressa, A.; Gao, Q.; Leal, M.F.; Bhamra, A.; Thornhill, A.; et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene 2020, 39, 4781–4797. [Google Scholar] [CrossRef]
- Chen, X.; Low, K.H.; Alexander, A.; Jiang, Y.; Karakas, C.; Hess, K.R.; Carey, J.P.W.; Bui, T.N.; Vijayaraghavan, S.; Evans, K.W.; et al. Cyclin E Overexpression Sensitizes Triple-Negative Breast Cancer to Wee1 Kinase Inhibition. Clin. Cancer Res. 2018, 24, 6594–6610. [Google Scholar] [CrossRef] [PubMed]
- Fallah, Y.; Demas, D.M.; Jin, L.; He, W.; Shajahan-Haq, A.N. Targeting WEE1 Inhibits Growth of Breast Cancer Cells That Are Resistant to Endocrine Therapy and CDK4/6 Inhibitors. Front. Oncol. 2021, 11, 681530. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Grant, S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin. Cancer Res. 2010, 16, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol. Life Sci. 2013, 70, 4009–4021. [Google Scholar] [CrossRef]
- Jin, J.; Fang, H.; Yang, F.; Ji, W.; Guan, N.; Sun, Z.; Shi, Y.; Zhou, G.; Guan, X. Combined Inhibition of ATR and WEE1 as a Novel Therapeutic Strategy in Triple-Negative Breast Cancer. Neoplasia 2018, 20, 478–488. [Google Scholar] [CrossRef]
- Stewart, A.; Song, J.; Pickard, L.; Muggiolu, G.; Sauvaigo, S.; Brandon, A.H.; Raynaud, F.; Banerji, U. Characterizing functional DNA damage and response caused by the combination of CHK1 and WEE1 inhibitors in ovarian and breast cancer models. BJC Rep. 2024, 2, 27. [Google Scholar] [CrossRef]
- Ha, D.H.; Min, A.; Kim, S.; Jang, H.; Kim, S.H.; Kim, H.J.; Ryu, H.S.; Ku, J.L.; Lee, K.H.; Im, S.A. Antitumor effect of a WEE1 inhibitor and potentiation of olaparib sensitivity by DNA damage response modulation in triple-negative breast cancer. Sci. Rep. 2020, 10, 9930. [Google Scholar] [CrossRef]
- Marincola, F.M.; Jaffee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of human solid tumors from T-cell recognition: Molecular mechanisms and functional significance. Adv. Immunol. 2000, 74, 181–273. [Google Scholar] [CrossRef]
- Teo, Z.L.; O’Connor, M.J.; Versaci, S.; Clarke, K.A.; Brown, E.R.; Percy, L.W.; Kuykhoven, K.; Mintoff, C.P.; Savas, P.; Virassamy, B.; et al. Combined PARP and WEE1 inhibition triggers anti-tumor immune response in BRCA1/2 wildtype triple-negative breast cancer. NPJ Breast Cancer 2023, 9, 68. [Google Scholar] [CrossRef]
- Moens, S.; Zhao, P.; Baietti, M.F.; Marinelli, O.; Van Haver, D.; Impens, F.; Floris, G.; Marangoni, E.; Neven, P.; Annibali, D.; et al. The mitotic checkpoint is a targetable vulnerability of carboplatin-resistant triple negative breast cancers. Sci. Rep. 2021, 11, 3176. [Google Scholar] [CrossRef]
- Patra, S.; Elahi, N.; Armorer, A.; Arunachalam, S.; Omala, J.; Hamid, I.; Ashton, A.W.; Joyce, D.; Jiao, X.; Pestell, R.G. Mechanisms Governing Metabolic Heterogeneity in Breast Cancer and Other Tumors. Front. Oncol. 2021, 11, 700629. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Kreso, A.; Jamieson, C.H. Cancer stem cells and self-renewal. Clin. Cancer Res. 2010, 16, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Giuffrida, R.; Raciti, G.; Puglisi, C.; Forte, S. Wee1 Kinase: A Potential Target to Overcome Tumor Resistance to Therapy. Int. J. Mol. Sci. 2021, 22, 689. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.A.; Alaa Edeen, M.; Shedid, E.M.; Kamal, A.S.S.; Warda, M.M.; Mamdouh, F.; Khedr, S.A.; Soltan, M.A.; Jeon, H.W.; Zaki, M.S.A.; et al. Targeting Cancer Stem Cells as the Key Driver of Carcinogenesis and Therapeutic Resistance. Int. J. Mol. Sci. 2023, 24, 1786. [Google Scholar] [CrossRef]
- Liu, H.; Lv, L.; Yang, K. Chemotherapy targeting cancer stem cells. Am. J. Cancer Res. 2015, 5, 880–893. [Google Scholar]
- Sun, D.; Li, C.; Zhang, F. MicroRNA-206 suppresses growth and metastasis of breast cancer stem cells via blocking EVI-1-mediated CALR expression. PLoS ONE 2022, 17, e0274919. [Google Scholar] [CrossRef]
