Whole-Exome Sequencing Analysis of Inflammatory Bowel Disease-Associated Serrated Dysplasia
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| CRC | Colorectal carcinoma |
| IBD | Inflammatory bowel disease |
| NOS | Not otherwise specified |
| PSC | Primary sclerosing cholangitis |
| SSL | Sessile serrated lesion |
| TSA | Traditional serrated adenoma |
| UC | Ulcerative colitis |
| WES | Whole-exome sequencing |
References
- Choi, W.T.; Yozu, M.; Miller, G.C.; Shih, A.R.; Kumarasinghe, P.; Misdraji, J.; Harpaz, N.; Lauwers, G.Y. Nonconventional Dysplasia in Patients with Inflammatory Bowel Disease and Colorectal Carcinoma: A Multicenter Clinicopathologic Study. Mod. Pathol. 2020, 33, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rabinovitch, P.S.; Mattis, A.N.; Lauwers, G.Y.; Choi, W.T. Non-conventional dysplasia in inflammatory bowel disease is more frequently associated with advanced neoplasia and aneuploidy than conventional dysplasia. Histopathology 2021, 78, 814–830. [Google Scholar] [CrossRef]
- Xiao, A.; Yozu, M.; Kővári, B.P.; Yassan, L.; Liao, X.; Salomao, M.; Westerhoff, M.; Sejben, A.; Lauwers, G.Y.; Choi, W.T. Nonconventional Dysplasia is Frequently Associated with Goblet Cell Deficient and Serrated Variants of Colonic Adenocarcinoma in Inflammatory Bowel Disease. Am. J. Surg. Pathol. 2024, 48, 691–698. [Google Scholar] [CrossRef]
- Bahceci, D.; Sejben, A.; Yassan, L.; Miller, G.; Liao, X.; Ko, H.M.; Salomao, M.; Yozu, M.; Lauwers, G.Y.; Choi, W.T. Inflammatory bowel disease-associated serrated lesions with dysplasia are frequently associated with advanced neoplasia: Supporting a unified classification approach. Histopathology 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Harpaz, N.; McBride, R.B.; Cui, M.; Ye, F.; Zhang, D.; Ullmann, T.A.; Polydorides, A. Serrated colorectal polyps in inflammatory bowel disease. Mod. Pathol. 2015, 28, 1584–1593. [Google Scholar] [CrossRef]
- Miller, G.C.; Liu, C.; Bettington, M.L.; Leggett, B.; Whitehall, V.L.J.; Rosty, C. Traditional serrated adenoma-like lesions in patients with inflammatory bowel disease. Hum. Pathol. 2020, 97, 19–28. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.E.; Nagtegaal, I.D.; Vos, S.; van der Prost, R.S.; van Herwaarden, Y.; Derikx, L.A.A.P.; Hoentjen, F. Increased Colorectal Neoplasia Risk in Patients with Inflammatory Bowel Disease and Serrated Polyps with Dysplasia. Dig. Dis. Sci. 2022, 67, 5647–5656. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Kunisaki, R.; Shibata, W.; Ajioka, Y.; Hirasawa, K.; Takase, A.; Chiba, S.; Inayama, Y.; Ueda, W.; Okawa, K.; et al. Serrated polyps in patients with ulcerative colitis: Unique clinicopathological and biological characteristics. PLoS ONE 2023, 18, e0282204. [Google Scholar] [CrossRef]
- Zhang, R.; Lauwers, G.Y.; Choi, W.T. Increased Risk of Non-Conventional and Invisible Dysplasias in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Disease. J. Crohns Colitis 2022, 16, 1825–1834. [Google Scholar] [CrossRef]
- Harpaz, N.; Goldblum, J.R.; Shepherd, N.A.; Riddell, R.H.; Rubio, C.A.; Vieth, M.; Wang, H.H.; Odze, R.D. Colorectal dysplasia in chronic inflammatory bowel disease: A contemporary consensus classification and interobserver study. Hum. Pathol. 2023, 138, 49–61. [Google Scholar] [CrossRef]
- Choi, W.T. Characteristics, Reporting, and Potential Clinical Significance of Nonconventional Dysplasia in Inflammatory Bowel Disease. Surg. Pathol. Clin. 2023, 16, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T.; Kővári, B.P.; Lauwers, G.Y. The Significance of Flat/Invisible Dysplasia and Nonconventional Dysplastic Subtypes in Inflammatory Bowel Disease: A Review of Their Morphologic, Clinicopathologic, and Molecular Characteristics. Adv. Anat. Pathol. 2022, 29, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T. Non-conventional dysplastic subtypes in inflammatory bowel disease: A review of their diagnostic characteristics and potential clinical implications. J. Pathol. Transl. Med. 2021, 55, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Kővári, B.; Brown, I.; Chaves, P.; Choi, W.T.; Clauditz, T.; Ghayouri, M.; Jiang, K.; Miller, G.C.; Nakanishi, Y.; et al. Non-conventional dysplasias of the tubular gut: A review and illustration of their histomorphological spectrum. Histopathology 2021, 78, 658–675. [Google Scholar] [CrossRef]
- Jung, P.; Kim, H.W.; Park, S.B.; Kang, D.H.; Choi, C.W.; Kim, S.J.; Nam, H.S.; Ryu, D.G.; Shin, D.H.; Na, J.Y.; et al. Clinical and endoscopic characteristics of sessile serrated lesions with dysplasia/carcinoma. Korean J. Intern. Med. 2023, 38, 349–361. [Google Scholar] [CrossRef]
- Cenaj, O.; Gibson, J.; Odze, R.D. Clinicopathologic and outcome study of sessile serrated adenomas/polyps with serrated versus intestinal dysplasia. Mod. Pathol. 2018, 31, 633–642. [Google Scholar] [CrossRef]
