Effect of Warfarin on Lifespan and Oxidative Stress Tolerance of Drosophila melanogaster
Abstract
1. Introduction
2. Results
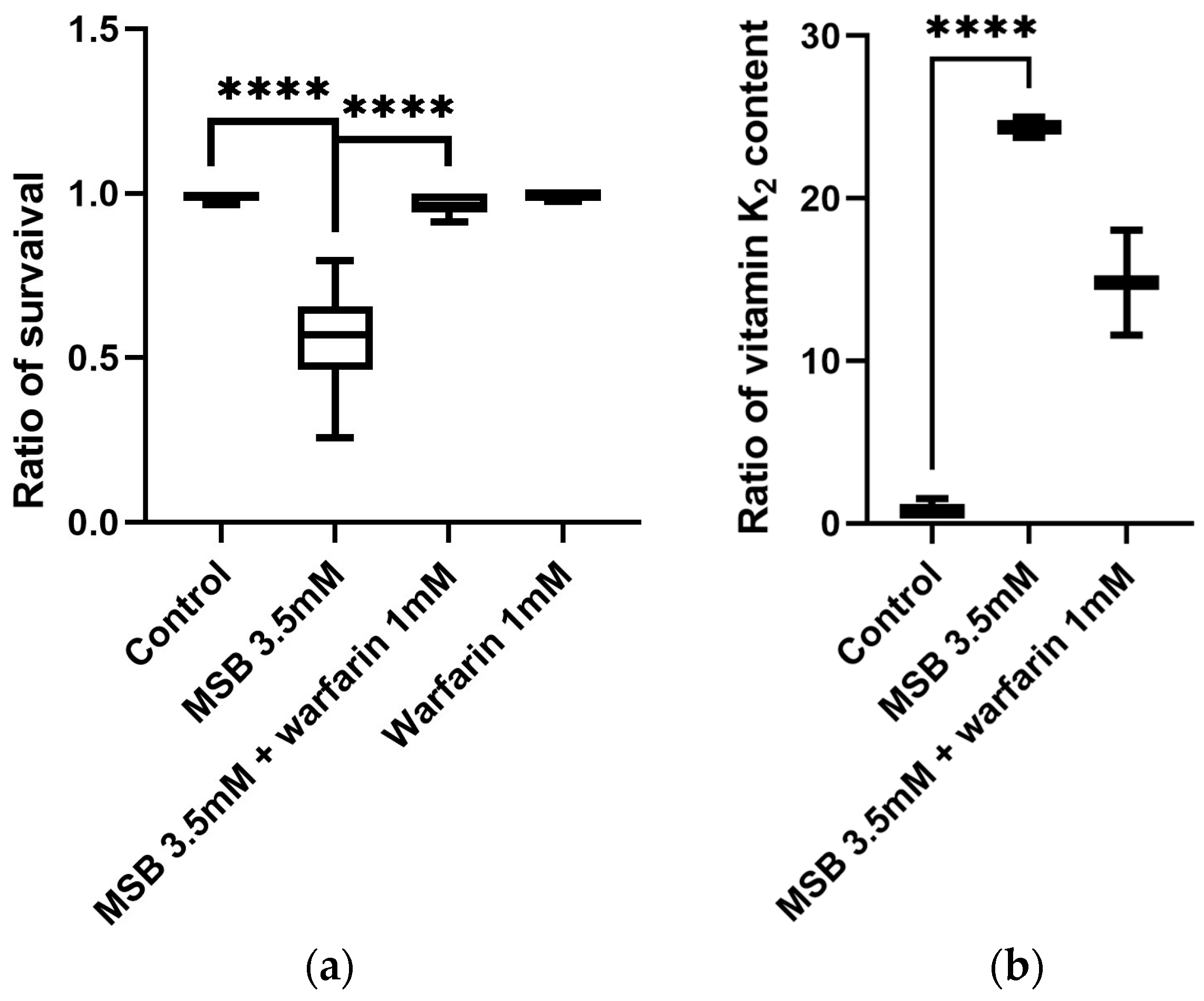
2.1. Concentration Effect of MSB and Warfarin on Survival of D. melanogaster
2.2. Vitamin K Content in D. melanogaster
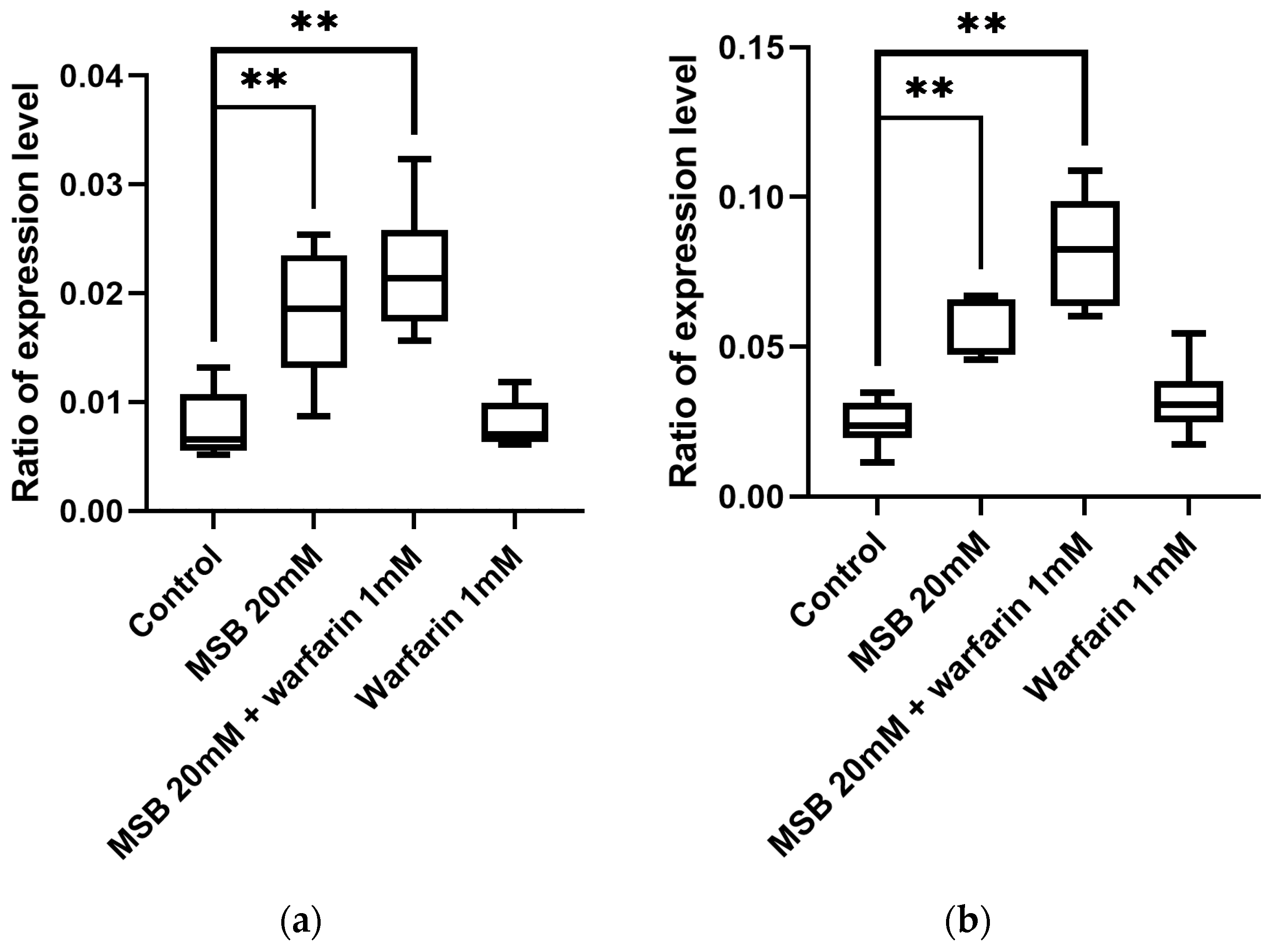
2.3. ROS Determination in Adult D. melanogaster Treated with High Doses of MSB
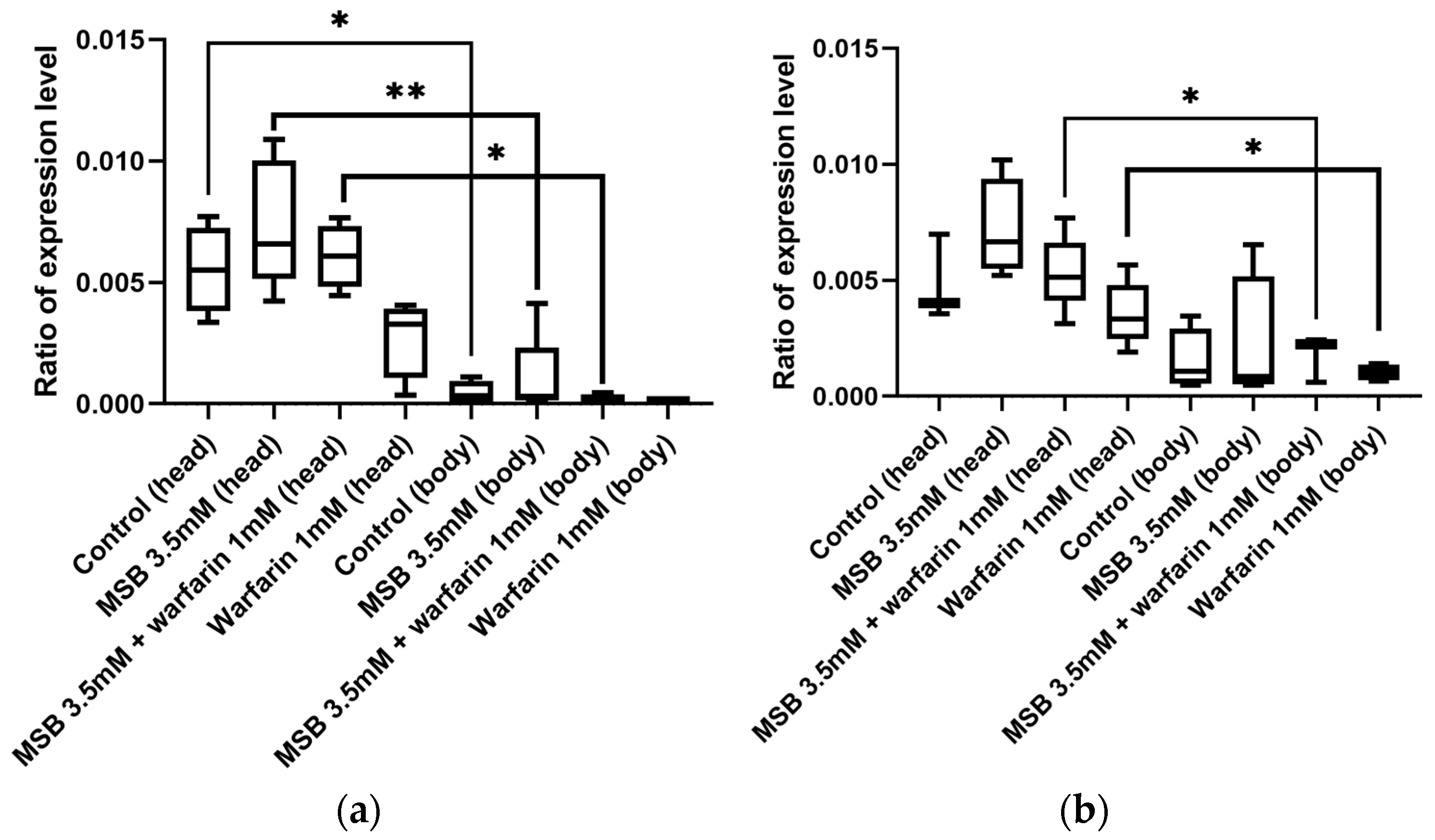
2.4. Gamma-Glutamyl Carboxylase Gene GC Expression in Adult D. melanogaster in Response to MSB and Warfarin
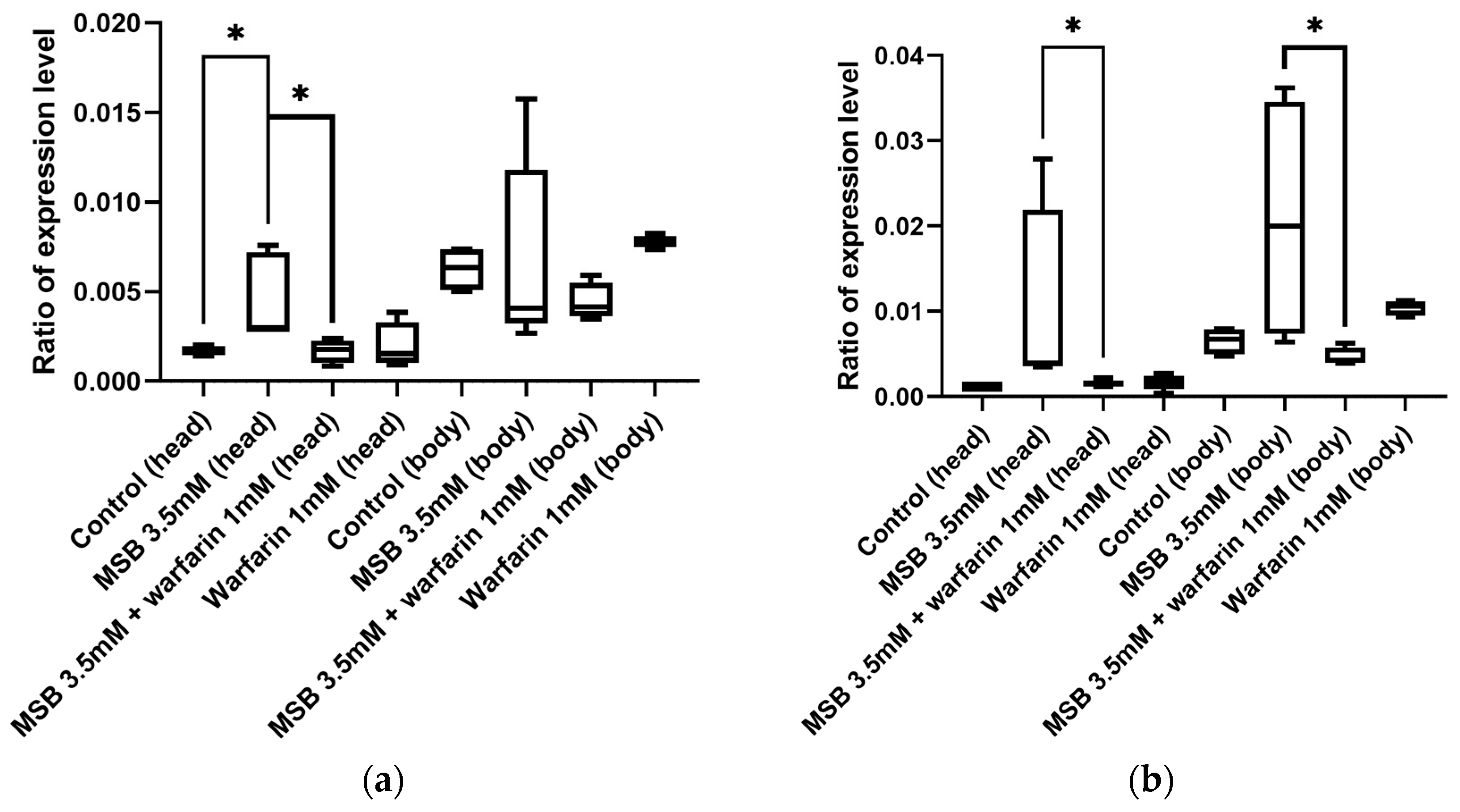
2.5. Expression Level of the Oxidative Stress Marker Gene hsp22 in D. melanogaster in Response to MSB and Warfarin
3. Discussion
4. Materials and Methods
4.1. Fruit Fly Cultivation Conditions and Viability Testing for Vitamin K and Warfarin Levels
4.2. Extraction of Vitamin K from D. melanogaster Adult Flies for Mass Spectrometry Analysis
4.3. LC-MS Analysis of Vitamin K of Fly Extract
4.4. Detection of ROS in D. melanogaster Homogenates by Electron Paramagnetic Resonance (EPR) Technique
4.5. Isolation of Total RNA and cDNA Synthesis
4.6. Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSB | Menadione sodium bisulfite |
| GGCX | Gamma-glutamyl carboxylase |
| VKOR | Vitamin K epoxide reductase |
| MRM | Multiple reaction monitoring |
| EPR | Electron paramagnetic resonance |
| ROS | Reactive oxygen species |
References
- Diachenko, A.I.; Rodin, I.A.; Krasnova, T.N.; Klychnikov, O.I.; Nefedova, L.N. The Role of Vitamin K in the Development of Neurodegenerative Diseases. Biochemistry 2024, 89, S57–S70. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Iwaki, M. Vitamin K1 (Phylloquinone) Restores the Turnover of FeS Centers in the Ether-Extracted Spinach PS I Particles. FEBS Lett. 1989, 243, 47–52. [Google Scholar] [CrossRef]