- Roscigno, G.; Cirella, A.; Affinito, A.; Quintavalle, C.; Scognamiglio, I.; Palma, F.; Ingenito, F.; Nuzzo, S.; De Micco, F.; Cuccuru, A.; et al. miR-216a Acts as a Negative Regulator of Breast Cancer by Modulating Stemness Properties and Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 2313. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, P.; Fan, H.; Wang, H.; Xu, W.; Zhou, W. Ultrasound reverses chemoresistance in breast cancer stem cell like cells by altering ABCG2 expression. Biosci. Rep. 2017, 37, BSR20171137. [Google Scholar] [CrossRef]
- Maximiano, S.; Magalhaes, P.; Guerreiro, M.P.; Morgado, M. Trastuzumab in the Treatment of Breast Cancer. BioDrugs 2016, 30, 75–86. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Merikhian, P.; Naseri, N.; Eisavand, M.R.; Farahmand, L. MUC1 is a potential target to overcome trastuzumab resistance in breast cancer therapy. Cancer Cell Int. 2022, 22, 110. [Google Scholar] [CrossRef]
- Engelmann, K.; Shen, H.; Finn, O.J. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 2008, 68, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Martin, A.R.; Demange, L.; Benhida, R. ATM, ATR, CHK1, CHK2 and WEE1 inhibitors in cancer and cancer stem cells. Medchemcomm 2017, 8, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, J.M.; Zhou, J.Y.; Wu, G.S. The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer. Cancers 2023, 15, 2752. [Google Scholar] [CrossRef] [PubMed]
- Garimella, S.V.; Rocca, A.; Lipkowitz, S. WEE1 inhibition sensitizes basal breast cancer cells to TRAIL-induced apoptosis. Mol. Cancer Res. 2012, 10, 75–85. [Google Scholar] [CrossRef]
- Antimetabolites. Available online: https://my.clevelandclinic.org/health/drugs/24790-antimetabolites (accessed on 5 June 2025).
- Rajeshkumar, N.V.; De Oliveira, E.; Ottenhof, N.; Watters, J.; Brooks, D.; Demuth, T.; Shumway, S.D.; Mizuarai, S.; Hirai, H.; Maitra, A.; et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin. Cancer Res. 2011, 17, 2799–2806. [Google Scholar] [CrossRef]
- Pitts, T.M.; Simmons, D.M.; Bagby, S.M.; Hartman, S.J.; Yacob, B.W.; Gittleman, B.; Tentler, J.J.; Cittelly, D.; Ormond, D.R.; Messersmith, W.A.; et al. Wee1 Inhibition Enhances the Anti-Tumor Effects of Capecitabine in Preclinical Models of Triple-Negative Breast Cancer. Cancers 2020, 12, 719. [Google Scholar] [CrossRef]
- Sokhi, S.; Lewis, C.W.; Bukhari, A.B.; Hadfield, J.; Xiao, E.J.; Fung, J.; Yoon, Y.J.; Hsu, W.H.; Gamper, A.M.; Chan, G.K. Myt1 overexpression mediates resistance to cell cycle and DNA damage checkpoint kinase inhibitors. Front. Cell Dev. Biol. 2023, 11, 1270542. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Carey, J.P.W.; Karakas, C.; Albarracin, C.; Sahin, A.A.; Arun, B.K.; Guray Durak, M.; Li, M.; Kohansal, M.; et al. Targeting Replicative Stress and DNA Repair by Combining PARP and Wee1 Kinase Inhibitors Is Synergistic in Triple Negative Breast Cancers with Cyclin E or BRCA1 Alteration. Cancers 2021, 13, 1656. [Google Scholar] [CrossRef]
- Aziz, D.; Portman, N.; Fernandez, K.J.; Lee, C.; Alexandrou, S.; Llop-Guevara, A.; Phan, Z.; Yong, A.; Wilkinson, A.; Sergio, C.M.; et al. Synergistic targeting of BRCA1 mutated breast cancers with PARP and CDK2 inhibition. NPJ Breast Cancer 2021, 7, 111. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Li, F.; Xiao, R. An update of predictive biomarkers related to WEE1 inhibition in cancer therapy. J. Cancer Res. Clin. Oncol. 2024, 150, 13. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Diplas, B.H.; Liu, S.; Liu, J.; Wang, D.; Lu, Y.; Zhu, Q.; Wu, J.; Wang, W.; et al. Genome-Wide CRISPR-Cas9 Screen Reveals Selective Vulnerability of ATRX-Mutant Cancers to WEE1 Inhibition. Cancer Res. 2020, 80, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, Z.; Zhang, L.; Wu, C.; Jin, Y.; Hwang, I.; Vladimirova, O.; Xu, L.; Yang, L.; Lu, B.; et al. ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. EMBO J. 2019, 38, e96659. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A. Targeting ATRX Loss through Inhibition of the Cell-Cycle Checkpoint Mediator WEE1. Cancer Res. 2020, 80, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers/ATRX. Available online: www.mycancergenome.org/content/gene/atrx (accessed on 5 June 2025).