- Bettington, M.; Walker, N.; Rosty, C.; Brown, I.; Clouston, A.; McKeone, D.; Pearson, S.A.; Leggett, B.; Whitehall, V. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut 2017, 66, 97–106. [Google Scholar] [CrossRef]
- Liu, C.; Walker, N.I.; Leggett, B.A.; Whitehall, V.L.; Bettington, M.L.; Rosty, C. Sessile serrated adenomas with dysplasia: Morphological patterns and correlations with MLH1 immunohistochemistry. Mod. Pathol. 2017, 30, 1728–1738. [Google Scholar] [CrossRef]
- Murakami, T.; Sakamoto, N.; Ritsuno, H.; Shibuya, T.; Osada, T.; Mitomi, H.; Yao, T.; Watanabe, S. Distinct endoscopic characteristics of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. Gastrointest. Endosc. 2017, 85, 590–600. [Google Scholar] [CrossRef]
- Lash, R.H.; Genta, R.M.; Schuler, C.M. Sessile serrated adenomas: Prevalence of dysplasia and carcinoma in 2139 patients. J. Clin. Pathol. 2010, 63, 681–686. [Google Scholar] [CrossRef]
- Murakami, T.; Akazawa, Y.; Yatagai, N.; Hiromoto, T.; Sasahara, N.; Saito, T.; Sakamoto, N.; Nagahara, A.; Yao, T. Molecular characterization of sessile serrated adenoma/polyps with dysplasia/carcinoma based on immunohistochemistry, next-generation sequencing, and microsatellite instability testing: A case series study. Diagn. Pathol. 2018, 13, 88. [Google Scholar] [CrossRef]
- Kawai, T.; Nyuya, A.; Mori, Y.; Tanaka, T.; Tanioka, H.; Yasui, K.; Toshima, T.; Taniguchi, F.; Shigeyashu, K.; Umeda, Y.; et al. Clinical and epigenetic features of colorectal cancer patients with somatic POLE proofreading mutations. Clin. Epigenetics 2021, 13, 117. [Google Scholar] [CrossRef]
- Hu, H.; Cai, W.; Wu, D.; Hu, W.; Wang, L.D.; Mao, J.; Zheng, S.; Ge, W. Ultra-mutated colorectal cancer patients with POLE driver mutations exhibit distinct clinical patterns. Cancer Med. 2021, 10, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Ansari, A.A.; Kim, J.; Kim, D.; Chun, S.M.; Kim, J.; Kim, T.W.; Park, I.; Yu, C.S.; Jang, S.J. The somatic POLE P286R mutation defines a unique subclass of colorectal cancer featuring hypermutation, representing a potential genomic biomarker for immunotherapy. Oncotarget 2016, 7, 68638–68649. [Google Scholar] [CrossRef]