- Kurosu, M.; Begari, E. Vitamin K2 in Electron Transport System: Are Enzymes Involved in Vitamin K2 Biosynthesis Promising Drug Targets? Molecules 2010, 15, 1531–1553. [Google Scholar] [CrossRef]
- Friedman, P.A.; Przysiecki, C.T. Vitamin K-Dependent Carboxylation. Int. J. Biochem. 1987, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ferland, G. The Discovery of Vitamin K and Its Clinical Applications. Ann. Nutr. Metab. 2012, 61, 213–218. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of Emerging Vitamin K-dependent Proteins: Growth Arrest-specific Protein 6, Gla-rich Protein and Periostin (Review). Int. J. Mol. Med. 2021, 47, 2. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Garrett, J.E.; Shetty, R.P.; Keate, T.; Walker, C.S.; Olivera, B.M. Gamma -Glutamyl Carboxylation: An Extracellular Posttranslational Modification That Antedates the Divergence of Molluscs, Arthropods, and Chordates. Proc. Natl. Acad. Sci. USA 2002, 99, 1264–1269. [Google Scholar] [CrossRef]
- Stafford, D.W. The Vitamin K Cycle. J. Thromb. Haemost. 2005, 3, 1873–1878. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Jin, D.-Y.; Stafford, D.W.; Pedersen, L.G.; Tie, J.-K. Warfarin and Vitamin K Epoxide Reductase: A Molecular Accounting for Observed Inhibition. Blood 2018, 132, 647–657. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic Therapy for VTE Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e419S–e496S. [Google Scholar] [CrossRef]