- Morel, K.L.; Sheahan, A.V.; Burkhart, D.L.; Baca, S.C.; Boufaied, N.; Liu, Y.; Qiu, X.; Canadas, I.; Roehle, K.; Heckler, M.; et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat. Cancer 2021, 2, 444–456. [Google Scholar] [CrossRef]
- Pfister, S.X.; Markkanen, E.; Jiang, Y.; Sarkar, S.; Woodcock, M.; Orlando, G.; Mavrommati, I.; Pai, C.C.; Zalmas, L.P.; Drobnitzky, N.; et al. Inhibiting WEE1 Selectively Kills Histone H3K36me3-Deficient Cancers by dNTP Starvation. Cancer Cell 2015, 28, 557–568. [Google Scholar] [CrossRef]
- Sun, L.; Moore, E.; Berman, R.; Clavijo, P.E.; Saleh, A.; Chen, Z.; Van Waes, C.; Davies, J.; Friedman, J.; Allen, C.T. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology 2018, 7, e1488359. [Google Scholar] [CrossRef]
- Friedman, J.; Morisada, M.; Sun, L.; Moore, E.C.; Padget, M.; Hodge, J.W.; Schlom, J.; Gameiro, S.R.; Allen, C.T. Inhibition of WEE1 kinase and cell cycle checkpoint activation sensitizes head and neck cancers to natural killer cell therapies. J. Immunother. Cancer 2018, 6, 59. [Google Scholar] [CrossRef]
- Sakamoto, K.; Goransson, O.; Hardie, D.G.; Alessi, D.R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: Effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E310–E317. [Google Scholar] [CrossRef]
- Casimiro, M.C.; Di Sante, G.; Di Rocco, A.; Loro, E.; Pupo, C.; Pestell, T.G.; Bisetto, S.; Velasco-Velazquez, M.A.; Jiao, X.; Li, Z.; et al. Cyclin D1 Restrains Oncogene-Induced Autophagy by Regulating the AMPK-LKB1 Signaling Axis. Cancer Res. 2017, 77, 3391–3405. [Google Scholar] [CrossRef]
- Zheng, B.; Jeong, J.H.; Asara, J.M.; Yuan, Y.Y.; Granter, S.R.; Chin, L.; Cantley, L.C. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell 2009, 33, 237–247. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Li, P.; Mao, X.; Li, W.; Yang, J.; Liu, P. Loss of LKB1 disrupts breast epithelial cell polarity and promotes breast cancer metastasis and invasion. J. Exp. Clin. Cancer Res. 2014, 33, 70. [Google Scholar] [CrossRef] [PubMed]
- Richer, A.L.; Cala, J.M.; O’Brien, K.; Carson, V.M.; Inge, L.J.; Whitsett, T.G. WEE1 Kinase Inhibitor AZD1775 Has Preclinical Efficacy in LKB1-Deficient Non-Small Cell Lung Cancer. Cancer Res. 2017, 77, 4663–4672. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Pai, S.; Mahmud, F. Haziness in leucovorin calcium for injection. Am. J. Hosp. Pharm. 1988, 45, 1278–1279. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, R.; Zhang, X.; Liang, Y.; Kong, F.; Wang, J.; Xu, Z. Loss of SETD2, but not H3K36me3, correlates with aggressive clinicopathological features of clear cell renal cell carcinoma patients. Biosci. Trends 2017, 11, 214–220. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.; Zhang, M.; Da, L.; Huang, W.; Zhang, C. Circular RNA circ_SETD2 represses breast cancer progression via modulating the miR-155-5p/SCUBE2 axis. Open Med. 2020, 15, 940–953. [Google Scholar] [CrossRef]
- Tsang, J.Y.; Lai, S.T.; Ni, Y.B.; Shao, Y.; Poon, I.K.; Kwan, J.S.; Chow, C.; Shea, K.H.; Tse, G.M. SETD2 alterations and histone H3K36 trimethylation in phyllodes tumor of breast. Breast Cancer Res. Treat. 2021, 187, 339–347. [Google Scholar] [CrossRef]
