Anti-Inflammatory Effects of Solanum tuberosum L. Polysaccharide and Its Limited Gene Expression Profile
Abstract
1. Introduction
2. Results
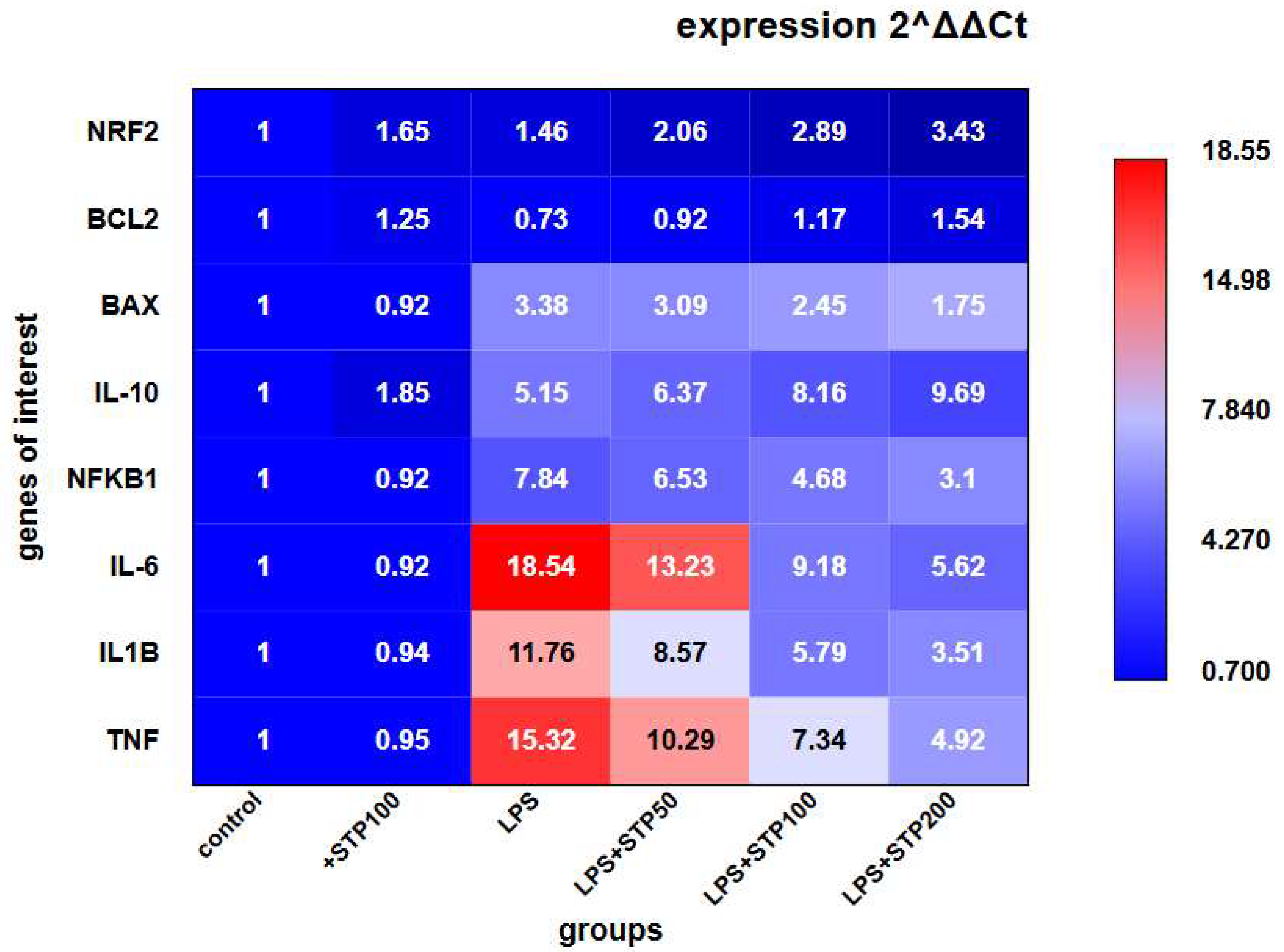
2.1. In Vitro Study of Anti-Inflammatory Activity
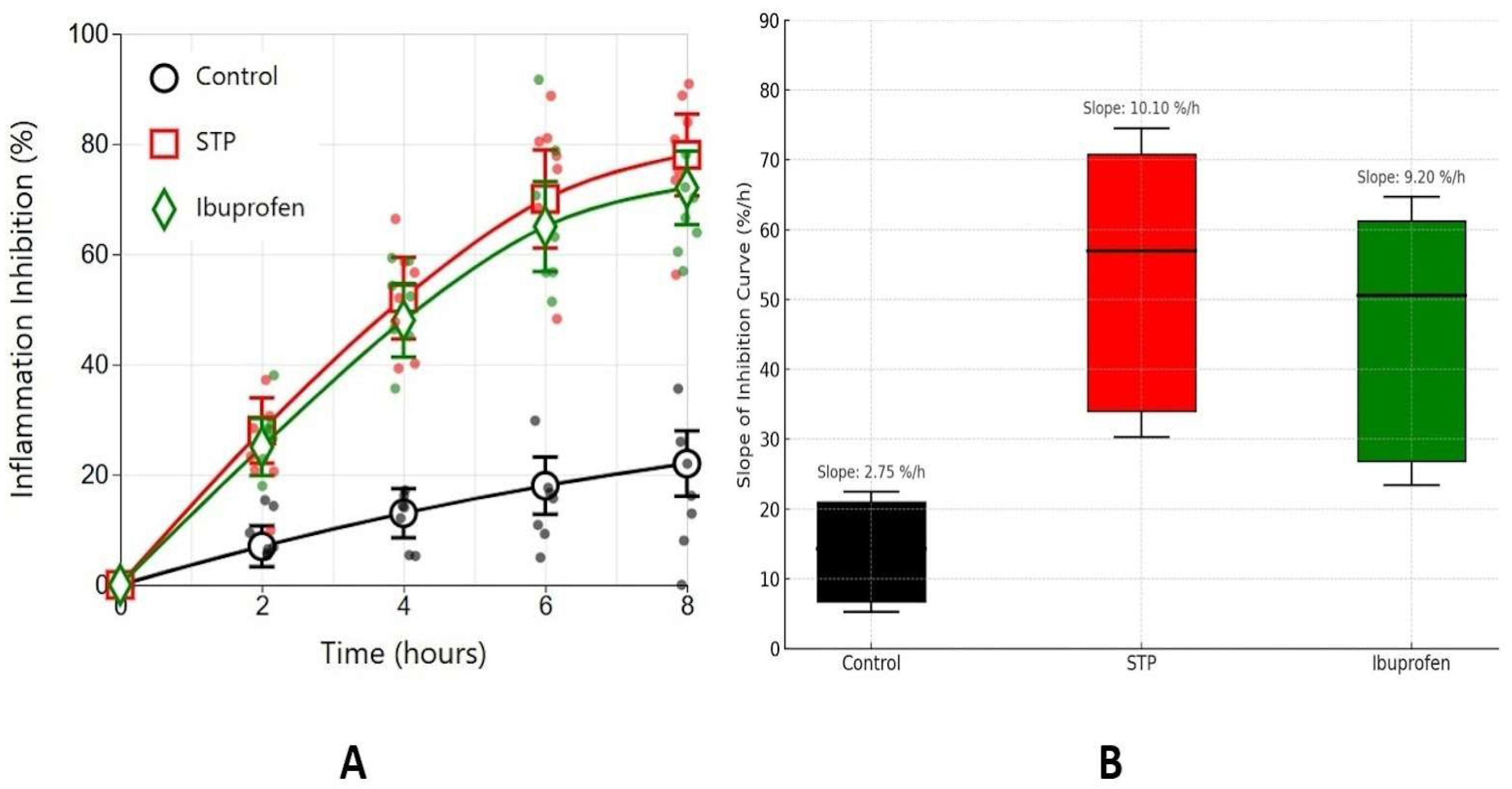