- Czogalla, K.J.; Biswas, A.; Höning, K.; Hornung, V.; Liphardt, K.; Watzka, M.; Oldenburg, J. Warfarin and Vitamin K Compete for Binding to Phe55 in Human VKOR. Nat. Struct. Mol. Biol. 2017, 24, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Matagrin, B.; Hodroge, A.; Montagut-Romans, A.; Andru, J.; Fourel, I.; Besse, S.; Benoit, E.; Lattard, V. New Insights into the Catalytic Mechanism of Vitamin K Epoxide Reductase (VKORC1)—The Catalytic Properties of the Major Mutations of rVKORC1 Explain the Biological Cost Associated to Mutations. FEBS Open Bio 2013, 3, 144–150. [Google Scholar] [CrossRef]
- Puckett, R.M.; Offringa, M. Prophylactic Vitamin K for Vitamin K Deficiency Bleeding in Neonates. Cochrane Database Syst. Rev. 2000, 2000, CD002776. [Google Scholar] [CrossRef]
- Goffart, S.; Tikkanen, P.; Michell, C.; Wilson, T.; Pohjoismäki, J.L.O.L.O. The Type and Source of Reactive Oxygen Species Influences the Outcome of Oxidative Stress in Cultured Cells. Cells 2021, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Halilovic, A.; Schmedt, T.; Benischke, A.-S.; Hamill, C.; Chen, Y.; Santos, J.H.; Jurkunas, U.V. Menadione-Induced DNA Damage Leads to Mitochondrial Dysfunction and Fragmentation During Rosette Formation in Fuchs Endothelial Corneal Dystrophy. Antioxid. Redox Signal 2016, 24, 1072–1083. [Google Scholar] [CrossRef]
- Jordan, K.W.; Craver, K.L.; Magwire, M.M.; Cubilla, C.E.; Mackay, T.F.C.; Anholt, R.R.H. Genome-Wide Association for Sensitivity to Chronic Oxidative Stress in Drosophila Melanogaster. PLoS ONE 2012, 7, e38722. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione Triggers Cell Death through ROS-Dependent Mechanisms Involving PARP Activation without Requiring Apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef]
- Li, J.; Zuo, X.; Cheng, P.; Ren, X.; Sun, S.; Xu, J.; Holmgren, A.; Lu, J. The Production of Reactive Oxygen Species Enhanced with the Reduction of Menadione by Active Thioredoxin Reductase. Metallomics 2019, 11, 1490–1497. [Google Scholar] [CrossRef]
