Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors
Abstract
1. Introduction
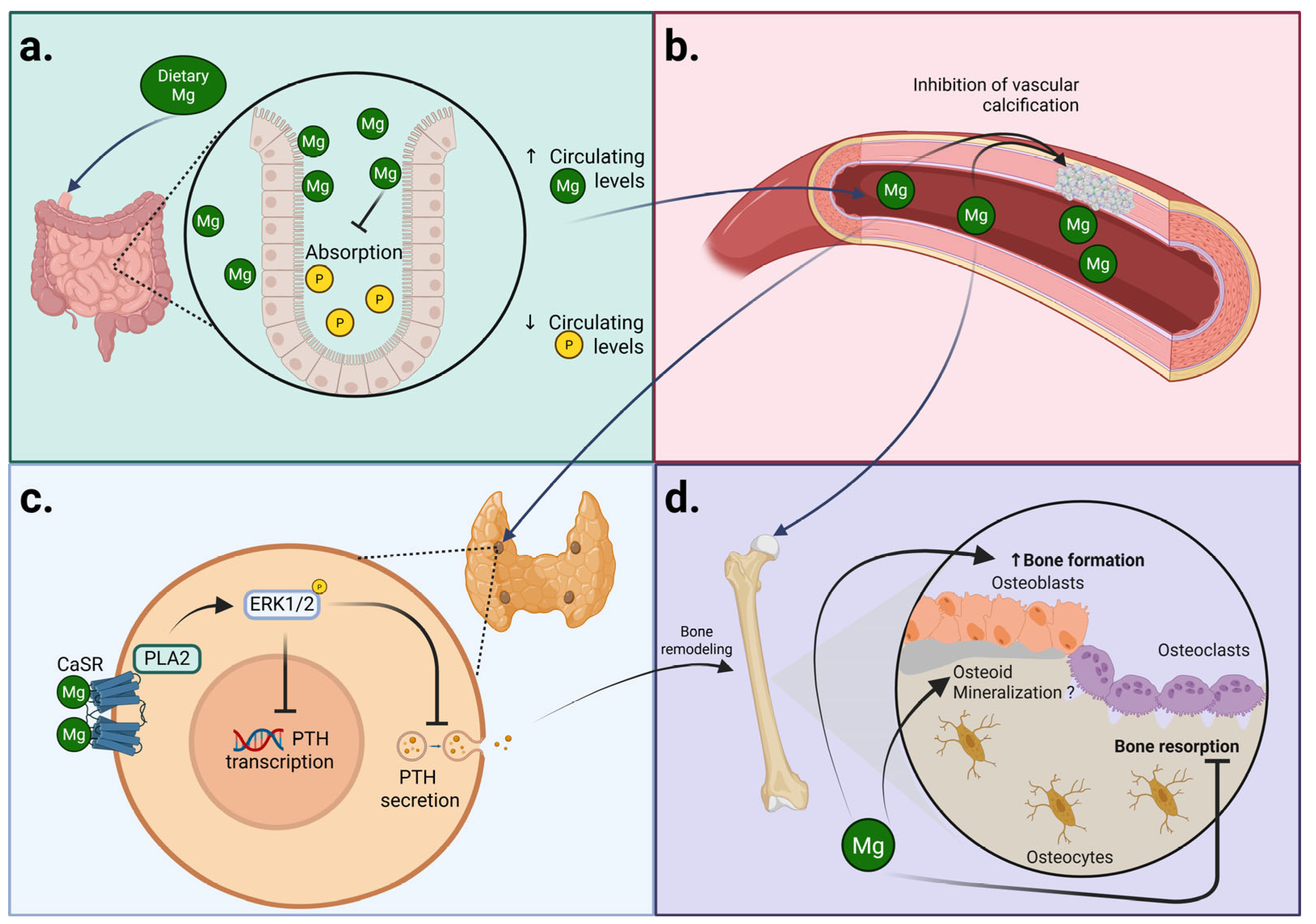
2. Magnesium and CKD-MBD
2.1. Magnesium and Secondary Hyperparathyroidism
2.1.1. Magnesium and Parathyroid Function
2.1.2. Magnesium and Clinical Management of Secondary Hyperparathyroidism
2.1.3. Risk of Hypermagnesemia
2.2. Magnesium and Bone Health
Magnesium Actions on Osteogenesis and Mineralization
2.3. Magnesium and Bone Homeostasis
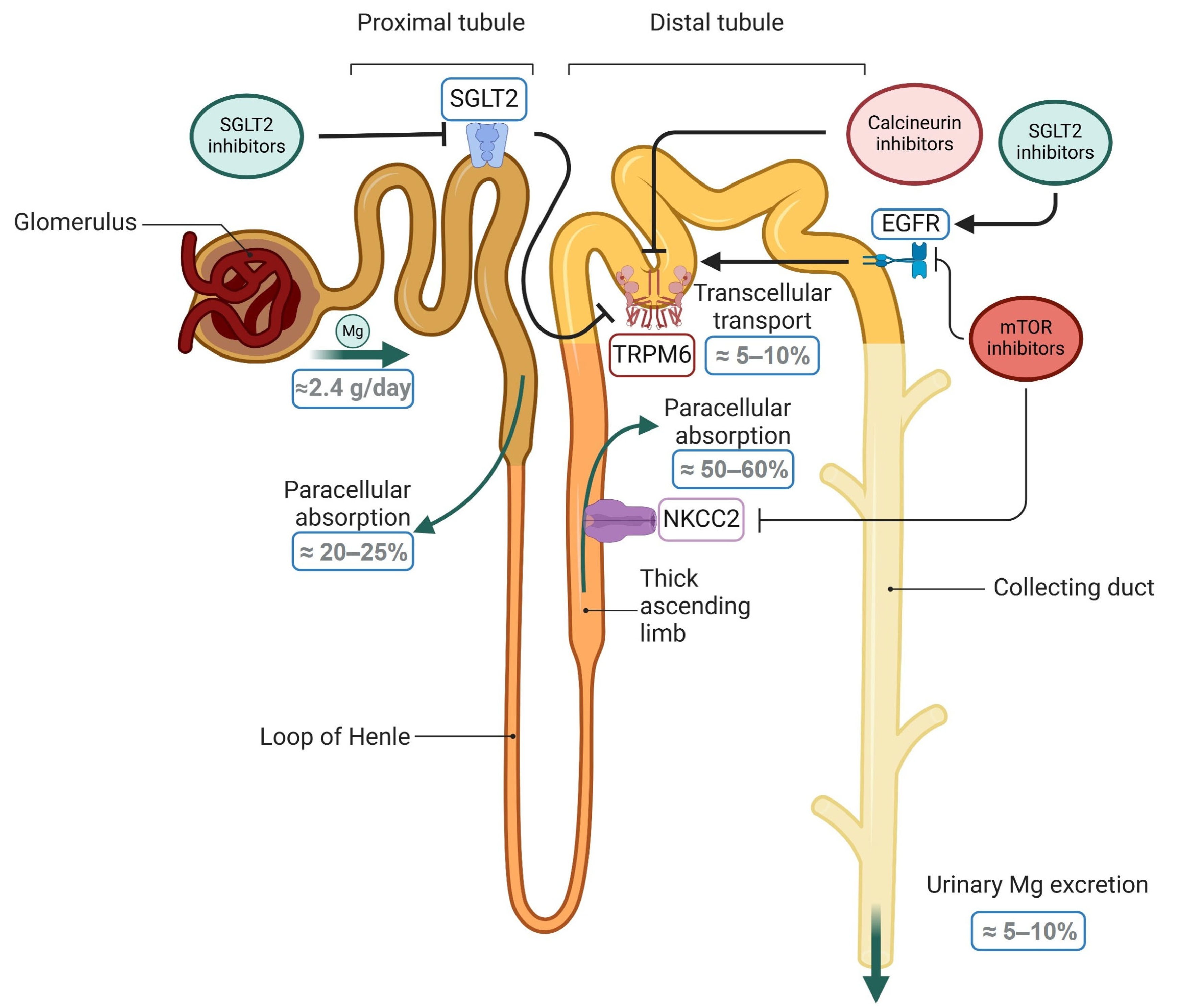
3. Magnesium in Renal Transplant Patients
4. Magnesium and SGLT2 Inhibitors
5. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Mg | Magnesium |
| P | Phosphorus |
| Ca | Calcium |
| CKD | Chronic kidney disease |
| KT | Kidney transplant |
| SGLT2 | Sodium-glucose co-transporter 2 |
| TRPM7 | Transient Receptor Potential Cation Channel Subfamily M Member 7 |
| TRPM6 | Transient Receptor Potential Cation Channel Subfamily M Member 6 |
| PTH | Parathyroid hormone |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| VDR | Vitamin D receptor |
| CaSR | Calcium-Sensing Receptor |
| P2X7 | P2X purinoceptor 7 |
| NMDA | N-methyl-D-aspartate |
| Magt1 | Magnesium Transporter 1 |
| SHPT | Secondary hyperparathyroidism |
| HD | Hemodialysis |
| TNFα | Tumor necrosis factor alpha |
| IL | Interleukin |
| BALP | bone alkaline phosphatase |
| TRAP5b | Tartrate-Resistant Acid Phosphatase 5 b |
| CPPs | Calciprotein particles |
| T2DM | Type 2 diabetes mellitus |
| mTOR | Mammalian target of rapamycin |
| CNIs | Calcineurin inhibitors |
| NKCC2 | sodium-potassium-2chloride cotransporter 2 |
| EGF | Epidermal growth factor |
| PTDM | Post-transplantation diabetes mellitus |
| PPIs | Proton pump inhibitors |
References
- Nadezhdin, K.D.; Correia, L.; Narangoda, C.; Patel, D.S.; Neuberger, A.; Gudermann, T.; Kurnikova, M.G.; Chubanov, V.; Sobolevsky, A.I. Structural Mechanisms of TRPM7 Activation and Inhibition. Nat. Commun. 2023, 14, 2639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, T.; Zou, J.; Miller, C.L.; Gorkhali, R.; Yang, J.-Y.; Schilmiller, A.; Wang, S.; Huang, K.; Brown, E.M.; et al. Structural Basis for Regulation of Human Calcium-Sensing Receptor by Magnesium Ions and an Unexpected Tryptophan Derivative Co-Agonist. Sci. Adv. 2016, 2, e1600241. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wang, L.; Fu, T.; Papasergi, M.; Yule, D.I.; Xia, H. Magnesium Acts as a Second Messenger in the Regulation of NMDA Receptor-Mediated CREB Signaling in Neurons. Mol. Neurobiol. 2020, 57, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Banerjee, R.; Marinelli, F.; Silberberg, S.; Faraldo-Gómez, J.D.; Hattori, M.; Swartz, K.J. Molecular Mechanisms of Human P2X3 Receptor Channel Activation and Modulation by Divalent Cation Bound ATP. eLife 2019, 8, e47060. [Google Scholar] [CrossRef]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second Messenger Role for Mg2+ Revealed by Human T-Cell Immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and Hormonal Effects of Magnesium Deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, Z.; Fu, R.; Zhou, T.; Long, C.; He, T.; Yang, D.; Li, Z.; Peng, S. Magnesium Supplementation Enhances mTOR Signalling to Facilitate Myogenic Differentiation and Improve Aged Muscle Performance. Bone 2021, 146, 115886. [Google Scholar] [CrossRef]
- Dickens, B.F.; Weglicki, W.B.; Li, Y.S.; Mak, I.T. Magnesium Deficiency in Vitro Enhances Free Radical-Induced Intracellular Oxidation and Cytotoxicity in Endothelial Cells. FEBS Lett. 1992, 311, 187–191. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium Metabolism and Its Disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Chen, Y.-T.; Kao, Z.-K.; Shih, C.-J.; Ou, S.-M.; Yang, C.-Y.; Yang, A.-H.; Lee, O.K.-S.; Tarng, D.-C. Magnesium Exposure Increases Hip Fracture Risks in Patients with Chronic Kidney Disease: A Population-Based Nested Case-Control Study. Osteoporos. Int. 2022, 33, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Menez, S.; Ding, N.; Grams, M.E.; Lutsey, P.L.; Heiss, G.; Folsom, A.R.; Selvin, E.; Coresh, J.; Jaar, B.G.; Matsushita, K. Serum Magnesium, Bone-Mineral Metabolism Markers and Their Interactions with Kidney Function on Subsequent Risk of Peripheral Artery Disease: The Atherosclerosis Risk in Communities Study. Nephrol. Dial. Transplant. 2020, 35, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Leenders, N.H.J.; Bos, C.; Hoekstra, T.; Schurgers, L.J.; Vervloet, M.G.; Hoenderop, J.G.J. Dietary Magnesium Supplementation Inhibits Abdominal Vascular Calcification in an Experimental Animal Model of Chronic Kidney Disease. Nephrol. Dial. Transplant. 2022, 37, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortiz, M.E.; Gómez-Delgado, F.; Arenas de Larriva, A.P.; Canalejo, A.; Gómez-Luna, P.; Herencia, C.; López-Moreno, J.; Rodríguez, M.; López-Miranda, J.; Almadén, Y. Serum Magnesium Is Associated with Carotid Atherosclerosis in Patients with High Cardiovascular Risk (CORDIOPREV Study). Sci. Rep. 2019, 9, 8013. [Google Scholar] [CrossRef]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated With Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association Between Circulating C-Reactive Protein and Muscle Mass in Women. J. Bone Miner. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Marino, A. Protective Role of Magnesium against Oxidative Stress on SO4= Uptake through Band 3 Protein in Human Erythrocytes. Cell Physiol. Biochem. 2019, 52, 1292–1308. [Google Scholar] [CrossRef]