2.2. Carrageenan-Induced Oedema Model
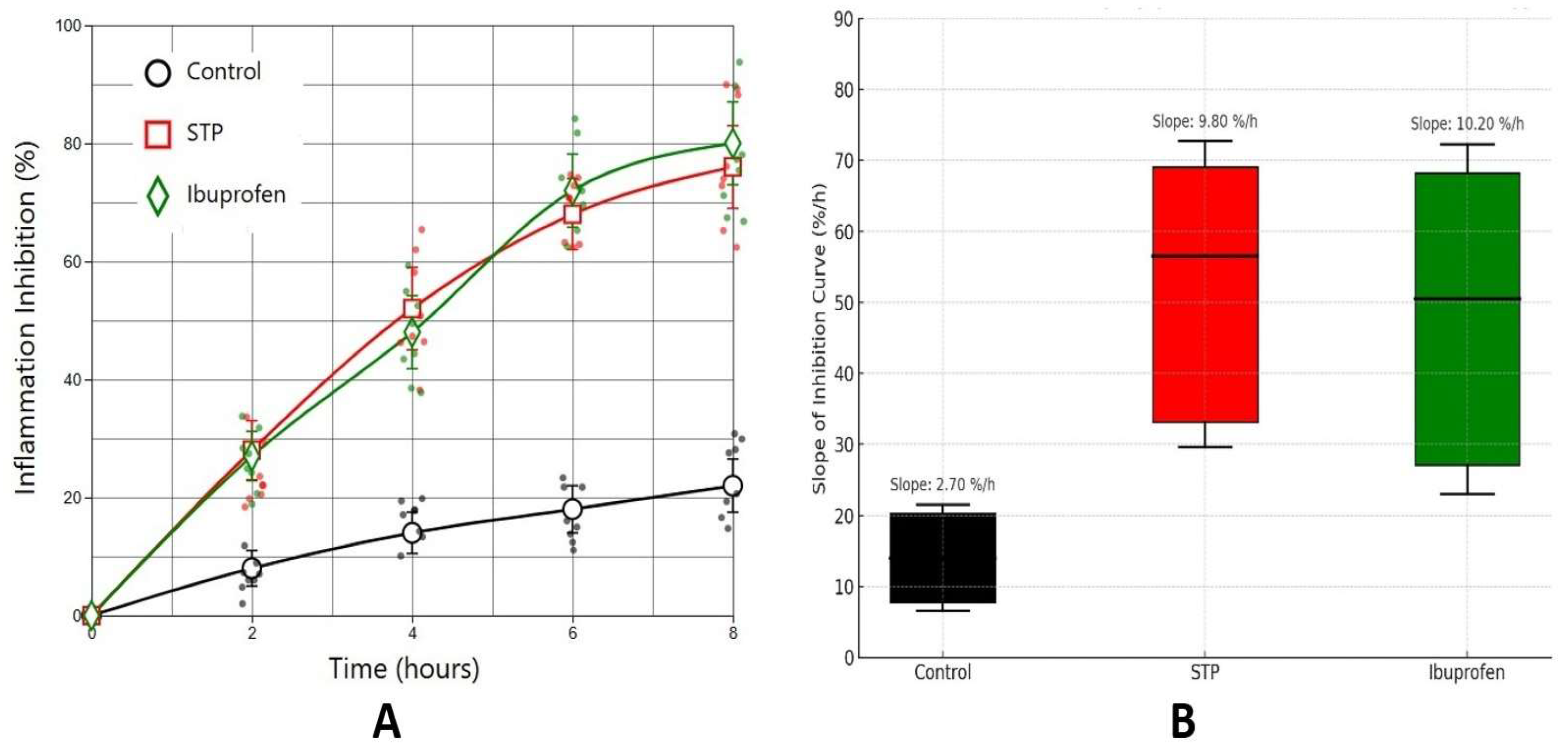
2.3. Air Pouch Granuloma Model
3. Discussion
4. Materials and Methods
4.1. Studied Substance
4.2. In Vivo Study of Anti-Inflammatory Activity
4.2.1. Experimental Animals
4.2.2. Carrageenan-Induced Oedema Model
- Control group—animals received a sterile 0.9% NaCl solution (n = 8);
- Experimental group—animals received a sterile 0.9% NaCl solution containing STP at a dose of 500 µg/rat (n = 8);
- Comparison group—animals received ibuprofen intraperitoneally at a dose of 100 mg/kg (n = 8).