- Majiene, D.; Kuseliauskyte, J.; Stimbirys, A.; Jekabsone, A. Comparison of the Effect of Native 1,4-Naphthoquinones Plumbagin, Menadione, and Lawsone on Viability, Redox Status, and Mitochondrial Functions of C6 Glioblastoma Cells. Nutrients 2019, 11, 1294. [Google Scholar] [CrossRef]
- Gul, S.; Maqbool, M.F.; Maryam, A.; Khan, M.; Shakir, H.A.; Irfan, M.; Ara, C.; Li, Y.; Ma, T. Vitamin K: A Novel Cancer Chemosensitizer. Biotechnol. Appl. Biochem. 2022, 69, 2641–2657. [Google Scholar] [CrossRef]
- Westhofen, P.; Watzka, M.; Marinova, M.; Hass, M.; Kirfel, G.; Müller, J.; Bevans, C.G.; Müller, C.R.; Oldenburg, J. Human Vitamin K 2,3-Epoxide Reductase Complex Subunit 1-like 1 (VKORC1L1) Mediates Vitamin K-Dependent Intracellular Antioxidant Function. J. Biol. Chem. 2011, 286, 15085–15094. [Google Scholar] [CrossRef]
- Mukai, K.; Morimoto, H.; Kikuchi, S.; Nagaoka, S. Kinetic Study of Free-Radical-Scavenging Action of Biological Hydroquinones (Reduced Forms of Ubiquinone, Vitamin K and Tocopherol Quinone) in Solution. Biochim. Biophys. Acta 1993, 1157, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamin K2 Is a Mitochondrial Electron Carrier That Rescues Pink1 Deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Ferland, G.; Johnson, M.A.; Poon, L.W.; Scott, T.M.; Barbey, A.K.; Barger, K.; Wang, X.-D.; Johnson, E.J. Concentrations of Circulating Phylloquinone, but Not Cerebral Menaquinone-4, Are Positively Correlated with a Wide Range of Cognitive Measures: Exploratory Findings in Centenarians. J. Nutr. 2020, 150, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Kasten, M.; Vos, M.; König, I.R.; Schmid, S.M.; Wilms, B.; Klein, C.; Brüggemann, N. The Use of Vitamin K2 in Patients With Parkinson’s Disease and Mitochondrial Dysfunction (PD-K2): A Theranostic Pilot Study in a Placebo-Controlled Parallel Group Design. Front. Neurol. 