In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin
Abstract
1. Introduction
2. Results
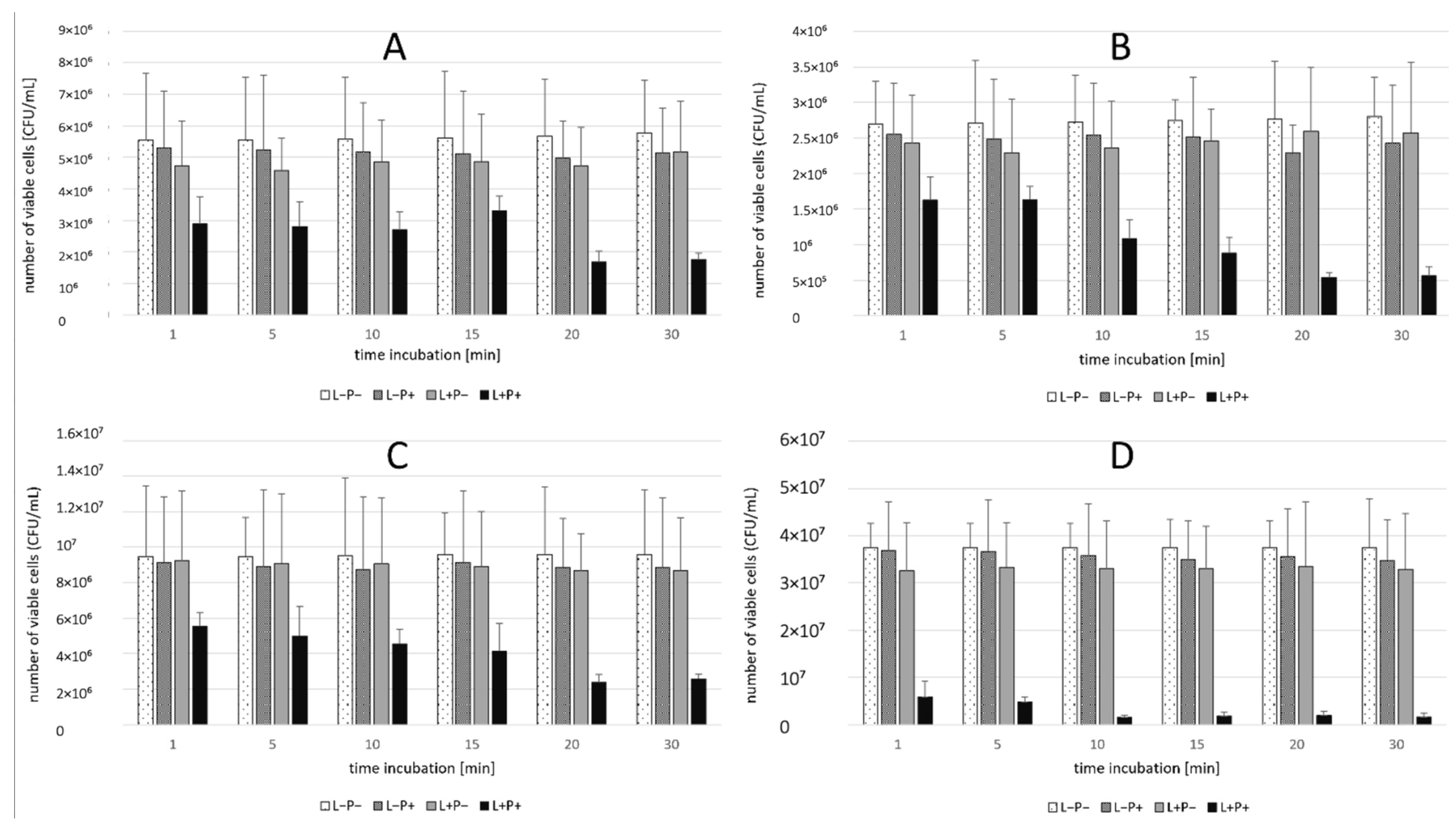
2.1. Phase 1 Results-Effect of Incubation Time
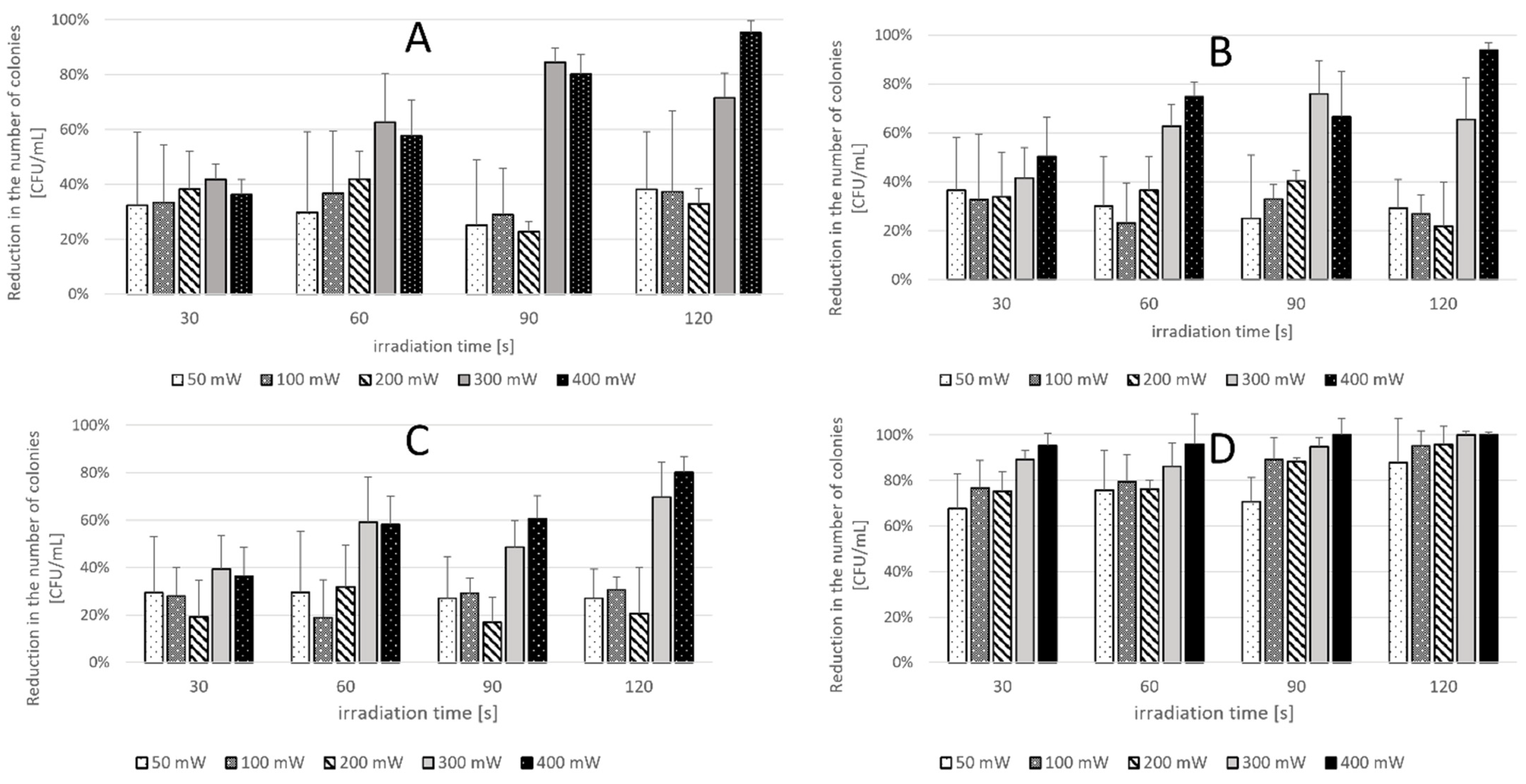
