Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium
Abstract
1. Introduction
2. MMPs as Biomarkers in Human Diseases
3. MMPs as Biomarkers in Epithelial Cell Differentiation
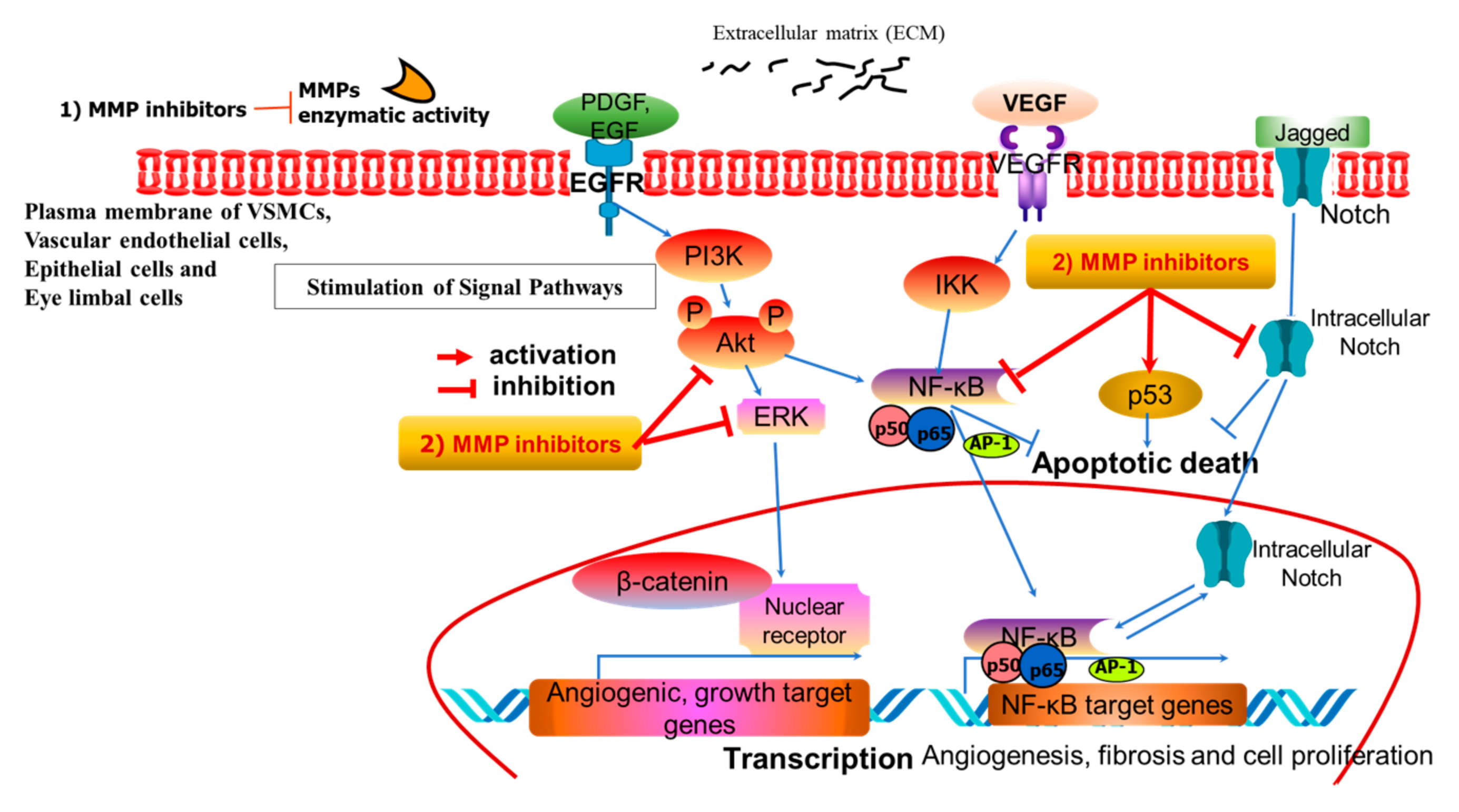
4. Functional Roles and Expression of MMPs in Vascular Diseases
5. MMPs in Pterygium as an Orphan Disease
5.1. MMPs in Intracellular Signaling of Pterygium
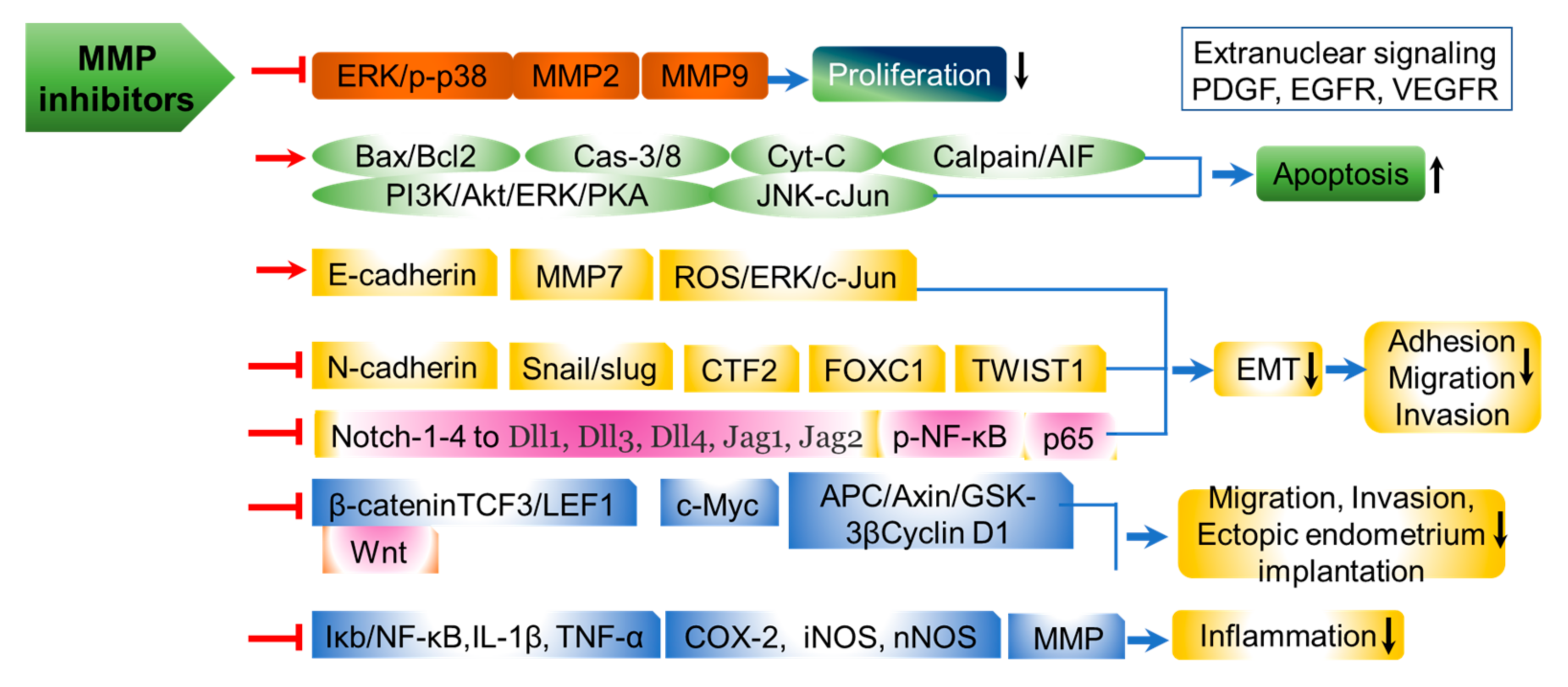
5.2. Prospective for MMP Inhibition in Pterygium Therapy
6. MMPs in Endometriosis
6.1. MMP Signaling in Endometriosis
6.2. MMP and TIMP Regulation in Endometriosis
7. Exploration and Development of MMP Inhibitory Agents as Candidates for Preventive and Therapeutic Drugs Targeting Vascular, Endometriosis, and Pterygium Diseases
7.1. Computational and Artificial Intelligence (AI)-Driven Approaches for the Development of MMP Inhibitory Agents Targeting the MMP S1 Pocket-Binding Hydrophobic Group
7.2. Chemical Synthetic MMP Inhibitors
7.3. Natural MMP-9 Inhibitory Agents
7.4. MMP Inhibitors Based on Multiple Molecular Targets of TIMP Domains
7.5. MMP Inhibitors Based on Natural Products
7.6. MMP-Targeting miRNA and Monoclonal Antibody (Mab)-Based MMP Inhibitors
8. The Representative Development Status Under Clinical Trials of Human MMP Therapeutics
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kim, I.S.; Yang, W.S.; Kim, C.H. Physiological Properties, Functions, and Trends in the Matrix Metalloproteinase Inhibitors in Inflammation-Mediated Human Diseases. Curr. Med. Chem. 2023, 30, 2075–2112. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Mu, X.; Bellayr, I.; Pan, H.; Choi, Y.; Li, Y. Regeneration of soft tissues is promoted by MMP1 treatment after digit amputation in mice. PLoS ONE 2013, 8, e59105. [Google Scholar] [CrossRef][Green Version]
- Bräuninger, H.; Krüger, S.; Bacmeister, L.; Nyström, A.; Eyerich, K.; Westermann, D.; Lindner, D. Matrix metalloproteinases in coronary artery disease and myocardial infarction. Basic Res. Cardiol. 2023, 118, 18. [Google Scholar] [CrossRef]
- Moon, S.K.; Cha, B.Y.; Lee, Y.C.; Ko, J.H.; Runge, M.S.; Patterson, C.; Kim, C.H. Age-related changes in matrix metalloproteinase-9 regulation in cultured mouse aortic smooth muscle cells. Exp. Gerontol. 2003, 39, 123–131. [Google Scholar] [CrossRef]
- Moon, S.K.; Jung, S.Y.; Choi, Y.-H.; Lee, Y.C.; Kim, C.-H. Platelet-derived growth factor induces p21/WAF1 promoter in vascular smooth muscle cells via activation of an Sp1 site. FEBS Lett. 2003, 552, 130–134. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, Y.K.; Chung, T.W.; Kim, J.R.; Moon, S.K.; Kim, C.H.; Park, Y.G. Regulation of matrix metalloproteinase-9 expression between gingival fibroblast cells from old and young rats. Biochem. Biophys. Res. Commun. 2009, 378, 152–156. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, T.W.; Choi, H.J.; Kwak, C.H.; Song, K.H.; Suh, S.J.; Kwon, K.M.; Chang, Y.C.; Park, Y.G.; Chang, H.W.; et al. Ganglioside GM3 participates in the TGF-β1-induced epithelial-mesenchymal transition of human lens epithelial cells. Biochem. J. 2013, 449, 241–251. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Pino, G.; Banh, A.; Nathu, Z.; Howchin, D.; Margetts, P.; Sivak, J.G.; West-Mays, J.A. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am. J. Pathol. 2006, 168, 69–79. [Google Scholar] [CrossRef]
- Tang, J.; Frey, J.M.; Wilson, C.L.; Moncada-Pazos, A.; Levet, C.; Freeman, M.; Rosenfeld, M.E.; Stanley, E.R.; Raines, E.W.; Bornfeldt, K.E. Neutrophil and Macrophage Cell Surface Colony-Stimulating Factor 1 Shed by ADAM17 Drives Mouse Macrophage Proliferation in Acute and Chronic Inflammation. Mol. Cell Biol. 2018, 38, e00103-18. [Google Scholar] [CrossRef]