2020, 11, 592104. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Clark, K.; Stevenson, B.J.; Rivier, J.E.; Olivera, B.M.; Golic, K.G.; Rong, Y.S. Biochemical Characterization of Drosophila Gamma-Glutamyl Carboxylase and Its Role in Fly Development. Insect Mol. Biol. 2006, 15, 147–156. [Google Scholar] [CrossRef]
- Li, T.; Yang, C.T.; Jin, D.; Stafford, D.W. Identification of a Drosophila Vitamin K-Dependent Gamma-Glutamyl Carboxylase. J. Biol. Chem. 2000, 275, 18291–18296. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Olivera, B.M.; Cruz, L.J.; Gray, W.R. Gamma-Carboxyglutamate in a Neuroactive Toxin. J. Biol. Chem. 1984, 259, 14343–14346. [Google Scholar] [CrossRef]
- Davies, M.J. Detection and Characterisation of Radicals Using Electron Paramagnetic Resonance (EPR) Spin Trapping and Related Methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron Paramagnetic Resonance Measurements of Reactive Oxygen Species by Cyclic Hydroxylamine Spin Probes. Antioxid. Redox Signal 2018, 28, 1433–1443. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Kirilyuk, I.A.; Voinov, M.; Grigor’ev, I.A. EPR Detection of Cellular and Mitochondrial Superoxide Using Cyclic Hydroxylamines. Free Radic. Res. 2011, 45, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Mockett, R.J.; Sohal, R.S.; Orr, W.C. Roles of Oxidative Stress in the Aging Process of Drosophila Melanogaster. In Oxidative Stress in Aging; Miwa, S., Beckman, K.B., Muller, F.L., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 111–128. ISBN 978-1-58829-991-8. [Google Scholar]
