Anoectochilus burmannicus Extract Rescues Aging-Related Phenotypes in Drosophila Susceptible to Oxidative Stress-Induced Senescence
Abstract
1. Introduction
2. Results
2.1. The Effect of Anoctochillus burmannicus Ethanolic Extract (ABE) on the Lifespan Extension and the Locomotor Activity Improvement in a Drosophila Aging Model
2.2. The Suppressive Effect of ABE on Age-Dependent Accumulation of Abnormal Protein Aggregates and Expression of a Marker Gene Induced by Oxidative Stress in Drosophila Adult Muscle
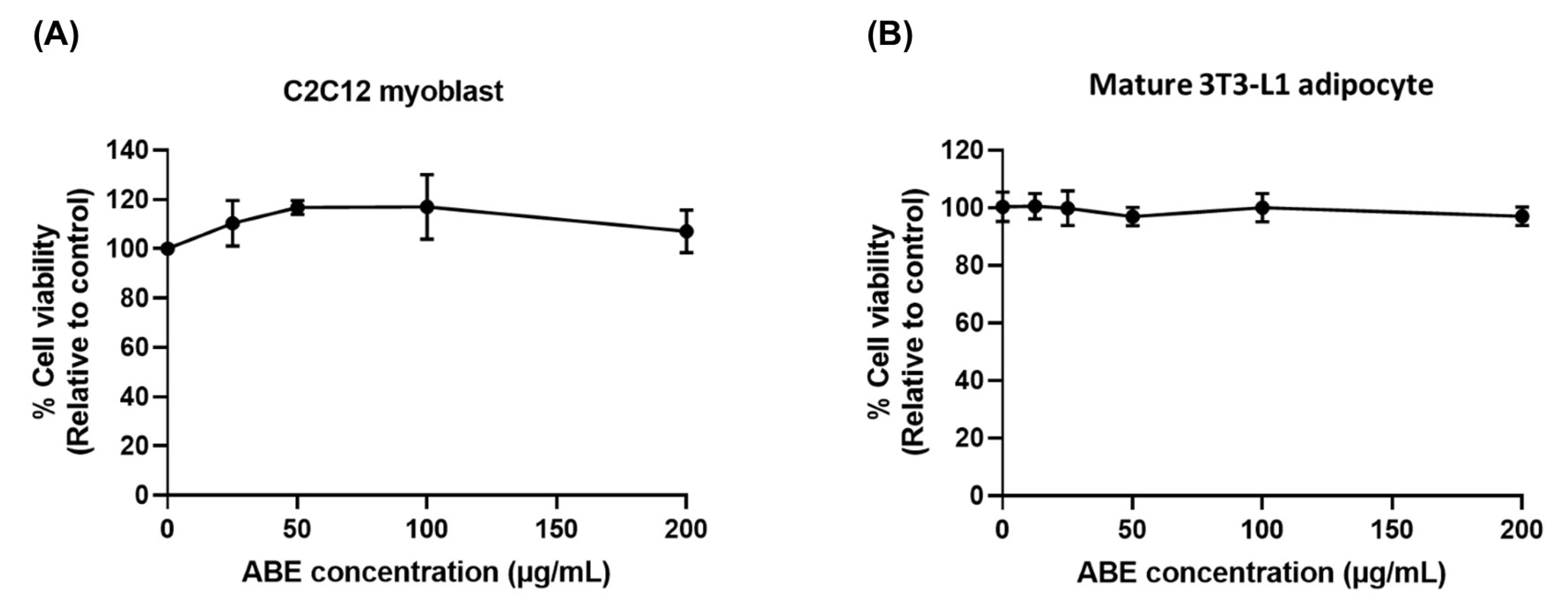
2.3. Cytotoxicity of ABE on C2C12 Myoblasts and 3T3-L1 Adipocytes
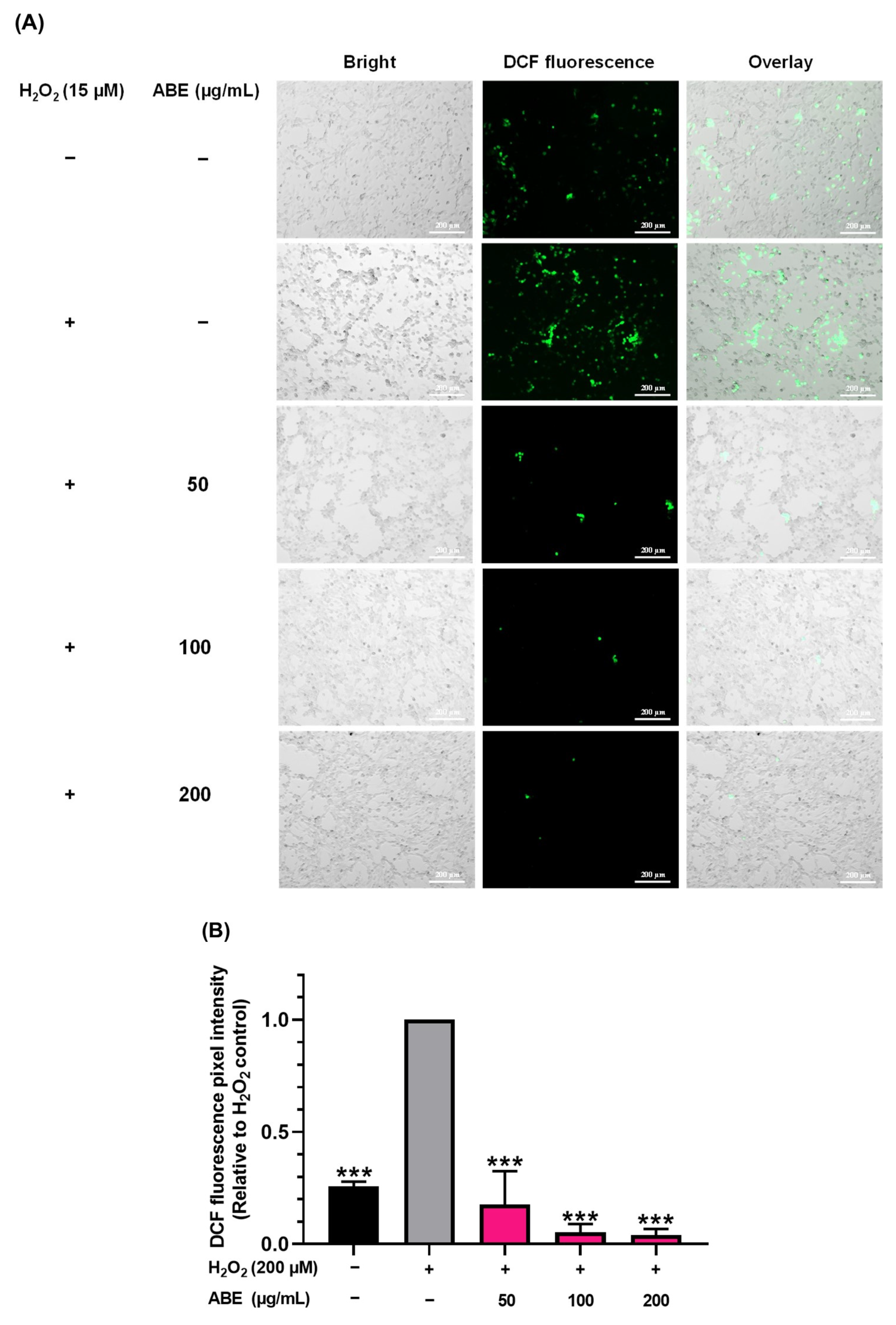
2.4. The Inhibitory Effect of ABE on Cellular ROS Production Induced by H2O2 in C2C12 Myoblasts
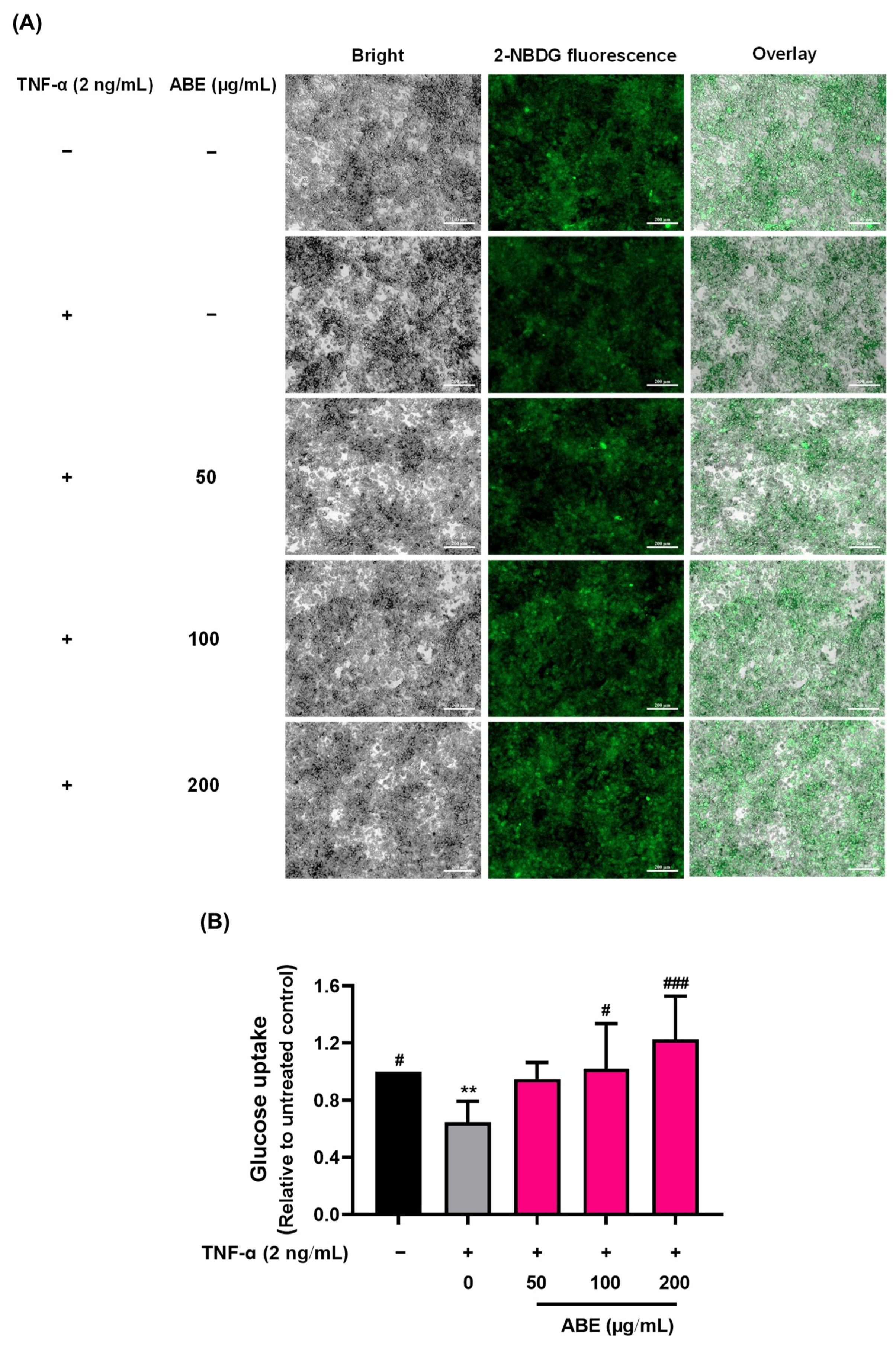
2.5. The Protective Effect of ABE on Inflammation-Induced Insulin Resistance in TNF-α Treated 3T3-L1 Adipocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Anoctochillus burmannicus Ethanolic Extract
4.3. Drosophila Melanogaster Stock
4.4. Lifespan Assay
4.5. Climbing Assay
4.6. Ubiquitinated Protein Aggregates Accumulation in the Muscle of the Adults
4.7. Oxidative Response Gene Expression by RT-qPCR
4.8. Cell Culture
4.8.1. Maturation of 3T3-L1 Adipocytes
4.8.2. Cell Culture of C2C12 Myoblast Cell Line
4.9. Cytotoxicity of Anoctochillus burmannicus Ethanolic Extract (ABE)
4.10. Detection of Intracellular ROS Levels in C2C12 Myoblast
4.11. Determination of Anti-Insulin Resistance Activity by Cellular Glucose Uptake Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging—Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Löw, P. The role of ubiquitin–proteasome system in ageing. Gen. Comp. Endocrinol. 2011, 172, 39–43. [Google Scholar] [CrossRef]
- Vernace, V.A.; Schmidt-Glenewinkel, T.; Figueiredo-Pereira, M.E. Aging and regulated protein degradation: Who has the UPPer hand? Aging Cell 2007, 6, 599–606. [Google Scholar] [CrossRef]
- Taylor, R.C.; Dillin, A. Aging as an Event of Proteostasis Collapse. Cold Spring Harb. Perspect. Biol. 2011, 3, a004440. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Schulz, J.; Mukherjee, A.; Park, K.-W.; Armijo, E.; Soto, C. Extensive accumulation of misfolded protein aggregates during natural aging and senescence. Front. Aging Neurosci. 2023, 14, 1090109. [Google Scholar] [CrossRef]
- Starke-Reed, P.E.; Oliver, C.N. Protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989, 275, 559–567. [Google Scholar] [CrossRef]