- Cheng, Y.; Zullo, A.R.; Yin, Y.; Shao, Y.; Liu, S.; Zeng-Treitler, Q.; Wu, W.-C. Nonprescription Magnesium Supplement Use and Risk of Heart Failure in Patients With Diabetes: A Target Trial Emulation. J. Am. Heart Assoc. 2025, 14, e038870. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia Is a Significant Predictor of Cardiovascular and Non-Cardiovascular Mortality in Patients Undergoing Hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef]
- Ferrè, S.; Li, X.; Adams-Huet, B.; Maalouf, N.M.; Sakhaee, K.; Toto, R.D.; Moe, O.W.; Neyra, J.A. Association of Serum Magnesium with All-Cause Mortality in Patients with and without Chronic Kidney Disease in the Dallas Heart Study. Nephrol. Dial. Transplant. 2018, 33, 1389–1396. [Google Scholar] [CrossRef]
- Xiong, J.; He, T.; Wang, M.; Nie, L.; Zhang, Y.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Serum Magnesium, Mortality, and Cardiovascular Disease in Chronic Kidney Disease and End-Stage Renal Disease Patients: A Systematic Review and Meta-Analysis. J. Nephrol. 2019, 32, 791–802. [Google Scholar] [CrossRef]
- Matias, P.J.; Ávila, G.; Domingos, D.; Gil, C.; Ferreira, A. Lower Serum Magnesium Levels Are Associated with a Higher Risk of Fractures and Vascular Calcifications in Hemodialysis Patients. Clin. Kidney J. 2025, 18, sfae381. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, A.D.; Tinnemans, P.T.; Shanahan, C.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. Magnesium Prevents Vascular Calcification in Vitro by Inhibition of Hydroxyapatite Crystal Formation. Sci. Rep. 2018, 8, 2069. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, A.D.; Eelderink, C.; Zeper, L.W.; Pasch, A.; Bakker, S.J.L.; de Borst, M.H.; Hoenderop, J.G.J.; de Baaij, J.H.F. Calciprotein Particle Inhibition Explains Magnesium-Mediated Protection against Vascular Calcification. Nephrol. Dial. Transplant. 2020, 35, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, A.; Guerrero, F.; Martinez-Moreno, J.M.; Madueño, J.A.; Herencia, C.; Peralta, A.; Almaden, Y.; Lopez, I.; Aguilera-Tejero, E.; Gundlach, K.; et al. Magnesium Inhibits Wnt/β-Catenin Activity and Reverses the Osteogenic Transformation of Vascular Smooth Muscle Cells. PLoS ONE 2014, 9, e89525. [Google Scholar] [CrossRef]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular Smooth Muscle Cell Differentiation to an Osteogenic Phenotype Involves TRPM7 Modulation by Magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef]
- Bressendorff, I.; Hansen, D.; Schou, M.; Kragelund, C.; Svensson, M.; Hashemi, B.; Kristensen, T.; Vrist, M.H.; Borg, R.; Tougaard, B.; et al. The Effect of Magnesium Supplementation on Vascular Calcification in CKD: A Randomized Clinical Trial (MAGiCAL-CKD). J. Am. Soc. Nephrol. 2023, 34, 886–894. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, R.; Li, G. Effect of Magnesium on Vascular Calcification in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Ren. Fail. 2023, 45, 2182603. [Google Scholar] [CrossRef]
- Zaslow, S.J.; Oliveira-Paula, G.H.; Chen, W. Magnesium and Vascular Calcification in Chronic Kidney Disease: Current Insights. Int. J. Mol. Sci. 2024, 25, 1155. [Google Scholar] [CrossRef]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and Characterization of an Extracellular Ca(2+)-Sensing Receptor from Bovine Parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef]
- Bourdeau, A.; Souberbielle, J.C.; Bonnet, P.; Herviaux, P.; Sachs, C.; Lieberherr, M. Phospholipase-A2 Action and Arachidonic Acid Metabolism in Calcium-Mediated Parathyroid Hormone Secretion. Endocrinology 1992, 130, 1339–1344. [Google Scholar] [CrossRef]
- Almadén, Y.; Canalejo, A.; Ballesteros, E.; Añón, G.; Cañadillas, S.; Rodríguez, M. Regulation of Arachidonic Acid Production by Intracellular Calcium in Parathyroid Cells: Effect of Extracellular Phosphate. J. Am. Soc. Nephrol. 2002, 13, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Garfia, B.; Cañadillas, S.; Canalejo, A.; Luque, F.; Siendones, E.; Quesada, M.; Almadén, Y.; Aguilera-Tejero, E.; Rodríguez, M. Regulation of Parathyroid Vitamin D Receptor Expression by Extracellular Calcium. J. Am. Soc. Nephrol. 2002, 13, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.J.; Lopez, I.; Canalejo, R.; Almaden, Y.; Martin, D.; Aguilera-Tejero, E.; Rodriguez, M. Direct Upregulation of Parathyroid Calcium-Sensing Receptor and Vitamin D Receptor by Calcimimetics in Uremic Rats. Am. J. Physiol. Renal Physiol. 2009, 296, F605–F613. [Google Scholar] [CrossRef] [PubMed]