- MacMillan, H.A.; Hughson, B.N. A High-Throughput Method of Hemolymph Extraction from Adult Drosophila without Anesthesia. J. Insect Physiol. 2014, 63, 27–31. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moysés, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K Plasma Levels Determination in Human Health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Tsugawa, N.; Nakagawa, K.; Suhara, Y.; Tanaka, K.; Uchino, Y.; Takeuchi, A.; Sawada, N.; Kamao, M.; Wada, A.; et al. Menadione (Vitamin K3) Is a Catabolic Product of Oral Phylloquinone (Vitamin K1) in the Intestine and a Circulating Precursor of Tissue Menaquinone-4 (Vitamin K2) in Rats. J. Biol. Chem. 2013, 288, 33071–33080. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Newman, P. Recent Trends in the Metabolism and Cell Biology of Vitamin K with Special Reference to Vitamin K Cycling and MK-4 Biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef]
- Oswald, M.C.; Brooks, P.S.; Zwart, M.F.; Mukherjee, A.; West, R.J.; Giachello, C.N.; Morarach, K.; Baines, R.A.; Sweeney, S.T.; Landgraf, M. Reactive Oxygen Species Regulate Activity-Dependent Neuronal Plasticity in Drosophila. eLife 2018, 7, e39393. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Colledge, C.J.; Walker, C.S.; Zhou, L.M.; Hillyard, D.R.; Olivera, B.M. Conantokin-G Precursor and Its Role in Gamma-Carboxylation by a Vitamin K-Dependent Carboxylase from a Conus Snail. J. Biol. Chem. 1998, 273, 5447–5450. [Google Scholar] [CrossRef]
- Holford, N.H. Clinical Pharmacokinetics and Pharmacodynamics of Warfarin. Understanding the Dose-Effect Relationship. Clin. Pharmacokinet. 1986, 11, 483–504. [Google Scholar] [CrossRef]