- Heijink, A.M.; Blomen, V.A.; Bisteau, X.; Degener, F.; Matsushita, F.Y.; Kaldis, P.; Foijer, F.; van Vugt, M.A. A haploid genetic screen identifies the G1/S regulatory machinery as a determinant of Wee1 inhibitor sensitivity. Proc. Natl. Acad. Sci. USA 2015, 112, 15160–15165. [Google Scholar] [CrossRef]
- Oza, A.M.; Estevez-Diz, M.; Grischke, E.M.; Hall, M.; Marme, F.; Provencher, D.; Uyar, D.; Weberpals, J.I.; Wenham, R.M.; Laing, N.; et al. A Biomarker-enriched, Randomized Phase II Trial of Adavosertib (AZD1775) Plus Paclitaxel and Carboplatin for Women with Platinum-sensitive TP53-mutant Ovarian Cancer. Clin. Cancer Res. 2020, 26, 4767–4776. [Google Scholar] [CrossRef]
- Kong, A.; Mehanna, H. WEE1 Inhibitor: Clinical Development. Curr. Oncol. Rep. 2021, 23, 107. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Hodakoski, C.; Barrows, D.; Mense, S.M.; Parsons, R.E. PTEN function: The long and the short of it. Trends Biochem. Sci. 2014, 39, 183–190. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, M.; Yang, J.; Lv, M.; Li, P.; Chen, Z.; Yang, J. Loss of PTEN expression in breast cancer: Association with clinicopathological characteristics and prognosis. Oncotarget 2017, 8, 32043–32054. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Wang, Y.; Ly, A.; Hosono, Y.; Murchie, E.; Walmsley, C.S.; Huynh, T.; Healy, C.; Peterson, R.; Yanase, S.; et al. PTEN Loss Mediates Clinical Cross-Resistance to CDK4/6 and PI3Kalpha Inhibitors in Breast Cancer. Cancer Discov. 2020, 10, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.; Suryo Rahmanto, A.; Johansson, H.; Franco, M.; Viiliainen, J.; Gazi, M.; Frings, O.; Fredlund, E.; Spruck, C.; Lehtio, J.; et al. PTEN and DNA-PK determine sensitivity and recovery in response to WEE1 inhibition in human breast cancer. Elife 2020, 9, e57894. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Katiyar, S.; Willmarth, N.E.; Liu, M.; Ma, X.; Flomenberg, N.; Lisanti, M.P.; Pestell, R.G. c-Jun induces mammary epithelial cellular invasion and breast cancer stem cell expansion. J. Biol. Chem. 2010, 285, 8218–8226. [Google Scholar] [CrossRef]
- Xu, L.; Ning, H.; Gu, L.; Wang, Q.; Lu, W.; Peng, H.; Cui, W.; Ying, B.; Ross, C.R.; Wilson, G.M.; et al. Tristetraprolin induces cell cycle arrest in breast tumor cells through targeting AP-1/c-Jun and NF-kappaB pathway. Oncotarget 2015, 6, 41679–41691. [Google Scholar] [CrossRef]
- Jiao, X.; Katiyar, S.; Liu, M.; Mueller, S.C.; Lisanti, M.P.; Li, A.; Pestell, T.G.; Wu, K.; Ju, X.; Li, Z.; et al. Disruption of c-Jun reduces cellular migration and invasion through inhibition of c-Src and hyperactivation of ROCK II kinase. Mol. Biol. Cell 2008, 19, 1378–1390. [Google Scholar] [CrossRef][Green Version]
- Shao, W.; Li, S.; Li, L.; Lin, K.; Liu, X.; Wang, H.; Wang, H.; Wang, D. Chemical genomics reveals inhibition of breast cancer lung metastasis by Ponatinib via c-Jun. Protein Cell 2019, 10, 161–177. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, S.T.; Zhu, R.; Li, W.; Gu, C.W.; Wei, S.H.; Xie, J.M.; Wu, H.R. Inhibition of proliferation of estrogen receptorpositive MCF7 human breast cancer cells by tamoxifen through cJun transcription factors. Mol. Med. Rep. 2013, 7, 1283–1287. [Google Scholar] [CrossRef]