- Oliver, C.; Ahn, B.; Moerman, E.; Goldstein, S.; Stadtman, E. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Stadtman, E.R.; Floyd, R.A.; Markesbery, W.R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10540–10543. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Agarwal, S.; Dubey, A.; Orr, W.C. Protein oxidative damage is associated with life expectancy of houseflies. Proc. Natl. Acad. Sci. USA 1993, 90, 7255–7259. [Google Scholar] [CrossRef]
- Friguet, B.; Bulteau, A.; Chondrogianni, N.; Conconi, M.; Petropoulos, I. Protein Degradation by the Proteasome and Its Implications in Aging. Ann. N. Y. Acad. Sci. 2000, 908, 143–154. [Google Scholar] [CrossRef]
- Noto, S. Perspectives on Aging and Quality of Life. Healthcare 2023, 11, 2131. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, R. Aging adipose tissue, insulin resistance, and type 2 diabetes. Biogerontology 2024, 25, 53–69. [Google Scholar] [CrossRef]
- Wu, C.; Yu, P.; Sun, R. Adipose tissue and age-dependent insulin resistance: New insights into WAT browning (Review). Int. J. Mol. Med. 2021, 47, 1–10. [Google Scholar] [CrossRef]
- Pitchakarn, P.; Inthachat, W.; Karinchai, J.; Temviriyanukul, P. Human Hazard Assessment Using Drosophila Wing Spot Test as an Alternative In Vivo Model for Genotoxicity Testing—A Review. Int. J. Mol. Sci. 2021, 22, 9932. [Google Scholar] [CrossRef]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Model. Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef]
- White, K.E.; Humphrey, D.M.; Hirth, F. The Dopaminergic System in the Aging Brain of Drosophila. Front. Neurosci. 2010, 4, 205. [Google Scholar] [CrossRef]
- Shafiq, K.; Sanghai, N.; Guo, Y.; Kong, J. Implication of post-translationally modified SOD1 in pathological aging. GeroScience 2021, 43, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Le, T.D.; Nakahara, Y.; Ueda, M.; Okumura, K.; Hirai, J.; Sato, Y.; Takemoto, D.; Tomimori, N.; Ono, Y.; Nakai, M.; et al. Sesamin suppresses aging phenotypes in adult muscular and nervous systems and intestines in a Drosophila senescence-accelerated model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Yifeng, Z.; Yoshihiro, H.I.; Nagi, K.; Masaki, F.; Eri, O.; Kenji, S. Phenethylamine in hot water extract of Chlorella pyrenoidosa expands lifespan of SOD1 mutant adults of Drosophila melanogaster at very low dose. J. Food Bioact. 2020, 9, 52–57. [Google Scholar] [CrossRef]