- Pancsa, T.; Vasas, B.; Melegh, Z.; Tóth, E.; Torday, L.; Sejben, A. POLE-Mutant Colon Adenocarcinoma-Case Presentation and Histopathological Evaluation. J. Gastrointest. Cancer 2024, 55, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Lenz, H.J.; Marshall, J.L.; Arguello, D.; Raghavan, D.; Korn, W.M.; Weinberg, W.A.; Poorman, K.; Heeke, A.L.; Philip, P.A.; et al. Impact of Patient Age on Molecular Alterations of Left-Sided Colorectal Tumors. Oncologist 2019, 24, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Tanteles, G.A.; Nicolaou, M.; Neocleous, V.; Shammas, C.; Loizidou, M.A.; Alexandrou, A.; Ellina, E.; Patsia, N.; Sismani, C.; Phylactou, L.A.; et al. Genetic screening of EXT1 and EXT2 in Cypriot families with hereditary multiple osteochondromas. J. Genet. 2015, 94, 749–754. [Google Scholar]
- Day, F.L.; Jorissen, R.N.; Lipton, L.; Mouradov, D.; Shakthianandeswaren, A.; Christie, M.; Li, S.; Tsui, C.; Tie, J.; Deasi, J.; et al. PIK3CA and PTEN gene and exon mutation-specific clinicopathologic and molecular associations in colorectal cancer. Clin. Cancer Res. 2013, 19, 3285–3296. [Google Scholar] [CrossRef]
- Wen, K.W.; Umetsu, S.E.; Goldblum, J.R.; Gill, R.M.; Kim, G.E.; Joseph, N.M.; Rabinovitch, P.S.; Kakar, S.; Lauwers, G.Y.; Choi, W.T. DNA flow cytometric and interobserver study of crypt cell atypia in inflammatory bowel disease. Histopathology 2019, 75, 578–588. [Google Scholar] [CrossRef]
- Choi, W.T.; Salomao, M.; Zhao, L.; Alpert, L.; Setia, N.; Liao, X.; Drage, M.G.; Westerhoff, M.; Cheng, J.; Lauwers, G.Y.; et al. Hypermucinous, Goblet Cell Deficient, and Crypt Cell Dysplasias in Inflammatory Bowel Disease are Often Associated with Flat/Invisible Endoscopic Appearance and Advanced Neoplasia on Follow-Up. J. Crohns Colitis 2022, 16, 98–108. [Google Scholar] [CrossRef]
- Bahceci, D.; Lauwers, G.Y.; Choi, W.T. Clinicopathologic features of undetected dysplasia found in total colectomy or proctocolectomy specimens of patients with inflammatory bowel disease. Histopathology 2022, 81, 183–191. [Google Scholar] [CrossRef]
- Zhang, R.; Rabinovitch, P.S.; Mattis, A.N.; Lauwers, G.Y.; Choi, W.T. DNA content abnormality frequently develops in the right/proximal colon in patients with primary sclerosing cholangitis and inflammatory bowel disease and is highly predictive of subsequent detection of dysplasia. Histopathology 2023, 83, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, E.D.; Wang, D.; Lauwers, G.Y.; Choi, W.T. Increased histologic inflammation is an independent risk factor for nonconventional