4.2.3. Air Pouch Granuloma Model
- Control group—animals received a sterile 0.9% NaCl solution (n = 8);
- Experimental group—animals received a sterile 0.9% NaCl solution containing STP at a dose of 500 µg/rat (n = 8);
- Comparison group—animals received intraperitoneally ibuprofen at a dose of 100 mg/kg (n = 8).
4.3. In Vitro Study of Anti-Inflammatory Activity
4.3.1. Cell Line and Culture Conditions
4.3.2. Differentiation of Monocytes into Macrophage-like Cells
4.3.3. Induction of Inflammation and Treatment with the Investigated Substance
4.3.4. Gene Expression Analysis by Real-Time Quantitative PCR
Primers Design
RNA Extraction and Reverse Transcription
Real-Time Quantitative PCR
Data Analysis and Quality Control
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| STP | Solanum tuberosum L. polysaccharide |
| cDNA | Complementary DNA |
| IQR | Interquartile range |
References
- Shen, Y.; Zhao, H.; Wang, X.; Wu, S.; Wang, Y.; Wang, C.; Zhang, Y.; Zhao, H. Unraveling the web of defense: The crucial role of polysaccharides in immunity. Front. Immunol. 2024, 15, 1406213. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally occurring immune modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Generalov, E.; Yakovenko, L. Receptor basis of biological activity of polysaccharides. Biophys. Rev. 2023, 15, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, Q.; Feng, J.; Zhang, X.; Li, T.; Liu, S.; Chen, Y.; Li, X.; Wu, Q.; Xue, Y.; et al. β-Glucan produced by Lentinus edodes suppresses breast cancer progression via the inhibition of macrophage M2 polarization by integrating autophagy and inflammatory signals. Immun. Inflamm. Dis. 2023, 11, e876. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signalling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Ayeka, P.A. Potential of mushroom compounds as immunomodulators in cancer immunotherapy: A review. Evid. Based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef]
- Arifkhodzhaev, A.O. Galactans and galactan-containing polysaccharides of higher plants. Chem. Nat. Compd. 2000, 36, 229–244. [Google Scholar] [CrossRef]
- Goellner, E.M.; Utermoehlen, J.; Kramer, R.; Classen, B. Structure of arabinogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinogalactans. Carbohydr. Polym. 2011, 86, 1739–1744. [Google Scholar] [CrossRef]
- Generalov, E.A. Study of the structure and immunoenhancing activity of glucan ADVA. Mosc. Univ. Phys. 2013, 68, 470–477. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan: Multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Generalov, E.A.; Levashova, N.T.; Sidorova, A.E.; Chumakov, P.M.; Yakovenko, L.V. An autowave model of the bifurcation behavior of transformed cells in response to polysaccharide. Biophysics 2017, 62, 717–721. [Google Scholar] [CrossRef]
- Generalova, L.V.; Laryushkin, D.P.; Leneva, I.A.; Ivanina, A.V.; Trunova, G.V.; Dolinnyi, S.V.; Generalov, E.A. Evaluation of the polysaccharide “Immeran” activity in Syrian hamsters’ model of SARS-CoV-2. Viruses 2024, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.W.; Li, S.S.; Ding, W.L.; Zhang, C.; Rehman, M.U.; Tareen, M.F.; Wang, L.; Huang, S.-C. From structure to function: A comprehensive overview of polysaccharide roles and applications. Food Front. 2025, 6, 15–39. [Google Scholar] [CrossRef]