- Cañadillas, S.; Canalejo, R.; Rodriguez-Ortiz, M.E.; Martinez-Moreno, J.M.; Estepa, J.C.; Zafra, R.; Perez, J.; Muñoz-Castañeda, J.R.; Canalejo, A.; Rodriguez, M.; et al. Upregulation of Parathyroid VDR Expression by Extracellular Calcium Is Mediated by ERK1/2-MAPK Signaling Pathway. Am. J. Physiol. Renal Physiol. 2010, 298, F1197–F1204. [Google Scholar] [CrossRef]
- Canalejo, R.; Canalejo, A.; Martinez-Moreno, J.M.; Rodriguez-Ortiz, M.E.; Estepa, J.C.; Mendoza, F.J.; Munoz-Castaneda, J.R.; Shalhoub, V.; Almaden, Y.; Rodriguez, M. FGF23 Fails to Inhibit Uremic Parathyroid Glands. J. Am. Soc. Nephrol. 2010, 21, 1125–1135. [Google Scholar] [CrossRef]
- Cholst, I.N.; Steinberg, S.F.; Tropper, P.J.; Fox, H.E.; Segre, G.V.; Bilezikian, J.P. The Influence of Hypermagnesemia on Serum Calcium and Parathyroid Hormone Levels in Human Subjects. N. Engl. J. Med. 1984, 310, 1221–1225. [Google Scholar] [CrossRef]
- Ferment, O.; Garnier, P.E.; Touitou, Y. Comparison of the Feedback Effect of Magnesium and Calcium on Parathyroid Hormone Secretion in Man. J. Endocrinol. 1987, 113, 117–122. [Google Scholar] [CrossRef]
- Habener, J.F.; Potts, J.T. Relative Effectiveness of Magnesium and Calcium on the Secretion and Biosynthesis of Parathyroid Hormone in Vitro. Endocrinology 1976, 98, 197–202. [Google Scholar] [CrossRef]
- Brown, E.M.; Thatcher, J.G.; Watson, E.J.; Leombruno, R. Extracellular Calcium Potentiates the Inhibitory Effects of Magnesium on Parathyroid Function in Dispersed Bovine Parathyroid Cells. Metab. Clin. Exp. 1984, 33, 171–176. [Google Scholar] [CrossRef]
- Shoback, D.M.; Thatcher, J.G.; Brown, E.M. Interaction of Extracellular Calcium and Magnesium in the Regulation of Cytosolic Calcium and PTH Release in Dispersed Bovine Parathyroid Cells. Mol. Cell Endocrinol. 1984, 38, 179–186. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, M.E.; Canalejo, A.; Herencia, C.; Martínez-Moreno, J.M.; Peralta-Ramírez, A.; Perez-Martinez, P.; Navarro-González, J.F.; Rodríguez, M.; Peter, M.; Gundlach, K.; et al. Magnesium Modulates Parathyroid Hormone Secretion and Upregulates Parathyroid Receptor Expression at Moderately Low Calcium Concentration. Nephrol. Dial. Transplant. 2014, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Mora, C.; Jiménez, A.; Torres, A.; Macía, M.; García, J. Relationship between Serum Magnesium and Parathyroid Hormone Levels in Hemodialysis Patients. Am. J. Kidney Dis. 1999, 34, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Mora, C.; Macia, M.; Garcia, J. Serum Magnesium Concentration Is an Independent Predictor of Parathyroid Hormone Levels in Peritoneal Dialysis Patients. Perit. Dial. Int. 1999, 19, 455–461. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H. Correlation of Serum Magnesium with Serum Parathormone Levels in Patients on Regular Hemodialysis. Saudi J. Kidney Dis. Transpl. 2006, 17, 344–350. [Google Scholar]
- Kuo, C.-Y.; Tsai, C.-H.; Lee, J.-J.; Cheng, S.-P. Abnormalities of Serum Magnesium Levels in Dialysis Patients Undergoing Parathyroidectomy. Updates Surg. 2024, 76, 1435–1441. [Google Scholar] [CrossRef]
- Oe, P.L.; Lips, P.; van der Meulen, J.; de Vries, P.M.; van Bronswijk, H.; Donker, A.J. Long-Term Use of Magnesium Hydroxide as a Phosphate Binder in Patients on Hemodialysis. Clin. Nephrol. 1987, 28, 180–185. [Google Scholar]
- Morinière, P.; Vinatier, I.; Westeel, P.F.; Cohemsolal, M.; Belbrik, S.; Abdulmassih, Z.; Hocine, C.; Marie, A.; Leflon, P.; Roche, D. Magnesium Hydroxide as a Complementary Aluminium-Free Phosphate Binder to Moderate Doses of Oral Calcium in Uraemic Patients on Chronic Haemodialysis: Lack of Deleterious Effect on Bone Mineralisation. Nephrol. Dial. Transplant. 1988, 3, 651–656. [Google Scholar] [CrossRef]
- O’Donovan, R.; Baldwin, D.; Hammer, M.; Moniz, C.; Parsons, V. Substitution of Aluminium Salts by Magnesium Salts in Control of Dialysis Hyperphosphataemia. Lancet 1986, 1, 880–882. [Google Scholar] [CrossRef]
- Spiegel, D.M.; Farmer, B.; Smits, G.; Chonchol, M. Magnesium Carbonate Is an Effective Phosphate Binder for Chronic Hemodialysis Patients: A Pilot Study. J. Ren. Nutr. 2007, 17, 416–422. [Google Scholar] [CrossRef]
- Tzanakis, I.P.; Papadaki, A.N.; Wei, M.; Kagia, S.; Spadidakis, V.V.; Kallivretakis, N.E.; Oreopoulos, D.G. Magnesium Carbonate for Phosphate Control in Patients on Hemodialysis. A Randomized Controlled Trial. Int. Urol. Nephrol. 2008, 40, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Delmez, J.A.; Kelber, J.; Norword, K.Y.; Giles, K.S.; Slatopolsky, E. Magnesium Carbonate as a Phosphorus Binder: A Prospective, Controlled, Crossover Study. Kidney Int. 1996, 49, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.M.; Farmer, B. Long-Term Effects of Magnesium Carbonate on Coronary Artery Calcification and Bone Mineral Density in Hemodialysis Patients: A Pilot Study. Hemodial. Int. 2009, 13, 453–459. [Google Scholar] [CrossRef] [PubMed]