- Suzuta, S.; Nishida, H.; Ozaki, M.; Kohno, N.; Le, T.D.; Inoue, Y.H. Metformin suppresses progression of muscle aging via activation of the AMP kinase-mediated pathways in Drosophila adults. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8039–8056. [Google Scholar] [CrossRef]
- Oka, S.; Hirai, J.; Yasukawa, T.; Nakahara, Y.; Inoue, Y.H. A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 2015, 16, 485–501. [Google Scholar] [CrossRef]
- Le, T.; Inoue, Y. Sesamin Activates Nrf2/Cnc-Dependent Transcription in the Absence of Oxidative Stress in Drosophila Adult Brains. Antioxidants 2021, 10, 924. [Google Scholar] [CrossRef]
- Zuo, Y.; Peng, C.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Chen, Z.-Y. Sesamin extends the mean lifespan of fruit flies. Biogerontology 2013, 14, 107–119. [Google Scholar] [CrossRef]
- Moneim, A.E.A.; Al-Quraishy, S.; Dkhil, M.A. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int. J. Nanomed. 2015, 10, 6741–6756. [Google Scholar] [CrossRef]
- Gogoi, K.; Borah, R.L.; Das, R.; Yonzone, R. Present Status of Orchid Species Diversity Resources of Joypur Reserve Forest of Dibrugarh District (Assam) of North East India. Int. J. Mod. Bot. 2012, 2, 47–67. [Google Scholar] [CrossRef]
- Ye, S.; Shao, Q.; Zhang, A. Anoectochilus roxburghii: A review of its phytochemistry, pharmacology, and clinical applications. J. Ethnopharmacol. 2017, 209, 184–202. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010, 4, 592–638. [Google Scholar]
- Bon, T.; Trieu, H.; Phung, D.; Nguyên, C.; Ha, D.; Anh, N.; Son, H.; Long, T.; Tuyen, P.; Ninh, V.; et al. Medicinal Plant, Anoectochilus: Distribution, Ecology, Commercial Value and Use in North Vietnam. J. Pharm. Res. Int 2020, 32, 84–92. [Google Scholar] [CrossRef]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances—An overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef]
- Zhang, F.-S.; Lv, Y.-L.; Zhao, Y.; Guo, S.-X. Promoting role of an endophyte on the growth and contents of kinsenosides and flavonoids of Anoectochilus formosanus Hayata, a rare and threatened medicinal Orchidaceae plant. J. Zhejiang Univ. B 2013, 14, 785–792. [Google Scholar] [CrossRef]
- Mukherjee, S.; Jagtap, S. Use of Orchids in treating Diabetes and related diseases: A review. J. Phytopharm. 2020, 9, 130–138. [Google Scholar] [CrossRef]
- Medicine, F.I.o.T.C. Record of Fujian Materia Medica; Fujian Science and Technology Press Fuzhou: Fuzhou, China, 1982. [Google Scholar]
- Du, X.-M.; Sun, N.-Y.; Tamura, T.; Mohri, A.; Sugiura, M.; Yoshizawa, T.; Irino, N.; Hayashi, J.; Shoyama, Y. Higher Yielding Isolation of Kinsenoside in Anoectochilus and Its Anti-hyperliposis Effect. Biol. Pharm. Bull. 2001, 24, 65–69. [Google Scholar] [CrossRef]