- Generalov, E.; Laryushkin, D.; Kritskaya, K.; Kulchenko, N.; Sinitsyn, A.; Yakovenko, L.; Generalova, L.; Belostotsky, N. Immune basis of therapeutic effects of Solanum tuberosum L. polysaccharide on chronic peptic ulcer healing. Pharmaceuticals 2025, 18, 502. [Google Scholar] [CrossRef]
- Generalov, E.A.; Yakovenko, L.V. Composition and mitogenic activity of polysaccharide from Solanum tuberosum L. Biofizika 2023, 68, 856–862. [Google Scholar] [CrossRef]
- Lin, Z.H.; Phan, S.N.; Tran, D.N.; Lu, M.K.; Lin, T.Y. Anti-inflammatory and anticancer effects of polysaccharides from Antrodia cinnamomea: A review. J. Chin. Med. Assoc. 2025, 88, 1–11. [Google Scholar] [CrossRef]
- Murgas, P.; Cornejo, F.A.; Merino, G.; von Bernhardi, R. SR-A regulates the inflammatory activation of astrocytes. Neurotox. Res. 2014, 25, 68–80. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.; Shi, S.; Bligh, S.A.; Li, Y.; Jiang, Y.; Huang, D.; Ke, Y.; Wang, S. A RG-II Type Polysaccharide Purified from Aconitum coreanum Alleviates Lipopolysaccharide-Induced Inflammation by Inhibiting the NF-κB Signal Pathway. PLoS ONE 2014, 9, e99697. [Google Scholar] [CrossRef]
- Herbert, J.-M.; Maffrand, J.-P. Effect of pentosan polysulphate, standard heparin and related compounds on protein kinase C activity. Biochim. Biophys. Acta 1991, 1091, 432–441. [Google Scholar] [CrossRef]
- Maiorov, S.A.; Laryushkin, D.P.; Kritskaya, K.A.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. The Role of Ion Channels and Intracellular Signalling Cascades in the Inhibitory Action of WIN 55,212-2 upon Hyperexcitation. Brain Sci. 2024, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Maiorov, S.A.; Zinchenko, V.P.; Laryushkin, D.P.; Tuleukhanov, S.T.; Kairat, B.K.; Kosenkov, A.M. Pharmacological inhibition of PLC and PKC triggers epileptiform activity in hippocampal neurons. Epilepsy Res. 2025, 214, 107570. [Google Scholar] [CrossRef] [PubMed]
- Generalova, L.V.; Kritskaya, K.A.; Laryushkin, D.P.; Generalov, E.A. Polysaccharide from Solanum tuberosum L. as a potential antiulcer drug. Biofizika 2024, 69, 1376–1381. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Lee, H.; Kim, G.Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Bae, T.; Hallis, S.P.; Kwak, M.K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]
- Delano, D.L.; Montesinos, M.C.; D’Eustachio, P.; Wiltshire, T.; Cronstein, B.N. An interaction between genetic factors and gender determines the magnitude of the inflammatory response in the mouse air pouch model of acute inflammation. Inflammation 2005, 29, 1–7. [Google Scholar] [CrossRef]
- Rossetti, A.C.; Paladini, M.S.; Trepci, A.; Mallien, A.; Riva, M.A.; Gass, P.; Molteni, R. Differential Neuroinflammatory Response in Male and Female Mice: A Role for BDNF. Front. Mol. Neurosci. 2019, 12, 166. [Google Scholar] [CrossRef]
- Kosyreva, A.M.; Dzhalilova, D.S.; Makarova, O.V.; Tsvetkov, I.S.; Zolotova, N.A.; Diatroptova, M.A.; Ponomarenko, E.A.; Mkhitarov, V.A.; Khochanskiy, D.N. Sex differences of inflammatory and immune response in pups of Wistar rats with SIRS. Sci. Rep. 2020, 10, 15884. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes. FAOLEX. Available online: https://www.fao.org/faolex/results/details/ru/c/LEX-FAOC098296/ (accessed on 15 January 2025).