- de Francisco, A.L.M.; Leidig, M.; Covic, A.C.; Ketteler, M.; Benedyk-Lorens, E.; Mircescu, G.M.; Scholz, C.; Ponce, P.; Passlick-Deetjen, J. Evaluation of Calcium Acetate/Magnesium Carbonate as a Phosphate Binder Compared with Sevelamer Hydrochloride in Haemodialysis Patients: A Controlled Randomized Study (CALMAG Study) Assessing Efficacy and Tolerability. Nephrol. Dial. Transplant. 2010, 25, 3707–3717. [Google Scholar] [CrossRef]
- Almaden, Y.; Canalejo, A.; Hernandez, A.; Ballesteros, E.; Garcia-Navarro, S.; Torres, A.; Rodriguez, M. Direct Effect of Phosphorus on PTH Secretion from Whole Rat Parathyroid Glands in Vitro. J. Bone Miner. Res. 1996, 11, 970–976. [Google Scholar] [CrossRef]
- McGonigle, R.J.; Weston, M.J.; Keenan, J.; Jackson, D.B.; Parsons, V. Effect of Hypermagnesemia on Circulating Plasma Parathyroid Hormone in Patients on Regular Hemodialysis Therapy. Magnesium 1984, 3, 1–7. [Google Scholar]
- Takahashi, S.; Okada, K.; Yanai, M. Magnesium and Parathyroid Hormone Changes to Magnesium-Free Dialysate in Continuous Ambulatory Peritoneal Dialysis Patients. Perit. Dial. Int. 1994, 14, 75–78. [Google Scholar] [CrossRef]
- Fang, L.; Tang, B.; Hou, D.; Meng, M.; Xiong, M.; Yang, J. Effect of Parathyroid Hormone on Serum Magnesium Levels: The Neglected Relationship in Hemodialysis Patients with Secondary Hyperparathyroidism. Ren. Fail. 2016, 38, 50–56. [Google Scholar] [CrossRef]
- Jain, N.; Reilly, R.F. Hungry Bone Syndrome. Curr. Opin. Nephrol. Hypertens. 2017, 26, 250–255. [Google Scholar] [CrossRef]
- Saha, H.; Harmoinen, A.; Pietilä, K.; Mörsky, P.; Pasternack, A. Measurement of Serum Ionized versus Total Levels of Magnesium and Calcium in Hemodialysis Patients. Clin. Nephrol. 1996, 46, 326–331. [Google Scholar]
- Navarro-González, J.F. Magnesium in Dialysis Patients: Serum Levels and Clinical Implications. Clin. Nephrol. 1998, 49, 373–378. [Google Scholar] [PubMed]
- Wallach, S. Relation of Magnesium to Osteoporosis and Calcium Urolithiasis. Magnes. Trace Elem. 1991, 10, 281–286. [Google Scholar] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An Update on Magnesium and Bone Health. Biometals 2021, 34, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Impact of Magnesium on Bone Health in Older Adults: A Systematic Review and Meta-Analysis. Bone 2021, 154, 116233. [Google Scholar] [CrossRef]
- Pendón-Ruiz de Mier, M.V.; Santamaría, R.; Moyano-Peregrín, C.; Gordillo, J.E.; Salmoral-Chamizo, A.; López-López, I.; Rodelo-Haad, C.; Valle, C.; Membrives-González, C.; López-Ruiz, D.J.; et al. Bone and Vascular Effects of Magnesium Supplements in CKD Patients (the MagicalBone Pilot Study). Nefrologia (Engl. Ed.) 2024, 44, 721–730. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium Basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Xing, J.H.; Soffer, E.E. Adverse Effects of Laxatives. Dis. Colon. Rectum 2001, 44, 1201–1209. [Google Scholar] [CrossRef]
- Touyz, R.M.; de Baaij, J.H.F.; Hoenderop, J.G.J. Magnesium Disorders. N. Engl. J. Med. 2024, 390, 1998–2009. [Google Scholar] [CrossRef]
- Rosanoff, A.; West, C.; Elin, R.J.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.-C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an Updated Standardization of Serum Magnesium Reference Ranges. Eur. J. Nutr. 2022, 61, 3697–3706. [Google Scholar] [CrossRef]
- Alfrey, A.C.; Miller, N.L. Bone Magnesium Pools in Uremia. J. Clin. Invest. 1973, 52, 3019–3027. [Google Scholar] [CrossRef]
- Aal-Hamad, A.H.; Al-Alawi, A.M.; Kashoub, M.S.; Falhammar, H. Hypermagnesemia in Clinical Practice. Medicina (Kaunas) 2023, 59, 1190. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.I.; Brown, P.W. The Effects of Magnesium on Hydroxyapatite Formation In Vitro from CaHPO4 and Ca4(PO4)2O at 37.4 °C. Calcif. Tissue Int. 1997, 60, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic Interplay between the Two Major Bone Minerals, Hydroxyapatite and Whitlockite Nanoparticles, for Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Jang, H.L.; Ahn, H.-Y.; Lee, H.K.; Park, J.; Lee, E.-S.; Lee, E.A.; Jeong, Y.-H.; Kim, D.-G.; Nam, K.T.; et al. Biomimetic Whitlockite Inorganic Nanoparticles-Mediated in Situ Remodeling and Rapid Bone Regeneration. Biomaterials 2017, 112, 31–43. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Qi, H.; Zhao, Q.; Liu, Y.; Zhang, Y. Dual Function of Magnesium in Bone Biomineralization. Adv. Healthc. Mater. 2019, 8, e1901030. [Google Scholar] [CrossRef]
- Díaz-Tocados, J.M.; Herencia, C.; Martínez-Moreno, J.M.; Montes de Oca, A.; Rodríguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almadén, Y.; Rodríguez, M.; et al. Magnesium Chloride Promotes Osteogenesis through Notch Signaling Activation and Expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef]