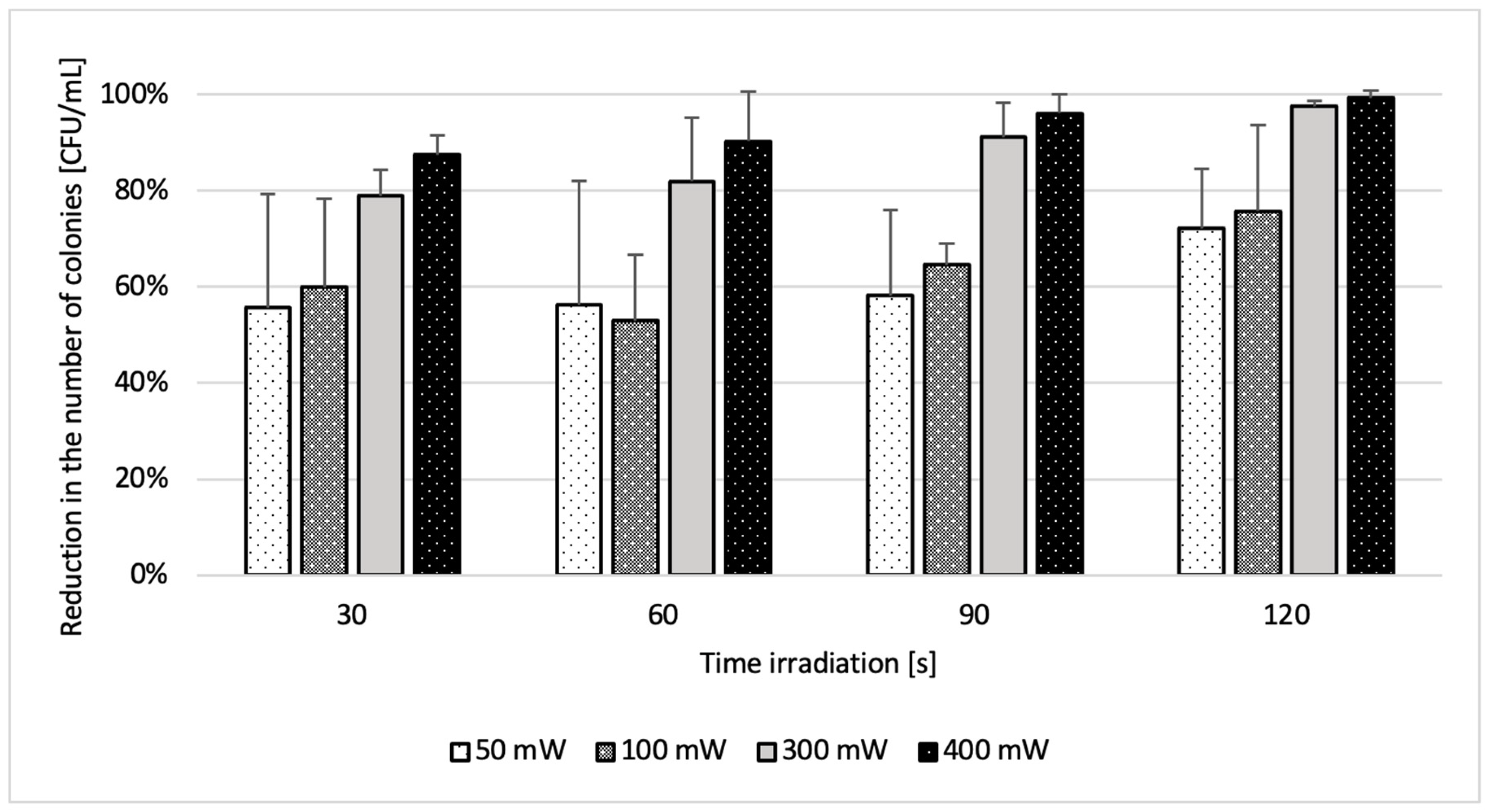
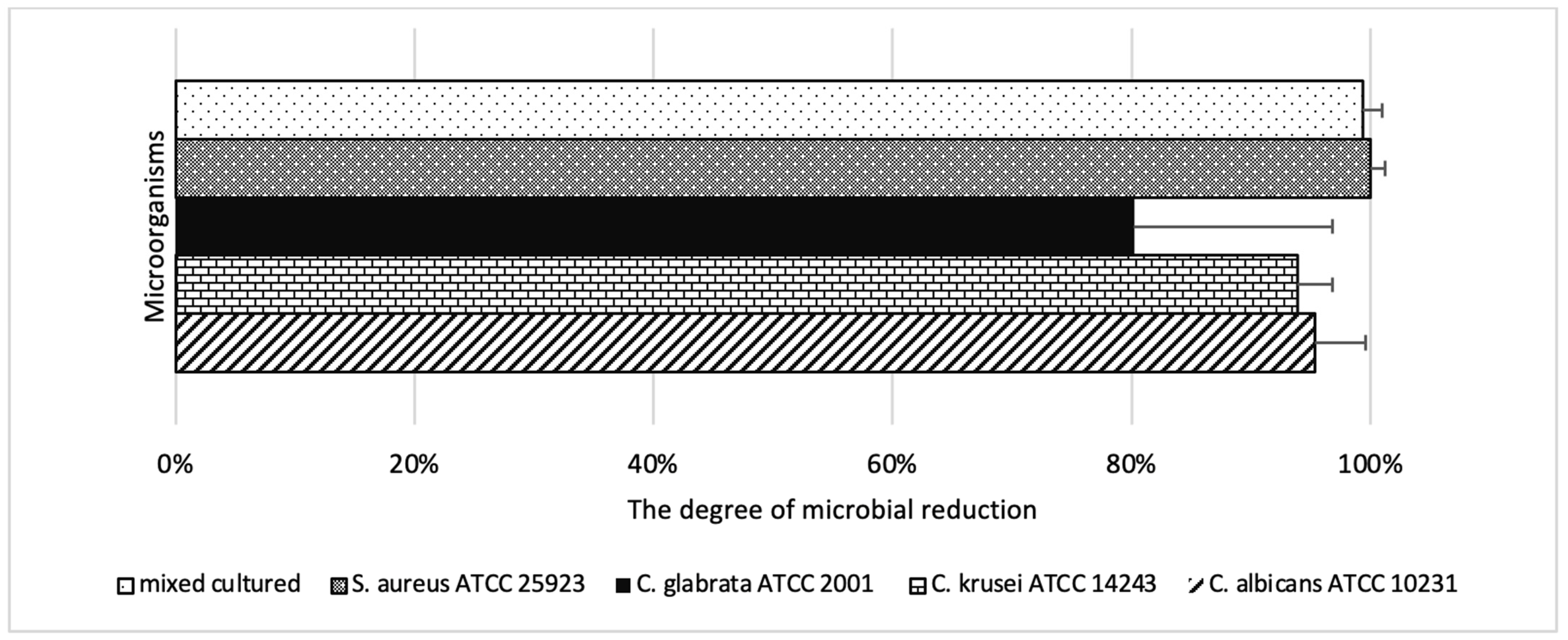
2.2. Phase 2 Results-Effect of Laser Parameters
3. Discussion
4. Materials and Methods
4.1. Reference Microbial Strains
4.2. Photosensitizer and Laser
4.3. First Phase of the Study
- (L+P+) aPDT group—suspension exposed to both photosensitizer and laser (n = 4);
- (L-P+) Photosensitizer group—suspension treated with the PS only, without laser exposure (n = 4);
- (L+P-) Light-only group—suspension exposed to laser only, without PS (n = 4);
- (L-P-) Control group—suspension not exposed to laser or PS (n = 4).