- Su, T.A.; Shihadih, D.S.; Cao, W.; Detomasi, T.C.; Heffern, M.C.; Jia, S.; Stahl, A.; Chang, C.J. A Modular Ionophore Platform for Liver-Directed Copper Supplementation in Cells and Animals. J. Am. Chem. Soc. 2018, 140, 13764–13774. [Google Scholar] [CrossRef] [PubMed]
- Smiljanic, K.; Dobutovic, B.; Obradovic, M.; Nikolic, D.; Marche, P.; Isenovic, E.R. Involvement of the ADAM 12 in thrombin-induced rat’s VSMCs proliferation. Curr. Med. Chem. 2011, 18, 3382–3386. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Glomset, J.A. Atherosclerosis and the arterial smooth muscle cell. Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 1973, 180, 1332–1339. [Google Scholar] [CrossRef]
- Peeters, W.; Moll, F.L.; Vink, A.; van der Spek, P.J.; de Kleijn, D.P.; de Vries, J.P.; Verheijen, J.H.; Newby, A.C.; Pasterkamp, G. Collagenase matrix metalloproteinase-8 expressed in atherosclerotic carotid plaques is associated with systemic cardiovascular outcome. Eur. Heart J. 2011, 32, 2314–2325. [Google Scholar] [CrossRef]
- Moon, S.K.; Cha, B.Y.; Kim, C.H. ERK1/2 mediates TNF-α-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kB and AP-1: Involvement of the Ras dependent pathway. Cell. Physiol. 2004, 198, 417. [Google Scholar] [CrossRef]
- Moon, S.K.; Kim, H.M.; Kim, C.H. PTEN Induces G1 Cell Cycle Arrest and Inhibits MMP-9 Expression via the Regulation of NF-kB and AP-1 in Vascular Smooth Muscle Cells. Arch. Biochem. Biophys. 2004, 421, 267–276. [Google Scholar] [CrossRef]
- Moon, S.K.; Jung, S.Y.; Choi, Y.H.; Lee, Y.C.; Patterson, C.; Kim, C.H. PDTCInduces G1 cell cycle arrest in vascular smooth muscle cells through inducing p21 Cip1 expression: Involvement of p38 mitogen activated protein kinase. J. Cell. Phys. 2004, 198, 310–323. [Google Scholar] [CrossRef]
- Martin-Lopez, J.; Perez-Rico, C.; Benito-Martinez, S.; PerezKohler, B.; Bujan, J.; Pascual, G. The role of the stromal extracellular matrix in the development of pterygium pathology: An update. J. Clin. Med. 2021, 10, 1530. [Google Scholar] [CrossRef]
- Mu, D.; Cambier, S.; Fjellbirkeland, L.; Baron, J.L.; Munger, J.S.; Kawakatsu, H.; Sheppard, D.; Broaddus, V.C.; Nishimura, S.L. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP–dependent activation of TGF-β1. J. Cell Biol. 2002, 157, 493–507. [Google Scholar] [CrossRef]
- Li, D.Q.; Lee, S.B.; Gunja-Smith, Z.; Liu, Y.; Solomon, A.; Meller, D.; Tseng, S.C. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch. Ophthalmol. 2001, 119, 71–80. [Google Scholar]
- Yang, S.F.; Lin, C.Y.; Yang, P.Y.; Chao, S.C.; Ye, Y.Z.; Hu, D.N. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.S.; Chang, W.S.; Chou, A.K.; Hsia, N.Y.; Hung, Y.W.; Lin, C.W.; Wu, C.W.; Huang, C.Y.; Wu, M.F.; Liao, C.H.; et al. The Association of MMP-8 Genotypes with Pterygium. Vivo 2018, 32, 41–46. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jung, J.C.; Gum, S.I.; Park, S.B.; Ma, J.Y.; Kim, Y.I.; Lee, K.W.; Park, Y.J. Inhibition of pterygium fibroblast migration and outgrowth by bevacizumab and cyclosporine A involves down-regulation of matrix metalloproteinases-3 and -13. PLoS ONE 2017, 12, e0169675. [Google Scholar] [CrossRef]
- Kim, H.D.; Choi, H.; Park, J.Y.; Kim, C.H. Distinct structural basis and catalytic classification of matrix metalloproteinases and their endogenous tissue inhibitors with glycosylation issue in cellular and tissue regulation. Arch. Biochem. Biophys. 2025, 23, 110436. [Google Scholar] [CrossRef]
- Bradley, J.C.; Yang, W.; Bradley, R.H.; Reid, T.W.; Schwab, I.R. The science of pterygia. Br. J. Ophthalmol. 2010, 94, 815–820. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. Active matrilysin (MMP-7) in human pterygia: Potential role in angiogenesis, Invest. Ophthalmol. Vis. Sci. 2001, 42, 1963–1968. [Google Scholar]