- Qiao, W.; Wong, K.H.M.; Shen, J.; Wang, W.; Wu, J.; Li, J.; Lin, Z.; Chen, Z.; Matinlinna, J.P.; Zheng, Y.; et al. TRPM7 Kinase-Mediated Immunomodulation in Macrophage Plays a Central Role in Magnesium Ion-Induced Bone Regeneration. Nat. Commun. 2021, 12, 2885. [Google Scholar] [CrossRef]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodríguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; Montes de Oca, A.; Vergara, N.; Steppan, S.; et al. Dietary Magnesium Supplementation Prevents and Reverses Vascular and Soft Tissue Calcifications in Uremic Rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Gray, R.W.; Omdahl, J.L.; Ghazarian, J.G.; DeLuca, H.F. 25-Hydroxycholecalciferol-1-Hydroxylase. Subcellular Location and Properties. J. Biol. Chem. 1972, 247, 7528–7532. [Google Scholar] [CrossRef]
- Mederle, O.A.; Balas, M.; Ioanoviciu, S.D.; Gurban, C.-V.; Tudor, A.; Borza, C. Correlations between Bone Turnover Markers, Serum Magnesium and Bone Mass Density in Postmenopausal Osteoporosis. Clin. Interv. Aging 2018, 13, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J.; Jiang, H. Association between Serum Level of Magnesium and Postmenopausal Osteoporosis: A Meta-Analysis. Biol. Trace Elem. Res. 2014, 159, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Farsinejad-Marj, M.; Saneei, P.; Esmaillzadeh, A. Dietary Magnesium Intake, Bone Mineral Density and Risk of Fracture: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2016, 27, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low Serum Magnesium Levels Are Associated with Increased Risk of Fractures: A Long-Term Prospective Cohort Study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef]
- Dimai, H.P.; Porta, S.; Wirnsberger, G.; Lindschinger, M.; Pamperl, I.; Dobnig, H.; Wilders-Truschnig, M.; Lau, K.H. Daily Oral Magnesium Supplementation Suppresses Bone Turnover in Young Adult Males. J. Clin. Endocrinol. Metab. 1998, 83, 2742–2748. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Hoshino, J.; Masakane, I. Magnesium and Risk of Hip Fracture among Patients Undergoing Hemodialysis. J. Am. Soc. Nephrol. 2018, 29, 991–999. [Google Scholar] [CrossRef]
- Aydin, H.; Deyneli, O.; Yavuz, D.; Gözü, H.; Mutlu, N.; Kaygusuz, I.; Akalin, S. Short-Term Oral Magnesium Supplementation Suppresses Bone Turnover in Postmenopausal Osteoporotic Women. Biol. Trace Elem. Res. 2010, 133, 136–143. [Google Scholar] [CrossRef]
- Carpenter, T.O.; DeLucia, M.C.; Zhang, J.H.; Bejnerowicz, G.; Tartamella, L.; Dziura, J.; Petersen, K.F.; Befroy, D.; Cohen, D. A Randomized Controlled Study of Effects of Dietary Magnesium Oxide Supplementation on Bone Mineral Content in Healthy Girls. J. Clin. Endocrinol. Metab. 2006, 91, 4866–4872. [Google Scholar] [CrossRef]
- Cowan, A.C.; Clemens, K.K.; Sontrop, J.M.; Dixon, S.N.; Killin, L.; Anderson, S.; Acedillo, R.R.; Bagga, A.; Bohm, C.; Brown, P.A.; et al. Magnesium and Fracture Risk in the General Population and Patients Receiving Dialysis: A Narrative Review. Can. J. Kidney Health Dis. 2023, 10, 20543581231154183. [Google Scholar] [CrossRef]
- Bressendorff, I.; Hansen, D.; Pasch, A.; Holt, S.G.; Schou, M.; Brandi, L.; Smith, E.R. The Effect of Increasing Dialysate Magnesium on Calciprotein Particles, Inflammation and Bone Markers: Post Hoc Analysis from a Randomized Controlled Clinical Trial. Nephrol. Dial. Transplant. 2021, 36, 713–721. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Biesen, W. Hypomagnesaemia in Kidney Transplantation. Transplant. Rev. 2015, 29, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Sankarasubbaiyan, S.; Gross, M.D.; Jeevanantham, V.; Monk, R.D. Tacrolimus-Associated Hypomagnesemia in Renal Transplant Recipients. Transplant. Proc. 2006, 38, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Santana, A.; Guerra, J.; Neves, M.; Nascimento, C.; Gonçalves, J.; da Costa, A.G. Serum Magnesium and Related Factors in Long-Term Renal Transplant Recipients: An Observational Study. Transplant. Proc. 2017, 49, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Margreiter, R. European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group Efficacy and Safety of Tacrolimus Compared with Ciclosporin Microemulsion in Renal Transplantation: A Randomised Multicentre Study. Lancet 2002, 359, 741–746. [Google Scholar] [CrossRef]
- Van de Cauter, J.; Sennesael, J.; Haentjens, P. Long-Term Evolution of the Mineral Metabolism after Renal Transplantation: A Prospective, Single-Center Cohort Study. Transplant. Proc. 2011, 43, 3470–3475. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Biesen, W.; Verbeke, F.; De Bacquer, D.; Peeters, P.; Vanholder, R. Posttransplantation Hypomagnesemia and Its Relation with Immunosuppression as Predictors of New-Onset Diabetes after Transplantation. Am. J. Transplant. 2009, 9, 2140–2149. [Google Scholar] [CrossRef]