4.4. Second Phase of the Study
- (L+P+) aPDT group—suspension exposed to both the photosensitizer and laser (n = 4);
- (L-P+) Photosensitizer group—suspension treated with the PS only, without laser exposure (n = 4);
- (L+P-) Light-only group—suspension exposed to laser only, without PS (n = 4);
- (L-P-) Control group—suspension not exposed to either the laser or PS (n = 4).
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Hertel, M.; Schmidt-Westhausen, A.M.; Strietzel, F.P. Local, Systemic, Demographic, and Health-Related Factors Influencing Pathogenic Yeast Spectrum and Antifungal Drug Administration Frequency in Oral Candidiasis: A Retrospective Study. Clin. Oral Investig. 2016, 20, 1477–1486. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Baumgardner, D.J. Oral Fungal Microbiota: To Thrush and Beyond. J. Patient-Cent. Res. Rev. 2019, 6, 252–261. [Google Scholar] [CrossRef]
- Serrano, J.; López-Pintor, R.M.; Ramírez, L.; Fernández-Castro, M.; Sanz, M.; Melchor, S.; Peiteado, D.; Hernández, G. Risk Factors Related to Oral Candidiasis in Patients with Primary Sjögren’s Syndrome. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e700–e705. [Google Scholar] [CrossRef]
- Suryana, K.; Suharsono, H.; Antara, I.G.P.J. Factors Associated with Oral Candidiasis in People Living with HIV/AIDS: A Case Control Study. HIV/AIDS Res. Palliat. Care 2020, 12, 33–39. [Google Scholar] [CrossRef]
- Yang, Y.L.; Lo, H.J. Mechanisms of Antifungal Agent Resistance. J. Microbiol. Immunol. Infect. 2001, 34, 79–86. [Google Scholar]
- Lu, S.-Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef]
- Kaur, R.; Dhakad, M.S.; Goyal, R.; Kumar, R. Emergence of Non-Albicans Candida Species and Antifungal Resistance in Intensive Care Unit Patients. Asian Pac. J. Trop. Biomed. 2016, 6, 455–460. [Google Scholar] [CrossRef]
- Quindós, G. Epidemiology of Candidemia and Invasive Candidiasis: A Changing Face. Rev. Iberoam. Micol. 2014, 31, 42–48. [Google Scholar] [CrossRef]
- Darwazeh, A.M.G.; Darwazeh, T.A. What Makes Oral Candidiasis a Recurrent Infection? A Clinical View. J. Mycol. 2014, 2014, 758394. [Google Scholar] [CrossRef]
- Supriya, H.; Harishchandra, R.; Suhasini, P.D.; Rajalekshmi, V. Pathogenic Mechanism of Candida albicans in Oral Mucosa—A Review. Int. J. Health Sci. Res. 2016, 1, 489–497. [Google Scholar]
- Capoor, M.R.; Nair, D.; Deb, M.; Verma, P.K.; Srivastava, L.; Aggarwal, P. Emergence of non-albicans Candida species and antifungal resistance in a tertiary care hospital. Jpn. J. Infect. Dis. 2005, 58, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Wibawa, T. The Role of Virulence Factors in Candida albicans Pathogenicity. J. Med. Sci. 2016, 48, 58–68. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans Biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Jebali, A.; Hajjar, F.H.E.; Pourdanesh, F.; Hekmatimoghaddam, S.; Kazemi, B.; Masoudi, A.; Daliri, K.; Sedighi, N. Silver and Gold Nanostructures: Antifungal Property of Different Shapes of These Nanostructures on Candida Species. Med. Mycol. 2014, 52, 65–72. [Google Scholar]
- Kubizna, M.; Dawiec, G.; Wiench, R. Efficacy of Curcumin-Mediated Antimicrobial Photodynamic Therapy on Candida spp.—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8136. [Google Scholar] [CrossRef]
- Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. [Google Scholar] [CrossRef]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida spp.—A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- Kuśka-Kiełbratowska, A.; Wiench, R.; Mertas, A.; Bobela, E.; Kiełbratowski, M.; Lukomska-Szymanska, M.; Tanasiewicz, M.; Skaba, D. Evaluation of the Sensitivity of Selected Candida Strains to Ozonated Water—An In Vitro Study. Medicina 2022, 58, 1731. [Google Scholar] [CrossRef]
- Schito, G.C. The Importance of the Development of Antibiotic Resistance in Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12, S3–S8. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24 (Suppl. S1), 14–28. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Law, S.K.; Leung, A.W.N.; Xu, C. Photodynamic Action of Curcumin and Methylene Blue against Bacteria and SARS-CoV-2—A Review. Pharmaceuticals 2024, 17, 34. [Google Scholar] [CrossRef]
- Law, S.; Lo, C.; Han, J.; Yang, F.; Leung, A.W.; Xu, C. Design, Synthesis and Characterization of Novel Curcumin Derivatives. Nat. Prod. Chem. Res. 2020, 8, 367. [Google Scholar]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2007; Volume 595, pp. 1–75. [Google Scholar]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Keivan, Z.; Fouladvand, M.; Ramedani, E.; Asayesh, G. Antibacterial Activity of Indium Curcumin and Indium Diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Martins, C.V.; da Silva, D.L.; Neres, A.T.; Magalhães, T.F.; Watanabe, G.A.; Modolo, L.V.; Sabino, A.A.; de Fátima, A.; de Resende, M.A. Curcumin as a Promising Antifungal of Clinical Interest. J. Antimicrob. Chemother. 2009, 63, 337–339. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Comeau, P.; Manso, A. A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pharmaceutics 2023, 15, 2707. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivatives as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. [Google Scholar] [CrossRef]
- Corbin, F. Pathogen Inactivation of Blood Components: Current Status and Introduction of an Approach Using Riboflavin as a Photosensitizer. Int. J. Hematol. 2002, 76, 253–257. [Google Scholar] [CrossRef]
- Reddy, H.L.; Dayan, A.D.; Cavagnaro, J.; Gad, S.; Li, J.; Goodrich, R.P. Toxicity Testing of a Novel Riboflavin-Based Technology for Pathogen Reduction and White Blood Cell Inactivation. Transfus. Med. Rev. 2008, 22, 133–153. [Google Scholar] [CrossRef]
- Ma, J.; Shi, H.; Sun, H.; Li, J.; Bai, Y. Antifungal Effect of Photodynamic Therapy Mediated by Curcumin on Candida albicans Biofilms In Vitro. Photodiagn. Photodyn. Ther. 2019, 27, 280–287. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the Photodynamic Effects of Curcumin Against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef]
- Imaizumi, U.; Inaba, K.; Kurahashi, A.; Kuroda, H.; Sanuki, T.; Yoshida, A.; Yoshino, F.; Hamada, N. Effectiveness of Curcumin-Based Antimicrobial Photodynamic Therapy Against Staphylococcus aureus. J. Oral Sci. 2023, 65, 270–274. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Huang, Y.; Qi, M.; Yan, H.; Li, W.; Zhuang, H. Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment Against Staphylococcus aureus and Its Application in Juices. Molecules 2022, 27, 7136. [Google Scholar] [CrossRef] [PubMed]