- Kudo, Y.; Iizuka, S.; Yoshida, M.; Tsunematsu, T.; Kondo, T.; Subarnbhesaj, A.; Deraz, E.M.; Siriwardena, S.B.S.M.; Tahara, H.; Ishimaru, N.; et al. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J. Biol. Chem. 2012, 287, 38716–38728. [Google Scholar] [CrossRef]
- Masitas, C.; Peng, Z.; Wang, M.; Konai, M.M.; Avila-Cobian, L.F.; Lemieux, L.; Hovanesian, J.; Grady, J.E.; Mobashery, S.; Chang, M. Matrix Metalloproteinase-14 as an Instigator of Fibrosis in Human Pterygium and Its Pharmacological Intervention. ACS Pharmacol. Transl. Sci. 2022, 5, 555–561. [Google Scholar] [CrossRef]
- Koranyi, G.; Artzen, D.; Seregard, S.; Kopp, E.D. Intraoperative mitomycin C versus autologous conjunctival autograft in surgery of primary pterygium with four-year follow-up. Acta Ophthalmol. 2012, 90, 266–270. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Chiang, C.C.; Yeh, K.T.; Lee, H.; Cheng, Y.W. Effect of TIMP-1 and MMP in pterygium invasion. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3462–3467. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, Q.; Zhao, C.; Yang, X.; Cun, Q.; Yang, W.; Zhang, Y.; Zhu, Y.; Zhong, H. The in vitro anti-fibrotic effect of Pirfenidone on human pterygium fibroblasts is associated with down-regulation of autocrine TGF-β and MMP-1. Int. J. Med. Sci. 2020, 17, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Motarjemizadeh, Q.; Aidenloo, N.S.; Sepehri, S. A comparative study of different concentrations of topical bevacizumab on the recurrence rate of excised primary pterygium: A short-term follow-up study. Int. Ophthalmol. 2016, 36, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Kim, C.D.; Lee, J.S. Effect of Bevacizumab on Human Tenon’s Fibroblasts Cultured from Primary and Recurrent Pterygium. Korean J. Physiol. Pharmacol. 2015, 19, 357–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ke, J.; Ye, J.; Li, M.; Zhu, Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules 2021, 11, 1739. [Google Scholar] [CrossRef]
- Harmsen, M.J.; Wong, C.F.C.; Mijatovic, V.; Griffioen, A.W.; Groenman, F.; Hehenkamp, W.J.K.; Huirne, J.A.F. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: A systematic review. Hum. Reprod. Update 2019, 25, 647–671. [Google Scholar] [CrossRef]
- Lang, G.A.; Yeaman, G.R. Autoantibodies in endometriosis sera recognize a Thomsen–Friedenreich-like carbohydrate antigen. J. Autoimmun. 2001, 16, 151–161. [Google Scholar] [CrossRef]
- Rossi, G.R.; Trindade, E.S.; Souza-Fonseca-Guimaraes, F. Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function. Front. Immunol. 2020, 11, 73. [Google Scholar] [CrossRef]
- Ito, T.K.; Ishii, G.; Saito, S.; Yano, K.; Hoshino, A.; Suzuki, T.; Ochiai, A. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 2009, 113, 2363–2369. [Google Scholar] [CrossRef]
- Shiraishi, K.; Mimura, K.; Kua, L.F.; Koh, V.; Siang, L.K.; Nakajima, S.; Fujii, H.; Shabbir, A.; Yong, W.P.; So, J.; et al. Inhibition of MMP activity can restore NKG2D ligand expression in gastric cancer, leading to improved NK cell susceptibility. J. Gastroenterol. 2016, 51, 1101–1111. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J.; Ding, X.; Sun, Y.; Cui, Z.; Liu, W. Tanshinone I Inhibits IL-1beta-Induced Apoptosis, Inflammation And Extracellular Matrix Degradation In Chondrocytes CHON-001 Cells And Attenuates Murine Osteoarthritis. Drug Des. Dev. Ther. 2019, 13, 3559–3568. [Google Scholar] [CrossRef]