- Negrea, L.; DeLozier, S.J.; Janes, J.L.; Rahman, M.; Dobre, M. Serum Magnesium and Cardiovascular Outcomes and Mortality in CKD: The Chronic Renal Insufficiency Cohort (CRIC). Kidney Med. 2021, 3, 183–192.e1. [Google Scholar] [CrossRef]
- Ishimura, E.; Okuno, S.; Yamakawa, T.; Inaba, M.; Nishizawa, Y. Serum Magnesium Concentration Is a Significant Predictor of Mortality in Maintenance Hemodialysis Patients. Magnes. Res. 2007, 20, 237–244. [Google Scholar]
- Lacson, E.; Wang, W.; Ma, L.; Passlick-Deetjen, J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am. J. Kidney Dis. 2015, 66, 1056–1066. [Google Scholar] [CrossRef]
- Shimohata, H.; Yamashita, M.; Ohgi, K.; Tsujimoto, R.; Maruyama, H.; Takayasu, M.; Hirayama, K.; Kobayashi, M. The Relationship between Serum Magnesium Levels and Mortality in Non-Diabetic Hemodialysis Patients: A 10-Year Follow-up Study. Hemodial. Int. 2019, 23, 369–374. [Google Scholar] [CrossRef]
- Nijenhuis, T.; Hoenderop, J.G.J.; Bindels, R.J.M. Downregulation of Ca(2+) and Mg(2+) Transport Proteins in the Kidney Explains Tacrolimus (FK506)-Induced Hypercalciuria and Hypomagnesemia. J. Am. Soc. Nephrol. 2004, 15, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.F.; Burdmann, E.A.; Fransechini, N.; Houghton, D.C.; Bennett, W.M. Comparison of Acute Rapamycin Nephrotoxicity with Cyclosporine and FK506. Kidney Int. 1996, 50, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Sanada, A.; Sawada, H.; Okude, C.; Tonegawa, C.; Sugatani, J. Decrease in Transient Receptor Potential Melastatin 6 mRNA Stability Caused by Rapamycin in Renal Tubular Epithelial Cells. Biochim. Biophys. Acta 2011, 1808, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.A.; de Bragança, A.C.; Shimizu, M.H.M.; Sanches, T.R.; Fortes, M.A.Z.; Giorgi, R.R.; Andrade, L.; Seguro, A.C. Rosiglitazone Prevents Sirolimus-Induced Hypomagnesemia, Hypokalemia, and Downregulation of NKCC2 Protein Expression. Am. J. Physiol. Renal Physiol. 2009, 297, F916–F922. [Google Scholar] [CrossRef]
- Sánchez-Fructuoso, A.I.; Santín Cantero, J.M.; Pérez Flores, I.; Valero San Cecilio, R.; Calvo Romero, N.; Vilalta Casas, R. Changes in Magnesium and Potassium Homeostasis after Conversion from a Calcineurin Inhibitor Regimen to an mTOR Inhibitor-Based Regimen. Transplant. Proc. 2010, 42, 3047–3049. [Google Scholar] [CrossRef]
- Wei, K.-Y.; van Heugten, M.H.; van Megen, W.H.; van Veghel, R.; Rehaume, L.M.; Cross, J.L.; Viel, J.J.; van Willigenburg, H.; Silva, P.H.I.; Danser, A.H.J.; et al. Calcineurin Inhibitor Effects on Kidney Electrolyte Handling and Blood Pressure: Tacrolimus versus Voclosporin. Nephrol. Dial. Transplant. 2024, 40, 151–163. [Google Scholar] [CrossRef]
- Azukaitis, K.; Ju, W.; Kirchner, M.; Nair, V.; Smith, M.; Fang, Z.; Thurn-Valsassina, D.; Bayazit, A.; Niemirska, A.; Canpolat, N.; et al. Low Levels of Urinary Epidermal Growth Factor Predict Chronic Kidney Disease Progression in Children. Kidney Int. 2019, 96, 214–221. [Google Scholar] [CrossRef]
- Chan, W.; Bosch, J.A.; Jones, D.; McTernan, P.G.; Inston, N.; Moore, S.; Kaur, O.; Phillips, A.C.; Borrows, R. Hypervolemia and Blood Pressure in Prevalent Kidney Transplant Recipients. Transplantation 2014, 98, 320–327. [Google Scholar] [CrossRef]
- Park, S.; Kang, E.; Park, S.; Kim, Y.C.; Han, S.S.; Ha, J.; Kim, D.K.; Kim, S.; Park, S.-K.; Han, D.J.; et al. Metabolic Acidosis and Long-Term Clinical Outcomes in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2017, 28, 1886–1897. [Google Scholar] [CrossRef]
- Maes, B.; Hadaya, K.; de Moor, B.; Cambier, P.; Peeters, P.; de Meester, J.; Donck, J.; Sennesael, J.; Squifflet, J.-P. Severe Diarrhea in Renal Transplant Patients: Results of the DIDACT Study. Am. J. Transplant. 2006, 6, 1466–1472. [Google Scholar] [CrossRef]
- Douwes, R.M.; Gomes-Neto, A.W.; Schutten, J.C.; van den Berg, E.; de Borst, M.H.; Berger, S.P.; Touw, D.J.; Hak, E.; Blokzijl, H.; Navis, G.; et al. Proton-Pump Inhibitors and Hypomagnesaemia in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2162. [Google Scholar] [CrossRef] [PubMed]
- Cundy, T.; Dissanayake, A. Severe Hypomagnesaemia in Long-Term Users of Proton-Pump Inhibitors. Clin. Endocrinol. 2008, 69, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Rondón, L.J.; Groenestege, W.M.T.; Rayssiguier, Y.; Mazur, A. Relationship between Low Magnesium Status and TRPM6 Expression in the Kidney and Large Intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R2001–R2007. [Google Scholar] [CrossRef] [PubMed]
- Holzmacher, R.; Kendziorski, C.; Michael Hofman, R.; Jaffery, J.; Becker, B.; Djamali, A. Low Serum Magnesium Is Associated with Decreased Graft Survival in Patients with Chronic Cyclosporin Nephrotoxicity. Nephrol. Dial. Transplant. 2005, 20, 1456–1462. [Google Scholar] [CrossRef]