- Trigo Gutierrez, J.K.; Zanatta, G.C.; Ortega, A.L.M.; Balastegui, M.I.C.; Sanita, P.V.; Pavarina, A.C.; Barbugli, P.A.; de Oliveira Mima, E.G. Encapsulation of Curcumin in Polymeric Nanoparticles for Antimicrobial Photodynamic Therapy. PLoS ONE 2017, 12, e0187418. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.G.; Chandran, R.; Altini, M.; Lemmer, J. Oral Candidosis in Relation to Oral Immunity. J. Oral Pathol. Med. 2014, 43, 563–569. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A Novel Photosensitization Treatment for the Inactivation of Fungal Spores and Cells Mediated by Curcumin. J. Photochem. Photobiol. B 2017, 173, 301–306. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of Clinical Isolates of Candida to Photodynamic Effects of Curcumin. Lasers Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef]
- Ito, T. Cellular and Subcellular Mechanisms of Photodynamic Action: The O2 Hypothesis as a Driving Force in Recent Research. Photochem. Photobiol. 1978, 28, 493–506. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, V. Photodynamic Antifungal Chemotherapy. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Calori, I.R.; de Oliveira, B.G.; Pavarina, A.C.; Gonçalves, R.S.; Caetano, W.; Tedesco, A.C.; Mima, E.G.O. Photo-Responsive Polymeric Micelles for the Light-Triggered Release of Curcumin Targeting Antimicrobial Activity. Front. Microbiol. 2023, 14, 1132781. [Google Scholar] [CrossRef]
- Sammarro Silva, K.J.; Lima, A.R.; Dias, L.D.; de Souza, M.; Nunes Lima, T.H.; Bagnato, V.S. Hydrogen Peroxide Preoxidation as a Strategy for Enhanced Antimicrobial Photodynamic Action Against Methicillin-Resistant Staphylococcus aureus. J. Water Health 2023, 21, 1922–1932. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Non-albicans Candida Species: Immune Response, Evasion Mechanisms, and New Plant-Derived Alternative Therapies. J. Fungi 2023, 9, 11. [Google Scholar] [CrossRef]
- Ksiezopolska, E.; Gabaldón, T. Evolutionary Emergence of Drug Resistance in Candida Opportunistic Pathogens. Genes 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C. Update on Antifungal Resistance in Aspergillus and Candida. Clin. Microbiol. Infect. 2014, 20, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products, and New Therapeutic Options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture Stomatitis: A Role for Candida Biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Andrade, M.C.; Ribeiro, A.P.; Dovigo, L.N.; Brunetti, I.L.; Giampaolo, E.T.; Bagnato, V.S.; Pavarina, A.C. Effect of Different Pre-Irradiation Times on Curcumin-Mediated Photodynamic Therapy Against Planktonic Cultures and Biofilms of Candida spp. Arch. Oral Biol. 2013, 58, 200–210. [Google Scholar] [CrossRef]
- Bakun, P.; Wysocki, M.; Stachowiak, M.; Musielak, M.; Dlugaszewska, J.; Mlynarczyk, D.T.; Sobotta, L.; Suchorska, W.M.; Goslinski, T. Quaternized Curcumin Derivative—Synthesis, Physicochemical Characteristics, and Photocytotoxicity, Including Antibacterial Activity After Irradiation with Blue Light. Molecules 2024, 29, 4536. [Google Scholar] [CrossRef]