- Sillem, M.; Prifti, S.; Koch, A.; Neher, M.; Jauckus, J.; Runnebaum, B. Regulation of matrix metalloproteinases and their inhibitors in uterine endometrial cells of patients with and without endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2001, 95, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.M.; Lai, Z.Z.; Ha, S.Y.; Yang, H.L.; Liu, L.B.; Wang, Y.; Shi, J.W.; Ruan, L.Y.; Ye, J.F.; Wu, J.N.; et al. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction 2020, 159, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Ma, J.; Peng, Y.; Sun, M.; Xu, K.; Wu, R.; Lin, J. Autocrine Production of Interleukin-34 Promotes the Development of Endometriosis through CSF1R/JAK3/STAT6 signaling. Sci. Rep. 2019, 9, 16781. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Jana, S.; DasMahapatra, P.; Swarnakar, S. EGFR-mediated matrix metalloproteinase-7 up-regulation promotes epithelial-mesenchymal transition via ERK1-AP1 axis during ovarian endometriosis progression. FASEB J. 2018, 32, 4560–4572. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, T.W.; Choi, H.J.; Han, J.H.; Choi, J.H.; Kim, C.H.; Ha, K.T. Increased α2-6 sialylation of endometrial cells contributes to the development of endometriosis. Exp. Mol. Med. 2018, 50, 164. [Google Scholar] [CrossRef]
- Fields, G.B. The rebirth of matrix metalloproteinase inhibitors: Moving beyond the dogma. Cells 2019, 8, 2. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, W.; Wang, L.; Shan, L.; Li, H.; Huang, J.; Sun, Q.; Zhang, W. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1). Eur. J. Med. Chem. 2013, 62, 148–157. [Google Scholar] [CrossRef]
- Umedera, K.; Yoshimori, A.; Bajorath, J.; Nakamura, H. Design of MMP-1 inhibitors via SAR transfer and experimental validation. Sci. Rep. 2022, 12, 20915. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Altintop, M.D.; Atli, O.; Sever, B.; Baysal, M.; Temel, H.E.; Demirci, F.; Ozdemir, A. Synthesis and Evaluation of A New Series of Thiazole Derivatives as Potential Antitumor Agents and MMP Inhibitors. Anticancer. Agents Med. Chem. 2017, 17, 674–681. [Google Scholar] [CrossRef]
- Turra, K.M.; Rivelli, D.P.; de Moraes Barros, S.B.; Pasqualoto, K.F.M. Predicting Novel Antitumor Agents: 3D-Pharmacophore Mapping of β-N-biaryl Ether Sulfonamide-Based Hydroxamates as Potentially MMP-2 Inhibitors. Mol. Inform. 2014, 9, 573–587. [Google Scholar] [CrossRef]
- Wang, P.F.; Qiu, H.Y.; Baloch, S.K.; Gong, H.B.; Wang, Z.C.; Zhu, H.L. Synthesis, Biological Evaluation, and Docking of Dihydropyrazole Sulfonamide Containing 2-hydroxyphenyl Moiety: A Series of Novel MMP-2 Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Shen, F.-Q.; Yang, M.-R.; You, L.-X.; Chen, L.-Z.; Zhu, H.-L.; Lu, Y.-D.; Kong, F.-L.; Wang, M.-H. Dihydropyrazothiazole derivatives as potential MMP-2/MMP-8 inhibitors for cancer therapy. Bioorg. Med. Chem. Lett. 2018, 28, 3816–3821. [Google Scholar] [CrossRef] [PubMed]
- Aouad, M.R.; Almehmadi, M.A.; Albelwi, F.F.; Teleb, M.; Tageldin, G.N.; Abu-Serie, M.M.; Hagar, M.; Rezki, N. Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation. Bioorg. Chem. 2022, 124, 105816. [Google Scholar] [CrossRef]
- Kreituss, I.; Rozenberga, E.; Zemītis, J.; Trapencieris, P.; Romanchikova, N.; Turks, M. Discovery of aziridine-triazole conjugates as selective MMP-2 inhibitors. Chem. Heterocycl. Compd. 2013, 49, 1108–1117. [Google Scholar] [CrossRef]
- Laghezza, A.; Luisi, G.; Caradonna, A.; Di Pizio, A.; Piemontese, L.; Loiodice, F.; Agamennone, M.; Tortorella, P. Vir1tual screening identification and chemical optimization of substituted 2-arylbenzimidazoles as new non-zinc-binding MMP-2 inhibitors. Bioorg. Med. Chem. 2020, 28, 115257. [Google Scholar] [CrossRef]
- Bertran, A.; Khomiak, D.; Konopka, A.; Rejmak, E.; Bulska, E.; Seco, J.; Kaczmarek, L.; Tarragó, T.; Prades, R. Design and synthesis of selective and blood-brain barrier-permeable hydroxamate-based gelatinase inhibitors. Bioorg. Chem. 2020, 94, 103365. [Google Scholar] [CrossRef]
- Fischer, T.; Senn, N.; Riedl, R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry 2019, 25, 7960–7980. [Google Scholar] [CrossRef]
- Matt, C.; Hess, T.; Benlian, A. Digital transformation strategies. Bus. Inf. Syst. Eng. 2015, 57, 339–343. [Google Scholar] [CrossRef]