- Sconce, E.; Avery, P.; Wynne, H.; Kamali, F. Vitamin K Supplementation Can Improve Stability of Anticoagulation for Patients with Unexplained Variability in Response to Warfarin. Blood 2007, 109, 2419–2423. [Google Scholar] [CrossRef]
- Schwab, M.; Schaeffeler, E. Warfarin Pharmacogenetics Meets Clinical Use. Blood 2011, 118, 2938–2939. [Google Scholar] [CrossRef]
- International Warfarin Pharmacogenetics Consortium; Klein, T.E.; Altman, R.B.; Eriksson, N.; Gage, B.F.; Kimmel, S.E.; Lee, M.-T.M.; Limdi, N.A.; Page, D.; Roden, D.M.; et al. Estimation of the Warfarin Dose with Clinical and Pharmacogenetic Data. N. Engl. J. Med. 2009, 360, 753–764. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrenova, A.; Klychnikov, O.; Ioutsi, V.; Rodin, I.; Luneva, O.; Nefedova, L. Effect of Warfarin on Lifespan and Oxidative Stress Tolerance of Drosophila melanogaster. Int. J. Mol. Sci. 2025, 26, 4808. https://doi.org/10.3390/ijms26104808
Lavrenova A, Klychnikov O, Ioutsi V, Rodin I, Luneva O, Nefedova L. Effect of Warfarin on Lifespan and Oxidative Stress Tolerance of Drosophila melanogaster. International Journal of Molecular Sciences. 2025; 26(10):4808. https://doi.org/10.3390/ijms26104808
Chicago/Turabian StyleLavrenova, Anna, Oleg Klychnikov, Vitaliy Ioutsi, Igor Rodin, Oksana Luneva, and Lidia Nefedova. 2025. "Effect of Warfarin on Lifespan and Oxidative Stress Tolerance of Drosophila melanogaster" International Journal of Molecular Sciences 26, no. 10: 4808. https://doi.org/10.3390/ijms26104808
APA StyleLavrenova, A., Klychnikov, O., Ioutsi, V., Rodin, I., Luneva, O., & Nefedova, L. (2025). Effect of Warfarin on Lifespan and Oxidative Stress Tolerance of Drosophila melanogaster. International Journal of Molecular Sciences, 26(10), 4808. https://doi.org/10.3390/ijms26104808