| Microorganism | Max Reduction (%) | Time (min) | Min Reduction (%) | Time (min) |
|---|---|---|---|---|
| S. aureus ATCC 25923 | 95.9 | 10 | 843 | 1 |
| C. krusei ATCC 14243 | 80.6 | 20 | 39.8 | 1 |
| C. glabrata ATCC 2001 | 75.3 | 20 | 41.5 | 1 |
| C. albicans ATCC 10231 | 70.4 | 20 | 41.3 | 15 |
| S. aureus + C. albicans mix | 89.1 | 20 | 72.7 | 1 |
| Microorganism | Max Reduction (%) | Power (mW) | Time (s) | Min Reduction (%) | Power (mW) | Time (s) |
|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 100.0 | 300–400 | 90–120 | 67.6 | 50 | 30 |
| C. albicans ATCC 10231 | 95.3 | 400 | 120 | 25.1 | 50 | 90 |
| C. krusei ATCC 14243 | 93.9 | 400 | 120 | 23.3 | 100 | 60 |
| C. glabrata ATCC 2001 | 80.2 | 400 | 120 | 19.0 | 100 | 60 |
| S. aureus + C. albicans mix | 99.3 | 400 | 120 | 52.9 | 100 | 60 |
| C. albicans ATCC 10231 | 50 mW | 100 mW | 200 mW | 300 mW | 400 mW |
|---|---|---|---|---|---|
| 30 s | 32.4% | 33.4% | 38.2% | 41.8% | 36.3% |
| 60 s | 29.7% | 36.8% | 41.9% | 62.6% | 57.7% |
| 90 s | 25.1% | 28.9% | 22.8% | 84.5% | 80.2% |
| 120 s | 38.2% | 37.4% | 32.9% | 71.6% | 95.3% |
| C. krusei ATCC 14243 | 50 mW | 100 mW | 200 mW | 300 mW | 400 mW |
|---|---|---|---|---|---|
| 30 s | 36.5% | 32.7% | 33.8% | 41.5% | 50.3% |
| 60 s | 30.1% | 23.3% | 36.5% | 62.7% | 74.7% |
| 90 s | 25.1% | 32.9% | 40.3% | 75.9% | 66.6% |
| 120 s | 29.3% | 26.8% | 21.9% | 65.6% | 93.9% |
| C. glabrata ATCC 2001 | 50 mW | 100 mW | 200 mW | 300 mW | 400 mW |
|---|---|---|---|---|---|
| 30 s | 29.4% | 28.0% | 19.3% | 39.4% | 36.5% |
| 60 s | 29.6% | 19.0% | 31.9% | 59.2% | 58.1% |
| 90 s | 27.0% | 29.2% | 16.9% | 48.5% | 60.7% |
| 120 s | 27.0% | 30.7% | 20.7% | 69.6% | 80.2% |
| Mixed Cultured | 50 mW | 100 mW | 200 mW | 300 mW | 400 mW |
|---|---|---|---|---|---|
| 30 s | 55.7% | 59.9% | 66.7% | 78.9% | 87.5% |
| 60 s | 56.3% | 52.9% | 57.8% | 81.9% | 90.3% |
| 90 s | 58.2% | 64.6% | 76.0% | 91.2% | 96.2% |
| 120 s | 72.1% | 75.8% | 81.8% | 97.6% | 99.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkaczyk, M.; Mertas, A.; Kuśka-Kiełbratowska, A.; Fiegler-Rudol, J.; Bobela, E.; Cisowska, M.; Morawiec, T.; Skaba, D.; Wiench, R. In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin. Int. J. Mol. Sci. 2025, 26, 5645. https://doi.org/10.3390/ijms26125645
Tkaczyk M, Mertas A, Kuśka-Kiełbratowska A, Fiegler-Rudol J, Bobela E, Cisowska M, Morawiec T, Skaba D, Wiench R. In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin. International Journal of Molecular Sciences. 2025; 26(12):5645. https://doi.org/10.3390/ijms26125645
Chicago/Turabian StyleTkaczyk, Marcin, Anna Mertas, Anna Kuśka-Kiełbratowska, Jakub Fiegler-Rudol, Elżbieta Bobela, Maria Cisowska, Tadeusz Morawiec, Dariusz Skaba, and Rafał Wiench. 2025. "In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin" International Journal of Molecular Sciences 26, no. 12: 5645. https://doi.org/10.3390/ijms26125645
APA StyleTkaczyk, M., Mertas, A., Kuśka-Kiełbratowska, A., Fiegler-Rudol, J., Bobela, E., Cisowska, M., Morawiec, T., Skaba, D., & Wiench, R. (2025). In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin. International Journal of Molecular Sciences, 26(12), 5645. https://doi.org/10.3390/ijms26125645