- Lim, H.; Hong, H.; Hwang, S.; Kim, S.J.; Seo, S.Y.; No, K.T. Identification of Novel Natural Product Inhibitors against Matrix Metalloproteinase 9 Using Quantum Mechanical Fragment Molecular Orbital-Based Virtual Screening Methods. Int. J. Mol. Sci. 2022, 23, 4438. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Hou, J.; Yao, Q.; Zhang, J. Multiple Receptor-Ligand Based Pharmacophore Modeling and Molecular Docking to Screen the Selective Inhibitors of Matrix Metalloproteinase-9 from Natural Products. J. Comput. Aided Mol. Des. 2017, 31, 625–641. [Google Scholar] [CrossRef]
- Johnson, J.L.; Fritsche-Danielson, R.; Behrendt, M.; Westin-Eriksson, A.; Wennbo, H.; Herslof, M.; Elebring, M.; George, S.J.; McPheat, W.L.; Jackson, C.L. Effect of broad-spectrum matrix metalloproteinase inhibition on atherosclerotic plaque stability. Cardiovasc. Res. 2006, 71, 586–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell, R.M.; Kunuthur, S.P.; Hendry, C.; Bruce-Hickman, D.; Davidson, S.; Yellon, D.M. Matrix metalloproteinase inhibition protects CyPD knockout mice independently of RISK/mPTP signalling: A parallel pathway to protection. Basic. Res. Cardiol. 2013, 108, 331. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.P.; de Castro Bras, L.E.; Patterson, N.L.; Bhowmick, M.; Flynn, E.R.; Asher, M.; Cannon, P.L.; Deleon-Pennell, K.Y.; Fields, G.B.; Lindsey, M.L. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J. Mol. Cell Cardiol. 2016, 100, 109–117. [Google Scholar] [CrossRef]
- Iyer, R.P.; Patterson, N.L.; Zouein, F.A.; Ma, Y.; Dive, V.; de Castro Bras, L.E.; Lindsey, M.L. Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution. Int. J. Cardiol. 2015, 185, 198–208. [Google Scholar] [CrossRef]
- Remacle, A.G.; Golubkov, V.S.; Shiryaev, S.A.; Dahl, R.; Stebbins, J.L.; Chernov, A.V.; Cheltsov, A.V.; Pellecchia, M.; Strongin, A.Y. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012, 72, 2339–2349. [Google Scholar] [CrossRef]
- Nuti, E.; Cantelmo, A.R.; Gallo, C.; Bruno, A.; Bassani, B.; Camodeca, C.; Tuccinardi, T.; Vera, L.; Orlandini, E.; Nencetti, S.; et al. N-O-Isopropyl Sulfonamido-Based Hydroxamates as Matrix Metalloproteinase Inhibitors: Hit Selection and in Vivo Antiangiogenic Activity. J. Med. Chem. 2015, 58, 7224–7240. [Google Scholar] [CrossRef]
- Brown, P.D. Clinical Studies with Matrix Metalloproteinase Inhibitors. APMIS. 1999, 107, 174–180. [Google Scholar] [CrossRef]
- Wojtowicz-Praga, S.M.; Dickson, R.B.; Hawkins, M.J. Matrix Metalloproteinase Inhibitors. Investig. New Drugs 1997, 15, 61–75. [Google Scholar] [CrossRef]
- Overall, C.M.; López-Otín, C. Strategies for MMP Inhibition in Cancer: Innovations for the Post-Trial Era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Bissett, D.; O’Byrne, K.J.; von Pawel, J.; Gatzemeier, U.; Price, A.; Nicolson, M.; Mercier, R.; Mazabel, E.; Penning, C.; Zhang, M.H.; et al. Phase III Study of Matrix Metalloproteinase Inhibitor Prinomastat in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 842–849. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.G.; Qiu, J.L.; Pang, T.; Wang, H.; Zhang, X.X. Sevoflurane inhibits the progression of PTC by downregulating miR-155. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6579–6587. [Google Scholar] [PubMed]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; Kim, C.H. Non-thermal atmospheric pressure plasma inhibits thyroid papillary cancer cell invasion via cytoskeletal modulation, altered MMP-2/-9/uPA activity. PLoS ONE 2014, 9, e92198. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Xue, J. Circular RNA DOCK1 downregulates microRNA-124 to induce the growth of human thyroid cancer cell lines. Biofactors 2020, 46, 591–599. [Google Scholar] [CrossRef]