- Du, X.-M.; Irino, N.; Furusho, N.; Hayashi, J.; Shoyama, Y. Pharmacologically active compounds in the Anoectochilus and Goodyera species. J. Nat. Med. 2008, 62, 132–148. [Google Scholar] [CrossRef]
- Shih, C.; Wu, Y.; Lin, W. Antihyperglycaemic And Anti-Oxidant Properties Of Anoectochilus Formosanus in Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 684–688. [Google Scholar] [CrossRef]
- Du, X.M.; Sun, N.Y.; Hayashi, J.; Chen, Y.; Sugiura, M.; Shoyama, Y. Hepatoprotective and antihyperliposis activities of in vitro cultured Anoectochilus formosanus. Phytother. Res. 2003, 17, 30–33. [Google Scholar] [CrossRef]
- Cui, S.-C.; Yu, J.; Zhang, X.-H.; Cheng, M.-Z.; Yang, L.-W.; Xu, J.-Y. Antihyperglycemic and antioxidant activity of water extract from Anoectochilus roxburghii in experimental diabetes. Exp. Toxicol. Pathol. 2013, 65, 485–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Ruan, H.; Pi, H.; Wu, J. Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J. Ethnopharmacol. 2007, 114, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Budluang, P.; Pitchakarn, P.; Ting, P.; Temviriyanukul, P.; Wongnoppawich, A.; Imsumran, A. Anti-inflammatory and anti-insulin resistance activities of aqueous extract from Anoectochilus burmannicus. Food Sci. Nutr. 2017, 5, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Karinchai, J.; Budluang, P.; Temviriyanukul, P.; Ting, P.; Nuchuchua, O.; Wongnoppavich, A.; Imsumran, A.; Pitchakarn, P. Bioassay-guided study of the anti-inflammatory effect of Anoectochilus burmannicus ethanolic extract in RAW 264.7 cells. J. Ethnopharmacol. 2021, 280, 114452. [Google Scholar] [CrossRef] [PubMed]
- Buacheen, P.; Karinchai, J.; Kammasit, N.; Temviriyanukul, P.; Butkinaree, C.; Watthana, S.; Wongnoppavich, A.; Imsumran, A.; Pitchakarn, P. Protective effect of Anoectochilus burmannicus extracts and its active compound, kinsenoside on adipocyte differentiation induced by benzyl butyl phthalate and bisphenol A. Sci. Rep. 2023, 13, 1–10. [Google Scholar] [CrossRef]
- Lévy, E.; El Banna, N.; Baïlle, D.; Heneman-Masurel, A.; Truchet, S.; Rezaei, H.; Huang, M.-E.; Béringue, V.; Martin, D.; Vernis, L. Causative Links between Protein Aggregation and Oxidative Stress: A Review. Int. J. Mol. Sci. 2019, 20, 3896. [Google Scholar] [CrossRef]
- Zou, S.; Meadows, S.; Sharp, L.; Jan, L.Y.; Jan, Y.N. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13726–13731. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. The oxidative damage theory of aging. Clin. Neurosci. Res. 2003, 2, 305–315. [Google Scholar] [CrossRef]