- Bauer, T.M.; Moore, K.N.; Rader, J.S.; Simpkins, F.; Mita, A.C.; Beck, J.T.; Hart, L.; Chu, Q.; Oza, A.; Tinker, A.V.; et al. A Phase Ib Study Assessing the Safety, Tolerability, and Efficacy of the First-in-Class Wee1 Inhibitor Adavosertib (AZD1775) as Monotherapy in Patients with Advanced Solid Tumors. Target. Oncol. 2023, 18, 517–530. [Google Scholar] [CrossRef]
- Maldonado, E.; Rathmell, W.K.; Shapiro, G.I.; Takebe, N.; Rodon, J.; Mahalingam, D.; Trikalinos, N.A.; Kalebasty, A.R.; Parikh, M.; Boerner, S.A.; et al. A Phase II Trial of the WEE1 Inhibitor Adavosertib in SETD2-Altered Advanced Solid Tumor Malignancies (NCI 10170). Cancer Res. Commun. 2024, 4, 1793–1801. [Google Scholar] [CrossRef]
- Fu, S.; Yao, S.; Yuan, Y.; Previs, R.A.; Elias, A.D.; Carvajal, R.D.; George, T.J.; Yuan, Y.; Yu, L.; Westin, S.N.; et al. Multicenter Phase II Trial of the WEE1 Inhibitor Adavosertib in Refractory Solid Tumors Harboring CCNE1 Amplification. J. Clin. Oncol. 2023, 41, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Scott, R. The WEE1 Inhibitor APR-1051 Shows Early Safety and Tolerability in Advanced, Mutated Solid Tumors. Available online: https://www.onclive.com/view/the-wee1-inhibitor-apr-1051-shows-early-safety-and-tolerability-in-advanced-mutated-solid-tumors (accessed on 5 June 2025).
- Keenan, T.E.; Li, T.; Vallius, T.; Guerriero, J.L.; Tayob, N.; Kochupurakkal, B.; Davis, J.; Pastorello, R.; Tahara, R.K.; Anderson, L.; et al. Clinical Efficacy and Molecular Response Correlates of the WEE1 Inhibitor Adavosertib Combined with Cisplatin in Patients with Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Falchook, G.S.; Wang, J.S.; Imedio, E.R.; Kumar, S.; Miah, K.; Mugundu, G.M.; Jones, S.F.; Spigel, D.R.; Hamilton, E.P. Open-Label, Multicenter, Phase I Study to Assess Safety and Tolerability of Adavosertib Plus Durvalumab in Patients with Advanced Solid Tumors. Target. Oncol. 2025, 20, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Leijen, S.; van Geel, R.M.; Pavlick, A.C.; Tibes, R.; Rosen, L.; Razak, A.R.; Lam, R.; Demuth, T.; Rose, S.; Lee, M.A.; et al. Phase I Study Evaluating WEE1 Inhibitor AZD1775 As Monotherapy and in Combination With Gemcitabine, Cisplatin, or Carboplatin in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2016, 34, 4371–4380. [Google Scholar] [CrossRef]
- Guertin, A.D.; Li, J.; Liu, Y.; Hurd, M.S.; Schuller, A.G.; Long, B.; Hirsch, H.A.; Feldman, I.; Benita, Y.; Toniatti, C.; et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol. Cancer Ther. 2013, 12, 1442–1452. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Booher, R.N.; Kraker, A.; Lawrence, T.; Leopold, W.R.; Sun, Y. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res. 2001, 61, 8211–8217. [Google Scholar]
- Du, X.; Li, J.; Luo, X.; Li, R.; Li, F.; Zhang, Y.; Shi, J.; He, J. Structure-activity relationships of Wee1 inhibitors: A review. Eur. J. Med. Chem. 2020, 203, 112524. [Google Scholar] [CrossRef]
- Panek, R.L.; Lu, G.H.; Klutchko, S.R.; Batley, B.L.; Dahring, T.K.; Hamby, J.M.; Hallak, H.; Doherty, A.M.; Keiser, J.A. In vitro pharmacological characterization of PD 166285, a new nanomolar potent and broadly active protein tyrosine kinase inhibitor. J. Pharmacol. Exp. Ther. 1997, 283, 1433–1444. [Google Scholar] [CrossRef]