- Dahl, R.; Titlestad, I.; Lindqvist, A.; Wielders, P.; Wray, H.; Wang, M.; Samuelsson, V.; Mo, J.; Holt, A. Effects of an oral MMP-9 and -12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: A randomised controlled trial. Pulm. Pharmacol. Ther. 2012, 25, 169–177. [Google Scholar] [CrossRef]
- Churg, A.; Wang, R.; Wang, X.; Onnervik, P.O.; Thim, K.; Wright, J.L. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax 2007, 62, 706–713. [Google Scholar] [CrossRef]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Kalva, S.; Azhagiya Singam, E.R.; Rajapandian, V.; Saleena, L.M.; Subramanian, V. Discovery of Potent Inhibitor for Matrix Metalloproteinase-9 by Pharmacophore Based Modeling and Dynamics Simulation Studies. J. Mol. Graph. Model. 2014, 49, 25–37. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, Y.H.; Kim, J.M.; Lee, H.; Park, W.K.; Lim, M.B.; Chu, Y.K.; Lo, E.H.; Lee, S.R. Green tea polyphenol (-)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J. Neurosci. Res. 2009, 87, 567–575. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Kang, C.H.; Choi, Y.H.; Kim, G.Y. Mangiferin inhibits tumor necrosis factor-alpha-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-kappaB activity. BMB Rep. 2015, 48, 559–564. [Google Scholar] [CrossRef]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Pavlickova, T.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol-Linoleate protects from exacerbated endothelial permeability via a drastic inhibition of the MMP-9 activity. Biosci. Rep. 2018, 38, BSR20171712. [Google Scholar] [CrossRef]
- Moon, S.K.; Cho, G.O.; Jung, S.Y.; Gal, S.W.; Kwon, T.K.; Lee, Y.C.; Madamanchi, N.R.; Kim, C.H. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: Role of ERK1/2, cell cycle regulation, and matrix metalloproteinase-9. Biochem. Biophys. Res. Commun. 2003, 301, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Chung, T.W.; Kang, S.K.; Suh, S.J.; Kim, J.K.; Chung, K.H.; Gu, Y.H.; Suzuki, I.; Kim, C.H. Caffeic acid phenyl ester in propolis is a strong inhibitor of matrix metalloproteinase-9 and invasion inhibitor: Isolation and identification. Clin. Chim. Acta. 2005, 362, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Malekipour, M.H.; Shirani, F.; Moradi, S.; Taherkhani, A. Cinnamic acid derivatives as potential matrix metalloproteinase-9 inhibitors: Molecular docking and dynamics simulations. Genom. Inform. 2023, 21, e9. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.F.L.; Hecht, F.; Cazarin, J.; Fortunato, R.S.; Vaisman, M.; Carvalho, D.P.; Ferreira, A.C.F. The flavonoid quercetin reduces cell migration and increases NIS and E-cadherin mRNA in the human thyroid cancer cell line BCPAP. Mol. Cell. Endocrinol. 2021, 529, 111266. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qi, B.; Yuan, D.; Dong, S.; Guo, D.; Zhang, C.; Yu, M. Suppression of PMA-induced tumor cell invasion and migration by ginsenoside Rg1 via the inhibition of NF-kappaB-dependent MMP-9 expression. Oncol. Rep. 2014, 32, 1779–1786. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Q.; Zhao, W.; Gong, Y.; Su, A.; Liu, F.; Liu, Y.; Li, Z.; Zhu, J. Ginsenoside Rg3 Inhibition of Thyroid Cancer Metastasis Is Associated with Alternation of Actin Skeleton. J. Med. Food. 2018, 21, 849–857. [Google Scholar] [CrossRef]
- Shirian, J.; Arkadash, V.; Cohen, I.; Sapir, T.; Radisky, E.S.; Papo, N.; Shifman, J.M. Converting a broad matrix metalloproteinase family inhibitor into a specific inhibitor of MMP-9 and MMP-14. FEBS Lett. 2018, 592, 1122–1134. [Google Scholar] [CrossRef]
- do Prado, A.F.; Bannwart, C.M.; Shinkai, V.M.T.; de Souza Lima, I.M.; Meschiari, C.A. Phyto-derived Products as Matrix Metalloproteinases Inhibitors in Cardiovascular Diseases. Curr. Hypertens. Rev. 2021, 17, 47–58. [Google Scholar] [CrossRef]