- Ali, S.S.; Xiong, C.; Lucero, J.; Behrens, M.M.; Dugan, L.L.; Quick, K.L. Gender differences in free radical homeostasis during aging: Shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 2006, 5, 565–574. [Google Scholar] [CrossRef]
- Rizvi, F.; Preston, C.C.; Emelyanova, L.; Yousufuddin, M.; Viqar, M.; Dakwar, O.; Ross, G.R.; Faustino, R.S.; Holmuhamedov, E.L.; Jahangir, A. Effects of Aging on Cardiac Oxidative Stress and Transcriptional Changes in Pathways of Reactive Oxygen Species Generation and Clearance. J. Am. Hear. Assoc. 2021, 10, e019948. [Google Scholar] [CrossRef]
- Bejma, J.; Ji, L.L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999, 87, 465–470. [Google Scholar] [CrossRef]
- Porto, M.L.; Rodrigues, B.P.; Menezes, T.N.; Ceschim, S.L.; Casarini, D.E.; Gava, A.L.; Pereira, T.M.C.; Vasquez, E.C.; Campagnaro, B.P.; Meyrelles, S.S. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J. Biomed. Sci. 2015, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Honma, T.; Tsuduki, T.; Sugawara, S.; Kitano, Y.; Ito, J.; Kijima, R.; Tsubata, M.; Nakagawa, K.; Miyazawa, T. Aging decreases antioxidant effects and increases lipid peroxidation in the Apolipoprotein E deficient mouse. J. Clin. Biochem. Nutr. 2013, 52, 234–240. [Google Scholar] [CrossRef]
- Adiga, U.; Adiga, S. Total Antioxidant Activity in Old age. Biomed. Res. 2008, 19, 185–186. [Google Scholar]
- Dasgupta, A.; Klein, K. Chapter 1—Introduction to Free Radicals and the Body’s Antioxidant Defense. In Antioxidants in Food, Vitamins and Supplements; Dasgupta, A., Klein, K., Eds.; Elsevier: San Diego, CA, USA, 2014; pp. 1–18. [Google Scholar]
- Zhang, Y.; Ikeno, Y.; Qi, W.; Chaudhuri, A.; Li, Y.; Bokov, A.; Thorpe, S.R.; Baynes, J.W.; Epstein, C.; Richardson, A.; et al. Mice Deficient in Both Mn Superoxide Dismutase and Glutathione Peroxidase-1 Have Increased Oxidative Damage and a Greater Incidence of Pathology but No Reduction in Longevity. J. Gerontol. Ser. A 2009, 64A, 1212–1220. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Lee, K.-Y.; Lee, H.-W.; Kim, J.-H.; Kim, T.-Y. SOD3 Variant, R213G, Altered SOD3 Function, Leading to ROS-Mediated Inflammation and Damage in Multiple Organs of Premature Aging Mice. Antioxid. Redox Signal. 2015, 23, 985–999. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Elchuri, S.; Oberley, T.D.; Qi, W.; Eisenstein, R.S.; Roberts, L.J.; Van Remmen, H.; Epstein, C.J.; Huang, T.-T. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2004, 24, 367–380. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Vlase, L.; Popa, D.-S. Antioxidants in Age-Related Diseases and Anti-Aging Strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef]