dysplasia in ulcerative colitis. Histopathology 2022, 81, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Lang-Schwarz, C.; Büttner-Herold, M.; Burian, S.; Erber, R.; Hartmann, A.; Jesinghaus, M.; Kamarádová, K.; Rubio, C.A.; Seitz, G.; Sterlacci, W.; et al. Morphological subtypes of colorectal low-grade intraepithelial neoplasia: Diagnostic reproducibility, frequency and clinical impact. J. Clin. Pathol. 2025, 78, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Sweeney, C.; Herrick, J.; Albertsen, H.; Levin, T.R.; Murtaugh, M.A.; Wolff, R.K.; Slattery, M.L. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005, 65, 6063–6069. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.K.; Jayachandran, P.; Koong, A.C.; Chang, D.T.; Kwok, S.; Ma, L.; Arber, D.A.; Balise, R.R.; Tubbs, R.R.; Shadrach, B.; et al. BRAF-mutated, microsatellite-stable adenocarcinoma of the proximal colon: An aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am. J. Surg. Pathol. 2012, 36, 744–752. [Google Scholar] [CrossRef]
- Bond, C.E.; Umapathy, A.; Ramsnes, I.; Greco, S.A.; Zhao, Z.Z.; Mallitt, K.A.; Buttenshaw, R.L.; Montgomery, G.W.; Leggett, B.A.; Whitehall, V.L.J.; et al. p53 mutation is common in microsatellite stable, BRAF mutant colorectal cancers. Int. J. Cancer 2012, 130, 1567–1576. [Google Scholar] [CrossRef]
- Linkowska, K.; Jawień, A.; Marszałek, A.; Malyarchuk, B.A.; Tońska, K.; Bartnik, E.; Skonieczna, K.; Grzybowski, T. Mitochondrial DNA Polymerase γ Mutations and Their Implications in mtDNA Alterations in Colorectal Cancer. Ann. Hum. Genet. 2015, 79, 320–328. [Google Scholar] [CrossRef]
- Maiuri, A.R.; Li, H.; Stein, B.D.; Tennessen, J.M.; O’Hagan, H.M. Inflammation-induced DNA methylation of DNA polymerase gamma alters the metabolic profile of colon tumors. Cancer Metab. 2018, 6, 9. [Google Scholar] [CrossRef]
- Balajthy, Z.; Szaszák, P.; Almási, S.; Lantos, T.; Sejben, A. Evaluation of dysplasias associated with inflammatory bowel disease-a single-center, retrospective, 5-year experience. Pathol. Oncol. Res. 2025, 31, 1612105. [Google Scholar] [CrossRef]
- Almási, S.; Balajthy, Z.; Baráth, B.; Török, Z.K.; Szaszák, P.; Lantos, T.; Kpvári, B.; Sejben, A. Examination of non-conventional dysplasias adjacent to colorectal adenocarcinoma in patients with IBD. Pathol. Oncol. Res. 2025, 30, 1611978. [Google Scholar] [CrossRef] [PubMed]