- Nanjan, P.; Nambiar, J.; Nair, B.G.; Banerji, A. Synthesis and discovery of (I-3, II-3)-biacacetin as a novel non-zinc binding inhibitor of MMP-2 and MMP-9. Bioorg. Med. Chem. 2015, 23, 3781–3787. [Google Scholar] [CrossRef]
- Scannevin, R.H.; Alexander, R.; Haarlander, T.M.; Burke, S.L.; Singer, M.; Huo, C.; Zhang, Y.M.; Maguire, D.; Spurlino, J.; Deckman, I.; et al. Discovery of a highly selective chemical inhibitor of matrix metalloproteinase-9 (MMP-9) that allosterically inhibits zymogen activation. J. Biol. Chem. 2017, 292, 17963–17974. [Google Scholar] [CrossRef]
- Nicolai, E.; Sinibaldi, F.; Sannino, G.; Laganà, G.; Basoli, F.; Licoccia, S.; Cozza, P.; Santucci, R.; Piro, M.C. Omega-3 and Omega-6 Fatty Acids Act as Inhibitors of the Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Activity. Protein J. 2017, 36, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Berton, A.; Rigot, V.; Huet, E.; Decarme, M.; Eeckhout, Y.; Patthy, L.; Godeau, G.; Hornebeck, W.; Bellon, G.; Emonard, H. Involvement of fibronectin type II repeats in the efficient inhibition of gelatinases A and B by long-chain unsaturated fatty acids. J. Biol. Chem. 2001, 276, 20458–20465. [Google Scholar] [CrossRef]
- Graf, B.L.; Cheng, D.M.; Esposito, D.; Shertel, T.; Poulev, A.; Plundrich, N.; Itenberg, D.; Dayan, N.; Lila, M.A.; Raskin, I. Compounds leached from quinoa seeds inhibit matrix metalloproteinase activity and intracellular reactive oxygen species. Int. J. Cosmet. Sci. 2015, 37, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Christina, V.S.; Sundaram, R.L.; Sivamurugan, V.; Kumar, D.T.; Mohanapriya, C.D.; Shailaja, V.L.; Thyagarajan, S.P.; Doss, C.G.P.; Gnanambal, K.M.E. Inhibition of MMP2-PEX by a novel ester of dihydroxy cinnamic and linoleic acid from the seagrass Cymodocea serrulata. Sci. Rep. 2021, 11, 11451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Tan, L.; Lin, S.; Long, M.; Peng, X. LncRNA-HCG18 regulates the viability, apoptosis, migration, invasion and epithelial-mesenchymal transition of papillary thyroid cancer cells via regulating the miR-106a-5p/PPP2R2A axis. Pathol. Res. Pract. 2021, 221, 153395. [Google Scholar] [CrossRef]
- Li, N.; Cui, M.; Yu, P.; Li, Q. Correlations of lncRNAs with cervical lymph node metastasis and prognosis of papillary thyroid carcinoma. OncoTargets Ther. 2019, 12, 1269–1278. [Google Scholar] [CrossRef]
- Du, H.T.; Du, L.L.; Tang, X.L.; Ge, H.Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1573–1579. [Google Scholar] [CrossRef]
- Shah, M.A.; Starodub, A.; Sharma, S.; Berlin, J.; Patel, M.; Wainberg, Z.A.; Chaves, J.; Gordon, M.; Windsor, K.; Brachmann, C.B.; et al. Andecaliximab/GS-5745 Alone and Combined with mFOLFOX6 in Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: Results from a Phase I Study. Clin. Cancer Res. 2018, 24, 3829–3837. [Google Scholar] [CrossRef]
- Tape, C.J.; Willems, S.H.; Dombernowsky, S.L.; Stanley, P.L.; Fogarasi, M.; Ouwehand, W.; McCafferty, J.; Murphy, G. Cross-domain inhibition of TACE ectodomain. Proc. Natl. Acad. Sci. USA 2011, 108, 5578–5583. [Google Scholar] [CrossRef] [PubMed]
- Mogharrabi, M.; Rahimi, H.R.; Hasanzadeh, S.; Dastani, M.; Kazemi-Oskuee, R.; Akhlaghi, S.; Soukhtanloo, M. The effects of nanomicelle of curcumin on the matrix metalloproteinase (MMP-2, 9) activity and expression in patients with coronary artery disease (CAD): A randomized controlled clinical trial. ARYA Atheroscler. 2020, 16, 136–145. [Google Scholar]