- Buacheen, P.; Karinchai, J.; Inthachat, W.; Butkinaree, C.; Jankam, C.; Wongnoppavich, A.; Imsumran, A.; Chewonarin, T.; Pimpha, N.; Temviriyanukul, P.; et al. The Toxicological Assessment of Anoectochilus burmannicus Ethanolic-Extract-Synthesized Selenium Nanoparticles Using Cell Culture, Bacteria, and Drosophila melanogaster as Suitable Models. Nanomaterials 2023, 13, 2804. [Google Scholar] [CrossRef]
- Phillips, J.P.; Campbell, S.D.; Michaud, D.; Charbonneau, M.; Hilliker, A.J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. USA 1989, 86, 2761–2765. [Google Scholar] [CrossRef]
- Phillips, J.P.; A Tainer, J.; Getzoff, E.D.; Boulianne, G.L.; Kirby, K.; Hilliker, A.J. Subunit-destabilizing mutations in Drosophila copper/zinc superoxide dismutase: Neuropathology and a model of dimer dysequilibrium. Proc. Natl. Acad. Sci. USA 1995, 92, 8574–8578. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, S.; De Lazzari, F.; Mrakic-Sposta, S.; Vezzoli, A.; Zordan, M.A.; Bisaglia, M.; Menti, G.M.; Meda, N.; Frighetto, G.; Bosco, G.; et al. Lifespan and ROS levels in different Drosophila melanogaster strains after 24 h hypoxia exposure. Biol. Open 2022, 11, bio059386. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y.; Zhang, J.; Fan, L.; Huang, Y.; Tan, T.; Ho, L. Artemisia argyi extract exerts antioxidant properties and extends the lifespan of Drosophila melanogaster. J. Sci. Food Agric. 2024, 104, 3926–3935. [Google Scholar] [CrossRef]
- Zakharenko, L.P.; Bobrovskikh, M.A.; Gruntenko, N.E.; Petrovskii, D.V.; Verevkin, E.G.; Putilov, A.A. Two Old Wild-Type Strains of Drosophila melanogaster Can Serve as an Animal Model of Faster and Slower Aging Processes. Insects 2024, 15, 329. [Google Scholar] [CrossRef]
- Martinez, V.; Javadi, C.; Ngo, E.; Ngo, L.; Lagow, R.; Zhang, B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev. Neurobiol. 2007, 67, 778–791. [Google Scholar] [CrossRef]
- Yusuf, A.O.; Danborno, B.; Bauchi, Z.M.; Sani, D.; Ndams, I.S. Aging impaired locomotor and biochemical activities in Drosophila melanogaster Oregon R (fruit fly) model. Exp. Gerontol. 2024, 197, 112593. [Google Scholar] [CrossRef]
- Neckameyer, W.S.; Woodrome, S.; Holt, B.; Mayer, A. Dopamine and senescence in Drosophila melanogaster. Neurobiol. Aging 2000, 21, 145–152. [Google Scholar] [CrossRef]