- Hod, T.; Isakov, O.; Patibandla, B.K.; Christopher, K.B.; Hershkoviz, R.; Schwartz, I.F.; Chandraker, A. Posttransplantation Hypomagnesemia as a Predictor of Better Graft Function after Transplantation. Kidney Blood Press. Res. 2020, 45, 982–995. [Google Scholar] [CrossRef]
- Miura, K.; Nakatani, T.; Asai, T.; Yamanaka, S.; Tamada, S.; Tashiro, K.; Kim, S.; Okamura, M.; Iwao, H. Role of Hypomagnesemia in Chronic Cyclosporine Nephropathy. Transplantation 2002, 73, 340–347. [Google Scholar] [CrossRef]
- Sharif, A.; Chakkera, H.; de Vries, A.P.J.; Eller, K.; Guthoff, M.; Haller, M.C.; Hornum, M.; Nordheim, E.; Kautzky-Willer, A.; Krebs, M.; et al. International Consensus on Post-Transplantation Diabetes Mellitus. Nephrol. Dial. Transplant. 2024, 39, 531–549. [Google Scholar] [CrossRef]
- Pham, P.-C.T.; Pham, P.-M.T.; Pham, S.V.; Miller, J.M.; Pham, P.-T.T. Hypomagnesemia in Patients with Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Biesen, W.; Vanholder, R. Hypomagnesaemia, the Kidney and the Vessels. Nephrol. Dial. Transplant. 2012, 27, 4003–4010. [Google Scholar] [CrossRef]
- Huang, J.W.; Famure, O.; Li, Y.; Kim, S.J. Hypomagnesemia and the Risk of New-Onset Diabetes Mellitus after Kidney Transplantation. J. Am. Soc. Nephrol. 2016, 27, 1793–1800. [Google Scholar] [CrossRef]
- van der Burgh, A.C.; Moes, A.; Kieboom, B.C.T.; van Gelder, T.; Zietse, R.; van Schaik, R.H.N.; Hesselink, D.A.; Hoorn, E.J. Serum Magnesium, Hepatocyte Nuclear Factor 1β Genotype and Post-Transplant Diabetes Mellitus: A Prospective Study. Nephrol. Dial. Transplant. 2020, 35, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, L.F.; Alessi, M.; Bertoldi, G.; Rossato, V.; Di Vico, V.; Nalesso, F.; Calò, L.A. Calcineurin-Inhibitor-Induced Hypomagnesemia in Kidney Transplant Patients: A Monocentric Comparative Study between Sucrosomial Magnesium and Magnesium Pidolate Supplementation. J. Clin. Med. 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; de Baaij, J.H.F. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kröse, J.L.; de Baaij, J.H.F. Magnesium Biology. Nephrol. Dial. Transplant. 2024, 39, 1965–1975. [Google Scholar] [CrossRef]
- Ayuk, J.; Gittoes, N.J.L. Treatment of Hypomagnesemia. Am. J. Kidney Dis. 2014, 63, 691–695. [Google Scholar] [CrossRef]
- Shah, C.V.; Sparks, M.A.; Lee, C.-T. Sodium/Glucose Cotransporter 2 Inhibitors and Magnesium Homeostasis: A Review. Am. J. Kidney Dis. 2024, 83, 648–658. [Google Scholar] [CrossRef]
- Giménez-Mascarell, P.; Schirrmacher, C.E.; Martínez-Cruz, L.A.; Müller, D. Novel Aspects of Renal Magnesium Homeostasis. Front. Pediatr. 2018, 6, 77. [Google Scholar] [CrossRef]
- Chen, L.; LaRocque, L.M.; Efe, O.; Wang, J.; Sands, J.M.; Klein, J.D. Effect of Dapagliflozin Treatment on Fluid and Electrolyte Balance in Diabetic Rats. Am. J. Med. Sci. 2016, 352, 517–523. [Google Scholar] [CrossRef]
- Ng, H.-Y.; Kuo, W.-H.; Tain, Y.-L.; Leung, F.-F.; Lee, W.-C.; Lee, C.-T. Effect of Dapagliflozin and Magnesium Supplementation on Renal Magnesium Handling and Magnesium Homeostasis in Metabolic Syndrome. Nutrients 2021, 13, 4088. [Google Scholar] [CrossRef]
- Dai, L.J.; Bapty, B.; Ritchie, G.; Quamme, G.A. Glucagon and Arginine Vasopressin Stimulate Mg2+ Uptake in Mouse Distal Convoluted Tubule Cells. Am. J. Physiol. 1998, 274, F328–F335. [Google Scholar] [CrossRef]
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thévenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W.J.; et al. Inhibition of the Glucose Transporter SGLT2 with Dapagliflozin in Pancreatic Alpha Cells Triggers Glucagon Secretion. Nat. Med. 2015, 21, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Thebault, S.; Alexander, R.T.; Tiel Groenestege, W.M.; Hoenderop, J.G.; Bindels, R.J. EGF Increases TRPM6 Activity and Surface Expression. J. Am. Soc. Nephrol. 2009, 20, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Bal, M.S.; Liu, J.; Yang, Z.; Rivera, C.; Wu, X.-R.; Hoenderop, J.G.J.; Bindels, R.J.M.; Marciano, D.K.; Wolf, M.T.F. Uromodulin Regulates Renal Magnesium Homeostasis through the Ion Channel Transient Receptor Potential Melastatin 6 (TRPM6). J. Biol. Chem. 2018, 293, 16488–16502. [Google Scholar] [CrossRef] [PubMed]
- Maeoka, Y.; McCormick, J.A. NaCl Cotransporter Activity and Mg2+ Handling by the Distal Convoluted Tubule. Am. J. Physiol. Renal Physiol. 2020, 319, F1043–F1053. [Google Scholar] [CrossRef]
- Cao, G.; Lee, K.P.; van der Wijst, J.; de Graaf, M.; van der Kemp, A.; Bindels, R.J.M.; Hoenderop, J.G.J. Methionine Sulfoxide Reductase B1 (MsrB1) Recovers TRPM6 Channel Activity during Oxidative Stress. J. Biol. Chem. 2010, 285, 26081–26087. [Google Scholar] [CrossRef]
- Verschuren, E.H.J.; Hoenderop, J.G.J.; Peters, D.J.M.; Arjona, F.J.; Bindels, R.J.M. Tubular Flow Activates Magnesium Transport in the Distal Convoluted Tubule. FASEB J. 2019, 33, 5034–5044. [Google Scholar] [CrossRef]