- Sandborn, W.J.; Bhandari, B.R.; Fogel, R.; Onken, J.; Yen, E.; Zhao, X.; Jiang, Z.; Ge, D.; Xin, Y.; Ye, Z.; et al. Randomised clinical trial: A phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis. Aliment. Pharmacol. Ther. 2016, 44, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Rudek, M.A.; Figg, W.D.; Dyer, V.; Dahut, W.; Turner, M.L.; Steinberg, S.M.; Liewehr, D.J.; Kohler, D.R.; Pluda, J.M.; Reed, E. Phase I clinical trial of oral COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J. Clin. Oncol. 2001, 19, 584–592. [Google Scholar] [CrossRef]
- Moon, S.K.; Cha, B.Y.; Lee, Y.C.; Kim, C.H. In vitro cellular aging is associated with enhanced proliferative capacity, G1 cell cycle modulation, and matrix metalloproteinase-9 regulation in mouse aortic smooth muscle cells. Arch Bichem. Biophys. 2003, 418, 39–48. [Google Scholar] [CrossRef]
- Chung, T.W.; Choi, H.; Lee, J.M.; Ha, S.H.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Chang, Y.C.; Ha, K.T.; Cho, S.H.; et al. Oldenlandia diffusa suppresses metastatic potential through inhibiting matrix metalloproteinase-9 and intercellular adhesion molecule-1 expression via p38 and ERK1/2 MAPK pathways and induces apoptosis in human breast cancer MCF-7 cells. J. Ethnopharmacol. 2017, 195, 309–317. [Google Scholar] [CrossRef]
- Cha, B.Y.; Park, C.J.; Lee, D.G.; Lee, Y.C.; Kim, D.W.; Kim, J.D.; Seo, W.G.; Moon, S.K.; Kim, C.H. Inhibitory Effect of Methanol Extract from Euonymus Alatus on Matrix Metalloproteinase-9. J. Ethnopharm. 2003, 85, 163–167. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwak, C.H.; Song, K.H.; Kwon, K.M.; Chung, T.W.; Cho, S.H.; Kim, Y.K.; Yoon, H.D.; Lee, Y.C.; Kim, D.S.; et al. Triple inhibitory activity of Cliona celata against TNF-α-induced matrix metalloproteinase-9 production via downregulated NF-κB and AP-1, enzyme activity, and migration potential. Inflammation 2012, 35, 736–745. [Google Scholar] [CrossRef]
- Park, J.; Ha, S.H.; Abekura, F.; Lim, H.; Magae, J.; Ha, K.T.; Chung, T.W.; Chang, Y.C.; Lee, Y.C.; Chung, E.; et al. 4-O-Carboxymethylascochlorin Inhibits Expression Levels of on Inflammation-Related Cytokines and Matrix Metalloproteinase-9 Through NF-κB/MAPK/TLR4 Signaling Pathway in LPS-Activated RAW264.7 Cells. Front. Pharmacol. 2019, 10, 304. [Google Scholar] [CrossRef]
- Ha, S.H.; Kwon, K.M.; Park, J.Y.; Abekura, F.; Lee, Y.C.; Chung, T.W.; Ha, K.T.; Chang, H.W.; Cho, S.H.; Kim, J.S.; et al. Esculentoside H inhibits colon cancer cell migration and growth through suppression of MMP-9 gene expression via NF-kB signaling pathway. J. Cell Beachem. 2019, 120, 9810–9819. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwak, C.H.; Chung, T.W.; Park, S.J.; Cheeeei, M.; Park, S.S.; Seo, C.S.; Son, J.K.; Chang, Y.C.; Park, Y.G.; et al. Pimaric acid from Aralia cordata has an inhibitory effect on TNF-α-induced MMP-9 production and HASMC migration via down-regulated NF-κB and AP-1. Chem. Biol. Interact. 2012, 199, 112–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Choi, Y.; Kim, H.-D.; Kuo, H.-H.; Chang, Y.-C.; Kim, C.-H. Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium. Int. J. Mol. Sci. 2025, 26, 5553. https://doi.org/10.3390/ijms26125553
Park J-Y, Choi Y, Kim H-D, Kuo H-H, Chang Y-C, Kim C-H. Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium. International Journal of Molecular Sciences. 2025; 26(12):5553. https://doi.org/10.3390/ijms26125553
Chicago/Turabian StylePark, Jun-Young, Yeonwoo Choi, Hee-Do Kim, Han-Hsi Kuo, Yu-Chan Chang, and Cheorl-Ho Kim. 2025. "Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium" International Journal of Molecular Sciences 26, no. 12: 5553. https://doi.org/10.3390/ijms26125553
APA StylePark, J.-Y., Choi, Y., Kim, H.-D., Kuo, H.-H., Chang, Y.-C., & Kim, C.-H. (2025). Matrix Metalloproteinases and Their Inhibitors in the Pathogenesis of Epithelial Differentiation, Vascular Disease, Endometriosis, and Ocular Fibrotic Pterygium. International Journal of Molecular Sciences, 26(12), 5553. https://doi.org/10.3390/ijms26125553