- OncoKB Database. Available online: https://www.oncokb.org/ (accessed on 9 June 2025).
- ClinVar Database. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 9 June 2025).
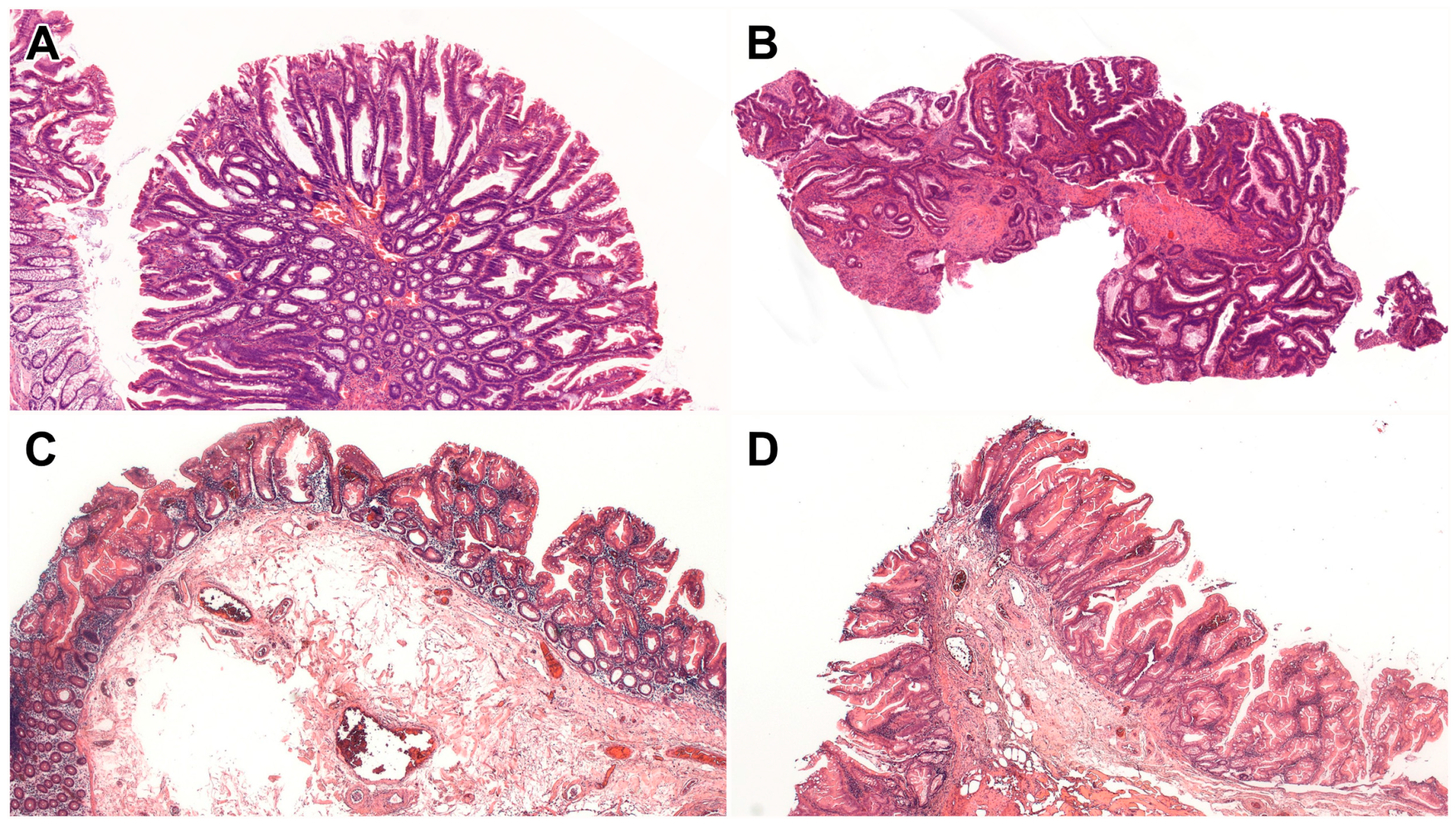
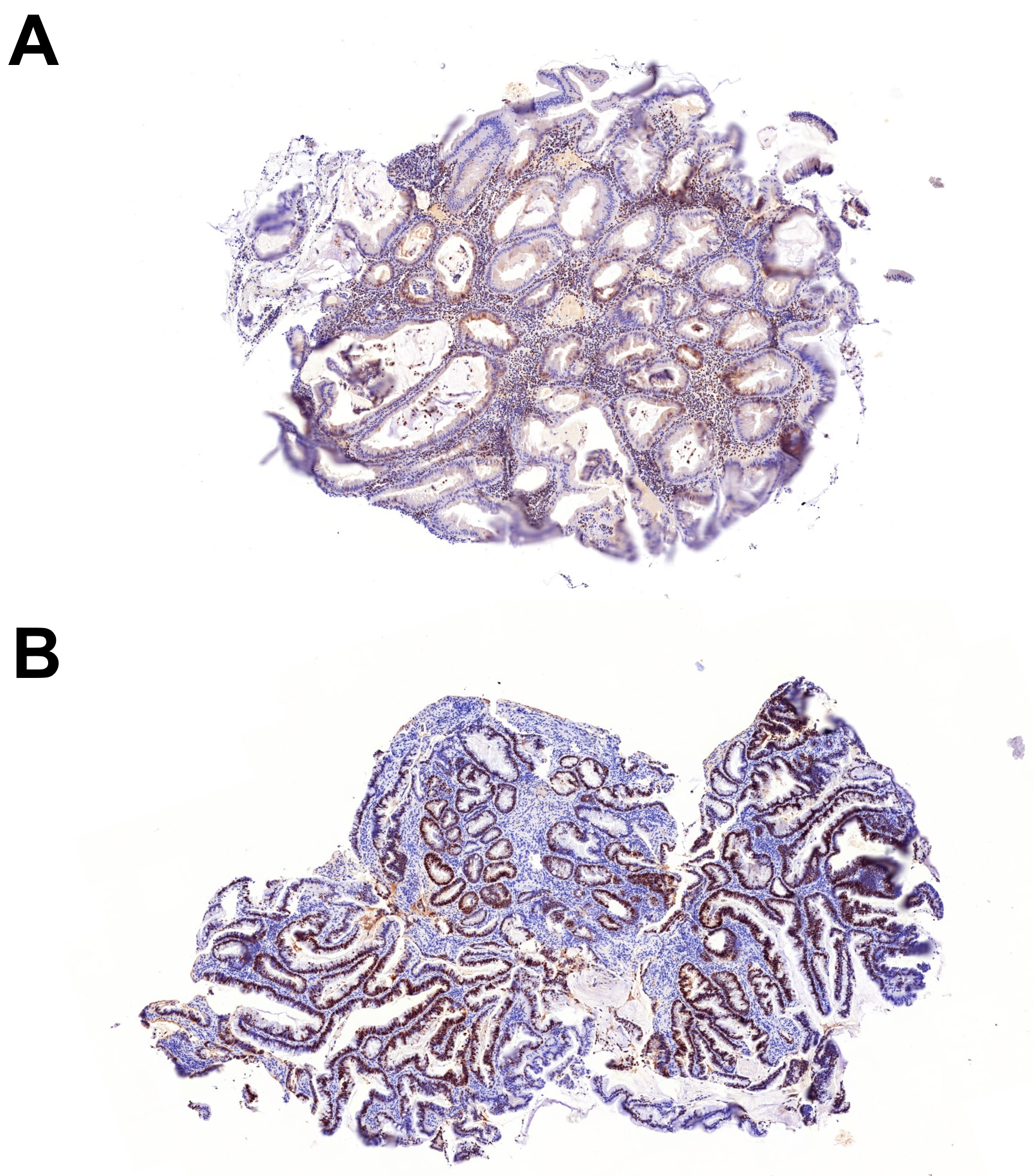
| Serrated dysplasia (n = 13, from 11 patients) | |
|---|---|
| Mean age (years, range) | 56 (35–71) |
| Male gender (%) | 8 (73%) |
| IBD subtype (%) | 10 UC (91%) 1 CD (9%) |
| Extent of IBD (%) | 7 pancolitis (64%) 4 left-sided (36%) |
| Mean duration of IBD (years, range) | 26 (5–59) |
| Primary sclerosing cholangitis (%) | 0 (0%) |
| Subtype of serrated dysplasia (%) | 5 SSL-like dysplasia (38%) 1 TSA-like dysplasia (8%) 6 serrated dysplasia, NOS (46%) 1 mixed SSL-like/TSA-like dysplasia (8%) |
| Location of dysplasia (%) | 9 left (69%) 4 right (31%) |
| Endoscopic appearance of dysplasia (%) | 11 polypoid (85%) 2 flat (15%) |
| Mean size of dysplasia (cm, range) | 0.8 (0.2–2.5) |
| Histologic grade of dysplasia (%) | 8 LGD (62%) 5 HGD (38%) |
| Association with conventional dysplasia (%) | 3 patients (27%) |
| Association with nonconventional dysplasia (%) | 3 patients (27%) |
| Association with CRC (%) | 5 patients (45%) |
| SSL-like Dysplasia (n = 5, from 5 Patients) | Serrated Dysplasia NOS (n = 6, from 5 Patients) | TSA-like Dysplasia (n = 1, from 1 Patient) | Mixed SSL-like/TSA-like Dysplasia (n = 1, from 1 Patient) | |
|---|---|---|---|---|
| Mean age (years, range) | 56 (35–71) | 52 (36–63) | 53 | 71 |
| Male gender (%) | 3 (60%) | 4 (80%) | 1 (100%) | 1 (100%) |
| IBD subtype (%) | 5 UC (100%) 0 CD (0%) | 4 UC (80%) 1 CD (20%) | 1 UC (100%) 0 CD (0%) | 1 UC (100%) 0 CD (0%) |
| Extent of IBD (%) | 4 Pancolitis (80%) 1 Left-sided (20%) | 3 Pancolitis (60%) 2 Left-sided (40%) | 0 Pancolitis (0%) 1 Left-sided (100%) | 1 Pancolitis (100%) 0 Left-sided (0%) |
| Mean duration of IBD (years, range) | 31 (5–59) | 30 (7–47) | 44 | 8 |
| Location of dysplasia (%) | 3 Left (60%) 2 Right (40%) | 5 Left (83%) 1 Right (17%) | 1 Left (100%) 0 Right (0%) | 0 Left (0%) 1 Right (100%) |
| Endoscopic appearance of dysplasia (%) | 5 Polypoid (100%) 0 Flat (0%) | 5 Polypoid (83%) 1 Flat (17%) | 1 Polypoid (100%) 0 Flat (0%) | 0 Polypoid (0%) 1 Flat (100%) |
| Mean size of dysplasia (cm, range) | 0.5 (0.2–0.9) | 1.0 (0.3–2.5) | 0.6 | 0.5 |
| Histologic grade of dysplasia (%) | 4 LGD (80%) 1 HGD (20%) | 3 LGD (50%) 3 HGD (50%) | 0 LGD (0%) 1 HGD (100%) | 1 LGD (100%) 0 HGD (0%) |