- Riemensperger, T.; Isabel, G.; Coulom, H.; Neuser, K.; Seugnet, L.; Kume, K.; Iché-Torres, M.; Cassar, M.; Strauss, R.; Preat, T.; et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 2010, 108, 834–839. [Google Scholar] [CrossRef]
- Olguín, H.J.; Guzmán, D.C.; García, E.H.; Mejía, G.B.; Bulteau, A.-L. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2016, 9730467. [Google Scholar] [CrossRef]
- Martin, I.; Jones, M.A.; Grotewiel, M. Manipulation of Sod1 expression ubiquitously, but not in the nervous system or muscle, impacts age-related parameters in Drosophila. FEBS Lett. 2009, 583, 2308–2314. [Google Scholar] [CrossRef]
- Martin, I.; Jones, M.A.; Rhodenizer, D.; Zheng, J.; Warrick, J.M.; Seroude, L.; Grotewiel, M. Sod2 knockdown in the musculature has whole-organism consequences in Drosophila. Free. Radic. Biol. Med. 2009, 47, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Demontis, F.; Perrimon, N. FOXO/4E-BP Signaling in Drosophila Muscles Regulates Organism-wide Proteostasis during Aging. Cell 2010, 143, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Landis, G.; Shen, J.; Tower, J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging 2012, 4, 768–789. [Google Scholar] [CrossRef]
- Ceci, R.; Maldini, M.; Olson, M.E.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Duranti, G. Moringa oleifera Leaf Extract Protects C2C12 Myotubes against H2O2-Induced Oxidative Stress. Antioxidants 2022, 11, 1435. [Google Scholar] [CrossRef]
- Ou, M.-Y.; Zhang, H.; Tan, P.-C.; Zhou, S.-B.; Li, Q.-F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Findeisen, H.M.; Pearson, K.J.; Gizard, F.; Zhao, Y.; Qing, H.; Jones, K.L.; Cohn, D.; Heywood, E.B.; de Cabo, R.; Bruemmer, D.; et al. Oxidative Stress Accumulates in Adipose Tissue during Aging and Inhibits Adipogenesis. PLoS ONE 2011, 6, e18532. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.; Lander, E. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Zhang, L.; Ebenezer, P.J.; Dasuri, K.; Fernandez-Kim, S.O.; Francis, J.; Mariappan, N.; Gao, Z.; Ye, J.; Bruce-Keller, A.J.; Keller, J.N. Aging is associated with hypoxia and oxidative stress in adipose tissue: Implications for adipose function. Am. J. Physiol. Metab. 2011, 301, E599–E607. [Google Scholar] [CrossRef]
- Han, C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef]