- Sen, T.; Ju, W.; Nair, V.; Ladd, P.; Menon, R.; Otto, E.A.; Pyle, L.; Vigers, T.; Nelson, R.G.; Arnott, C.; et al. Sodium Glucose Co-Transporter 2 Inhibition Increases Epidermal Growth Factor Expression and Improves Outcomes in Patients with Type 2 Diabetes. Kidney Int. 2023, 104, 828–839. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated Serum Magnesium Associated with SGLT2 Inhibitor Use in Type 2 Diabetes Patients: A Meta-Analysis of Randomised Controlled Trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef]
- Zhang, J.; Huan, Y.; Leibensperger, M.; Seo, B.; Song, Y. Comparative Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Serum Electrolyte Levels in Patients with Type 2 Diabetes: A Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Kidney360 2022, 3, 477–487. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Tsimihodimos, V.; Liamis, G.; Elisaf, M.S. SGLT2 Inhibitors-Induced Electrolyte Abnormalities: An Analysis of the Associated Mechanisms. Diabetes Metab. Syndr. 2018, 12, 59–63. [Google Scholar] [CrossRef]
- Ray, E.C.; Boyd-Shiwarski, C.R.; Liu, P.; Novacic, D.; Cassiman, D. SGLT2 Inhibitors for Treatment of Refractory Hypomagnesemia: A Case Report of 3 Patients. Kidney Med. 2020, 2, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Heleno, C.T.; Miranda, H.; Gotera, N.; Kloecker, G. The Use of Amiloride and Sodium-Glucose Cotransporter 2 Inhibitors in Cisplatin-Induced Hypomagnesemia: A Case Report and Review of the Literature. Cureus 2024, 16, e62546. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Omer, A.; Trivedi, N. Improvement in Serum Magnesium Levels With Sodium-Glucose Cotransporter 2 Inhibitors. JCEM Case Rep. 2023, 1, luac018. [Google Scholar] [CrossRef] [PubMed]
- Toto, R.D.; Goldenberg, R.; Chertow, G.M.; Cain, V.; Stefánsson, B.V.; Sjöström, C.D.; Sartipy, P. Correction of Hypomagnesemia by Dapagliflozin in Patients with Type 2 Diabetes: A Post Hoc Analysis of 10 Randomized, Placebo-Controlled Trials. J. Diabetes Complications 2019, 33, 107402. [Google Scholar] [CrossRef]
- Gilbert, R.E.; Mende, C.; Vijapurkar, U.; Sha, S.; Davies, M.J.; Desai, M. Effects of Canagliflozin on Serum Magnesium in Patients With Type 2 Diabetes Mellitus: A Post Hoc Analysis of Randomized Controlled Trials. Diabetes Ther. 2017, 8, 451–458. [Google Scholar] [CrossRef]
- Shah, C.V.; Robbins, T.S.; Sparks, M.A. Sodium-Glucose Cotransporter 2 Inhibitors and Management of Refractory Hypomagnesemia Without Overt Urinary Magnesium Wasting: A Report of 2 Cases. Kidney Med. 2022, 4, 100533. [Google Scholar] [CrossRef]
- Shah, C.V.; Hammad, N.; Bhasin-Chhabra, B.; Rashidi, A. SGLT2 Inhibitors in Management of Severe Hypomagnesemia in Patients Without Diabetes: A Report of 4 Cases. Kidney Med. 2023, 5, 100697. [Google Scholar] [CrossRef]
- Song, C.C.; Brown, A.; Winstead, R.; Yakubu, I.; Demehin, M.; Kumar, D.; Gupta, G. Early Initiation of Sodium-Glucose Linked Transporter Inhibitors (SGLT-2i) and Associated Metabolic and Electrolyte Outcomes in Diabetic Kidney Transplant Recipients. Endocrinol. Diabetes Metab. 2021, 4, e00185. [Google Scholar] [CrossRef]
- Sánchez Fructuoso, A.I.; Bedia Raba, A.; Banegas Deras, E.; Vigara Sánchez, L.A.; Valero San Cecilio, R.; Franco Esteve, A.; Cruzado Vega, L.; Gavela Martínez, E.; González Garcia, M.E.; Saurdy Coronado, P.; et al. Sodium-Glucose Cotransporter-2 Inhibitor Therapy in Kidney Transplant Patients with Type 2 or Post-Transplant Diabetes: An Observational Multicentre Study. Clin. Kidney J. 2023, 16, 1022–1034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Tocados, J.M.; Lloret, M.J.; Domínguez-Coral, J.D.; Tinoco Aranda, A.P.; Fayos de Arizón, L.; Massó Jiménez, E.; Bover, J.; Valdivielso, J.M.; Rodríguez-Ortiz, M.E. Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors. Int. J. Mol. Sci. 2025, 26, 5657. https://doi.org/10.3390/ijms26125657
Díaz-Tocados JM, Lloret MJ, Domínguez-Coral JD, Tinoco Aranda AP, Fayos de Arizón L, Massó Jiménez E, Bover J, Valdivielso JM, Rodríguez-Ortiz ME. Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors. International Journal of Molecular Sciences. 2025; 26(12):5657. https://doi.org/10.3390/ijms26125657
Chicago/Turabian StyleDíaz-Tocados, Juan Miguel, Maria Jesús Lloret, Juan Diego Domínguez-Coral, Adria Patricia Tinoco Aranda, Leonor Fayos de Arizón, Elisabet Massó Jiménez, Jordi Bover, José Manuel Valdivielso, and María Encarnación Rodríguez-Ortiz. 2025. "Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors" International Journal of Molecular Sciences 26, no. 12: 5657. https://doi.org/10.3390/ijms26125657
APA StyleDíaz-Tocados, J. M., Lloret, M. J., Domínguez-Coral, J. D., Tinoco Aranda, A. P., Fayos de Arizón, L., Massó Jiménez, E., Bover, J., Valdivielso, J. M., & Rodríguez-Ortiz, M. E. (2025). Magnesium Balance in Chronic Kidney Disease: Mineral Metabolism, Immunosuppressive Therapies and Sodium-Glucose Cotransporter 2 Inhibitors. International Journal of Molecular Sciences, 26(12), 5657. https://doi.org/10.3390/ijms26125657