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar] [PubMed]
- Whiteley, P.E.; Dalrymple, S.A. Models of inflammation: Carrageenan-induced paw edema in the rat. Curr. Protoc. Pharmacol. 1998, 5.4.1–5.4.3. [Google Scholar] [CrossRef]
- Colville-Nash, P.; Lawrence, T. Air-pouch models of inflammation and modifications for the study of granuloma-mediated cartilage degradation. Methods Mol. Biol. 2003, 225, 181–189. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef]
- Kritskaya, K.A.; Fedotova, E.I.; Nadeev, A.D.; Berezhnov, A.V. Silver Bullet of Acidification: Studying Anti-PD Neuroprotective Mechanisms of Transient pH-Decrease. Biocell 2025, 49, 451–464. [Google Scholar] [CrossRef]
| Gene (Protein) | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| TNF (TNF-α) | CCTCTCTCTAATCAGCCCTCTG | GAGGACCTGGGAGTAGATGAG |
| IL1B (IL-1β) | ATGATGGCTTATTACAGTGGCA | GTCGGAGATTCGTAGCTGGA |
| IL6 | ACTCACCTCTTCAGAACGAAT | CCATCTTTGGAAGGTTCAGGTTG |
| NFKB1 | AACAGAGAGGATTTCGTTTCC | TTTGACCTGAGGGTAAGACTTCT |
| IL10 | GACTTTAAGGGTTACCTGGGT | TCACATGCGCCTTGATGTCTG |
| NRF2 | TTCCCGGTCACATCGAGAG | TCCTGTTGCATACCGTCTAAATC |
| BAX | CCCGAGAGGTCTTTTTCCGAG | CCAGCCCATGATGGTTCTGAT |
| BCL2 | GGTGGGGTCATGTGTGTGG | CGGTTCAGGTACTCAGTCATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generalov, E.; Grigoryan, I.; Minaichev, V.; Sinitsyna, O.; Yakovenko, L.; Sinitsyn, A.; Generalova, L. Anti-Inflammatory Effects of Solanum tuberosum L. Polysaccharide and Its Limited Gene Expression Profile. Int. J. Mol. Sci. 2025, 26, 5562. https://doi.org/10.3390/ijms26125562
Generalov E, Grigoryan I, Minaichev V, Sinitsyna O, Yakovenko L, Sinitsyn A, Generalova L. Anti-Inflammatory Effects of Solanum tuberosum L. Polysaccharide and Its Limited Gene Expression Profile. International Journal of Molecular Sciences. 2025; 26(12):5562. https://doi.org/10.3390/ijms26125562
Chicago/Turabian StyleGeneralov, Evgenii, Ilya Grigoryan, Vladislav Minaichev, Olga Sinitsyna, Leonid Yakovenko, Arkady Sinitsyn, and Liubov Generalova. 2025. "Anti-Inflammatory Effects of Solanum tuberosum L. Polysaccharide and Its Limited Gene Expression Profile" International Journal of Molecular Sciences 26, no. 12: 5562. https://doi.org/10.3390/ijms26125562
APA StyleGeneralov, E., Grigoryan, I., Minaichev, V., Sinitsyna, O., Yakovenko, L., Sinitsyn, A., & Generalova, L. (2025). Anti-Inflammatory Effects of Solanum tuberosum L. Polysaccharide and Its Limited Gene Expression Profile. International Journal of Molecular Sciences, 26(12), 5562. https://doi.org/10.3390/ijms26125562