- Ahmed, B.; Si, H. The Aging of Adipocytes Increases Expression of Pro-Inflammatory Cytokines Chronologically. Metabolites 2021, 11, 292. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Chang, Q.; Wang, J.; Liu, J.; Lv, Y.; Wang, T.T.Y.; Gao, B.; Zhang, Y.; Yu, L.L. Inhibitory Effect of Piceatannol on TNF-α-Mediated Inflammation and Insulin Resistance in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2017, 65, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Al-Regaiey, K. Crosstalk between adipogenesis and aging: Role of polyphenols in combating adipogenic-associated aging. Immun. Ageing 2024, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Le, T.D.; Inoue, Y.H. Downregulating Mitochondrial DNA Polymerase γ in the Muscle Stimulated Autophagy, Apoptosis, and Muscle Aging-Related Phenotypes in Drosophila Adults. Biomolecules 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Yuk, H.J.; Kim, D.S. Effect of Jakyakgamcho-Tang Extracts on H2O2-Induced C2C12 Myoblasts. Molecules 2021, 26, 215. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buacheen, P.; Karinchai, J.; Inthachat, W.; Butkinaree, C.; Wongnoppavich, A.; Imsumran, A.; Temviriyanukul, P.; Inoue, Y.H.; Pitchakarn, P. Anoectochilus burmannicus Extract Rescues Aging-Related Phenotypes in Drosophila Susceptible to Oxidative Stress-Induced Senescence. Int. J. Mol. Sci. 2025, 26, 5694. https://doi.org/10.3390/ijms26125694
Buacheen P, Karinchai J, Inthachat W, Butkinaree C, Wongnoppavich A, Imsumran A, Temviriyanukul P, Inoue YH, Pitchakarn P. Anoectochilus burmannicus Extract Rescues Aging-Related Phenotypes in Drosophila Susceptible to Oxidative Stress-Induced Senescence. International Journal of Molecular Sciences. 2025; 26(12):5694. https://doi.org/10.3390/ijms26125694
Chicago/Turabian StyleBuacheen, Pensiri, Jirarat Karinchai, Woorawee Inthachat, Chutikarn Butkinaree, Ariyaphong Wongnoppavich, Arisa Imsumran, Piya Temviriyanukul, Yoshihiro H. Inoue, and Pornsiri Pitchakarn. 2025. "Anoectochilus burmannicus Extract Rescues Aging-Related Phenotypes in Drosophila Susceptible to Oxidative Stress-Induced Senescence" International Journal of Molecular Sciences 26, no. 12: 5694. https://doi.org/10.3390/ijms26125694
APA StyleBuacheen, P., Karinchai, J., Inthachat, W., Butkinaree, C., Wongnoppavich, A., Imsumran, A., Temviriyanukul, P., Inoue, Y. H., & Pitchakarn, P. (2025). Anoectochilus burmannicus Extract Rescues Aging-Related Phenotypes in Drosophila Susceptible to Oxidative Stress-Induced Senescence. International Journal of Molecular Sciences, 26(12), 5694. https://doi.org/10.3390/ijms26125694







_Bonness.jpeg)