| Association with conventional dysplasia (%) | 1 Patient with TA-like dysplasia (20%) | 1 Patient with TVA-like dysplasia (20%) | 0 Patient (0%) | 1 Patient with TA-like dysplasia (100%) |
| Association with nonconventional dysplasia (%) | 1 Patient with mixed SSL-like/TSA-like dysplasia (20%) | 2 Patients with hypermucinous dysplasia (40%) 1 Patient with goblet cell-deficient dysplasia (20%) | 0 Patient (0%) | 1 Patient with SSL-like dysplasia (100%) |
| Association with CRC (%) | 1 Patient (20%) | 3 Patients (60%) | 1 Patient (100%) | 0 Patient (0%) |
| Patient # | Subtype | Pathogenic Mutations | Likely Pathogenic Mutations |
|---|---|---|---|
| 1 | SSL-like dysplasia | BRAF, ATR | KMT2C |
| 3 | SSL-like dysplasia | EXT1 | POLE, CDKN1B |
| 4 | Serrated dysplasia NOS | - | KMT2C, MAX, CDC6 |
| 5 | SSL-like dysplasia | KRAS, PTEN, TSC1 | MLH1, DICER1, HIF1A, ASXL1, ZNF292, EXT1, ACVR2A, KMT2C, MGA, SETD1B, TGFBR2 |
| 6 (lesion #1) | SSL-like dysplasia | MUTYH, MADD | SERPINB4 |
| 6 (lesion #2) | Mixed SSL-like/TSA-like dysplasia | MUTYH, MADD | - |
| 8 | Serrated dysplasia NOS | - | TP53, BRAF, MPL, EXT1 |
| 11 | Serrated dysplasia NOS | POLG | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balajthy, Z.; Almási, S.; Lantos, T.; Kuthi, L.; Deftereos, G.; Choi, W.-T.; Sejben, A. Whole-Exome Sequencing Analysis of Inflammatory Bowel Disease-Associated Serrated Dysplasia. Int. J. Mol. Sci. 2025, 26, 5704. https://doi.org/10.3390/ijms26125704
Balajthy Z, Almási S, Lantos T, Kuthi L, Deftereos G, Choi W-T, Sejben A. Whole-Exome Sequencing Analysis of Inflammatory Bowel Disease-Associated Serrated Dysplasia. International Journal of Molecular Sciences. 2025; 26(12):5704. https://doi.org/10.3390/ijms26125704
Chicago/Turabian StyleBalajthy, Zsófia, Szintia Almási, Tamás Lantos, Levente Kuthi, Georgios Deftereos, Won-Tak Choi, and Anita Sejben. 2025. "Whole-Exome Sequencing Analysis of Inflammatory Bowel Disease-Associated Serrated Dysplasia" International Journal of Molecular Sciences 26, no. 12: 5704. https://doi.org/10.3390/ijms26125704
APA StyleBalajthy, Z., Almási, S., Lantos, T., Kuthi, L., Deftereos, G., Choi, W.-T., & Sejben, A. (2025). Whole-Exome Sequencing Analysis of Inflammatory Bowel Disease-Associated Serrated Dysplasia. International Journal of Molecular Sciences, 26(12), 5704. https://doi.org/10.3390/ijms26125704