- Palmer, B.D.; Thompson, A.M.; Booth, R.J.; Dobrusin, E.M.; Kraker, A.J.; Lee, H.H.; Lunney, E.A.; Mitchell, L.H.; Ortwine, D.F.; Smaill, J.B.; et al. 4-Phenylpyrrolo [3,4-c]carbazole-1,3(2H,6H)-dione inhibitors of the checkpoint kinase Wee1. Structure-activity relationships for chromophore modification and phenyl ring substitution. J. Med. Chem. 2006, 49, 4896–4911. [Google Scholar] [CrossRef]
- Huang, P.Q.; Boren, B.C.; Hegde, S.G.; Liu, H.; Unni, A.K.; Abraham, S.; Hopkins, C.D.; Paliwal, S.; Samatar, A.A.; Li, J.; et al. Discovery of ZN-c3, a Highly Potent and Selective Wee1 Inhibitor Undergoing Evaluation in Clinical Trials for the Treatment of Cancer. J. Med. Chem. 2021, 64, 13004–13024. [Google Scholar] [CrossRef]
- Lin, C.C.; Grewal, J.S.; Sommerhalder, D.; Strauss, J.F.; Bai, L.Y.; Shen, L.; Yeh, Y.M.; Hsieh, C.Y.; Cai, S.X.; Tian, Y.E.; et al. A phase 1 dose-escalation and -expansion study of IMP7068, a WEE1 inhibitor, in patients with advanced solid tumors. In Proceedings of the ASCO Annual Meeting, Chicago, IL, USA, 2 June 2022. [Google Scholar]
- Cai, S.X.; Ma, N.; Wang, X.; Jiang, Y.; Zhang, H.; Guo, M.; Zhou, R.; Tian, Y.E. Abstract 3091: Discovery and development of a potent and highly selective WEE1 inhibitor IMP7068. Cancer Res 2023, 83, 3091. [Google Scholar] [CrossRef]
- Yang, C.; Li, Z.; Li, Q.; Xia, Y.; Chan, C.C.; Yuan, X.; Wang, Y.; Chen, S.; Qian, W. Preclinical evaluation of SC0191, a small molecule inhibitor of Wee1 kinase. In Proceedings of the 2020 ASCO Annual Meeting, Virtually, 25 May 2020. [Google Scholar]
- Aprea Announces Preclinical Data Supporting Highly Differentiated WEE1 Inhibitor, ATRN-1051, Relative to Other WEE1 Inhibitors. Available online: https://www.globenewswire.com/news-release/2023/09/11/2740731/0/en/Aprea-Announces-Preclinical-Data-Sup-porting-Highly-Differentiated-WEE1-Inhibitor-ATRN-1051-Relative-To-Other-WEE1-Inhibitors.html (accessed on 5 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Harish, R.; Elahi, N.; Saini, S.; Telia, A.; Kundlas, M.; Koroleva, A.; Umoh, I.N.; Lota, M.; Bilkhu, M.; et al. Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update. Int. J. Mol. Sci. 2025, 26, 5701. https://doi.org/10.3390/ijms26125701
Zhang Z, Harish R, Elahi N, Saini S, Telia A, Kundlas M, Koroleva A, Umoh IN, Lota M, Bilkhu M, et al. Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update. International Journal of Molecular Sciences. 2025; 26(12):5701. https://doi.org/10.3390/ijms26125701
Chicago/Turabian StyleZhang, Zhao, Ritika Harish, Naveed Elahi, Sawanjit Saini, Aamir Telia, Manjit Kundlas, Allexes Koroleva, Israel N. Umoh, Manpreet Lota, Meha Bilkhu, and et al. 2025. "Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update" International Journal of Molecular Sciences 26, no. 12: 5701. https://doi.org/10.3390/ijms26125701
APA StyleZhang, Z., Harish, R., Elahi, N., Saini, S., Telia, A., Kundlas, M., Koroleva, A., Umoh, I. N., Lota, M., Bilkhu, M., Kawaiah, A., Allala, M. R., Leukeu, A., Nebuwa, E., Sharifi, N., Ashton, A. W., Jiao, X., & Pestell, R. G. (2025). Targeting WEE1 Kinase for Breast Cancer Therapeutics: An Update. International Journal of Molecular Sciences, 26(12), 5701. https://doi.org/10.3390/ijms26125701